Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics
Abstract
1. Introduction
2. Results
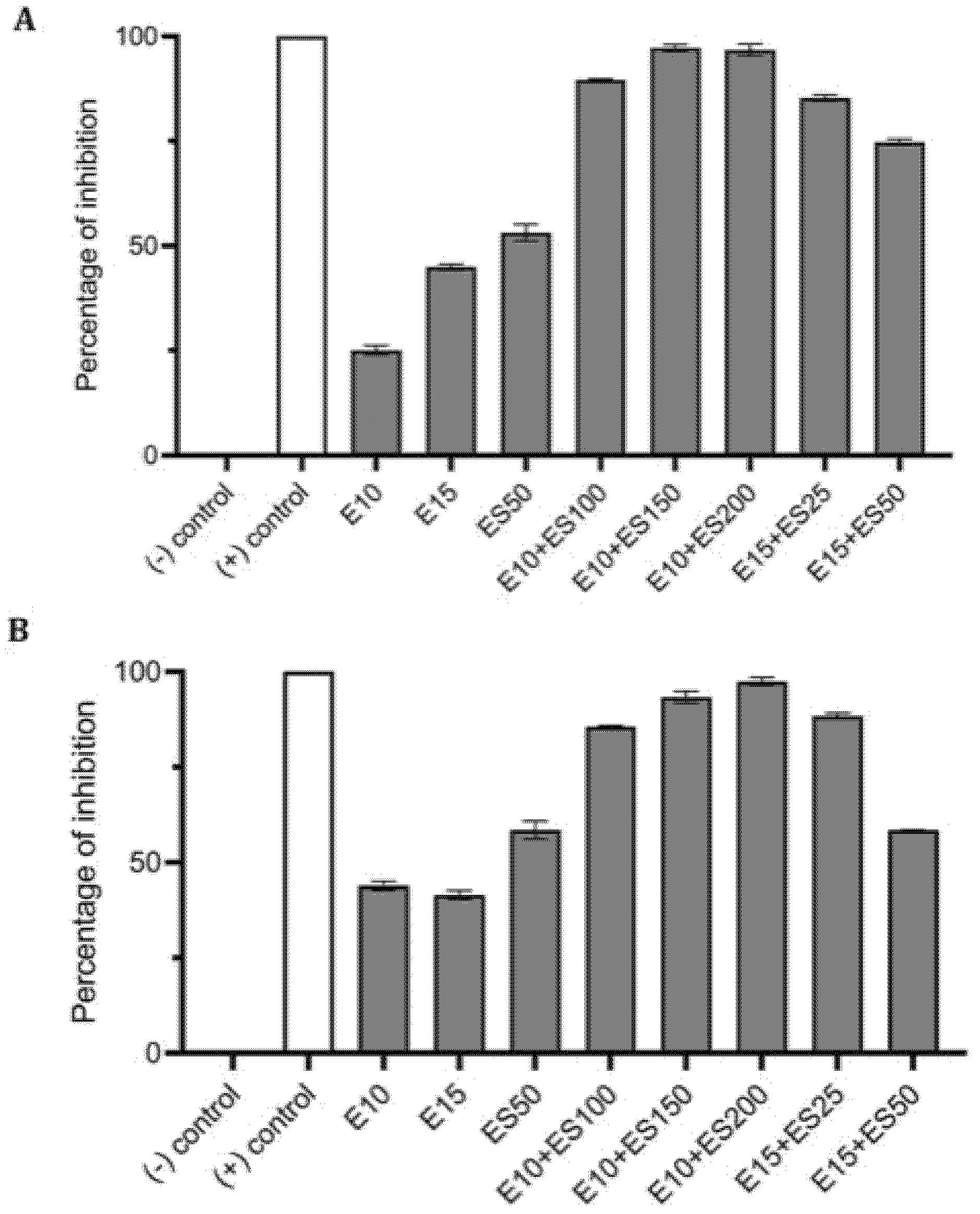
2.1. The Effect of Erythromycin and EGCG-S on E. coli Biofilm Formation
2.2. The Effect of Erythromycin and EGCG-S on M. smegmatis Biofilm Formation
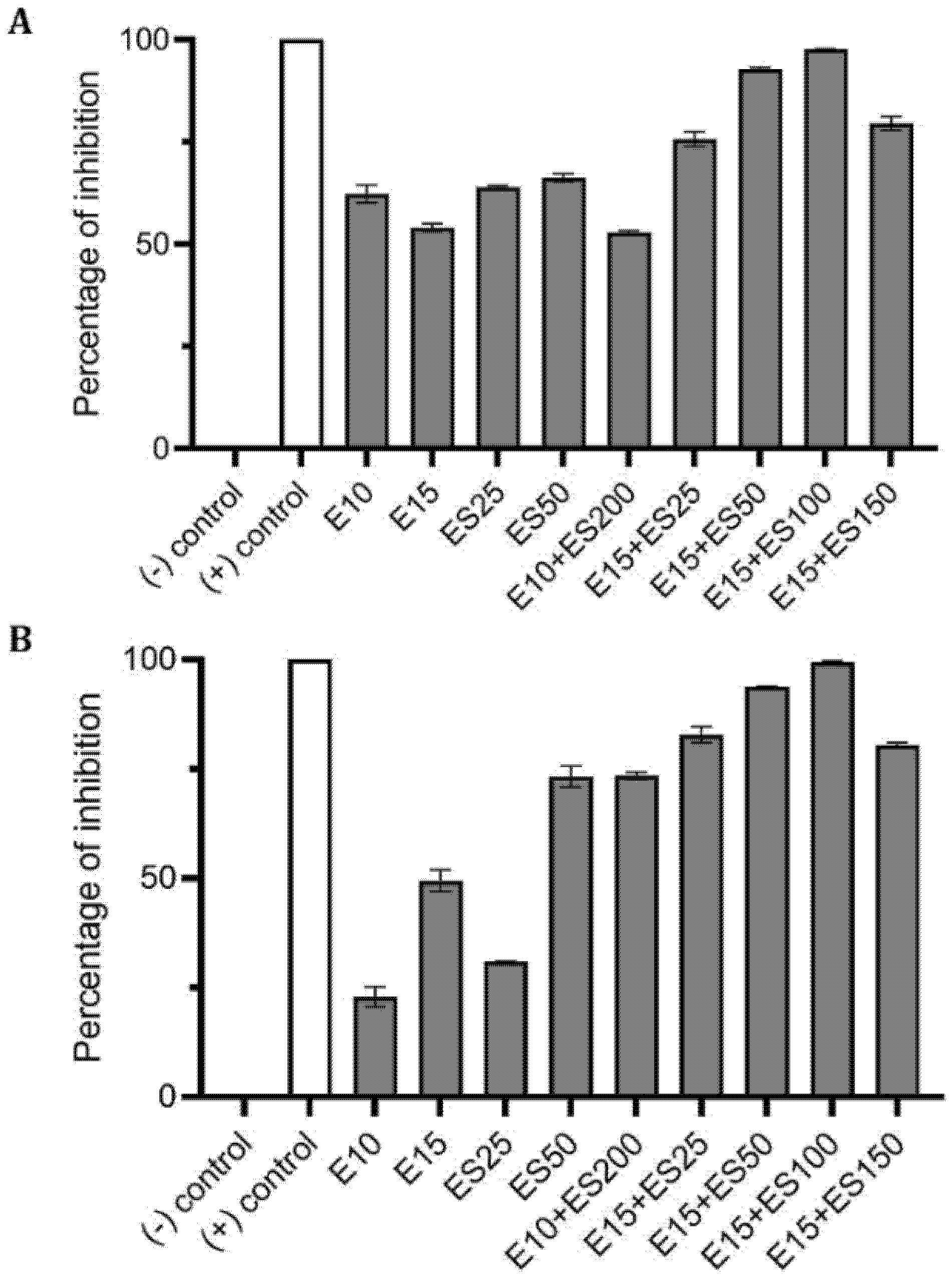
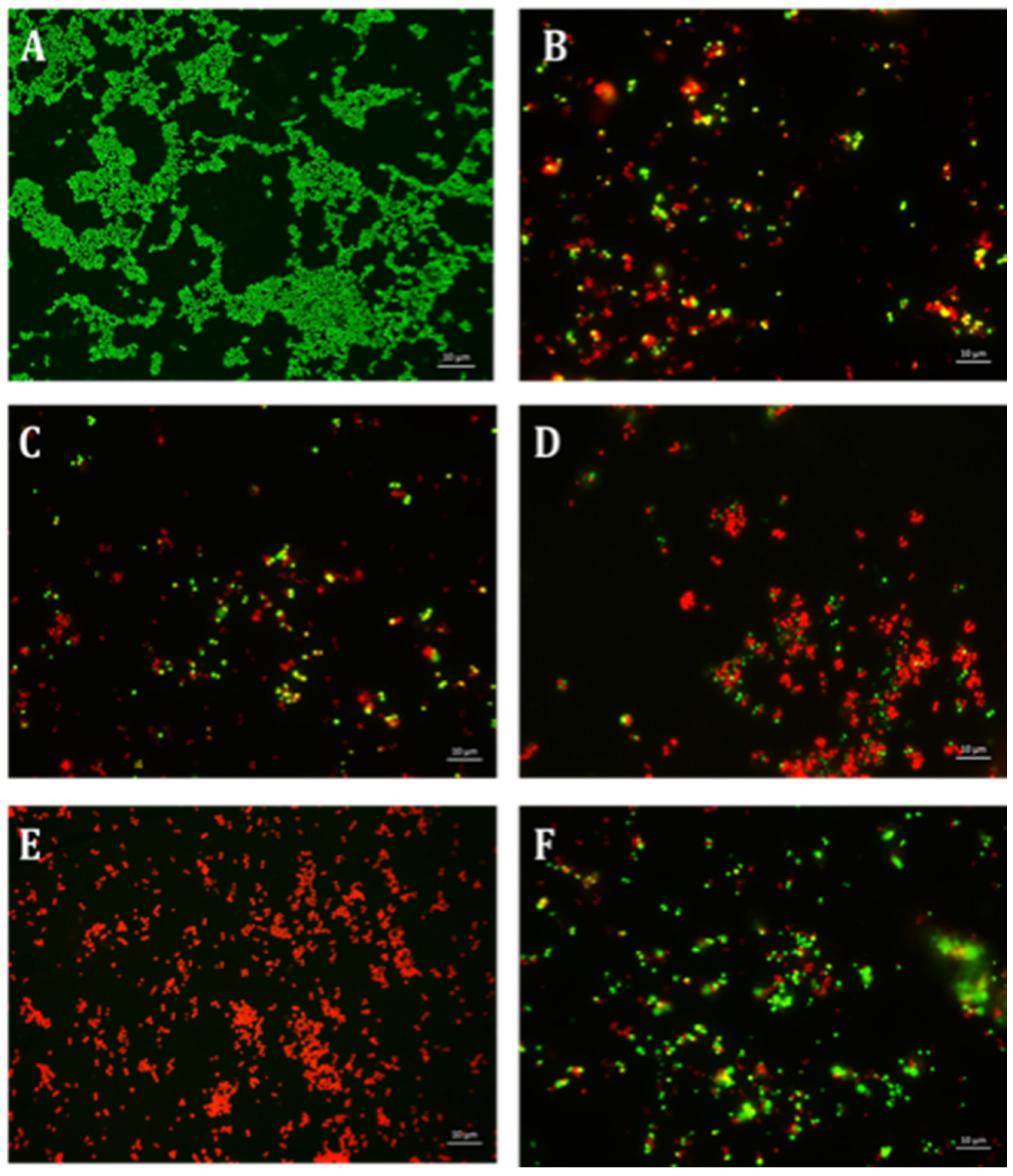
2.3. The Effect of Erythromycin and EGCG-S on P. aeruginosa Biofilm Formation
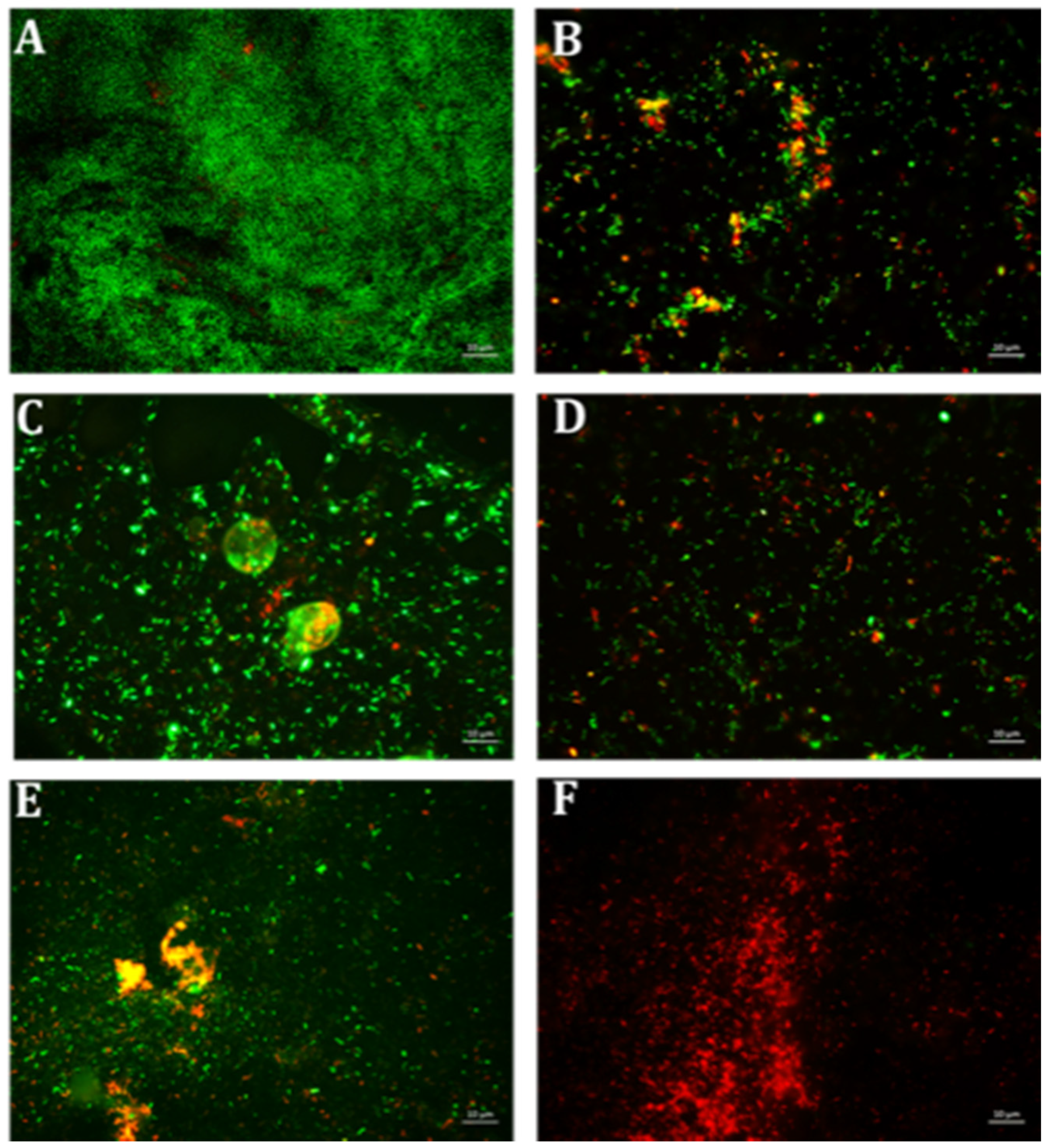
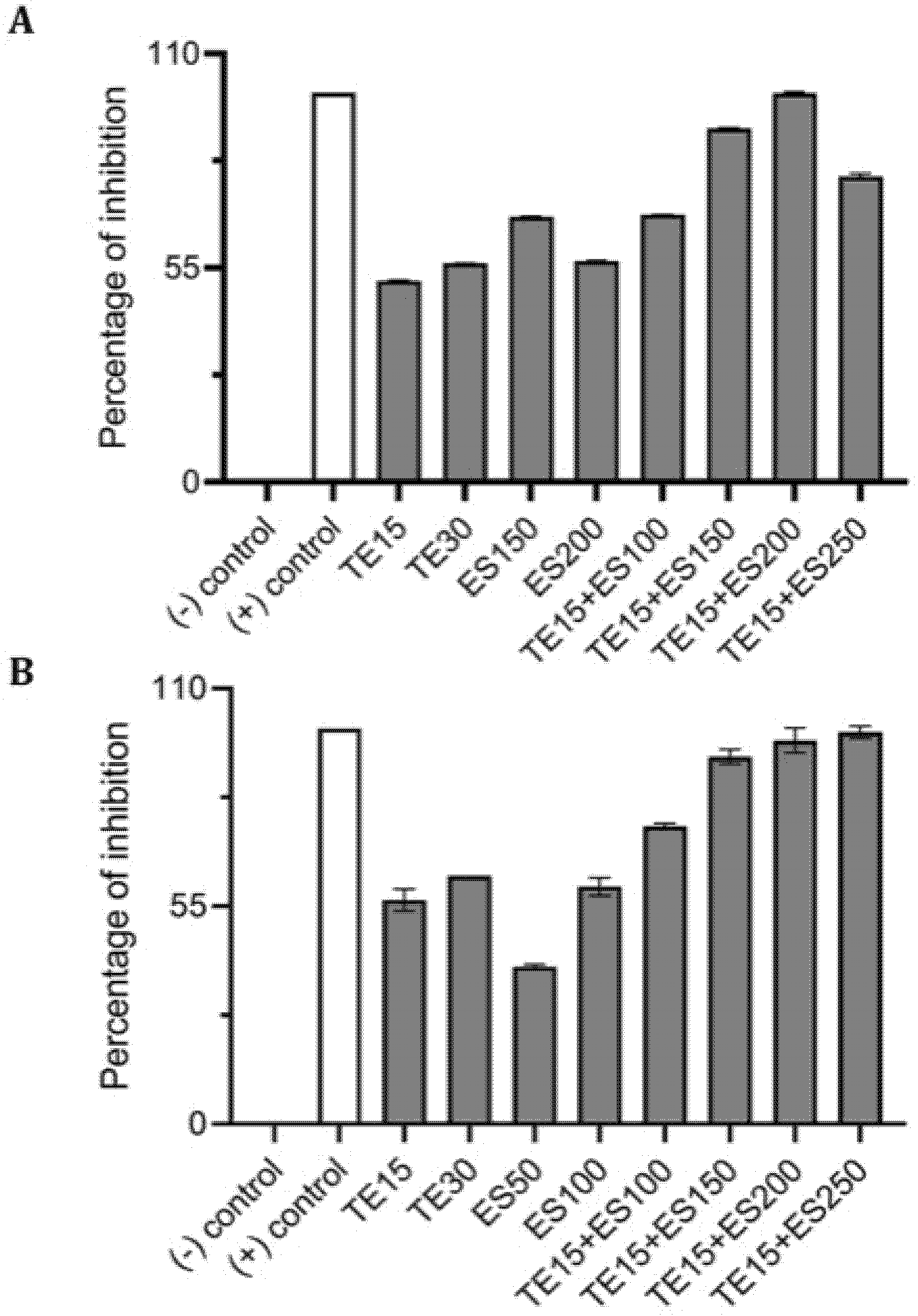
2.4. Synergistic Inhibitory Effect of Tetracycline and EGCG-S on Staphylococcus spp.
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures
4.2. EGCG-S and Antibiotic Formulations
4.3. Quantitative Absorbance-Based Biofilm Measurement (Crystal Violet Assay)
4.4. Quantitative Fluorescence-Based Biofilm Measurement (Resazurin Assay)
4.5. Quantitative Growth-Based Cell Viability Measurement (Colony Forming Unit Assay)
