Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey
Abstract
1. Introduction
2. Results
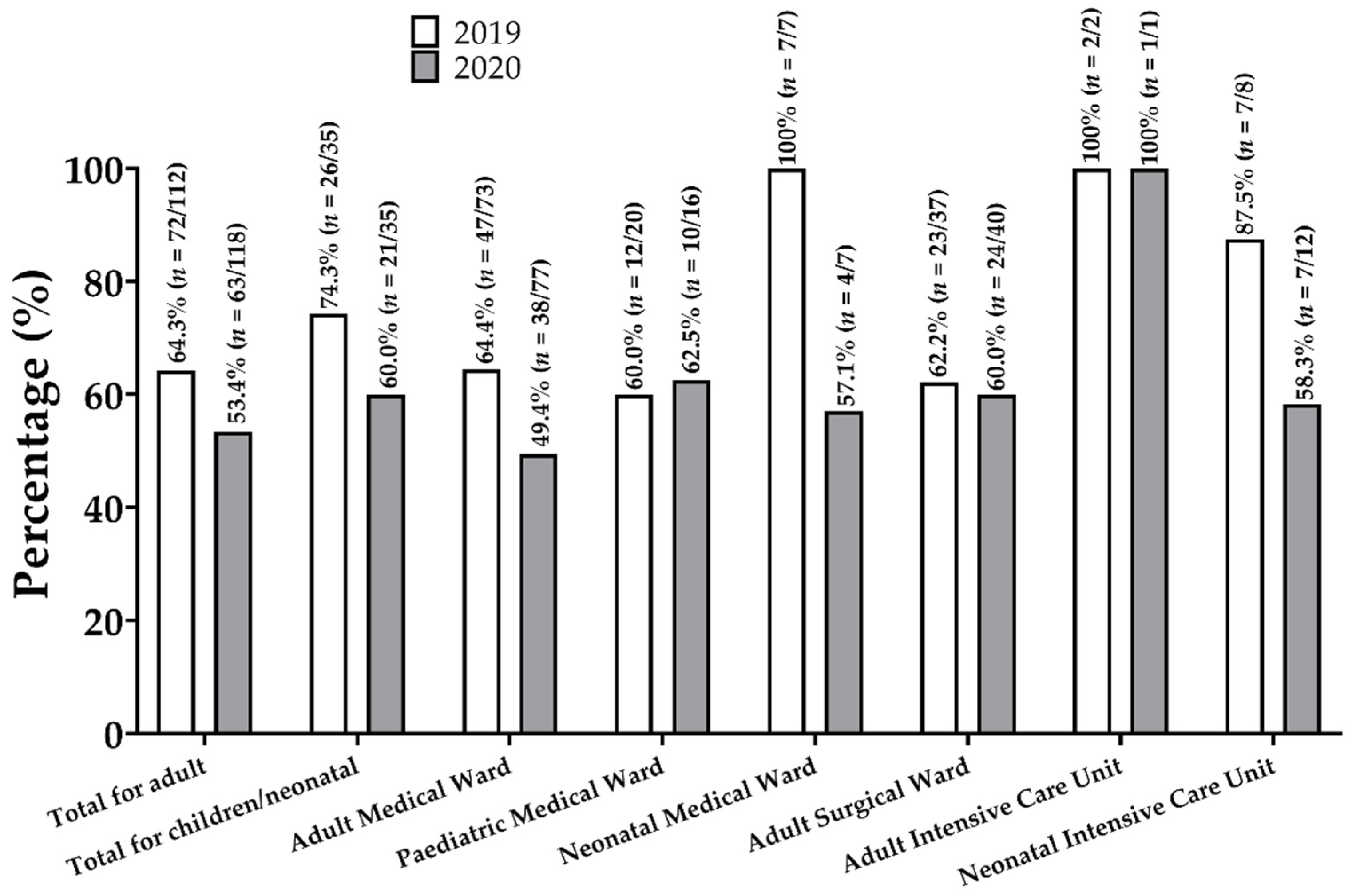
2.1. Prevalence of Antibiotic Prescriptions
2.2. Types of Antimicrobials Prescribed
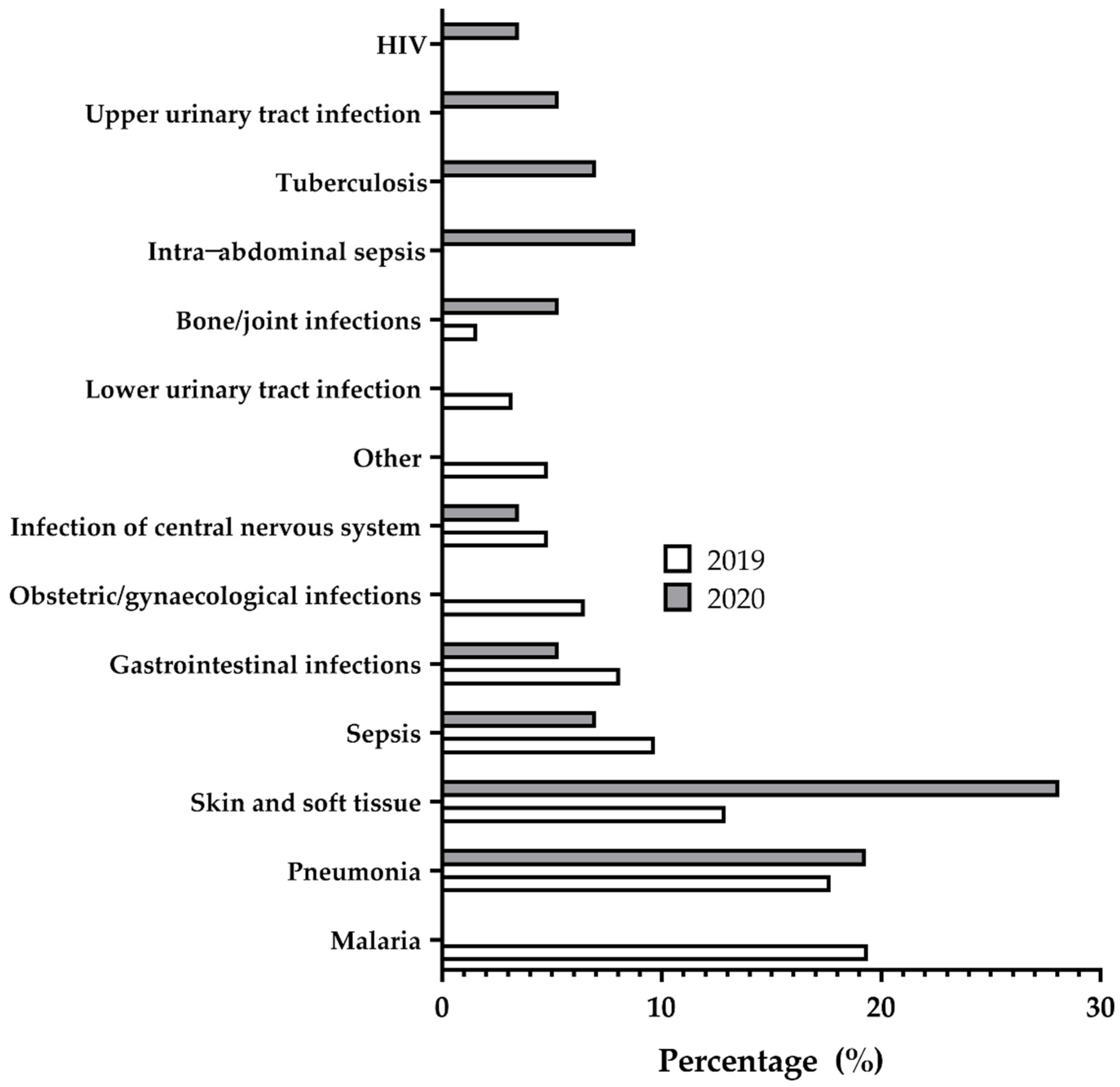
2.3. Common Diagnoses for Antimicrobials Use
2.4. Patterns of Antibiotics Use
2.5. Quality Indicators for Prescribing
3. Discussion
3.1. Prevalence
3.2. Types of Antibiotics Prescribed
3.3. Commonly Diagnosed Diseases
3.4. Quality Indicators
3.5. Implications for Practice
4. Materials and Methods
4.1. Study Settings and Design
4.2. Data Collection
4.3. Data Handling and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ECDC. Annual Epidemiological Report on Communicable Diseases in Europe 2010. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1011_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf (accessed on 11 February 2021).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; Aboderin, O.A.; Byarugaba, D.K.; Ojo, K.K.; Opintan, J.A. Growing Problem of Multidrug-Resistant Enteric Pathogens in Africa. Emerg. Infect. Dis. 2007, 13, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.A.J.W.; Laxminarayan, R.; Saam, M.; Van Belkum, A.; Pittet, D.; for the World Healthcare-Associated Infections Resistance Forum Participants. Antimicrobial resistance: One world, one fight! Antimicrob. Resist. Infect. Control. 2015, 4, 49. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017. Available online: https://www.who.int/docs/default-source/searo/amr/global-antimicrobial-resistance-surveillance-system-(glass)-report-early-implementation-2016-2017.pdf?sfvrsn=ea19cc4a_2 (accessed on 11 February 2021).
