New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus
Abstract
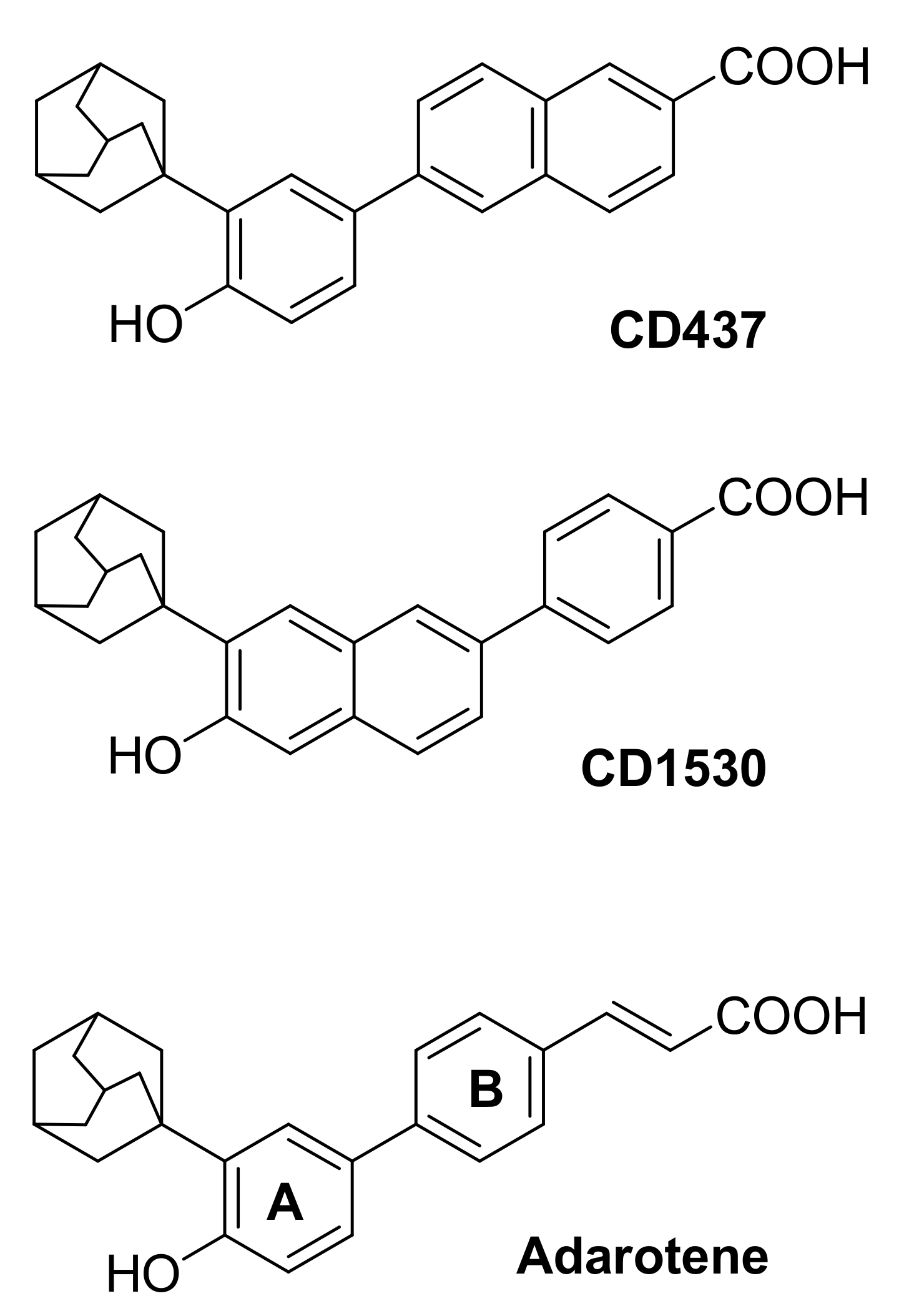
1. Introduction
2. Results and Discussion
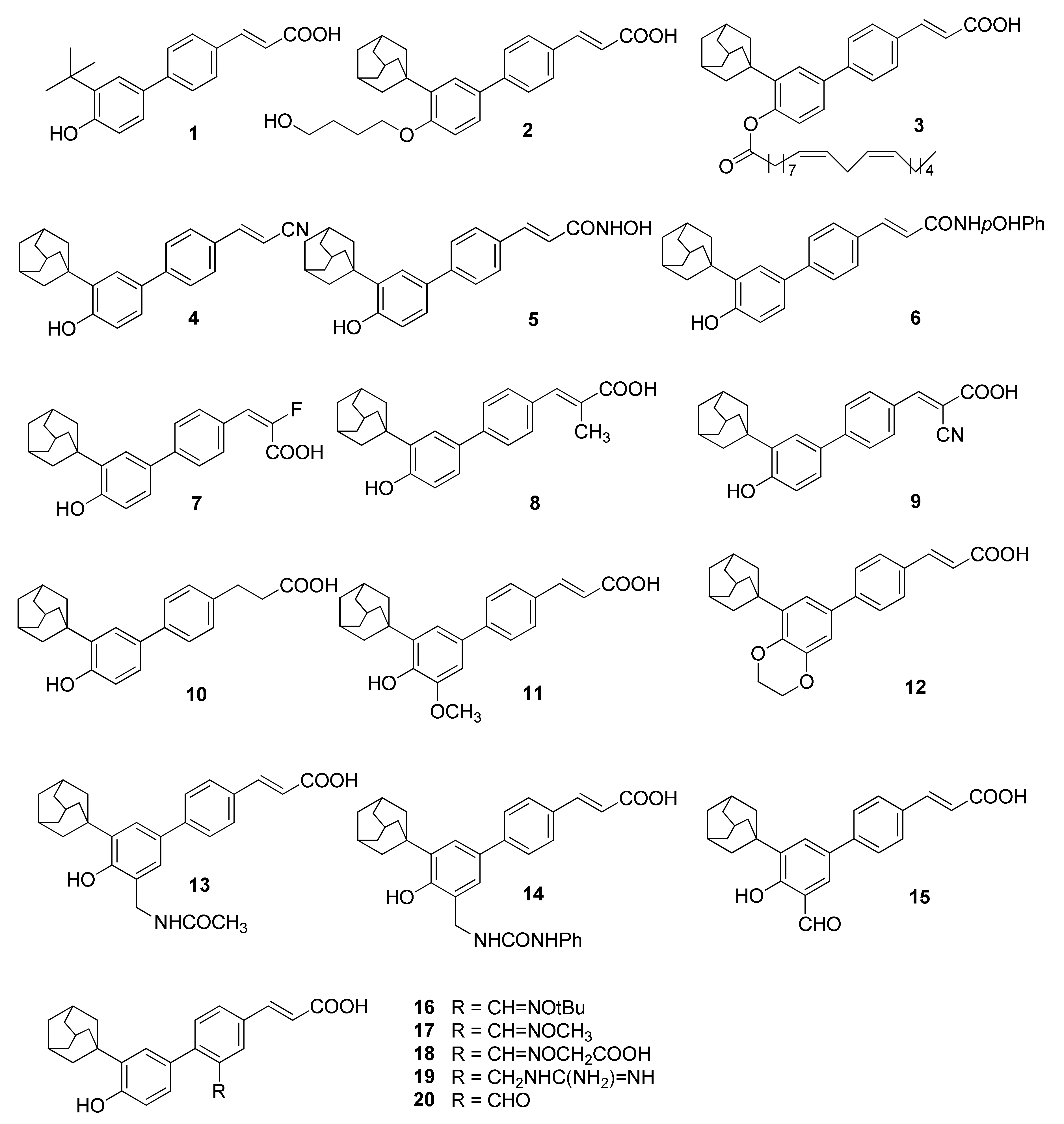
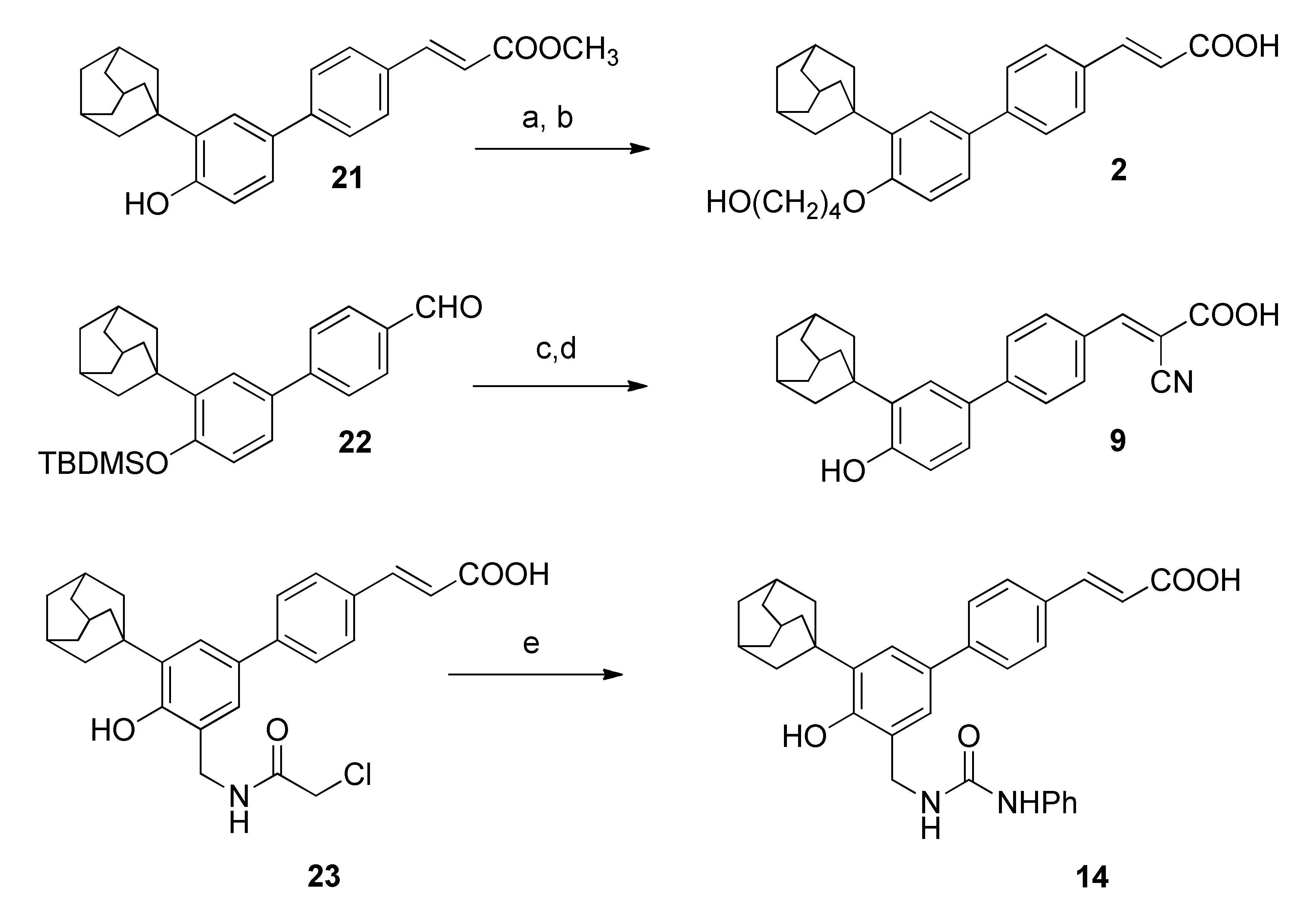
2.1. Chemistry
