Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant
Abstract
1. Introduction
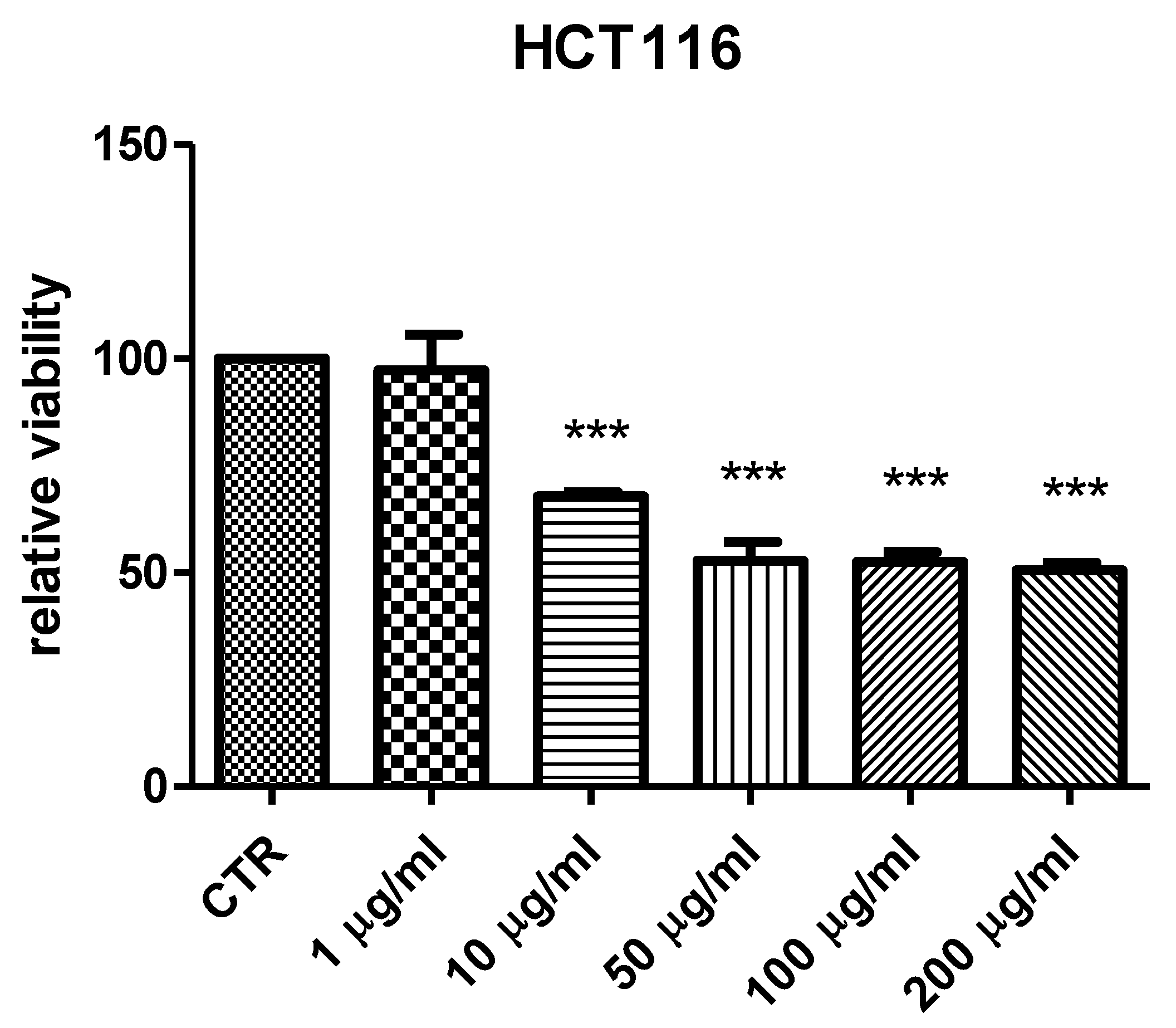
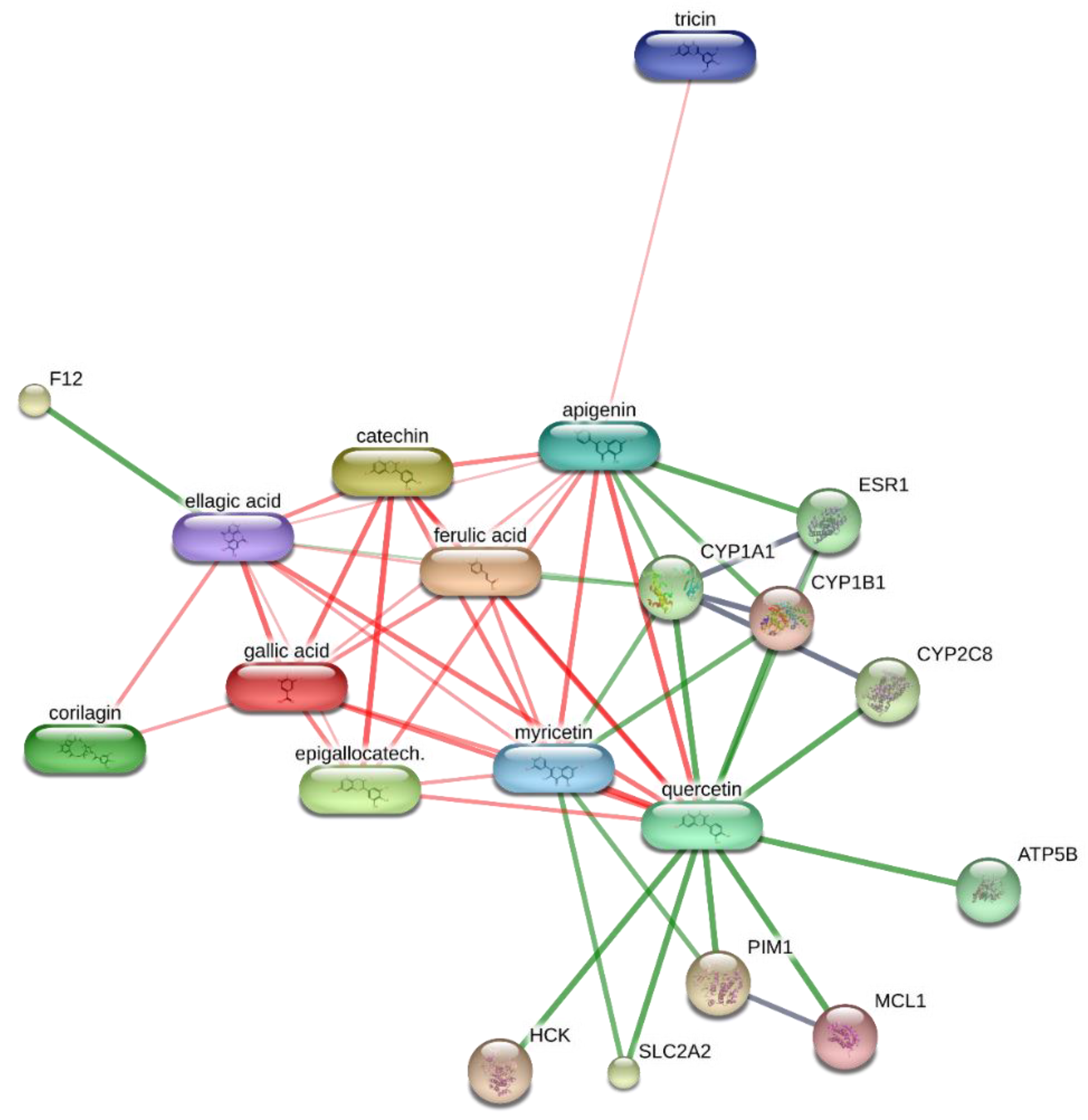
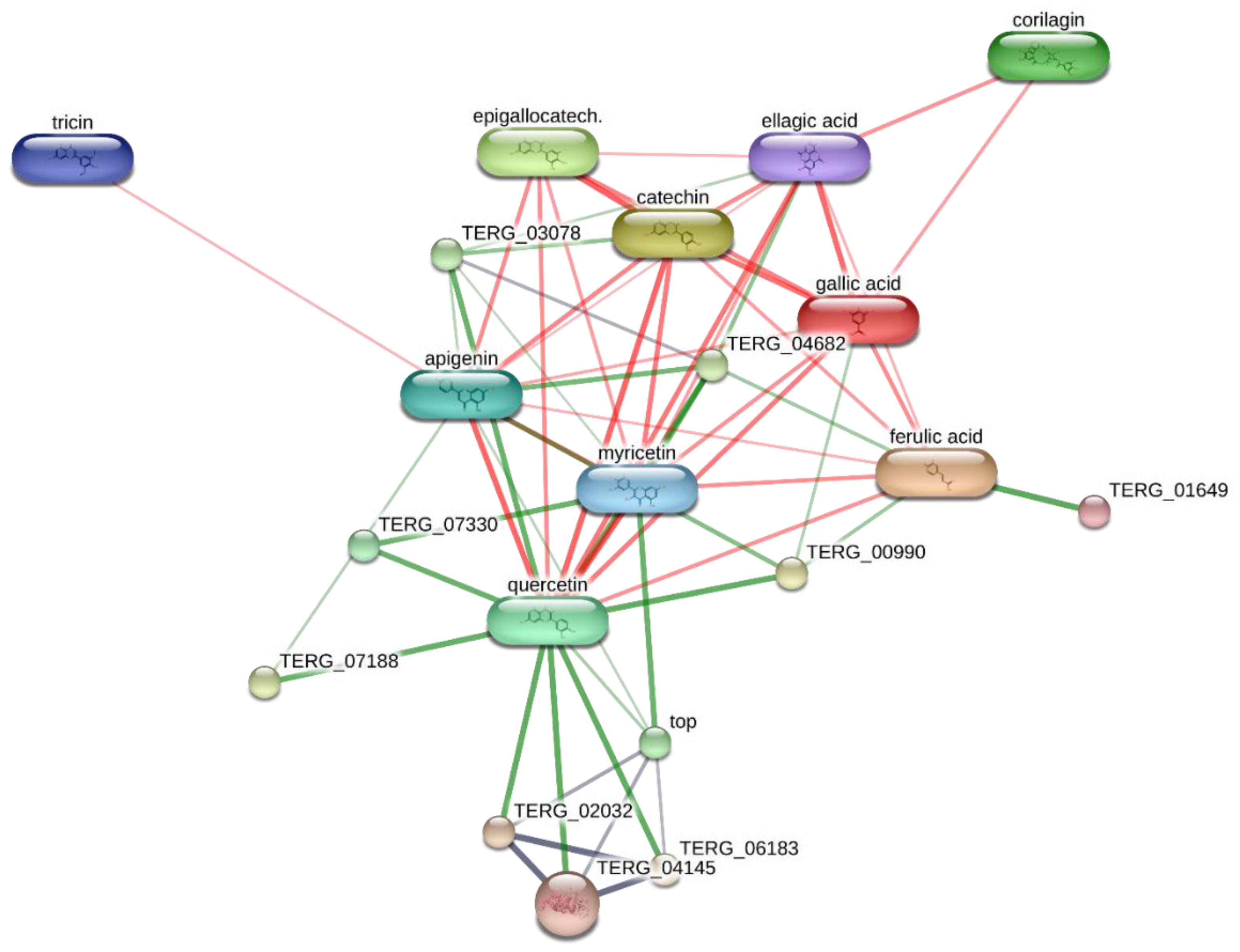
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material Used and Extracts’ Preparation
3.2. Spectrophotometric Assays for Total Phenolics and Flavonoids
3.3. Chromatographic Separation
3.4. Antioxidant and Enzyme Inhibition Assays
3.5. Artemia salina Lethality Test
3.6. Cell Cultures and Viability Test
3.7. Antibacterial and Antifungal Activities
3.8. Bioinformatics
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njamen, D.; Nkeh-Chungag, B.N.; Tsala, E.; Fomum, Z.T.; Mbanya, J.C.; Ngufor, G.F. Effect of Bridelia ferruginea (Euphorbiaceae) leaf extract on sucrose-induced glucose intolerance in rats. Trop. J. Pharm. Res. 2012, 11, 759–765. [Google Scholar] [CrossRef]
- Afolayan, M.; Srivedavyasasri, R.; Asekun, O.T.; Familoni, O.B.; Ross, S.A. Chemical and biological studies on Bridelia ferruginea grown in Nigeria. Nat. Prod. Res. 2019, 33, 287–291. [Google Scholar] [CrossRef]
- Ngueyem, T.; Brusotti, G.; Caccialanza, G.; Finzi, P.V. The genus Bridelia: A phytochemical and ethnopharmacological review. J. Ethnopharmacol. 2009, 124, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Adetutu, A.; Morgan, W.A.; Corcoran, O. Antibacterial, antioxidant and fibroblast growth stimulation activity of crude extracts of Bridelia ferruginea leaf, a wound-healing plant of Nigeria. J. Ethnopharmacol. 2011, 133, 116–119. [Google Scholar] [CrossRef]
- Olajide, O.A.; Aderogba, M.A.; Okorji, U.P.; Fiebich, B.L. Bridelia ferruginea produces antineuroinflammatory activity through inhibition of nuclear factor-kappa B and p38 MAPK signalling. Evid. Based Complementary Altern. Med. 2012, 2012, 546873. [Google Scholar] [CrossRef]
- Akuodor, G.; Mbah, C.; Essien, A.; Akpan, J.; Ezeokpo, B.; Iwuanyanwu, T.; Osunkwo, U. Ulcer-protective and antidiarrhoeal effects of the aqueous stem bark extract of Bridelia ferruginea in rodents. Pharmacologia 2012, 3, 591–597. [Google Scholar] [CrossRef][Green Version]
- Jose, R.; Kayode, J. The Effect of Bridelia ferruginea Bark extracts on some pathogenic micro-organisms. Ethnobot. Leafl. 2009, 2009, 8. [Google Scholar]
- Mbah, C.C.; Akuodor, G.C.; Anyalewechi, N.A.; Iwuanyanwu, T.C.; Osunkwo, U.A. In vivo antiplasmodial activities of aqueous extract of Bridelia ferruginea stem bark against Plasmodium berghei berghei in mice. Pharm. Biol. 2012, 50, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Owoseni, A.A.; Ayanbamiji, T.; Ajayi, Y.O.; Ewegbenro, I.B. Antimicrobial and phytochemical analysis of leaves and bark extracts from Bridelia ferruginea. Afr. J. Biotechnol. 2010, 9, 1031–1036. [Google Scholar] [CrossRef]
