Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic
Abstract
1. Introduction
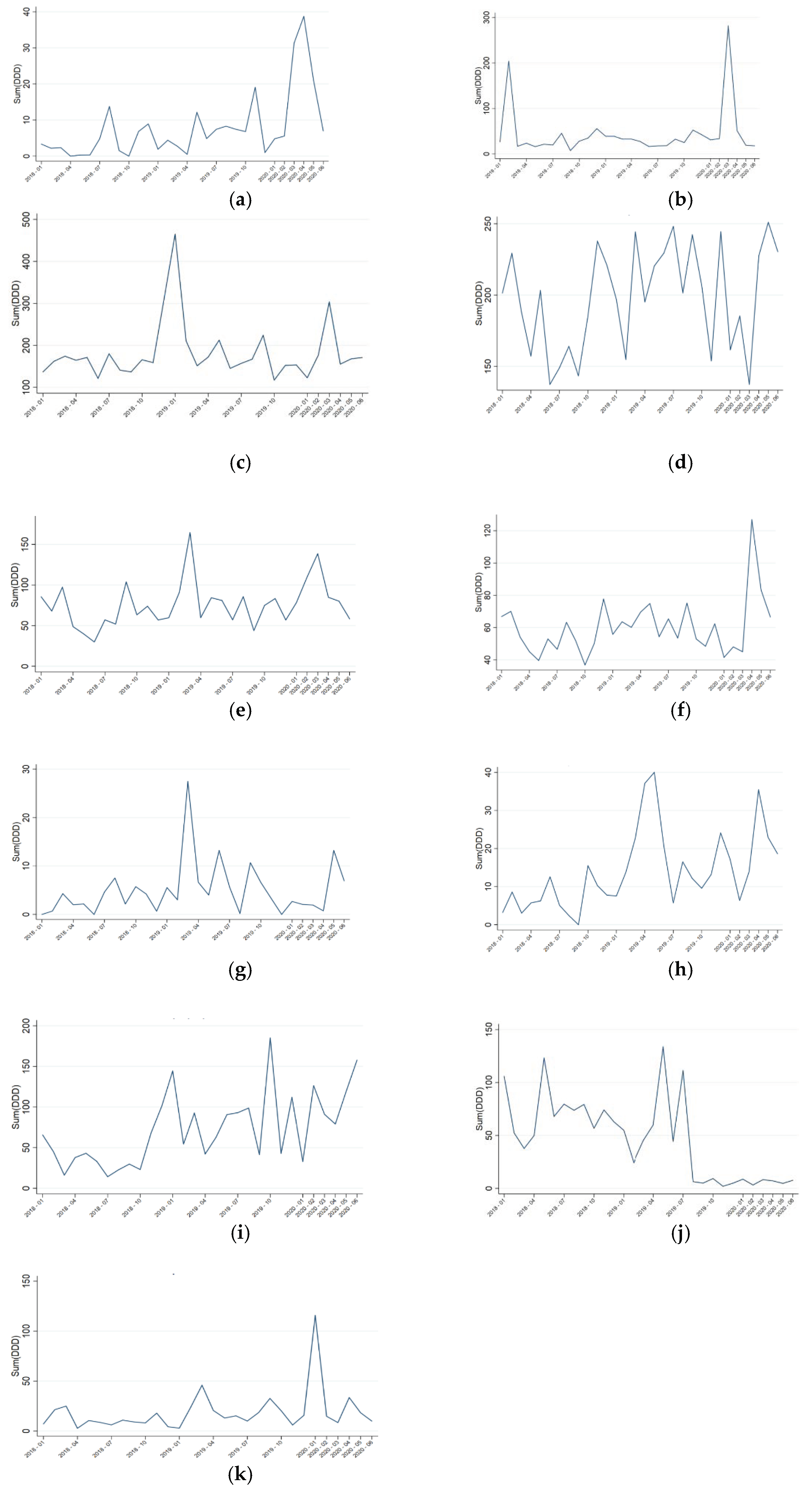
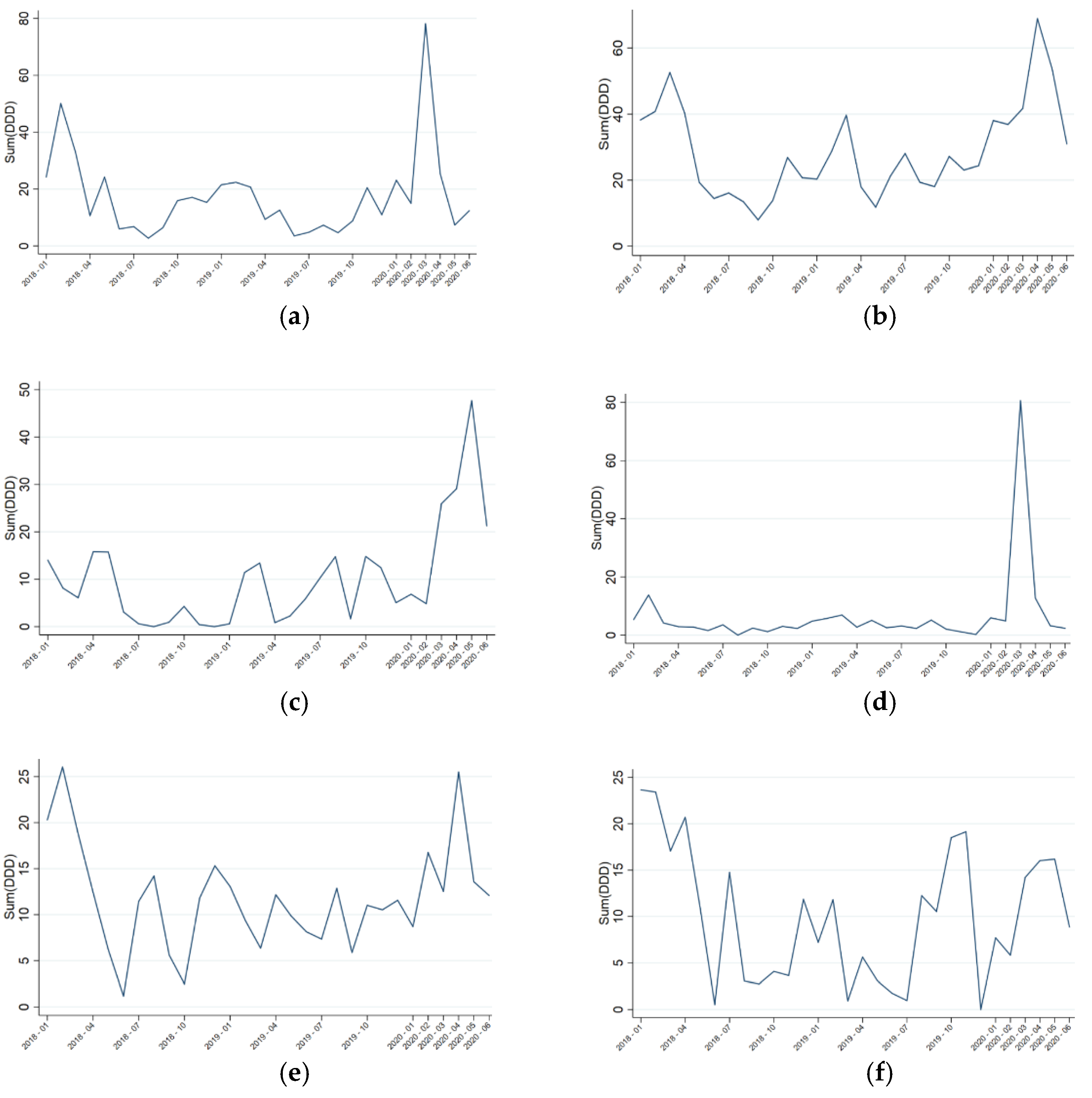
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Hospital Setting
4.2. Study Period and Study Population
4.3. Impact of the COVID-19 Pandemic
4.4. Antimicrobial Consumption
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Programas de Optimización de Uso de los Antibióticos (PROA) | PRAN. Available online: https://www.resistenciaantibioticos.es/es/programas-de-optimizacion-de-uso-de-los-antibioticos-proa (accessed on 18 December 2020).
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health & Human Services—CDC: New York, NY, USA, 2019.
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Beović, B.; Pulcini, C.; Tacconelli, E.; Hulscher, M.; Cookson, B.; Ashiru-Oredope, D.; Barcs, I.; Blix, H.S.; Buyle, F.; et al. ESCMID generic competencies in antimicrobial prescribing and stewardship: Towards a European consensus. Clin. Microbiol. Infect. 2019, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Paño-Pardo, J.R.; Alvarez-Rocha, L.; Asensio, Á.; Calbo, E.; Cercenado, E.; Cisneros, J.M.; Cobo, J.; Delgado, O.; Garnacho-Montero, J.; et