blaNDM and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China
Abstract
1. Introduction
2. Results
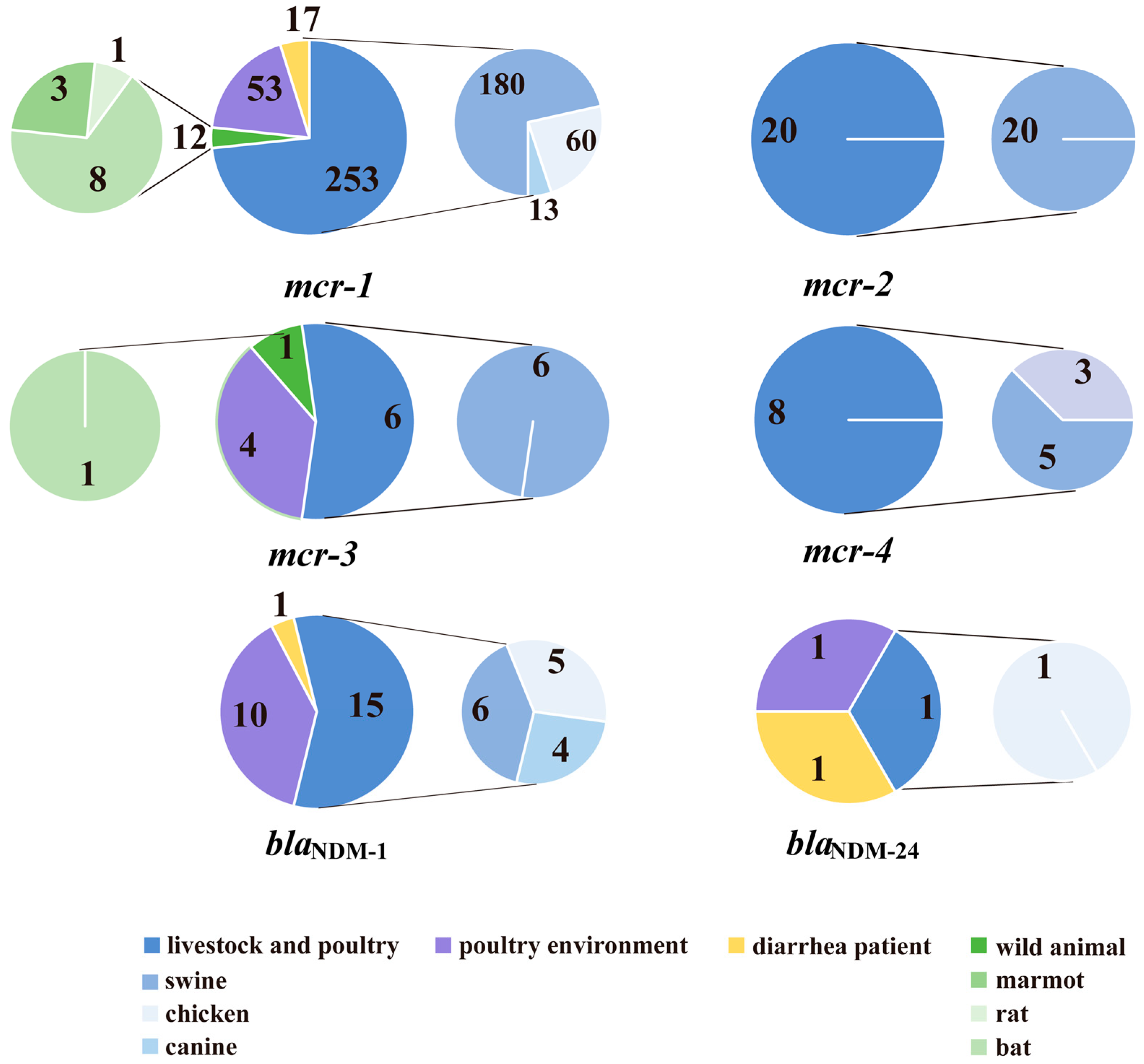
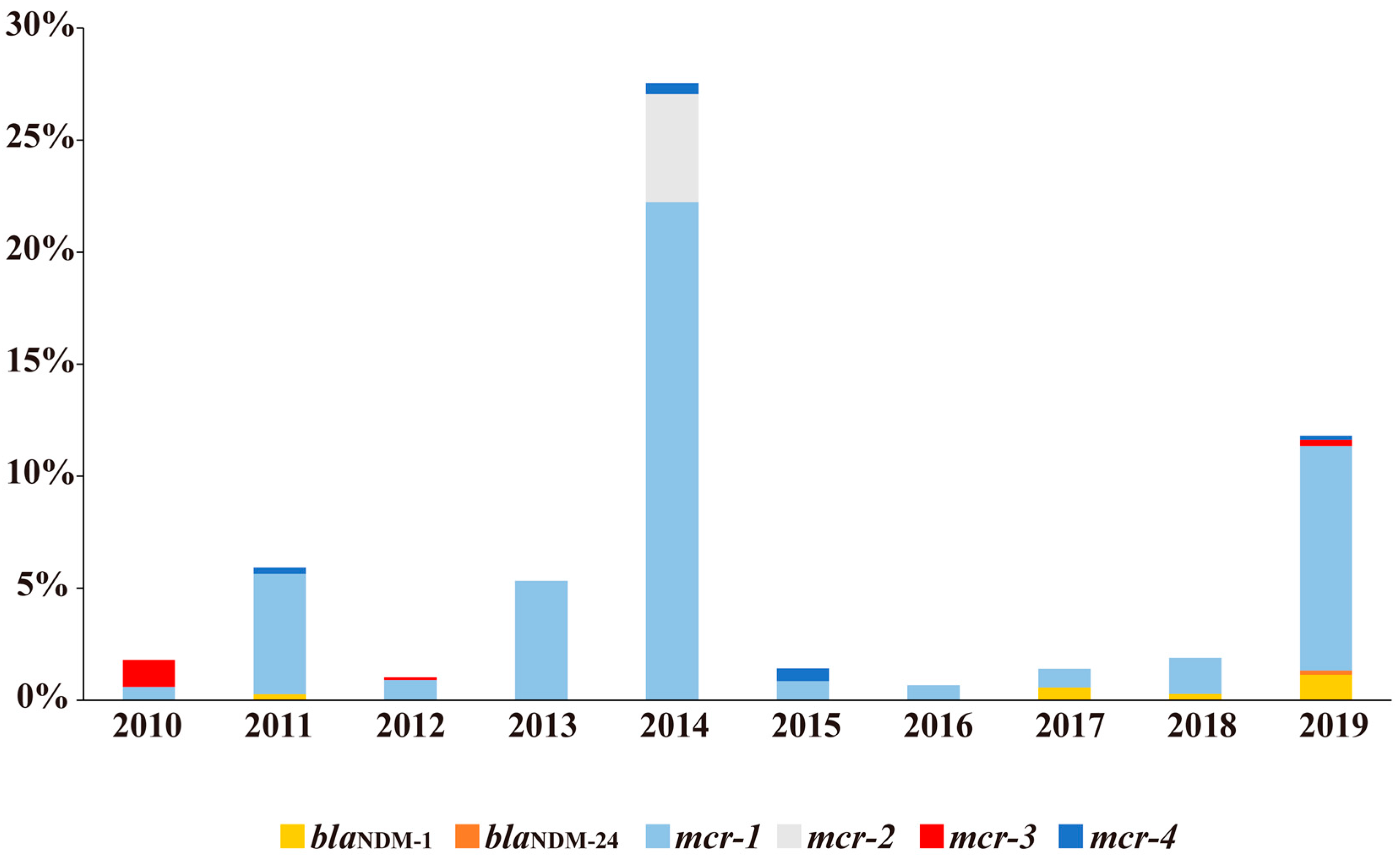
2.1. Distributions of blaNDM and mcr
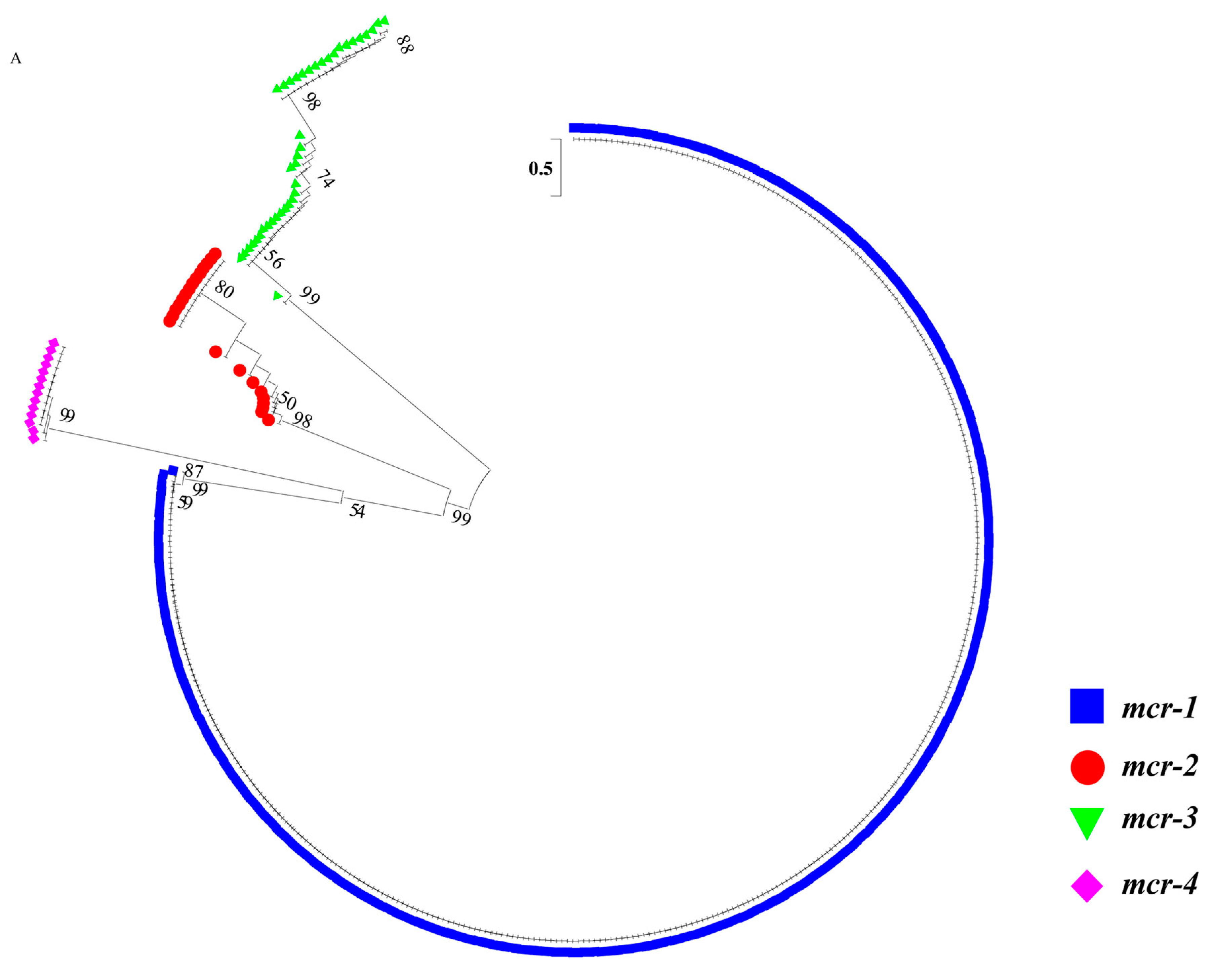
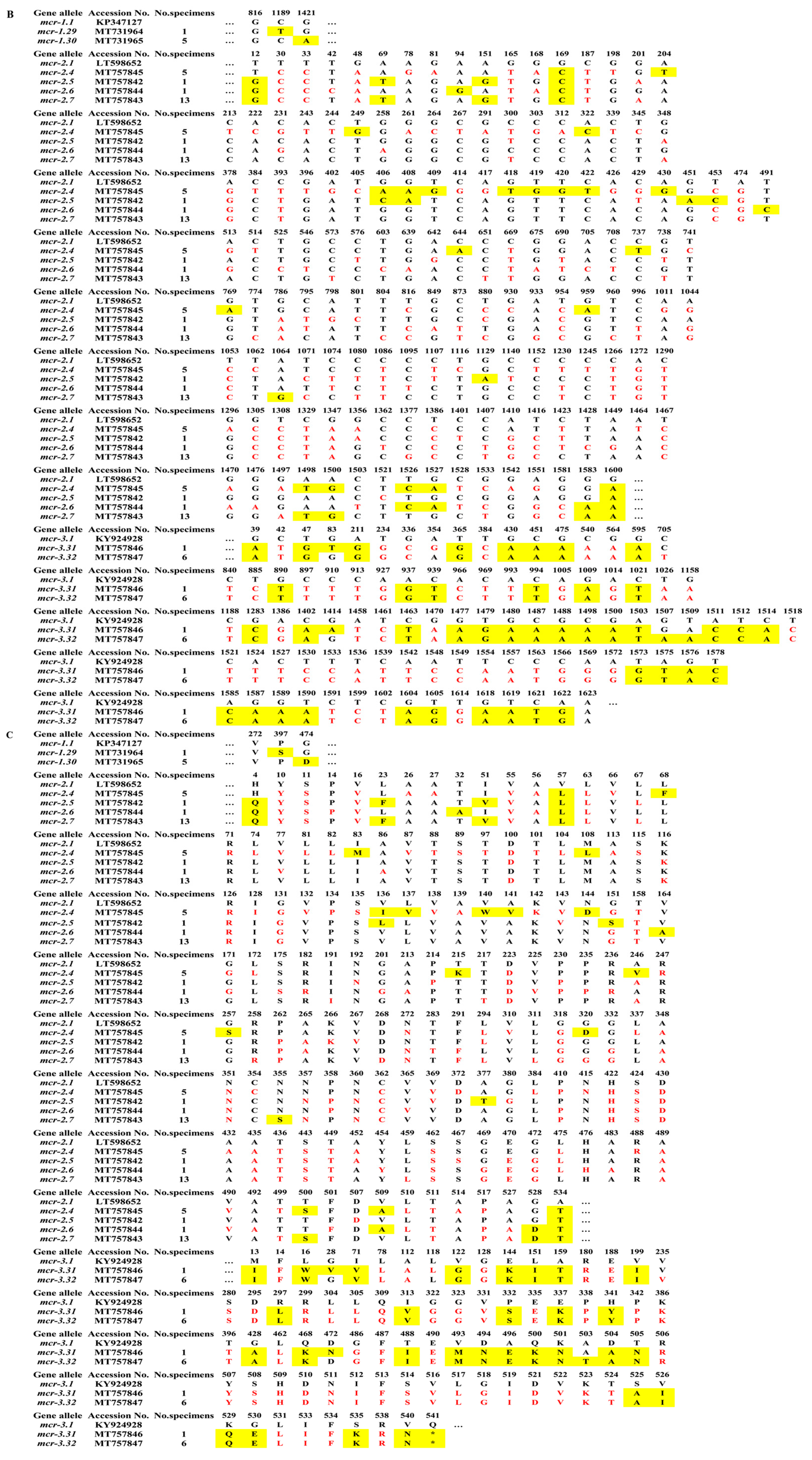
2.2. Sequence Analysis of blaNDM and mcr
2.3. Distribution of Coexisting Genes/Genotypes and New Subtypes
3. Discussion
4. Materials and Methods
4.1. Gut Specimen Sources
4.2. blaNDM and mcr-1 to mcr-5 Screening of Gut Specimens and Sequence Analysis
4.3. Statistical Analysis
4.4. Nucleotide Sequence Accession Numbers
4.5. Ethics Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; Bagaria, J.; Butt, F.; Balakrishnan, R.; Chaudhary, U.; Doumith, M.; Giske, C.G.; Irfan, S.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010, 10, 597–602. [Google Scholar] [CrossRef]
- Kaase, M.; Nordmann, P.; Wichelhaus, T.A.; Gatermann, S.G.; Bonnin, R.A.; Poirel, L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 2011, 66, 1260–1262. [Google Scholar] [CrossRef]
