Conservative Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
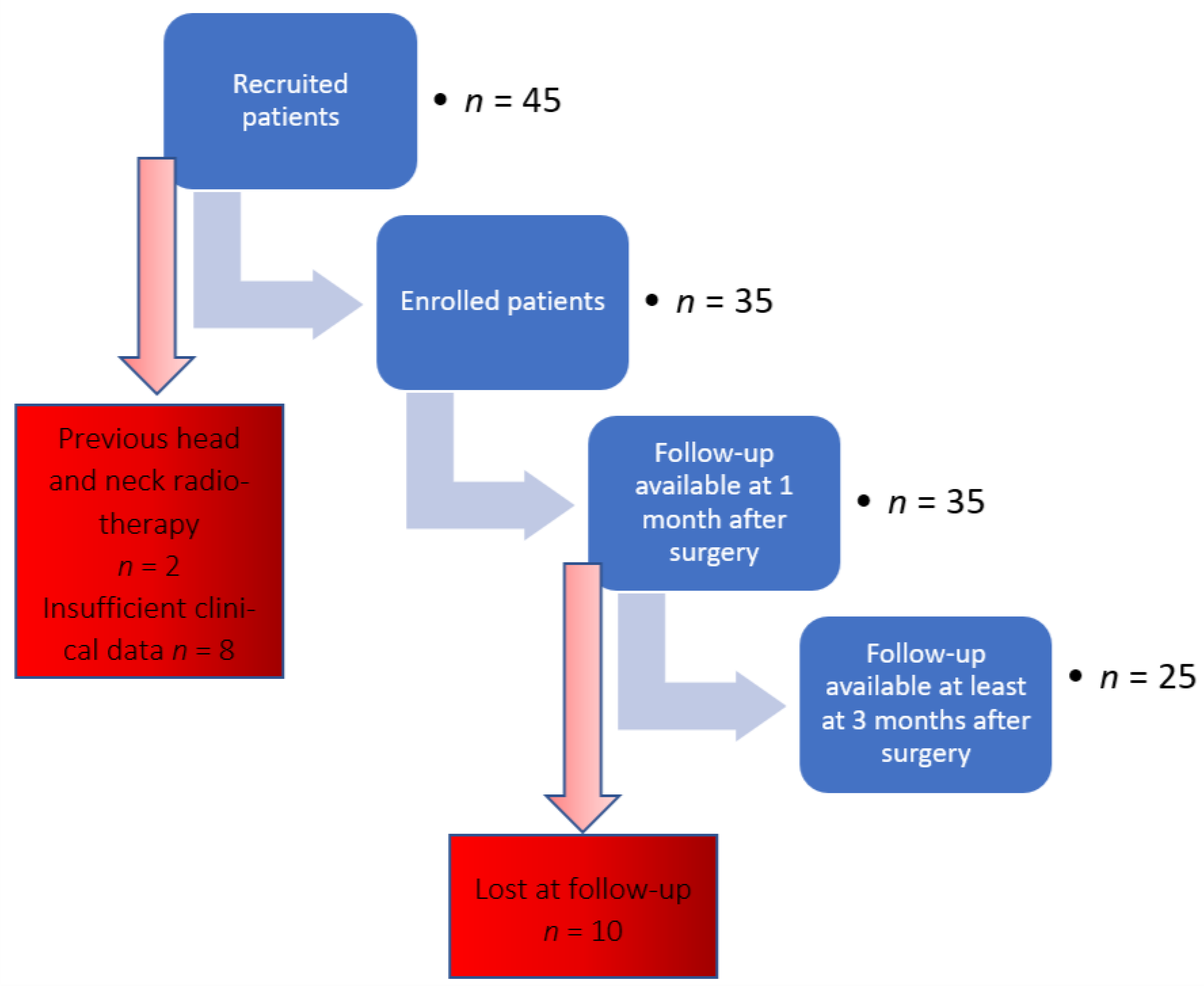
2.1.1. Study Design and Patient Population
2.1.2. Eligibility Criteria
2.1.3. Treatment Intervention
2.2. Data Collection
2.3. Outcomes
2.3.1. Primary Outcomes: Clinical Healing
- Short-term healing—A patient was defined as “healed at short-term”, if presenting, for at least 1 month after sequestrectomy or spontaneous exfoliation of necrotic bone, the following clinical picture: absence of exposed necrotic bone or bone that can be probed through a fistula; absence of purulent drainage; absence of edema and stimulated pain; complete mucosal coverage of the surgical site.
- Long-term healing—A patient was defined as “healed at long-term”, if presenting the same clinical picture described above but lasting for at least 3 months after sequestrectomy or spontaneous exfoliation of bone sequestration.