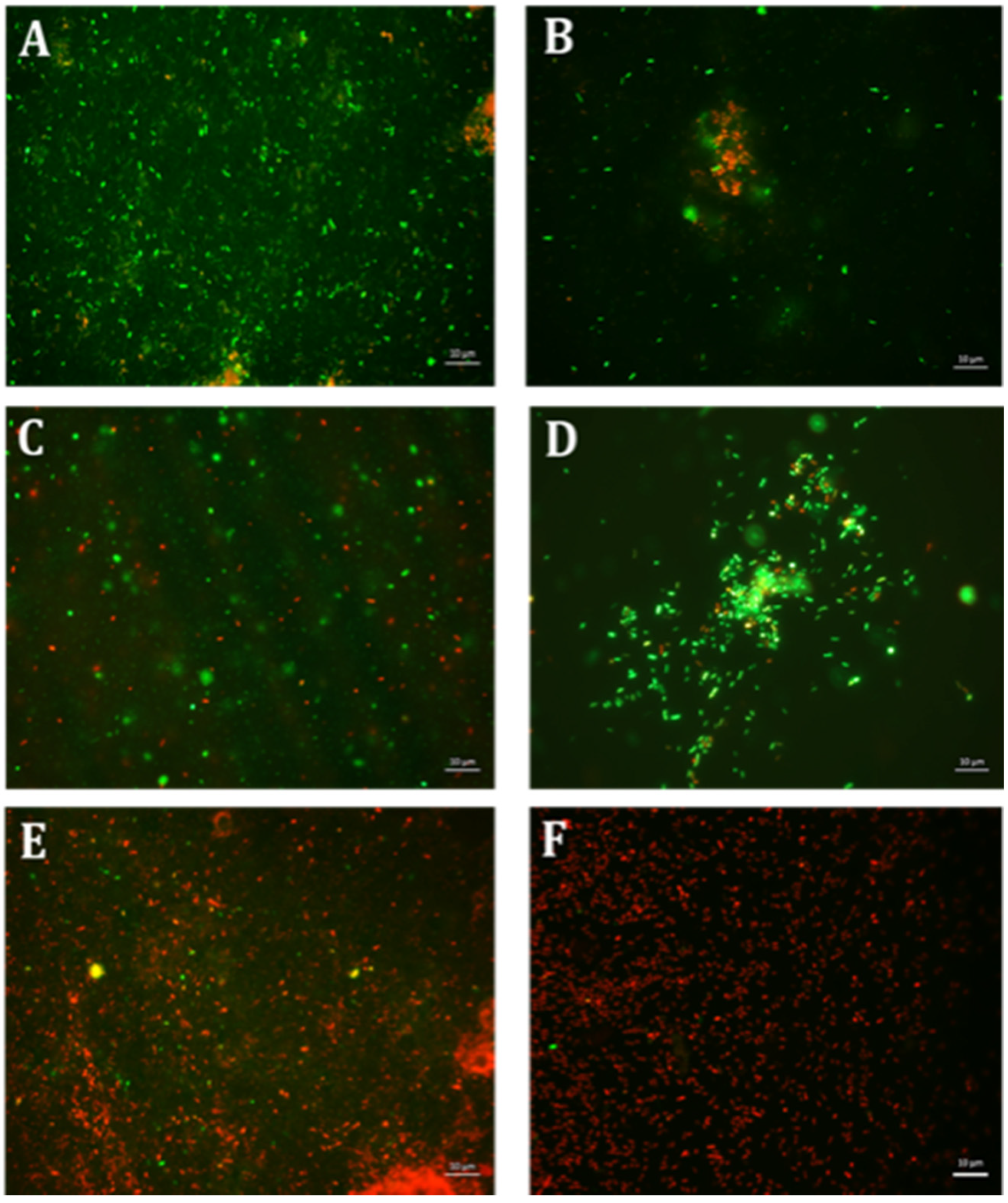
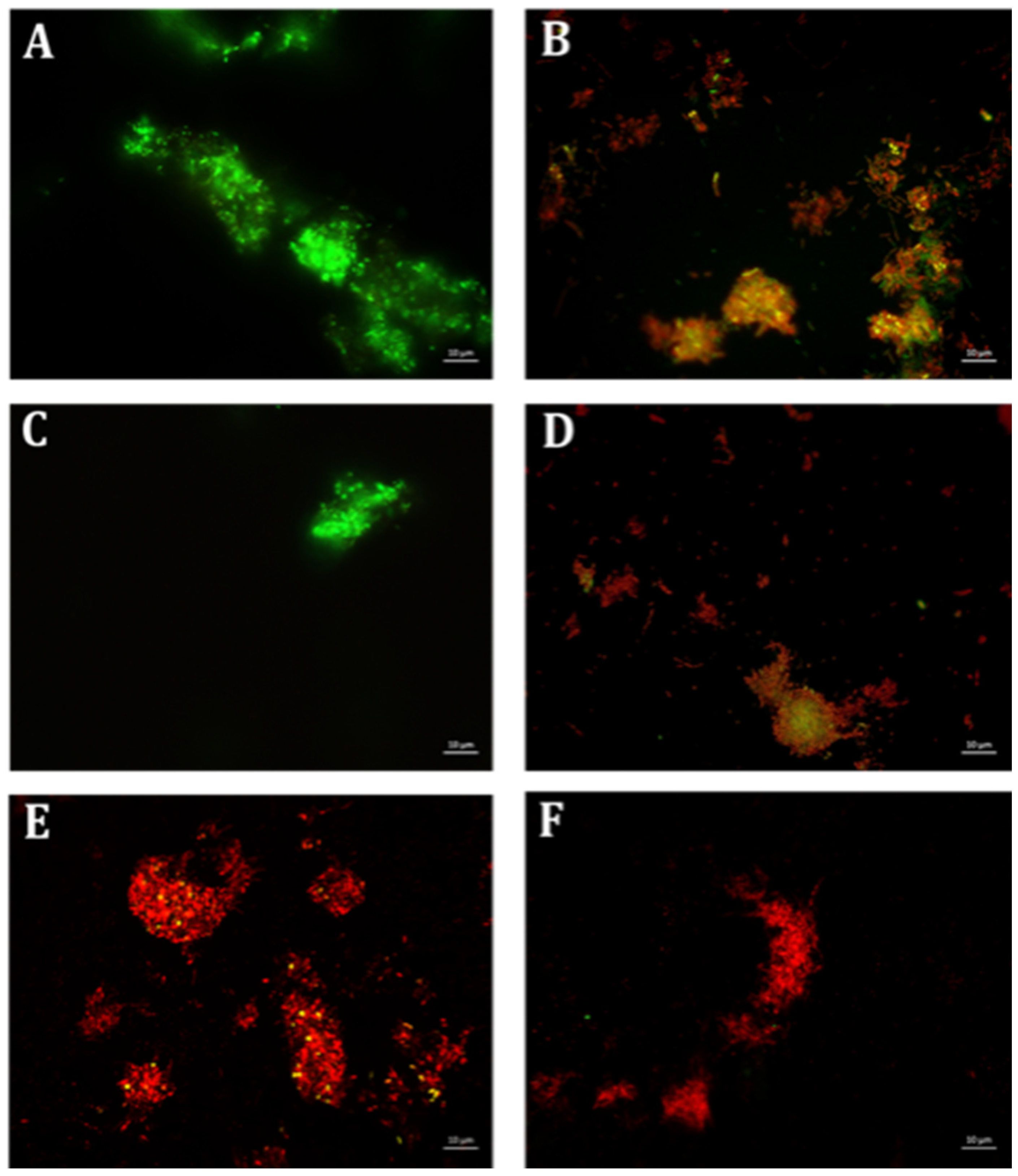
4.6. Qualitative Microscopy-Based Cell Viability Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Romling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Akerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- O’Gara, J.P.; Humphreys, H. Staphylococcus epidermidis biofilms: Importance and implications. J. Med. Microbiol. 2001, 50, 582–587. [Google Scholar] [CrossRef]
- Ojha, A.; Anand, M.; Bhatt, A.; Kremer, L.; Jacobs, W.R., Jr.; Hatfull, G.F. GroEL1: A dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 2005, 123, 861–873. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, G.; Wu, Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complement. Med. 2014, 4, 17–23. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Andritsos, N.D.; Kabolis, D.; Kapsokefalou, M.; Drosinos, E.H. Antioxidant and antimicrobial properties of tea and aromatic plant extracts against bacterial foodborne pathogens: A comparative evaluation. Curr. Top. Nutraceutical Res. 2016, 14, 133–141. [Google Scholar]
- Min, K.J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C., Jr.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef]
- Paterson, I.; Anderson, E.A. The renaissance of natural products as drug candidates. Science 2005, 310, 451–453. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef]
- Haghjoo, B.; Lee, L.H.; Habiba, U.; Tahir, H.; Olabi, M.; Chu, T. The synergistic effects of green tea polyphenols and antibiotics against potential pathogens. Adv. Biosci. Biotechnol. 2013, 4, 959–967. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin resistance in Staphylococcus aureus: Mechanisms and modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Larente, J.; Morin, M.P.; Grenier, D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Stenvang, M.; Dueholm, M.S.; Vad, B.S.; Seviour, T.; Zeng, G.; Geifman-Shochat, S.; Sondergaard, M.T.; Christiansen, G.; Meyer, R.L.; Kjelleberg, S.; et al. Epigallocatechin Gallate Remodels Overexpressed Functional Amyloids in Pseudomonas aeruginosa and Increases Biofilm Susceptibility to Antibiotic Treatment. J. Biol. Chem. 2016, 291, 26540–26553. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Widyaningrum, N.; Fudholi, A.; Sudarsono, P.; Setyowati, E.P. Stability of epigallocatechin gallate (EGCG) from green tea (Camellia sinensis) and its antibacterial activity against Staphylococcus epidermidis ATCC 35984 and Propionibacterium acnes ATCC 6919. Asian J. Biol. Sci. 2015, 8, 93–101. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Landis-Piwowar, K.; Chan, T.H.; Dou, Q.P. Green tea polyphenols as proteasome inhibitors: Implication in chemoprevention. Curr. Cancer Drug Targets 2011, 11, 296–306. [Google Scholar] [CrossRef]