- Cuzon, G.; Bonnin, R.A.; Nordmann, P. First identification of novel NDM carbapenemase, NDM-7, in Escherichia coli in France. PLoS ONE 2013, 8, e61322. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Miyoshi-Akiyama, T.; Dahal, R.K.; Sah, M.K.; Ohara, H.; Kirikae, T.; Pokhrel, B.M. NDM-8 metallo-β-lactamase in a multidrug-resistant Escherichia coli strain isolated in Nepal. Antimicrob. Agents Chemother. 2013, 57, 2394–2396. [Google Scholar] [CrossRef]
- Zou, D.; Huang, Y.; Zhao, X.; Liu, W.; Dong, D.; Li, H.; Wang, X.; Huang, S.; Wei, X.; Yan, X.; et al. A novel New Delhi metallo-beta-lactamase variant, NDM-14, isolated in a Chinese Hospital possesses increased enzymatic activity against carbapenems. Antimicrob. Agents Chemother. 2015, 59, 2450–2453. [Google Scholar] [CrossRef]
- Shrestha, B.; Tada, T.; Miyoshi-Akiyama, T.; Shimada, K.; Ohara, H.; Kirikae, T.; Pokhrel, B.M. Identification of a novel NDM variant, NDM-13, from a multidrug-resistant Escherichia coli clinical isolate in Nepal. Antimicrob. Agents Chemother. 2015, 59, 5847–5850. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, Y.; McNally, A.; Zong, Z. blaNDM-21, a new variant of blaNDM in an Escherichia coli clinical isolate carrying blaCTX-M-55 and rmtB. J. Antimicrob. Chemother. 2018, 73, 2336–2339. [Google Scholar] [CrossRef]