- Cimanga, K.; Ying, L.; De Bruyne, T.; Apers, S.; Cos, P.; Hermans, N.; Bakana, P.; Tona, L.; Kambu, K.; Kalenda, D. Radical scavenging and xanthine oxidase inhibitory activity of phenolic compounds from Bridelia ferruginea stem bark. J. Pharm. Pharmacol. 2001, 53, 757–761. [Google Scholar] [CrossRef]
- Pettit, G.R.; Searcy, J.D.; Tan, R.; Cragg, G.M.; Melody, N.; Knight, J.C.; Chapuis, J.-C. Antineoplastic Agents. 585. Isolation of Bridelia ferruginea Anticancer Podophyllotoxins and Synthesis of 4-Aza-podophyllotoxin Structural Modifications1. J. Nat. Prod. 2016, 79, 507–518. [Google Scholar] [CrossRef]
- Bakoma, B.; Berké, B.; Eklu-Gadegbeku, K.; Agbonon, A.; Aklikokou, K.; Gbeassor, M.; Creppy, E.E.; Moore, N. Acute and sub-chronic (28 days) oral toxicity evaluation of hydroethanolic extract of Bridelia ferruginea Benth root bark in male rodent animals. Food Chem. Toxicol. 2013, 52, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Alfa, A.A.; Ayodeji, O.A.; Teru, G.A.D.; Tijani, K.B. Studies on the Phytochemical Compounds in the Ethanolic Leaf Extract (ELE), Ethanolic Bark Extract (EBE) and Ethanolic Root Extract (ERE) of Bridelia ferruginea Benth (Euphorbiaceae). Asian J. Biochem. Genet. Mol. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Oladejo, A.A.; Anjorin, F.F.; Okesola, M.A. Bridelia ferruginea Leaf Fractions Ameliorates Helminth Infections. Clin. Med. 2019, 1, 1003. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Sangeetha, K.S.; Umamaheswari, S.; Reddy, C.U.M.; Kalkura, S.N. Flavonoids: Therapeutic potential of natural pharmacological agents. Int. J. Pharm. Sci. Res. 2016, 7, 3924. [Google Scholar]
- Halliwell, B. Antioxidant Activity and Other Biological Effects of Flavonoids; Rice-Evans, C., Ed.; International Congress and Symposium Series—Royal Society of Medicine; Royal Society of Medicine Press Ltd.: London, UK, 2000; pp. 13–23. [Google Scholar]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem. Biophys. Res. Commun. 2000, 279, 692–699. [Google Scholar] [CrossRef]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Khan, N.; Al Daghri, N.M.; Al Ajlan, A.S.; Alokail, M.S. The use of natural and derived sources of flavonoids and antioxidants in Saudi Arabia. Integr. Food Nutr. Metab. 2014, 1, 100–106. [Google Scholar]
- Fidrianny, I.; Suhendy, H.; Insanu, M. Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia. Asian Pac. J. Trop. Biomed. 2018, 8, 25. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, L.D.O.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of total phenolic and flavonoid contents of Brazilian honeys with colour and antioxidant capacity. Int. J. Food Prop. 2014, 17, 65–76. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Olaide, O.; Omotade, O.; Olufemi, O.; Amos, O.; Bashir, A. In-vitro antioxidant activities of the stem bark extract fractions of Bridelia ferruginea. J. Biol. Agric. Healthc. 2014, 4, 1–7. [Google Scholar]