- Chu, T.; Lee, L.H.; Aponte, T.; Lopez, S.; Lalata, G.; Herrera, G.; Yussof, A.; Dickinson, D.; Hsu, S. Sporicidal activity of novel formulations containing lipophilic epigallocatechin-3-gallate and natural ingredients. Microbiol. Infect. Dis. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Chu, T.; Lee, L.H.; Yussof, A.; Lopez, S.; Herrera, G.; Luna, P.; Uddin, M.; Wu, L.; Murzaku, J.; Dickinson, D.; et al. Enhanced sporicidal activity of alcohol and epigallocatechin-palmitate-based hand hygiene formulations comprised of plant-derived compounds. J. Biosci. Med. 2020, 8, 89–99. [Google Scholar] [CrossRef]
- Ali, B.; Lee, L.H.; Laskar, N.; Shaikh, N.; Tahir, H.; Hsu, S.; Newby, R.; Valsechi-Diaz, J.; Chu, T. Modified green tea polyphenols, EGCG-S and LTP, inhibit endospore in three Bacillus spp. Adv. Microbiol. 2017, 7, 175–187. [Google Scholar] [CrossRef]
- Melok, A.L.; Lee, L.H.; Mohamed Yussof, S.A.; Chu, T. Green Tea Polyphenol Epigallocatechin-3-Gallate-Stearate Inhibits the Growth of Streptococcus mutans: A Promising New Approach in Caries Prevention. Dent. J. 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Yussof, A.; Habiba, U.; Liaw, D.; Chu, T.; Lee, L.H. Epigallocatechin gallate-stearate enhances the efficacy of antibiotics. Open J. Med. Microbiol. 2019, 9, 77–94. [Google Scholar] [CrossRef][Green Version]
- Karigoudar, R.M.; Karigoudar, M.H.; Wavare, S.M.; Mangalgi, S.S. Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J. Lab. Physicians 2019, 11, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Suman, E.; Jose, J.; Varghese, S.; Kotian, M.S. Study of biofilm production in Escherichia coli causing urinary tract infection. Indian J. Med. Microbiol. 2007, 25, 305–306. [Google Scholar] [CrossRef][Green Version]
- Bhunu, B.; Mautsa, R.; Mukanganyama, S. Inhibition of biofilm formation in Mycobacterium smegmatis by Parinari curatellifolia leaf extracts. BMC Complement. Altern. Med. 2017, 17, 285. [Google Scholar] [CrossRef]
- Kaur, P.; Ghosh, A.; Krishnamurthy, R.V.; Bhattacharjee, D.G.; Achar, V.; Datta, S.; Narayanan, S.; Anbarasu, A.; Ramaiah, S. A high-throughput cidality screen for Mycobacterium tuberculosis. PLoS ONE 2015, 10, e0117577. [Google Scholar] [CrossRef][Green Version]
- Esteban, J.; Garcia-Coca, M. Mycobacterium Biofilms. Front. Microbiol. 2017, 8, 2651. [Google Scholar] [CrossRef]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Gaston, J.R.; Kocak, B.R.; Coburn, S.L.; Lee, S.; Pilewski, J.M.; Myerburg, M.M.; Bomberger, J.M. Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6133255/ (accessed on 19 January 2021).