- Kaye, K.S.; Pogue, J.M. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacotherapy 2015, 35, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Walsh, T.R.; Liu, D.; Shen, Z.; Zhang, R.; Yin, W.; Yao, H.; Li, J.; Shen, J. Plasmid-Mediated Novel bla(NDM-17) Gene Encoding a Carbapenemase with Enhanced Activity in a Sequence Type 48 Escherichia coli Strain. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Wang, X.; Liu, D.; Ke, Y.; Wang, Y.; Shen, J. Novel Variant of New Delhi Metallo-β-lactamase, NDM-20, in Escherichia coli. Front. Microbiol. 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2016, 21. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio 2017, 8. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Li, Y.X.; Lei, C.W.; Zhang, A.Y.; Wang, H.N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Zhou, Q.; He, W.; Lin, Q.; Yang, J.; Liu, J.H. mcr-1 and plasmid prevalence in Escherichia coli from livestock. Lancet Infect. Dis. 2020, 20, 1126. [Google Scholar] [CrossRef]
- Fan, R.; Li, C.; Duan, R.; Qin, S.; Liang, J.; Xiao, M.; Lv, D.; Jing, H.; Wang, X. Retrospective Screening and Analysis of mcr-1 and bla (NDM) in Gram-Negative Bacteria in China, 2010–2019. Front. Microbiol. 2020, 11, 121. [Google Scholar] [CrossRef]