- Gravel, D.; Taylor, G.; Ofner, M.; Johnston, L.; Loeb, M.; Roth, V.; Stegenga, J.; Bryce, E.; The Canadian Nosocomial Infection Surveillance Program; Matlow, A. Point prevalence survey for healthcare-associated infections within Canadian adult acute-care hospitals. J. Hosp. Infect. 2007, 66, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Global-PPS. Available online: https://www.global-pps.com (accessed on 23 November 2020).
- Goossens, H. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 2009, 15, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Iosifidis, E.; Antachopoulos, C.; Tsivitanidou, M.; Katragkou, A.; Farmaki, E.; Tsiakou, M.; Kyriazi, T.; Sofianou, D.; Roilides, E. Differential Correlation between Rates of Antimicrobial Drug Consumption and Prevalence of Antimicrobial Resistance in a Tertiary Care Hospital in Greece. Infect. Control Hosp. Epidemiol. 2008, 29, 615–622. [Google Scholar] [CrossRef] [PubMed]
- ECTMIH 2017 (Poster N°5P95): Global Point Prevalence Survey on Antimicrobial Use and Resistance (Global-PPS): Implications for Antibiotic Stewardship Programme for Komfo Anokye Teaching Hospital in Ghana. Available online: https://www.global-pps.com/wp-content/uploads/ECTMIH-2017-5P95-Ghana.pdf (accessed on 11 February 2021).
- Labi, A.-K.; Obeng-Nkrumah, N.; Nartey, E.T.; Bjerrum, S.; Adu-Aryee, N.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Afriyie, D.K.; A Sefah, I.; Sneddon, J.; Malcolm, W.; McKinney, R.; Cooper, L.; Kurdi, A.; Godman, B.; Seaton, R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC Antimicrob. Resist. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Umeokonkwo, C.D.; Madubueze, U.C.; Onah, C.K.; Okedo-Alex, I.N.; Adeke, A.S.; Versporten, A.; Goossens, H.; Igwe-Okomiso, D.; Okeke, K.; Azuogu, B.N.; et al. Point prevalence survey of antimicrobial prescription in a tertiary hospital in South East Nigeria: A call for improved antibiotic stewardship. J. Glob. Antimicrob. Resist. 2019, 17, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Okoth, C.; Opanga, S.; Okalebo, F.; Oluka, M.; Kurdi, A.B.; Godman, B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 2018, 46, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Andoh-Adjei, F.-X.; Van Der Wal, R.; Nsiah-Boateng, E.; Asante, F.A.; Van Der Velden, K.; Spaan, E. Does a provider payment method affect membership retention in a health insurance scheme? A mixed method study of Ghana’s capitation payment for primary care. BMC Health Serv. Res. 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Kusama, Y.; Muraki, Y.; Mochizuki, T.; Kurai, H.; Gu, Y.; Ohmagari, N. Relationship between drug formulary and frequently used cephalosporins, macrolides and quinolones in Japanese hospitals. J. Infect. Chemother. 2020, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- WHO. The 2019 WHO AWaRe Classification of Antibiotics for EVALUATION and monitoring of Use. Available online: https://apps.who.int/iris/handle/10665/327957 (accessed on 11 February 2021).