al. Programas de optimización de uso de antimicrobianos (PROA) en hospitales españoles: Documento de consenso GEIH-SEIMC, SEFH y SEMPSPH. Enferm. Infecc. Microbiol. Clin. 2012, 30. [Google Scholar] [CrossRef] [PubMed]
- Guisado-Gil, A.B.; Infante-Domínguez, C.; Peñalva, G.; Praena, J.; Roca, C.; Navarro-Amuedo, M.D.; Aguilar-Guisado, M.; Espinosa-Aguilera, N.; Poyato-Borrego, M.; Romero-Rodríguez, N.; et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics 2020, 9, 816. [Google Scholar] [CrossRef]
- Youngs, J.; Wyncoll, D.; Hopkins, P.; Arnold, A.; Ball, J.; Bicanic, T. Improving antibiotic stewardship in COVID-19: Bacterial co-infection is less common than with influenza. J. Infect. 2020, 81, e55–e57. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin. Infect. Dis. 2019, 68, 895–902. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020. [Google Scholar] [CrossRef]
- Adarsh Bhimraj, A.; Morgan, R.L.; Hirsch Shumaker, A.; Lavergne, V.; Baden, L.; Chi-Chung Cheng, V.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of patients with COVID-19. Idsa 2020. Available online: https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt-v3.6.0.pdf (accessed on 21 January 2021).