2.2. Antimicrobial Activity
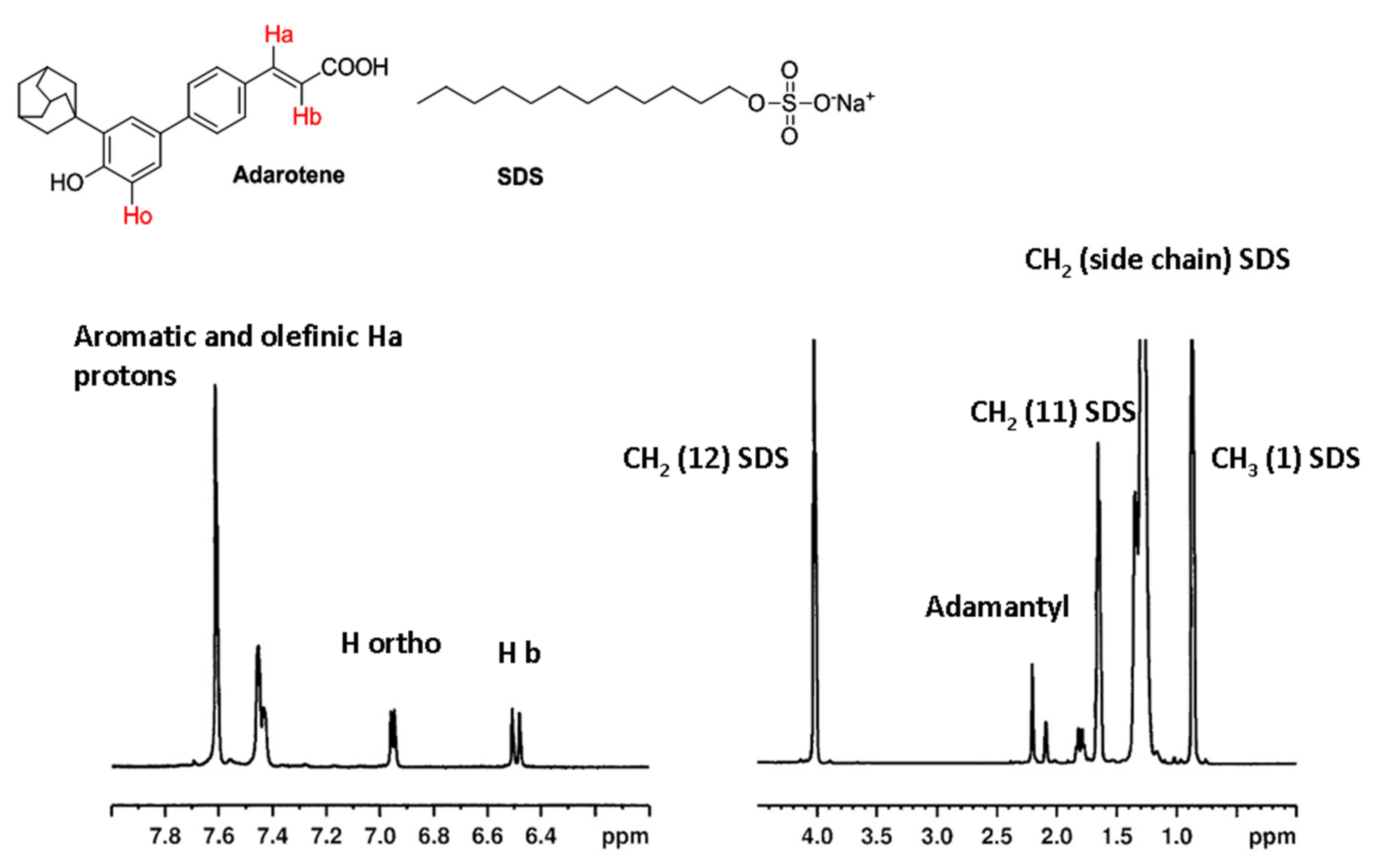
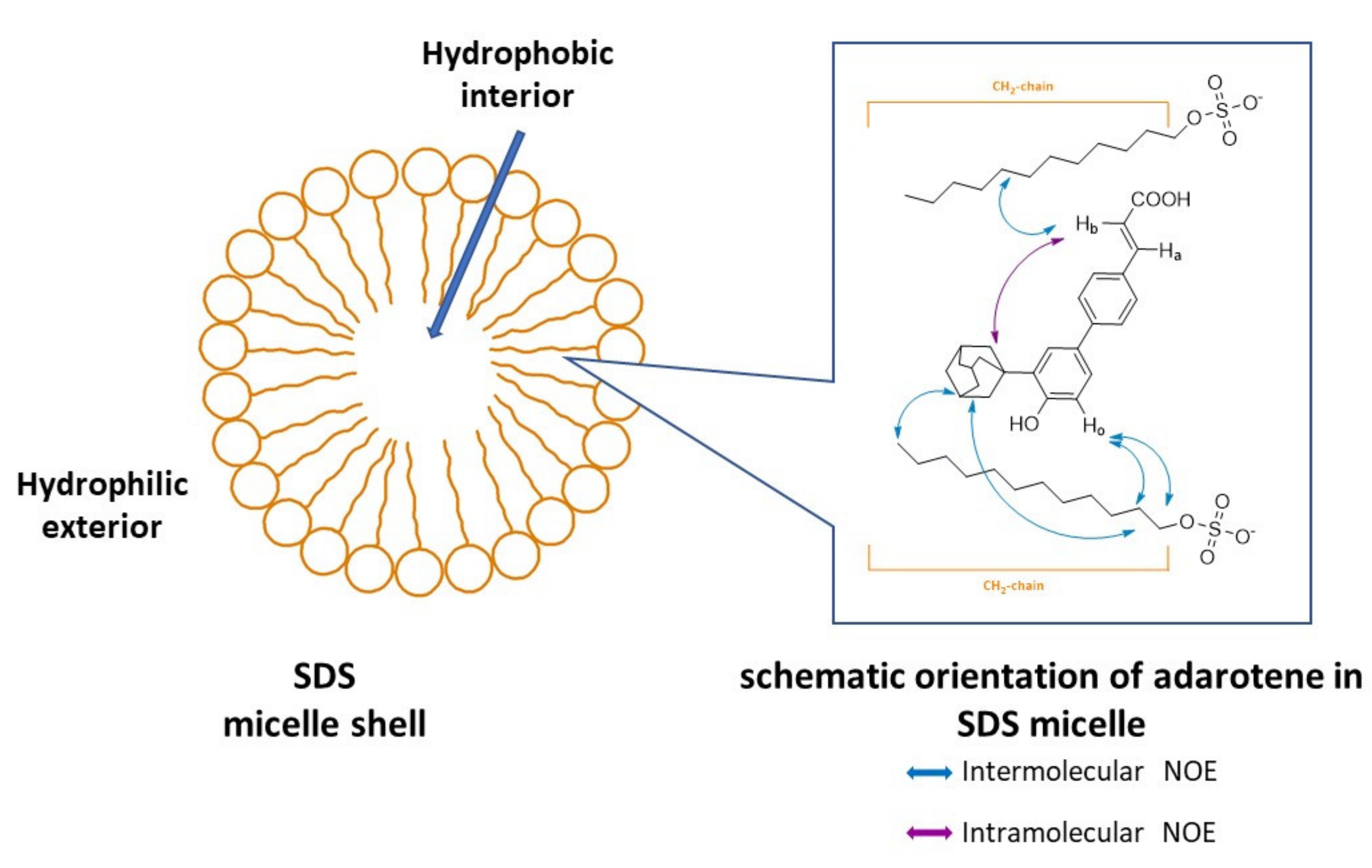
2.3. NMR Investigation
3. Materials and Methods
3.1. Chemistry. General Information
3.2. NMR Investigation on the Interaction of Adarotene with a Model of Microorganism Membrane
3.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K., Jr.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ’20 Progress—Development of new drugs active against gram-negative bacilli: An update from the infectious diseases society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Arena, F.; Giani, T. 138—Mechanisms of Antibacterial Resistance. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1181–1196.e1. [Google Scholar] [CrossRef]
- Arias, C.C.; Murray, B.E. A new antibiotic and the evolution of resistance. N. Engl. J. Med. 2015, 372, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final report and recommendations. Rev. Antimicrob. Resist. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 10 December 2020).