- Abbey, M.; Afagbedzi, S.K.; Afriyie-Mensah, J.; Antwi-Agyei, D.; Atengble, K.; Badoe, E.; Batchelor, J.; Donkor, E.S.; Esena, R.; Goka, B.Q.; et al. Pneumonia in Ghana—A need to raise the profile. Int. Health 2018, 10, 4–7. [Google Scholar] [CrossRef] [PubMed]
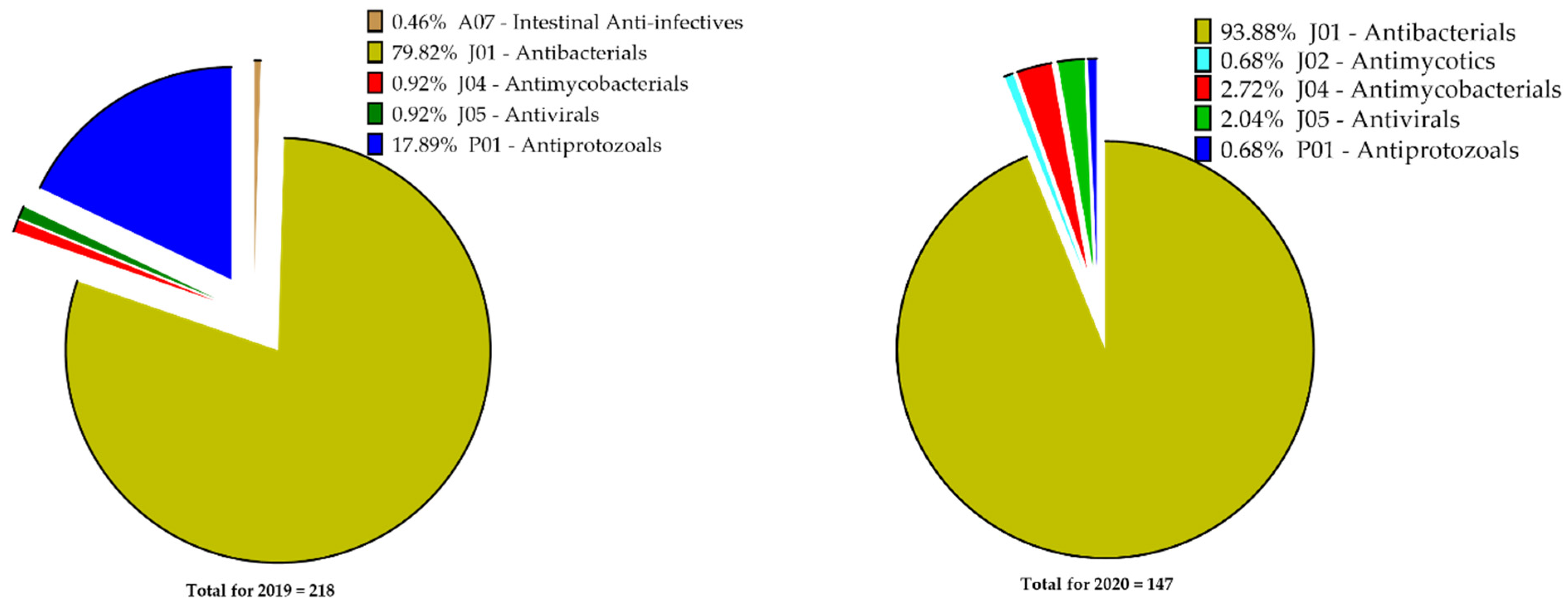
| Anatomical Therapeutic Chemical (ATC) Classification | Class/Sub-Class of Antimicrobials | Proportion of Antimicrobials in July 2019 | Proportion of Antimicrobials in January 2020 |
|---|---|---|---|
| A07—Intestinal Anti-infectives | Number of Antimicrobials and Patients | N = 1 | - |
| Antibiotics | 100% (n = 1/1) | - | |
| J01—Antibacterials for systemic use | Number of Antimicrobials and Patients | N = 174; 88 patients | N = 138; 82 patients |
| Tetracyclines | 1.1% (n = 2/174) | - | |
| Penicillins | 21.3% (n = 37/174) | 22.5% (n = 31/138) | |
| a. Penicillins with extended spectrum | 37.8% (n = 14/37) | 51.6% (n = 16/31) | |
| b. β-lactamase = sensitive penicillins | 21.6% (n = 8/37) | 3.2% (n = 1/31) | |
| c. β-lactamase = resistant penicillins | 29.7% (n = 11/37) | 45.2% (n = 14/31) | |
| d. Combinations of penicillins, including β-lactamase inhibitors | 10.8% (n = 4/37) | - | |
| Other β-lactams | 39.1% (n = 68/174) | 26.1% (n = 36/138) | |
| a. First-generation cephalosporins | - | - | |
| b. Second-generation cephalosporins | 51.5% (n = 35/68) | 41.7% (n = 15/36) | |
| c. Third-generation cephalosporins | 47.1% (n = 32/68) | 58.3% (n = 21/36) | |
| d. Fourth-generation cephalosporins | - | - | |
| e. Carbapenems | 1.5% (n = 1/68) | - | |
| Sulfonamides/trimethoprim | 1.7% (n = 3/174) | 5.8% (n = 8/138) | |
| Macrolides/liconsamides/streptogramins | 8.0% (n = 14/174) | 13.8% (n = 19/138) | |
| Aminoglycosides | 2.9% (n = 5/174) | 2.2% (n = 3/138) | |
| Quinolones | 8.0% (n = 14/174) | 10.1% (n = 14/138) | |
| Other antibacterials | 17.8% (n = 31/174) | 19.6% (n = 27/138) | |
| J02—Antimycotics for systemic use | Number of Antimicrobials and Patients | - | N = 1; 1 patient |
| Triazole derivatives | - | 100% (n = 1/1) | |
| J04—Antimycobacterials | Number of Antimicrobials and Patients | N = 2; 2 patients | N = 4; 4 patients |
| Other drugs for tuberculosis | - | 25.0% (n = 1/4) | |
| Combinations of drugs for tuberculosis | 100% (n = 2/2) | 75.0% (n = 3/4) | |
| J05—Antivirals | Number of Antimicrobials and Patients | N = 2; 2 patients | N = 3; 1 patient |