- Philippe, B.; Gnogbo, B.; Souleymane, M.; Francis, Y.; David, N.; Joseph, D. Evaluation of the antioxidant activity of total aqueous extract of Bridelia ferruginea benth. (Euphorbiaceae). World J. Pharm. Sci. 2015, 3, 2125–2134. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.J.R.; Perry, N.S.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Orhan, I.; Şener, B.; Choudhary, M.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Sinan, K.I.; Bene, K.; Zengin, G.; Orlando, G.; Menghini, L.; Veschi, S.; Chiavaroli, A.; Recinella, L.; Brunetti, L. Bridelia speciosa Müll. Arg. Stem bark Extracts as a Potential Biomedicine: From Tropical Western Africa to the Pharmacy Shelf. Antioxidants 2020, 9, 128. [Google Scholar] [CrossRef]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.-M.; Sun, Y.; Wang, L.-F.; Wang, H.; Zhang, Y.-J.; Li, H.-Y.; Zhuang, P.-W.; Yang, Z. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Muddathir, A.; Yamauchi, K.; Batubara, I.; Mohieldin, E.; Mitsunaga, T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. South Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Rocha, S.; Sousa, A.; Ribeiro, D.; Correia, C.M.; Silva, V.L.; Santos, C.M.; Silva, A.M.; Araújo, A.N.; Fernandes, E.; Freitas, M. A study towards drug discovery for the management of type 2 diabetes mellitus through inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by chalcone derivatives. Food Funct. 2019, 10, 5510–5520. [Google Scholar] [CrossRef]
- Olaokun, O.O.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complement. Altern. Med. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Camberos, E.; Lazcano-Díaz, E.; Flores-Fernandez, J.M.; Owolabi, M.S.; Allen, K.; Villanueva-Rodríguez, S. Evaluation of the inhibition of carbohydrate hydrolyzing enzymes, the antioxidant activity, and the polyphenolic content of Citrus limetta peel extract. Sci. World J. 2014, 2014, 121760. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef]
- Picot, C.M.N.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory Potential of Five Traditionally Used Native Antidiabetic Medicinal Plants on α-Amylase, α-Glucosidase, Glucose Entrapment, and Amylolysis Kinetics In Vitro. Adv. Pharmacol. Sci. 2014, 2014, 739834. [Google Scholar]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Bakoma, B.; Berké, B.; Eklu-Gadegbeku, K.; Aboudoulatif, D.; Agbonon, A.; Aklikokou, K.; Gbeassor, M.; Moore, N. Total Phenolic Content, Antioxidant Activity and in vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia of Bridelia ferruginea Extracts. Res. J. Phytochem. 2012, 6, 120–126. [Google Scholar] [CrossRef][Green Version]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour Technol 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Bhandari, M.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Shanmugam, S.; Sreerama, Y.N.; Malleshi, N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar]