- Cui, L.; Lei, L.; Lv, Y.; Zhang, R.; Liu, X.; Li, M.; Zhang, F.; Wang, Y. blaNDM-1-producing multidrug-resistant Escherichia coli isolated from a companion dog in China. J. Glob. Antimicrob. Resist. 2018, 13, 24–27. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Li, Y.X.; Song, T.; Yang, Y.X.; Jiang, W.; Zhang, A.Y.; Guo, X.Y.; Liu, B.H.; Wang, Y.X.; Lei, C.W.; et al. Colistin Resistance Gene mcr-1 and Its Variant in Escherichia coli Isolates from Chickens in China. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Perry, J.D.; Naqvi, S.H.; Mirza, I.A.; Alizai, S.A.; Hussain, A.; Ghirardi, S.; Orenga, S.; Wilkinson, K.; Woodford, N.; Zhang, J.; et al. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J. Antimicrob. Chemother. 2011, 66, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Bachiri, T.; Lalaoui, R.; Bakour, S.; Allouache, M.; Belkebla, N.; Rolain, J.M.; Touati, A. First Report of the Plasmid-Mediated Colistin Resistance Gene mcr-1 in Escherichia coli ST405 Isolated from Wildlife in Bejaia, Algeria. Microb. Drug Resist. (Larchmt. N. Y.) 2018, 24, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Swift, B.M.C.; Bennett, M.; Waller, K.; Dodd, C.; Murray, A.; Gomes, R.L.; Humphreys, B.; Hobman, J.L.; Jones, M.A.; Whitlock, S.E.; et al. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci. Total Environ. 