- Stable MRONJ clinical picture—A patient was “stable” when, at the last available follow-up visit, showing clinical evidence of MRONJ, with the same stage seen during the first visit.
- Worsened MRONJ clinical picture—A patient was “worsened” when, at the last available follow-up visit, showing clinical evidence of MRONJ, with a worse stage than found at first diagnosis.
- Improved MRONJ clinical picture—A patient was “improved” when, at the last available follow-up visit, showing clinical evidence of MRONJ, with a better stage than the one of the first diagnosis.
2.3.2. Secondary Outcomes: Rate of MRONJ Recurrence
Recurrence
Adverse Effects
2.4. Statistical Analysis
2.5. Ethical Approval
2.6. STROBE Statement
3. Results
3.1. Primary Outcomes
3.1.1. Short-Term Healing
3.1.2. Long-Term Healing
3.2. Secondary Outcomes
3.2.1. Recurrences
3.2.2. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, R.; Costa, L.; Ripamonti, C.I.; Niepel, D.; Santini, D. Improving quality of life in patients with advanced cancer: Targeting metastatic bone pain. Eur. J. Cancer 2017, 71, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, D.; Soundia, A.; Gkouveris, I.; Dry, S.M.; Aghaloo, T.L.; Tetradis, S. Development of Medication-Related Osteonecrosis of the Jaw After Extraction of Teeth With Experimental Periapical Disease. J. Oral Maxillofac. Surg. 2019, 77, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.; Odabaşı, O.; Aliyev, T.; Tatar, B. Risk factors of medication-related osteonecrosis of the jaw: A retrospective study in a Turkish subpopulation. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 108–115. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management From the International Task Force on ONJ. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Van Cann, T.; Loyson, T.; Verbiest, A.; Clement, P.M.; Bechter, O.; Willems, L.; Spriet, I.; Coropciuc, R.-G.; Politis, C.; Vandeweyer, R.O.; et al. Incidence of medication-related osteonecrosis of the jaw in patients treated with both bone resorption inhibitors and vascular endothelial growth factor receptor tyrosine kinase inhibitors. Support. Care Cancer 2018, 26, 869–878. [Google Scholar] [CrossRef]
- Capocci, M.; Romeo, U.; Guerra, F.; Mannocci, A.; Tenore, G.; Annibali, S.; Ottolenghi, L. Medication-related osteonecrosis of the jaws (MRONJ) and quality of life evaluation: A pilot study. Clin. Ter. 2017, 168, 253. [Google Scholar]
- Karna, H.; Gonzalez, J.; Radia, H.S.; Sedghizadeh, P.P.; Enciso, R. Risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: A systematic review with meta-analyses. Oral Oncol. 2018, 85, 15–23. [Google Scholar] [CrossRef]
- Coropciuc, R.; Grisar, K.; Aerden, T.; Schol, M.; Schoenaers, J.; Politis, C. Medication-related osteonecrosis of the jaw in oncological patients with skeletal metastases: Conservative treatment is effective up to stage 2. Br. J. Oral Maxillofac. Surg. 2017, 55, 787–792. [Google Scholar] [CrossRef] [PubMed]
- El-Rabbany, M.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Surgical Management of Medication-Related Osteonecrosis of the Jaw Is Associated With Improved Disease Resolution: A Retrospective Cohort Study. J. Oral Maxillofac. Surg. 2019, 77, 1816–1822. [Google Scholar] [CrossRef]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw. J. Am. Dent. Assoc. 2017, 148, 584–594.e2. [Google Scholar] [CrossRef]
- Ristow, O.; Rückschloß, T.; Müller, M.; Berger, M.; Kargus, S.; Pautke, C.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J. Cranio-Maxillofac. Surg. 2019, 47, 491–499. [Google Scholar] [CrossRef]
- Hadaya, D.; Soundia, A.; Freymiller, E.; Grogan, T.; Elashoff, D.; Tetradis, S.; Aghaloo, T.L. Nonsurgical Management of Medication-Related Osteonecrosis of the Jaws Using Local Wound Care. J. Oral Maxillofac. Surg. 2018, 76, 2332–2339. [Google Scholar] [CrossRef]
- Graziani, F.; Vescovi, P.; Campisi, G.; Favia, G.; Gabriele, M.; Gaeta, G.M.; Gennai, S.; Goia, F.; Miccoli, M.; Peluso, F.; et al. Resective Surgical Approach Shows a High Performance in the Management of Advanced Cases of Bisphosphonate-Related Osteonecrosis of the Jaws: A Retrospective Survey of 347 Cases. J. Oral Maxillofac. Surg. 2012, 70, 2501–2507. [Google Scholar] [CrossRef]