- Inter Agency Coordination Group on AMR. AMR Framework for Action Supported by the IACG. Working Document. McKinsey & Company. 2017. Available online: http://www.who.int/antimicrobial-resistance/interagency-coordination-group/20170818_AMR_FfA_v01.pdf (accessed on 10 December 2020).
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe—Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017; ECDC: Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf (accessed on 10 December 2020).
- Cassini, A.; Diaz Högberg, L.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Skov Simonsen, G.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Ali, J.; Rafiq, Q.A.; Ratcliffe, E. Antimicrobial resistance mechanisms and potential synthetic treatments. Future Sci. OA 2018, 4, FSO290. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Magrini, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 10 December 2020).
- Kim, W.; Zhu, W.; Hendricks, G.L.; Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Dallavalle, S.; Nannei, R.; Carella, S.; De Zani, D.; Merlini, L.; Penco, S.; Garattini, E.; Giannini, G.; Pisano, C.; et al. Synthesis and structure−activity relationships of a new series of retinoid-Related biphenyl-4-ylacrylic acid endowed with antiproliferative and proapoptotic activity. J. Med. Chem. 2005, 48, 4931–4946. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Dallavalle, S.; Nannei, R.; Merlini, L.; Penco, S.; Giannini, G.; Pisano, C.; Vesci, L.; Ferrara, F.F.; Zuco, V.; et al. Synthesis and structure-activity relationships of new antiproliferative and proapoptotic retinoid-related biphenyl-4-yl-acrylic acids. Bioorg. Med. Chem. 2007, 15, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Musso, L.; Giannini, G.; Zuco, V.; De Cesare, M.; Zunino, F.; Dallavalle, S. Influence of the adamantyl moiety on the activity of biphenylacrylohydroxamic acid-based HDAC inhibitors. Eur. J. Med. Chem. 2014, 79, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Brunetti, T.; Battistuzzi, G.; Alloatti, D.; Quattrociocchi, G.; Cima, M.G.; Merlini, L.; Dallavalle, S.; Cincinelli, R.; Nannei, R.; et al. New retinoid derivatives as back-ups of Adarotene. Bioorg. Med. Chem. 2012, 20, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Musso, L.; Guglielmi, M.B.; La Porta, I.; Fucci, A.; D’Andrea, L.E.; Cardile, F.; Colelli, F.; Signorino, G.; Darwiche, N.; et al. Novel adamantyl retinoid-related molecules with POLA1 inhibitory activity. Bioorg. Chem. 2020, 104, 104253. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.V.; Kim, W.; Escobar, I.E.; Mylonakis, E.; Wuest, W.M. Structure−activity relationship and anticancer profile of second-generation anti-MRSA synthetic retinoids. ACS Med. Chem. Lett. 2020, 11, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatullin, I.Z.; Galiullina, L.F.; Klochkova, E.A.; Latfullin, I.A.; Aganov, A.V.; Klochkov, V.V. Structural studies of pravastatin and simvastatin and their complexes with SDS micelles by NMR spectroscopy. J. Mol. Struct. 2016, 1105, 25–29. [Google Scholar] [CrossRef]
- Usachev, K.S.; Filippov, A.V.; Filippova, E.A.; Antzutkin, O.N.; Klochkov, V.V. Solution structures of Alzheimer’s amyloid Aβ13–23 peptide: NMR studies in solution and in SDS. J. Mol. Struct. 2013, 1049, 436–440. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Standard: M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Standard: M26; Methods for Determining Bactericidal Activity of Antimicrobial Agents, 1st ed.; CLSI: Wayne, PA, USA, 1999. [Google Scholar]