2019, 649, 12–20. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Elshafiee, E.A.; Khalefa, H.S.; Kadry, M.; Hamza, D.A. Evidence of colistin resistance genes (mcr-1 and mcr-2) in wild birds and its public health implication in Egypt. Antimicrob. Resist. Infect. Control 2019, 8, 197. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet (Lond. Engl.) 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Mather, A.E.; Matthews, L.; Mellor, D.J.; Reeve, R.; Denwood, M.J.; Boerlin, P.; Reid-Smith, R.J.; Brown, D.J.; Coia, J.E.; Browning, L.M.; et al. An ecological approach to assessing the epidemiology of antimicrobial resistance in animal and human populations. Proc. Biol. Sci. 2012, 279, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, Y.; Shen, Y.; Shen, J.; Wu, C. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect. Dis. 2016, 16, 293. [Google Scholar] [CrossRef]
- Fajardo, A.; Martínez, J.L. Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 2008, 11, 161–167. [Google Scholar] [CrossRef]
- Bunce, J.T.; Hellyer, P. Antibiotic resistance and antibiotic prescribing by dentists in England 2007-2016. Br. Dent. J. 2018, 225, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Li, Y.; Paudyal, N.; Pan, H.; Li, X.; Xie, S.; Rajkovic, A.; Feng, Y.; Fang, W.; Rankin, S.C.; et al. Global Burden of Colistin-Resistant Bacteria: Mobilized Colistin Resistance Genes Study (1980–2018). Microorganisms 2019, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Ward, M.J.; Muloi, D.; Bettridge, J.M.; Robinson, T.P.; Kariuki, S.; Ogendo, A.; Kiiru, J.; Imboma, T.; Kang’ethe, E.K.; et al. Clinically relevant antimicrobial resistance at the wildlife-livestock-human interface in Nairobi: An epidemiological study. Lancet Planet. Health 2019, 3, e259–e269. [Google Scholar] [CrossRef]
- Paudyal, N.; Pan, H.; Wu, B.; Zhou, X.; Zhou, X.; Chai, W.; Wu, Q.; Li, S.; Li, F.; Gu, G.; et al. Persistent Asymptomatic Human Infections by Salmonella enterica Serovar Newport in China. mSphere 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Cao, J.; Bi, Y.; Lv, N.; Liu, F.; Liang, S.; Shi, Y.; Jiao, X.; Gao, G.F.; et al. Antibiotic resistance gene reservoir in live poultry markets. J. Infect. 2019, 78, 445–453. [Google Scholar] [CrossRef]
- Ministry of Agriculture, People’s Republic of China. No. 2428 Announcement. 2016. Available online: http://www.moa.gov.cn/nybgb/2016/dibaqi/201712/t20171219_6102822.htm (accessed on 9 October 2020).