| Nucleosides and nucleotides, excluding reverse transcriptase inhibitors | 50.0% (n = 1/2) | - | |
| Nucleosides and nucleotides reverse transcriptase inhibitors | - | 66.7% (n = 2/3) | |
| Combinations of antivirals for treatment of HIV infections | 50.0% (n = 1/2) | ||
| Other antivirals | - | 33.3% (n = 1/3) | |
| P01—Antiprotozoals | Number of Antimicrobials and Patients | N = 39; 31 patients | N = 1; 1 patient |
| Nitroimidazole derivatives | 53.8% (n = 21/39) | - | |
| Antimalarials | 46.2% (n = 18/39) | 100% (n = 1/1) | |
| a. Artemisinin and derivatives—plain | 38.9% (n = 7/18) | 100% (n = 1/1) | |
| b. Combinations of artemisinin and derivatives | 61.1% (n = 11/18) | - |
| Disease Condition | Antibiotics Used in July 2019 | Antibiotics Used in January 2020 |
|---|---|---|
| Sepsis in adults and children | 8 antibiotics in 6 patients | 6 antibiotics in 4 patients |
| Ceftriaxone (50%, n = 4/8) | Ceftriaxone (50%, n = 3/6) | |
| Cefuroxime (12.5%, n = 1/8) | Metronidazole (33.3%, n = 2/6) | |
| Ciprofloxacin (12.5%, n = 1/8) | Gentamicin (16.7%, n = 1/6) | |
| Meropenem (12.5%, n = 1/8) | ||
| Metronidazole (12.5%, n = 1/8) | ||
| Pneumonia in adults and children | 15 antibiotics in 11 patients | 15 antibiotics in 11 patients |
| Amoxicillin (33.3%, n = 5/15) | Amoxicillin (54.5%, n = 6/11) | |
| Ceftriaxone (33.3%, n = 5/15) | Ceftriaxone (6.7%, n = 1/15) | |
| Azithromycin (20.0%, n = 3/15) | Azithromycin (13.3%, n = 2/15) | |
| Cefuroxime (6.7%, n = 1/15) | Metronidazole (20.0%, n = 3/15) | |
| Sulfamethoxazole and trimethoprim (6.7%, n = 1/15) | Sulfamethoxazole and trimethoprim (13.3%, n = 2/15) | |
| Medical prophylaxis in adults and children | 8 antibiotics in 5 patients | 4 antibiotics in 4 patients |
| Amoxicillin and enzyme inhibitor (12.5%, n = 1/8) | Amoxicillin (50.0%, n = 2/4) | |
| Azithromycin (12.5%, n = 1/8) | Cefuroxime (25.0%, n = 1/4) | |
| Ceftriaxone (12.5%, n = 1/8) | Sulfamethoxazole and trimethoprim (25.0%, n = 1/4) | |
| Cefuroxime (12.5%, n = 1/8) | ||
| Ciprofloxacin (12.5%, n = 1/8) | ||
| Metronidazole intravenous infusion IV (12.5%, n = 1/8) | ||
| Metronidazole oral (12.5%, n = 1/8) | ||
| Sulfamethoxazole and trimethoprim (12.5%, n = 1/8) | ||
| Surgical prophylaxis in adults and children | 84 antibiotics in 32 patients | 30 antibiotics in 19 patients |
| Cefuroxime (35.7%, n = 30/84) | Cefuroxime (26.7%, n = 8/30) | |