- Ojo, O.; Basiru, A.; Olayide, I.; Adewale, O.F.; Olasehinde, O. Ethyl acetate Fraction of Bark of Bridelia ferruginea inhibits Carbohydrate Hydrolyzing Enzymes associated with type 2 Diabetes (α-glucosidase and α-amylase). Adv. Biores. 2016, 7, 126–133. [Google Scholar]
- Ferrante, C.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics 2020, 9, 611. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ding, S.; Zhao, C.; Gu, X.; He, X.; Huang, K.; Luo, Y.; Liang, Z.; Tian, H.; Xu, W. Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 2015, 162, 7–13. [Google Scholar] [CrossRef]
- Iguidbashian, J.P.; Parekh, J.D.; Kukrety, S.; Andukuri, V.G. Campylobacter jejuni and Pseudomonas coinfection in the setting of ulcerative colitis. Case Rep. 2018. [Google Scholar] [CrossRef]
- Trojanowska, D.; Zwolińska-Wcisło, M.; Tokarczyk, M.; Kosowski, K.; Mach, T.; Budak, A. The Role of “Candida” in inflammatory bowel disease: Estimation of transmission of “C. albicans” fungi in gastrointestinal tract based on genetic affinity between strains. Med. Sci. Monit. 2010, 16, CR451-7. [Google Scholar] [PubMed]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Research article toxicity assessment of different solvent extracts of the medicinal plant, phragmanthera capitata (Sprengel) Balle on Brine Shrimp (Artemia salina). Int. J. Pharmacol. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Larsen, C.A.; Dashwood, R.H. (−)-Epigallocatechin-3-gallate inhibits Met signaling, proliferation, and invasiveness in human colon cancer cells. Arch. Biochem. Biophys. 2010, 501, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Moseley, V.R.; Morris, J.; Knackstedt, R.W.; Wargovich, M.J. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer Res. 2013, 33, 5325–5333. [Google Scholar]
- Yang, H.; He, K.; Dong, W.; Fang, J.; Zhong, S.; Tang, L.; Long, L. PIM-1 may function as an oncogene in cervical cancer via activating the EGFR signaling. Int. J. Biol. Markers 2020, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mosieniak, G.; Sliwinska, M.A.; Alster, O.; Strzeszewska, A.; Sunderland, P.; Piechota, M.; Was, H.; Sikora, E. Polyploidy formation in doxorubicin-treated cancer cells can favor escape from senescence. Neoplasia 2015, 17, 882–893. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, L.; Li, F.; Xu, Y.; Lv, S.; Wang, B. Simultaneous dermatophytosis and keratomycosis caused by Trichophyton interdigitale infection: A case report and literature review. BMC Infect. Dis. 2019, 19, 983. [Google Scholar] [CrossRef] [PubMed]
- Isa-Isa, R.; Arenas, R.; Isa, M. Inflammatory tinea capitis: Kerion, dermatophytic granuloma, and mycetoma. Clin. Dermatol. 2010, 28, 133–136. [Google Scholar] [CrossRef]