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, 18–19. [Google Scholar] [CrossRef]
- Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 3 August 2020).
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 21 January 2021).
- Ginsburg, A.S.; Klugman, K.P. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob. Health 2020, 8, e1453–e1454. [Google Scholar] [CrossRef]
- Zhu, N.J.; McLeod, M.; McNulty, C.A.M.; Lecky, D.M.; Holmes, A.H.; Ahmad, R. Trends in Antibiotic Prescribing in Out-of-Hours Primary Care in England from January 2016 to June 2020 to Understand Behaviours during the First Wave of COVID-19. Antibiotics 2021, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Abelenda-Alonso, G.; Padullés, A.; Rombauts, A.; Gudiol, C.; Pujol, M.; Alvarez-Pouso, C.; Jodar, R.; Carratalà, J. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infect. Control Hosp. Epidemiol. 2020, 41, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zorn, B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Kollef, M.H.; Timsit, J.F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Mayoral, T.N.; Gómez, M.A.M.; Rosselló, G.A.M.; Fuertes, L.P.; Benito, E.C.; García, A.M.M.; Martín, A.B.M.; Domingo, A.O. Infección bacteriana/fúngica en pacientes con COVID-19 ingresados en un hospital de tercer nivel de Castilla y León, España. Enferm. Infecc. Microbiol. Clin. 2020. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Mph, B.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; Macfadden, D.R.; Langford Pharmd, B.J.; et al. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 10. [Google Scholar] [CrossRef]
- Beovic, B.; Dousak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A “snapshot” Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef]
- Koehler, P.; Meis, J.F.; Ostrosky-zeichner, L.; Böll, B.; Cornely, O.A.; Eichenauer, D.A. Covid-19/Influenza-Associated Pulmonary Aspergillosis—Management; Uniklinik Köln: Köln, Germany, 2020; pp. 6–7. [Google Scholar] [CrossRef]
- Khor, W.P.; Olaoye, O.; D’arcy, N.; Krockow, E.M.; Elshenawy, R.A.; Rutter, V.; Ashiru-Oredope, D. The need for ongoing antimicrobial stewardship during the COVID-19 pandemic and actionable recommendations. Antibiotics 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.S.; Wolf, I.; Schwei, R.J.; Chen, D.; Lepak, A.J.; Schulz, L.T.; Safdar, N. Antibiotic prescribing patterns for COVD-19 in two emergency departments with rapid procalcitonin. Infect. Control Hosp. Epidemiol. 2020, 2019, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.Y.; Kanitkar, T.; Richardson, C.J.; Lanzman, M.; Stone, Z.; Mahungu, T.; Mack, D.; Wey, E.Q.; Lamb, L.; Balakrishnan, I.; et al. Exclusion of bacterial co-infection in COVID-19 using baseline inflammatory markers and their response to antibiotics Claire. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Álvarez-Lerma, F.; Grau, S.; Echeverría-Esnal, D.; Martínez-Alonso, M.; Gracia-Arnillas, M.P.; Horcajada, J.P.; Masclans, J.R. A Before-and-After Study of the Effectiveness of an Antimicrobial Stewardship Program in Critical Care. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Horcajada, J.P.; Grau, S.; Echeverría, D.; Padullés, A.; Gimenez, M.; Oliva, G.; Boix, L.; Ferrer, R.; Limón, E.; et al. INFORME DADES 2018 Monitoratge estandarditzat del consum hospitalari d’antimicrobians Programa VINCat Vigilància de les Infeccions Nosocomials als Hospitals de Catalunya; Departamento de Salud Generalidad de Cataluña: Barcelona, Spain, 2019.
- VINCat Program. Programa de Vigilància de les Infeccions Nosocomials a Catalunya. Generalitat de Catalunya. Departament de Salut. Available online: http://catsalut.gencat.cat/ca/proveidors-professionals/vincat/ (accessed on 25 June 2017).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 132. https://doi.org/10.3390/antibiotics10020132
Grau S, Echeverria-Esnal D, Gómez-Zorrilla S, Navarrete-Rouco ME, Masclans JR, Espona M, Gracia-Arnillas MP, Duran X, Comas M, Horcajada JP, et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics. 2021; 10(2):132. https://doi.org/10.3390/antibiotics10020132
Chicago/Turabian StyleGrau, Santiago, Daniel Echeverria-Esnal, Silvia Gómez-Zorrilla, Maria Eugenia Navarrete-Rouco, Joan Ramon Masclans, Merce Espona, Maria Pilar Gracia-Arnillas, Xavier Duran, Merce Comas, Juan Pablo Horcajada, and et al. 2021. "Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic" Antibiotics 10, no. 2: 132. https://doi.org/10.3390/antibiotics10020132
APA StyleGrau, S., Echeverria-Esnal, D., Gómez-Zorrilla, S., Navarrete-Rouco, M. E., Masclans, J. R., Espona, M., Gracia-Arnillas, M. P., Duran, X., Comas, M., Horcajada, J. P., & Ferrández, O. (2021). Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics, 10(2), 132. https://doi.org/10.3390/antibiotics10020132