- Walsh, T.R.; Toleman, M.A. The emergence of pan-resistant Gram-negative pathogens merits a rapid global political response. J. Antimicrob. Chemother. 2012, 67, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, J.R.; Patrawalla, A.; Chen, L.; Chavda, K.D.; Mathema, B.; Vinnard, C.; Dever, L.L.; Kreiswirth, B.N. Colistin- and Carbapenem-Resistant Escherichia coli Harboring mcr-1 and blaNDM-5, Causing a Complicated Urinary Tract Infection in a Patient from the United States. mBio 2016, 7. [Google Scholar] [CrossRef]
- Delgado-Blas, J.F.; Ovejero, C.M.; Abadia-Patiño, L.; Gonzalez-Zorn, B. Coexistence of mcr-1 and blaNDM-1 in Escherichia coli from Venezuela. Antimicrob. Agents Chemother. 2016, 60, 6356–6358. [Google Scholar] [CrossRef]
- Uchida, H.; Tada, T.; Sugahara, Y.; Kato, A.; Miyairi, I.; Kirikae, T. A clinical isolate of Escherichia coli co-harbouring mcr-1 and blaNDM-5 in Japan. J. Med Microbiol. 2018, 67, 1047–1049. [Google Scholar] [CrossRef]
- Jousset, A.B.; Bernabeu, S.; Bonnin, R.A.; Creton, E.; Cotellon, G.; Sauvadet, A.; Naas, T.; Dortet, L. Development and validation of a multiplex polymerase chain reaction assay for detection of the five families of plasmid-encoded colistin resistance. Int. J. Antimicrob. Agents 2019, 53, 302–309. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Liu, F.; Cao, J.; Lv, N.; Zhu, B.; Zhang, G.; Gao, G.F. Integrated metagenomic and metatranscriptomic profiling reveals differentially expressed resistomes in human, chicken, and pig gut microbiomes. Environ. Int. 2020, 138, 105649. [Google Scholar] [CrossRef]
| Source | No. Specimens | mcr (%) * | blaNDM (%) * | mcr and blaNDM (%) |
|---|---|---|---|---|
| Livestock and poultry | 1823 | 14.81 a | 0.88 a | 0.38 |
| Poultry environments | 350 | 16.00 a | 3.14 b | 0.86 |
| Diarrhea patients | 1186 | 1.43 b | 0.17 c | - |
| Wild animals | 3632 | 0.36 c | - | - |
| Total | 6991 | 5.09 | 0.41 | 0.14 |
| Gene | Genotype | Livestock and Poultry | Poultry Environments | Diarrhea Patient | Wild Animals | Total |
|---|---|---|---|---|---|---|
| blaNDM or mcr | blaNDM-1 | 9 | 8 | 1 | 18 | |
| blaNDM-24 | 1 | 1 | ||||
| mcr-1.1 | 228 | 48 | 17 | 12 | 305 | |
| mcr-1.29 * | 1 | 1 | ||||
| mcr-1.30 * | 1 | 1 | ||||
| mcr-2.4 * | 3 | 3 | ||||
| mcr-2.6 * | 1 | 1 | ||||
| mcr-3.18 | 2 | 2 | ||||
| mcr-3.3 | 1 | 1 | ||||
| mcr-3.31 * | 1 | 1 | ||||
| mcr-3.32 * | 6 | 6 | ||||
| mcr-4.3 | 7 | 7 | ||||
| mcr-1.1, mcr-2.4 * | 2 | 2 | ||||
| mcr-1.1, mcr-2.5 * | 1 | 1 | ||||
| mcr-1.1, mcr-2.7 * | 9 | 9 | ||||
| mcr-1.1, mcr-3.3 | 1 | 1 | ||||
| mcr-1.1, mcr-4.3 | 1 | 1 | ||||
| mcr-1.30 *, mcr-2.7 * | 4 | 4 | ||||
| blaNDM and mcr | blaNDM-1, mcr-1.1 | 6 | 2 | 8 | ||
| blaNDM-24, mcr-1.1 | 1 | 1 | 2 | |||
| Total | 279 | 64 | 19 | 13 | 375 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, D.; Duan, R.; Fan, R.; Mu, H.; Liang, J.; Xiao, M.; He, Z.; Qin, S.; Yang, J.; Jing, H.; et al. blaNDM and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China. Antibiotics 2021, 10, 233. https://doi.org/10.3390/antibiotics10030233
Lv D, Duan R, Fan R, Mu H, Liang J, Xiao M, He Z, Qin S, Yang J, Jing H, et al. blaNDM and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China. Antibiotics. 2021; 10(3):233. https://doi.org/10.3390/antibiotics10030233
Chicago/Turabian StyleLv, Dongyue, Ran Duan, Rong Fan, Hui Mu, Junrong Liang, Meng Xiao, Zhaokai He, Shuai Qin, Jinchuan Yang, Huaiqi Jing, and et al. 2021. "blaNDM and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China" Antibiotics 10, no. 3: 233. https://doi.org/10.3390/antibiotics10030233
APA StyleLv, D., Duan, R., Fan, R., Mu, H., Liang, J., Xiao, M., He, Z., Qin, S., Yang, J., Jing, H., Wang, Z., & Wang, X. (2021). blaNDM and mcr-1 to mcr-5 Gene Distribution Characteristics in Gut Specimens from Different Regions of China. Antibiotics, 10(3), 233. https://doi.org/10.3390/antibiotics10030233