- Bottari, N.B.; Lopes, L.Q.S.; Pizzuti, K.; dos Santos Alves, C.F.; Corrêa, M.S.; Bolzan, L.P.; Zago, A.; de Almeida Vaucher, R.; Boligon, A.A.; Giongo, J.L. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb. Pathog. 2017, 104, 190–195. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic mode of action of catechin, vanillic and protocatechuic acids to inhibit the adhesion of uropathogenic Escherichia coli on silicone surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complementary Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Katanić Stanković, J.S.; Ceylan, R.; Zengin, G.; Matić, S.; Jurić, T.; Diuzheva, A.; Jeko, J.; Cziáky, Z.; Aktumsek, A. Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition. Ind. Crop. Prod. 2020, 144, 112053. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Zengin, G.; Locatelli, M.; Macedonio, G.; Orlando, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018, 107, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L. Effects of kisspeptin-10 on hypothalamic neuropeptides and neurotransmitters involved in appetite control. Molecules 2018, 23, 3071. [Google Scholar] [CrossRef]
| Parts | Solvents | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|
| Leaves | EA | 26.90 ± 1.12 e | 29.71 ± 0.82 b |
| MeOH | 103.94 ± 2.00 c | 42.31 ± 0.39 a | |
| Water | 85.05 ± 0.58 d | 24.37 ± 0.13 c | |
| Stem bark | EA | 26.07 ± 0.48 e | 2.62 ± 0.23 d |
| MeOH | 193.58 ± 0.98 a | 2.45 ± 0.35 d | |
| Water | 187.84 ± 1.88 b | 2.05 ± 0.17 d |
| No. | Name | Formula | Stem Bark-EA | Stem Bark-MeOH | Stem Bark-Water | Leaves-EA | Leaves-MeOH | Leaves-Water |
|---|---|---|---|---|---|---|---|---|
| 1 | Epigallocatechin-(7-O-4′)-gallocatechin or isomer | C30H26O13 | − | + | − | − | − | − |
| 2 1 | Gallic acid (3,4,5-Trihydroxybenzoic acid) | C7H6O5 | + | + | + | − | + | + |
| 3 | Gallocatechin | C15H14O7 | + | + | + | − | + | + |
| 4 | Pantothenic acid | C9H17NO5 | + | + | + | − | + | + |
| 5 | Epigallocatechin-(7-O-4′)-gallocatechin or isomer | C30H26O13 | − | − | + | − | − | − |
| 6 | Prodelphinidin C | C30H26O13 | − | + | + | − | − | − |
| 7 | Epigallocatechin-(7-O-4′)-gallocatechin or isomer | C30H26O13 | − | + | + | − | − | − |
| 8 | Kynurenic acid | C10H7NO3 | − | − | − | − | + | + |
| 9 1 | Catechin | C15H14O6 | − | + | + | − | + | + |
| 10 1 | Epigallocatechin | C15H14O7 | + | + | + | − | + | + |
| 11 | Epigallocatechin-(7-O-4′)-gallocatechin or isomer | C30H26O13 | − | + | + | − | − | − |
| 12 1 | Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | − | − | − | + | + | + |
| 13 1 | Epigallocatechin-3-O-gallate (Teatannin II) | C22H18O11 | + | + | + | − | + | − |
| 14 | 5-O-(4-Coumaroyl)quinic acid | C16H18O8 | − | − | + | − | − | − |
| 15 | Dihydrokaempferol-O-hexoside | C21H22O11 | − | + | + | − | − | − |
| 16 1 | Epicatechin | C15H14O6 | + | + | + | − | + | + |
| 17 | Corilagin | C27H22O18 | + | + | + | − | − | − |
| 18 | 5-O-Feruloylquinic acid | C17H20O9 | − | − | + | − | − | − |
| 19 1 | Epicatechin-3-O-gallate | C22H18O10 | − | + | + | − | − | − |
| 20 1 | Ferulic acid | C10H10O4 | + | + | + | − | − | − |