- Silva, L.F.; Curra, C.; Munerato, M.S.; DeAntoni, C.C.; Matsumoto, M.A.; Cardoso, C.L.; Curi, M.M. Surgical management of bisphosphonate-related osteonecrosis of the jaws: Literature review. Oral Maxillofac. Surg. 2016, 20, 9–17. [Google Scholar] [CrossRef]
- Carlson, E.R.; Basile, J.D. The Role of Surgical Resection in the Management of Bisphosphonate-Related Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2009, 67, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rupel, K.; Ottaviani, G.; Gobbo, M.; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Biasotto, M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014, 50, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, S.; Puzzo, S.; Palermo, F.; Verzì, P. Treatment of bisphosphonate-related osteonecrosis of the jaws: Presentation of a protocol and an observational longitudinal study of an Italian series of cases. Br. J. Oral Maxillofac. Surg. 2012, 50, 425–429. [Google Scholar] [CrossRef]
- Lodi, G.; Sardella, A.; Salis, A.; Demarosi, F.; Tarozzi, M.; Carrassi, A. Tooth Extraction in Patients Taking Intravenous Bisphosphonates: A Preventive Protocol and Case Series. J. Oral Maxillofac. Surg. 2010, 68, 107–110. [Google Scholar] [CrossRef]
- Magremanne, M.; Reychler, H. Pentoxifylline and Tocopherol in the Treatment of Yearly Zoledronic Acid–Related Osteonecrosis of the Jaw in a Corticosteroid-Induced Osteoporosis. J. Oral Maxillofac. Surg. 2014, 72, 334–337. [Google Scholar] [CrossRef]
- Epstein, M.S.; Wicknick, F.W.; Epstein, J.B.; Berenson, J.R.; Gorsky, M. Management of bisphosphonate-associated osteonecrosis: Pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 593–596. [Google Scholar] [CrossRef]
- Owosho, A.A.; Estilo, C.L.; Huryn, J.M.; Yom, S.K. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: An observational retrospective study of initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 455–459. [Google Scholar] [CrossRef]
- Landesberg, R.; Woo, V.; Cremers, S.; Cozin, M.; Marolt, D.; Vunjak-Novakovic, G.; Kousteni, S.; Raghavan, S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011, 1218, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.S.; Correia, J.A.; Salvado, F.; Caldas, C.; Santos, N.; Capelo, A.; Palmela, P. Relevant factors for treatment outcome and time to healing in medication-related osteonecrosis of the jaws—A retrospective cohort study. J. Cranio-Maxillofac. Surg. 2017, 45, 1736–1742. [Google Scholar] [CrossRef]
- Rosella, D.; Papi, P.; Pompa, G.; Capogreco, M.; De Angelis, F.; Di Carlo, S. Dental students’ knowledge of medication-related osteonecrosis of the jaw. Eur. J. Dent. 2017, 11, 461–468. [Google Scholar] [CrossRef][Green Version]
- Al-Eid, R.; Alduwayan, T.; Bin Khuthaylah, M.; Al Shemali, M. Dentists’ knowledge about medication-related osteonecrosis of the jaw and its management. Heliyon 2020, 6, e04321. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Acharya, S.; Vineetha, R.; Nikhil, K. Awareness About Medication-Related Osteonecrosis of the Jaw Among Dental Professionals: A Multicentre Study. Oral Health Prev. Dent. 2020, 18, 1–5. [Google Scholar]
- Mücke, T.; Koschinski, J.; Deppe, H.; Wagenpfeil, S.; Pautke, C.; Mitchell, D.A.; Wolff, K.-D.; Hölzle, F. Outcome of treatment and parameters influencing recurrence in patients with bisphosphonate-related osteonecrosis of the jaws. J. Cancer Res. Clin. Oncol. 2010, 137, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, S.; Troeltzsch, M.; Hafner, S.; Kaeppler, G.; Mast, G.; Otto, S. Surgical treatment of medication-related osteonecrosis of the upper jaw: Case series. Oral Dis. 2019, 25, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Reich, W.; Bilkenroth, U.; Schubert, J.; Wickenhauser, C.; Eckert, A.W. Surgical treatment of bisphosphonate-associated osteonecrosis: Prognostic score and long-term results. J. Cranio-Maxillofac. Surg. 2015, 43, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Trental-Compressa a Rilascio Modificato (Pentoxifillina). Available online: https://www.codifa.it/farmaci/t/trental-compressa-a-rilascio-modificato-pentoxifillina-vasodilatatori-periferici (accessed on 20 December 2020).