| Metronidazole (44.0%, n = 37/84) | Metronidazole (33.3%, n = 10/30) | |
| Ciprofloxacin (8.3%, n = 7/84) | Ciprofloxacin (10%, n = 3/30) | |
| Amoxicillin (6.0%, n = 5/84) | Amoxicillin (20.0%, n = 6/30) | |
| Gentamicin (2.4%, n = 2/84) | Ceftriaxone (3.3%, n = 1/30) | |
| Surgical prophylaxis of the gastrointestinal tract in adults and children | 10 antibiotics in 5 patients | 8 antibiotics in 4 patients |
| Metronidazole (50.0%, n = 5/10) | Metronidazole (37.5%, n = 3/8) | |
| Ciprofloxacin (40.0%, n = 4/10) | Ciprofloxacin (37.5%, n = 3/8) | |
| Cefuroxime (10.0%, n = 1/10) | Amoxicillin (25.0%, n = 2/8) |
| July 2019, N (%) | January 2020, N (%) | Statistical Parameters | |||||
|---|---|---|---|---|---|---|---|
| Medical | Surgical | ICU | Medical | Surgical | ICU | χ2, p-Value | |
| Reasons in medical notes | 62 (68.1) | 52 (78.8) | 17 (100) | 66 (95.7) | 50 (98.0) | 17 (100) | 2.758, 0.2519 |
| Guidelines missing | 4 (4.4) | 22 (33.3) | 0 (0) | 6 (8.7) | 20 (39.2) | 0 (0) | - |
| Guideline compliant | 36 (73.5) | 13 (59.1) | 8 (88.9) | 33 (76.7) | 11 (68.8) | 8 (100) | 0.2510, 0.8821 |
| Stop/review date | 86 (94.5) | 66 (100) | 17 (100) | 69 (100) | 51 (100) | 17 (100) | 0.08619, 0.9578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodoo, C.C.; Orman, E.; Alalbila, T.; Mensah, A.; Jato, J.; Mfoafo, K.A.; Folitse, I.; Hutton-Nyameaye, A.; Okon Ben, I.; Mensah-Kane, P.; et al. Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics 2021, 10, 199. https://doi.org/10.3390/antibiotics10020199
Dodoo CC, Orman E, Alalbila T, Mensah A, Jato J, Mfoafo KA, Folitse I, Hutton-Nyameaye A, Okon Ben I, Mensah-Kane P, et al. Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics. 2021; 10(2):199. https://doi.org/10.3390/antibiotics10020199
Chicago/Turabian StyleDodoo, Cornelius C., Emmanuel Orman, Thelma Alalbila, Adelaide Mensah, Jonathan Jato, Kwadwo A. Mfoafo, Isaac Folitse, Araba Hutton-Nyameaye, Inemesit Okon Ben, Paapa Mensah-Kane, and et al. 2021. "Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey" Antibiotics 10, no. 2: 199. https://doi.org/10.3390/antibiotics10020199
APA StyleDodoo, C. C., Orman, E., Alalbila, T., Mensah, A., Jato, J., Mfoafo, K. A., Folitse, I., Hutton-Nyameaye, A., Okon Ben, I., Mensah-Kane, P., Sarkodie, E., Kpokiri, E., Ladva, M., Awadzi, B., & Jani, Y. (2021). Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics, 10(2), 199. https://doi.org/10.3390/antibiotics10020199