| 21 | Ellagic acid-O-hexoside | C20H16O13 | + | + | + | − | − | − |
| 22 | Myricetin-O-hexoside | C21H20O13 | − | − | − | + | + | + |
| 23 1 | Vitexin (Apigenin-8-C-glucoside) | C21H20O10 | − | − | + | + | + | + |
| 24 | Methylellagic acid-O-hexoside | C21H18O13 | + | + | + | − | − | − |
| 25 | Myricitrin (Myricetin-3-O-rhamnoside) | C21H20O12 | − | + | + | − | + | − |
| 26 | Isovitexin (Apigenin-6-C-glucoside) | C21H20O10 | + | + | + | + | + | + |
| 27 | Coatline A or isomer | C21H24O10 | − | + | + | − | + | − |
| 28 | Hexahydroxy(iso)flavanone | C15H12O8 | − | − | − | − | + | + |
| 29 | Ellagic acid-O-pentoside | C19H14O12 | + | + | + | − | − | − |
| 30 1 | Rutin (Quercetin-3-O-rutinoside) | C27H30O16 | − | − | − | + | + | + |
| 31 | Eschweilenol C (Ellagic acid-4-O-rhamnoside) | C20H16O12 | + | + | + | − | − | − |
| 32 | Ellagic acid | C14H6O8 | + | + | + | − | + | + |
| 33 | Methoxy-pentahydroxy(iso)flavone-O-rhamnosylhexoside isomer 1 | C28H32O17 | − | − | − | − | + | + |
| 34 | Quercetin-O-malonylhexoside | C23H22O13 | − | − | − | − | + | + |
| 35 | Methoxy-pentahydroxy(iso)flavone-O-rhamnosylhexoside isomer 2 | C28H32O17 | − | − | − | − | + | + |
| 36 1 | Myricetin (3,3′,4′,5,5′,7-Hexahydroxyflavone) | C15H10O8 | − | + | + | − | + | + |
| 37 | Kaempferol-O-rhamnosylhexoside | C27H30O15 | − | − | − | − | + | + |
| 38 | Tricin-O-hexoside | C23H24O12 | − | + | − | − | − | − |
| 39 | Tricin-O-hexoside isomer 1 | C23H24O12 | − | − | − | + | + | + |
| 401 | Quercitrin (Quercetin-3-O-rhamnoside) | C21H20O11 | − | − | − | + | + | + |
| 41 | N-trans-Feruloyltyramine | C18H19NO4 | − | − | − | − | + | − |
| 42 | Ducheside A (3-O-Methylellagic acid-4′-O-xyloside) | C20H16O12 | + | + | + | − | − | − |
| 43 | Kaempferol-3-O-rutinoside (Nicotiflorin) | C27H30O15 | − | − | − | + | + | + |
| 44 | Dimethoxy-tetrahydroxy(iso)flavone-O-hexoside | C23H24O13 | + | + | + | + | + | + |
| 45 | Tricin-O-hexoside isomer 2 | C23H24O12 | − | − | − | + | + | + |
| 46 | Trihydroxy-trimethoxy(iso)flavone-O-hexoside isomer 1 | C24H26O13 | − | − | − | + | + | + |
| 47 | Isorhamnetin-3-O-rutinoside (Narcissin) | C28H32O16 | − | − | − | + | + | + |
| 48 | 3-O-Methylellagic acid-O-rhamnoside | C21H18O12 | + | + | + | − | − | − |
| 49 | Dihydroxy(iso)flavone-C-hexoside | C21H20O9 | − | + | + | − | + | + |
| 50 | Pentahydroxy(iso)flavanone | C15H12O7 | − | − | − | − | + | + |
| 51 | 3-O-Methylellagic acid | C15H8O8 | + | + | + | − | − | − |
| 52 | Eschweilenol A or isomer | C20H10O11 | − | + | − | − | − | − |
| 53 | Dimethoxy-trihydroxy(iso)flavone-O-hexoside | C23H24O12 | − | − | − | − | + | + |
| 54 | Dihydroactinidiolide | C11H16O2 | − | − | − | + | − | − |
| 55 | Trihydroxy-trimethoxy(iso)flavone-O-hexoside isomer 2 | C24H26O13 | − | − | − | − | + | + |
| 56 1 | Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | C15H10O7 | + | + | + | + | + | + |