- Levison, M.E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, T.; Ma, X.X.; Watanabe, S.; Kreiswirth, B.N.; Etienne, J.; Hiramatsu, K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: Rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 2007, 51, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Dutka-Malen, S.; Evers, S.; Courvalin, P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 1995, 33, 24–27. [Google Scholar] [CrossRef] [PubMed]

| S. aureus Strain 1 | E. faecalis Strain 1 | ||||
|---|---|---|---|---|---|
| cpd | IC50 a (µM) | MIC [MBC] g | MIC [MBC] g | ||
| (µg/mL) | (µM) | (µg/mL) | (µM) | ||
| Adarotene | 0.23 ± 0.08 b | 8 [16] | 21 [42] | 1 [32] | 3 [85] |
| 1 | 30 b | 64 | 216 | >256 | >864 |
| 2 | >10 c | 256 | 573 | 128 | 287 |
| 3 | 7.8 ± 0.7 f | 32 | 50 | 128 | 201 |
| 4 | 8.3 ± 1.4 b | 128 [128] | 361 [361] | 32 [128] | 90 [360] |
| 5 | 1.1 ± 0.6 d | 64 | 164 | >256 | >657 |
| 6 | 6.6 ± 0.5 b | >256 | >549 | >256 | >550 |
| 7 | >3 b | 4 [16] | 10 [40] | 4 [16] | 10 [40] |
| 8 | 7.19 ± 1.27 b | 2 [8] | 5 [20] | 1 [8] | 3 [21] |
| 9 | >10 f | 2 [4] | 5 [10] | 1 [4] | 2 [10] |
| 10 | 48.42 ± 0.88 b | 16 [16] | 42 [42] | 16 [32] | 42 [85] |
| 11 | 0.52 ± 0.07 e | 16 [32] | 40 [80] | 8 | 20 |
| 12 | 0.23 ± 0.07 e | 4 | 9 | 2 | 5 |
| 13 | 1.24 ± 0.07 e | 8 [16] | 18 [36] | 4 [16] | 9 [36] |
| 14 | >10 f | 8 [8] | 15 [15] | 4 [16] | 8 [31] |
| 15 | 1.64 ± 0.03 e | 64 | 159 | 64 | 159 |
| 16 | >10 c | 4 [4] | 8 [8] | 2 [4] | 4 [8] |
| 17 | >10 c | 4 [4] | 9 [9] | 8 [16] | 19 [38] |
| 18 | >10 c | 128 | 269 | 128 | 269 |
| 19 | >10 c | 32 [64] | 72 [144] | 32 [64] | 72 [144] |
| 20 | 3.2 ± 0.2 c | 2 [32] | 5 [80] | 8 | 20 |
| S. aureus ATCC 25923 | S. aureus Strain 2 | E. faecalis ATCC 51299 | E. faecalis Strain 2 | |||||
|---|---|---|---|---|---|---|---|---|
| cpd | MIC [MBC] a | |||||||
| (µg/mL) | (µM) | (µg/mL) | (µM) | (µg/mL) | (µM) | (µg/mL) | (µM) | |
| Adarotene | 8 [16] | 21 [42] | 4 [8] | 10 [21] | 2 [32] | 5 [84] | 4 [32] | 10 [84] |
| 2 | 256 | 572 | 256 | 572 | 64 | 143 | 128 | 286 |
| 16 | 2 [4] | 4 [8] | 2 [2] | 4 [4] | 2 [8] | 4 [16] | 2 [8] | 4 [16] |
| 17 | 2 [4] | 5 [10] | 4 [32] | 10 [74] | 8 [32] | 18 [74] | 8 [16] | 18 [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Princiotto, S.; Mazzini, S.; Musso, L.; Arena, F.; Dallavalle, S.; Pisano, C. New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus. Antibiotics 2021, 10, 126. https://doi.org/10.3390/antibiotics10020126
Princiotto S, Mazzini S, Musso L, Arena F, Dallavalle S, Pisano C. New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus. Antibiotics. 2021; 10(2):126. https://doi.org/10.3390/antibiotics10020126
Chicago/Turabian StylePrinciotto, Salvatore, Stefania Mazzini, Loana Musso, Fabio Arena, Sabrina Dallavalle, and Claudio Pisano. 2021. "New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus" Antibiotics 10, no. 2: 126. https://doi.org/10.3390/antibiotics10020126
APA StylePrinciotto, S., Mazzini, S., Musso, L., Arena, F., Dallavalle, S., & Pisano, C. (2021). New Antimicrobials Based on the Adarotene Scaffold with Activity against Multi-Drug Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus. Antibiotics, 10(2), 126. https://doi.org/10.3390/antibiotics10020126