- Tofthagen, C. Threats to Validity in Retrospective Studies. J. Adv. Pract. Oncol. 2012, 3, 181–183. [Google Scholar] [CrossRef]







| Demographic and Clinical Data | Number of Patients (%) |
|---|---|
| Gender | |
| -Male | 11 (31.4%) |
| -Female | 24 (68.6%) * |
| Age, years | Years |
| Range | 51–93 |
| Mean, SD | 73.46 ± 9.29 |
| Concomitant cancer therapies | |
| -Steroids | 4 (11.4%) |
| -Chemotherapy | 6 (17.1%) |
| -Steroids and Chemotherapy | 4 (11.4%) |
| -No steroids, No Chemotherapy | 21 (60%) |
| Primary disease requiring anti-resorptive drugs | |
| -Breast cancer | 10 (28.6%) |
| -Prostate cancer | 4 (11.4%) |
| -Multiple myeloma | 7 (20%) |
| -Osteoporosis | 14 (40%) |
| Type of drug associated with MRONJ | |
| Zolendronate | 17 (48.5%) * |
| Alendronate | 9 (25.7%) |
| Denosumab | 2 (5.7%) |
| Alendronate + Denosumab | 2 (5.7%) |
| Alendronate + Risendronate | 1(2.9%) |
| Alendronate + Zolendronate | 1(2.9%) |
| Alendronate + Ibandronate | 1(2.9%) |
| Ibandronate + Clodronate | 1 (2.9%) |
| Zolendronate + Denosumab | 1 (2.9%) |
| Stage of MRONJ | |
| -Stage I | 6 (17.1%) |
| -Stage II | 28 (80%) |
| -Stage III | 1 (2.9%) * |
| MRONJ localization | |
| Maxilla | 12 (34.2%) |
| Mandible | 24 (68.5%) * ψ |
| MRONJ-Related Therapy | Months |
|---|---|
| Duration of therapy | |
| -Zoledronate | 34.29 ± 33.42 |
| -Alendronate | 79.42 ± 63.33 |
| -Denosumab | 15 ± 7.94 |
| Suspension of drug | |
| -Zoledronate | 8.53 ± 20.21 |
| -Alendronate | 13.15 ± 19.58 |
| -Denosumab | 0.8 ± 1.1 |
| Age (Years) | Cause of Anti-Resorptive Treatment | Gender | Type of MRONJ-Related Drug | UCONNS Score | Stage of MRONJ | Site of MRONJ | Outcomes |
|---|---|---|---|---|---|---|---|
| 68 | Cancer | Female | Alendronate | 12 | Stage II | Mandible | Healed |
| 65 | Cancer | Female | Zoledronate | 23 | Stage II | Mandible | Worsened |
| 53 | Cancer | Female | Zoledronate | 31 | Stage II | Mandible | Stable |
| 51 | Cancer | Female | Zoledronate-Denosumab | 24 | Stage II | Maxilla | Worsened |
| 93 | Osteoporosis | Female | Alendronate-Denosumab | 13 | Stage II | Maxilla/Mandible | Healed |
| 85 | Cancer | Female | Zoledronate | 26 | Stage II | Maxilla | Healed |
| 65 | Cancer | Male | Denosumab | 22 | Stage II | Mandible | Healed |
| 90 | Osteoporosis | Female | Alendronate | 8 | Stage II | Maxilla | Healed |
| 77 | Cancer | Male | Zoledronate | 21 | Stage II | Mandible | Healed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varoni, E.M.; Lombardi, N.; Villa, G.; Pispero, A.; Sardella, A.; Lodi, G. Conservative Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Retrospective Cohort Study. Antibiotics 2021, 10, 195. https://doi.org/10.3390/antibiotics10020195
Varoni EM, Lombardi N, Villa G, Pispero A, Sardella A, Lodi G. Conservative Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Retrospective Cohort Study. Antibiotics. 2021; 10(2):195. https://doi.org/10.3390/antibiotics10020195
Chicago/Turabian StyleVaroni, Elena M., Niccolò Lombardi, Giulio Villa, Alberto Pispero, Andrea Sardella, and Giovanni Lodi. 2021. "Conservative Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Retrospective Cohort Study" Antibiotics 10, no. 2: 195. https://doi.org/10.3390/antibiotics10020195
APA StyleVaroni, E. M., Lombardi, N., Villa, G., Pispero, A., Sardella, A., & Lodi, G. (2021). Conservative Management of Medication-Related Osteonecrosis of the Jaws (MRONJ): A Retrospective Cohort Study. Antibiotics, 10(2), 195. https://doi.org/10.3390/antibiotics10020195