| 57 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | − | − | − | + | + | − |
| 58 | 3,3′-Di-O-methylellagic acid | C16H10O8 | + | + | + | − | − | − |
| 59 | Methoxy-tetrahydroxy(iso)flavone | C16H12O7 | + | + | + | − | − | − |
| 60 | Methoxy-tetrahydroxy(iso)flavone isomer 1 | C16H12O7 | − | − | − | + | + | + |
| 61 1 | Kaempferol (3,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | − | − | − | + | + | + |
| 62 | Methoxy-tetrahydroxy(iso)flavone isomer 2 | C16H12O7 | − | − | − | + | + | + |
| 63 1 | Tricin (3′,5′-Dimethoxy-4′,5,7-trihydroxyflavone) | C17H14O7 | + | + | + | + | + | + |
| 64 | Salcolin A (Tricin-4′-O-(erythro-β-guaiacylglyceryl)ether) | C27H26O11 | − | − | − | − | + | − |
| 65 | Methoxy-trihydroxy(iso)flavone isomer 1 | C16H12O6 | − | − | − | + | + | − |
| 66 | Dimethoxy-tetrahydroxy(iso)flavone | C17H14O8 | − | − | + | − | − | − |
| 67 | 3,3′,4-Tri-O-methylellagic acid | C17H12O8 | + | + | + | − | − | − |
| 68 | Methoxy-trihydroxy(iso)flavone isomer 2 | C16H12O6 | − | − | − | + | + | − |
| 69 | Dimethoxy-trihydroxy(iso)flavone isomer 1 | C17H14O7 | + | − | − | − | − | − |
| 70 | Salcolin B (Tricin-4′-O-(threo-β-guaiacylglyceryl)ether) | C27H26O11 | − | − | − | − | + | − |
| 71 | 3,3′,4,4′-Tetra-O-methylellagic acid | C18H14O8 | + | + | + | + | + | + |
| 72 | Dimethoxy-trihydroxy(iso)flavone isomer 2 | C17H14O7 | + | − | − | − | − | − |
| 73 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | − | − | − | + | + | − |
| 74 | Hydroxy-tetramethoxy(iso)flavone isomer 1 | C19H18O7 | − | − | − | + | + | + |
| 75 | Hydroxy-tetramethoxy(iso)flavone isomer 2 | C19H18O7 | − | − | − | + | + | + |
| Parts | Solvents | DPPH | ABTS | CUPRAC | FRAP | Phosphomolybdenum | Metal Chelating |
|---|---|---|---|---|---|---|---|
| (mg TE/g) | (mmol TE/g) | (mg EDTAE/g) | |||||
| Leaves | EA | 10.71 ± 0.78 f | 2.97 ± 0.66 f | 69.51 ± 0.90 f | 30.97 ± 0.66 f | 2.61 ± 0.11 c | 19.57 ± 0.50 b |
| MeOH | 197.38 ± 0.51 b | 345.15 ± 4.27 b | 395.81 ± 12.02 b | 256.72 ± 3.39 b | 3.17 ± 0.07 b | 12.41 ± 1.28 d | |
| Water | 146.91 ± 1.77 c | 247.75 ± 3.05 c | 328.08 ± 3.79 c | 204.92 ± 1.91 c | 2.24 ± 0.07 cd | 17.83 ± 0.17 b | |
| Stem bark | EA | 31.68 ± 0.46 e | 22.98 ± 0.93 e | 81.54 ± 1.30 e | 38.57 ± 0.37 e | 2.03 ± 0.15 d | 26.59 ± 0.25 a |
| MeOH | 491.59 ± 0.37 a | 804.22 ± 5.03 a | 1066.93 ± 12.02 a | 633.44 ± 10.13 a | 7.11 ± 0.39 a | 8.70 ± 0.33 e | |
| Water | 95.26 ± 1.41 d | 118.34 ± 4.38 d | 241.37 ± 0.78 d | 136.26 ± 0.40 d | 6.92 ± 0.20 a | 14.44 ± 0.94 c | |
| Parts | Solvents | AChE | BChE | Tyrosinase | Amylase | Glucosidase |
|---|---|---|---|---|---|---|
| (mg GALAE/g) | (mg KAE/g) | (mmol ACAE/g) | ||||
| Leaves | EA | 2.45 ± 0.30 d | 6.21 ± 0.17 c | 103.13 ± 1.23 d | 0.94 ± 0.01 c | 6.24 ± 0.29 b |
| MeOH | 4.64 ± 0.08 b | 9.27 ± 1.08 b | 150.71 ± 0.57 b | 1.38 ± 0.03 a | na | |
| Water | 2.39 ± 0.05 d | 3.15 ± 0.54 d | 46.04 ± 3.47 e | 0.21 ± 0.02 d | na | |
| Stem bark | EA | 4.37 ± 0.17 b | 7.70 ± 1.54 bc | 106.70 ± 1.41 d | 0.91 ± 0.04 c | 6.68 ± 0.01 a |
| MeOH | 5.18 ± 0.04 a | 12.79 ± 0.93 a | 157.07 ± 0.37 a | 1.35 ± 0.05 a | na | |
| Water | 3.36 ± 0.10 c | 6.36 ± 0.03 c | 123.23 ± 1.15 c | 1.06 ± 0.04 b | na | |
| Bacteria (ID Strain) | Minimum Inhibitory Concentration (MIC) * µg/mL |
|---|---|
| Gram− | |
| Escherichia coli (ATCC 10536) | 2.48 (1.562–3.125) |
| Escherichia coli (PeruMycA 2) | 2.48 (1.562–3.125) |
| Escherichia coli (PeruMycA 3) | 62.99 (50–100) |
| Pseudomonas aeruginosa (PeruMyc 5) | >200 |
| Salmonella typhy (PeruMyc 7) | 125 (100–200) |
| Gram+ | |
| Bacillus cereus (PeruMycA 4) | 39.68 (25–50) |
| Bacillus subtilis (PeruMyc 6) | 31.50 (25–50) |
| Staphylococcus aureus (ATCC 6538) | 62.99 (50–100) |
| Dermatophytes (ID Strain) | Minimum Inhibitory Concentration (MIC) * µg/mL |
|---|---|
| Trichophyton mentagrophytes (CCF 4823) | 31.49 (25–50) |
| Trichophyton tonsurans (4834) | 19.84 (12.5–25) |
| Trichophyton rubrum (4933) | 9.92 (6.25–12.5) |
| Arthroderma quadrifidum (5792) | 4.96 (3.125–6.25) |
| Tricophyton mentagrophytes (5930) | 19.84 (12.5–25) |
| Nannizia gypseum (6261) | 9.92 (6.25–12.5) |
| Arthroderma currei (5207) | 9.92 (6.25–12.5) |
| Arthroderma insingulare (5417) | 7.87 (6.25–12.5) |
| Yeasts (ID Strain) | |
| Candida tropicalis (6148) | 62.99 (50–100) |
| Candida albicans (6379) | 62.99 (50–100) |
| Candida parapsilosis (6551) | 31.49 (25–50) |
| Candida albicans (6183) | 62.99 (50–100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahomoodally, M.F.; Jugreet, S.; Sinan, K.I.; Zengin, G.; Ak, G.; Ceylan, R.; Jekő, J.; Cziáky, Z.; Angelini, P.; Angeles Flores, G.; et al. Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant. Antibiotics 2021, 10, 223. https://doi.org/10.3390/antibiotics10020223
Mahomoodally MF, Jugreet S, Sinan KI, Zengin G, Ak G, Ceylan R, Jekő J, Cziáky Z, Angelini P, Angeles Flores G, et al. Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant. Antibiotics. 2021; 10(2):223. https://doi.org/10.3390/antibiotics10020223
Chicago/Turabian StyleMahomoodally, Mohamad Fawzi, Sharmeen Jugreet, Kouadio Ibrahime Sinan, Gokhan Zengin, Gunes Ak, Ramazan Ceylan, József Jekő, Zoltán Cziáky, Paola Angelini, Giancarlo Angeles Flores, and et al. 2021. "Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant" Antibiotics 10, no. 2: 223. https://doi.org/10.3390/antibiotics10020223
APA StyleMahomoodally, M. F., Jugreet, S., Sinan, K. I., Zengin, G., Ak, G., Ceylan, R., Jekő, J., Cziáky, Z., Angelini, P., Angeles Flores, G., Venanzoni, R., Di Simone, S. C., Menghini, L., Orlando, G., Ferrante, C., Etienne, O. K., & Tacchini, M. (2021). Pharmacological Potential and Chemical Characterization of Bridelia ferruginea Benth.—A Native Tropical African Medicinal Plant. Antibiotics, 10(2), 223. https://doi.org/10.3390/antibiotics10020223