- Sandberg, M.E.; Schellmann, D.; Brunhofer, G.; Erker, T.; Busygin, I.; Leino, R.; Vuorela, P.M.; Fallarero, A. Pros and cons of using resazurin staining for quantification of viable Staphylococcus aureus biofilms in a screening assay. J. Microbiol. Methods 2009, 78, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef]
- Mariscal, A.; Lopez-Gigosos, R.M.; Carnero-Varo, M.; Fernandez-Crehuet, J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biotechnol. 2009, 82, 773–783. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Evaluation of the efficacy of disinfection procedures against Burkholderia cenocepacia biofilms. J. Hosp. Infect. 2008, 70, 361–368. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Arita-Morioka, K.I.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef]
- Blanco, A.R.; Sudano-Roccaro, A.; Spoto, G.C.; Nostro, A.; Rusciano, D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob. Agents Chemother. 2005, 49, 4339–4343. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Asahi, Y.; Noiri, Y.; Miura, J.; Maezono, H.; Yamaguchi, M.; Yamamoto, R.; Azakami, H.; Hayashi, M.; Ebisu, S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014, 116, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the sigma(E)-dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Naito, A.; Nakayama, T. Solid-state NMR analysis of the orientation and dynamics of epigallocatechin gallate, a green tea polyphenol, incorporated into lipid bilayers. Magn. Reson. Chem. 2008, 46, 174–177. [Google Scholar] [CrossRef]
- Cui, Y.; Kim, S.H.; Kim, H.; Yeom, J.; Ko, K.; Park, W.; Park, S. AFM probing the mechanism of synergistic effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) with cefotaxime against extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli. PLoS ONE 2012, 7, e48880. [Google Scholar] [CrossRef]
- Hoshino, N.; Kimura, T.; Yamaji, A.; Ando, T. Damage to the cytoplasmic membrane of Escherichia coli by catechin-copper (II) complexes. Free Radic. Biol. Med. 1999, 27, 1245–1250. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Sun, T.; Qin, B.; Gao, M.; Yin, Y.; Wang, C.; Zang, S.; Li, X.; Zhang, C.; Xin, Y.; Jiang, T. Effects of epigallocatechin gallate on the cell-wall structure of Mycobacterial smegmatis mc2155. Nat. Prod. Res. 2015, 29, 2122–2124. [Google Scholar] [CrossRef]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, P.; Pallares, I.; Navarro, S.; Ventura, S. Dissecting the contribution of Staphylococcus aureus alpha-phenol-soluble modulins to biofilm amyloid structure. Sci. Rep. 2016, 6, 34552. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapun-Araiz, B.; Ziemyte, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef] [PubMed]
- Najarzadeh, Z.; Mohammad-Beigi, H.; Nedergaard Pedersen, J.; Christiansen, G.; Sonderby, T.V.; Shojaosadati, S.A.; Morshedi, D.; Stromgaard, K.; Meisl, G.; Sutherland, D.; et al. Plant Polyphenols Inhibit Functional Amyloid and Biofilm Formation in Pseudomonas Strains by Directing Monomers to Off-Pathway Oligomers. Biomolecules 2019, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Besingi, R.N.; Wenderska, I.B.; Senadheera, D.B.; Cvitkovitch, D.G.; Long, J.R.; Wen, Z.T.; Brady, L.J. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology 2017, 163, 488–501. [Google Scholar] [CrossRef]
- Bikels-Goshen, T.; Landau, E.; Saguy, S.; Shapira, R. Staphylococcal strains adapted to epigallocathechin gallate (EGCG) show reduced susceptibility to vancomycin, oxacillin and ampicillin, increased heat tolerance, and altered cell morphology. Int. J. Food Microbiol. 2010, 138, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Teng, Z.; Li, M.; Niu, X.; Wang, J.; Deng, X. Epigallocatechin gallate inhibits Streptococcus pneumoniae virulence by simultaneously targeting pneumolysin and sortase A. J. Cell. Mol. Med. 2017, 21, 2586–2598. [Google Scholar] [CrossRef]
- Dey, D.; Ghosh, S.; Ray, R.; Hazra, B. Polyphenolic Secondary Metabolites Synergize the Activity of Commercial Antibiotics against Clinical Isolates of beta-Lactamase-producing Klebsiella pneumoniae. Phytother. Res. 2016, 30, 272–282. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Hoiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- O’May, C.; Ciobanu, A.; Lam, H.; Tufenkji, N. Tannin derived materials can block swarming motility and enhance biofilm formation in Pseudomonas aeruginosa. Biofouling 2012, 28, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (-)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorg. Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Dickinson, D.; Hsu, S. Lipid-soluble green tea polyphenols: Stabilized for effective formulation. In Handbook of Green Tea and Health Research, 1st ed.; Mckinley, H., Jamieson, M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 45–62. [Google Scholar]
- Chen, P.; Tan, Y.; Sun, D.; Zheng, X.M. A novel long-chain acyl-derivative of epigallocatechin-3-O-gallate prepared and purified from green tea polyphenols. J. Zhejiang Univ. Sci. 2003, 4, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Cruz, C.D.; Palmer, J.; Fletcher, G.C.; Flint, S. Biofilm formation of the L. monocytogenes strain 15G01 is influenced by changes in environmental conditions. J. Microbiol. Methods 2015, 119, 189–195. [Google Scholar] [CrossRef] [PubMed]



| Treatments (μg/mL) | CFU/mL (Mean ± SD) | Log Reduction | Avg % Inhibition |
|---|---|---|---|
| E0+ES0 | (1.81 ± 0.02) × 106 | 0 | 0 |
| E10 | (1.34 ± 0.09) × 106 | 0.13 | 27.01 |
| E15 | (1.10 ± 0.03) × 106 | 0.22 | 40.52 |
| ES50 | (8.72 ± 0.90) × 105 | 0.32 | 53.39 |
| E10+ES100 | (3.00 ± 0.23) × 105 | 0.78 | 86.07 |
| E10+ES150 | (9.00 ± 0.43) × 104 | 1.30 | 98.04 |
| E10+ES200 | (1.10 ± 0.15) × 105 | 1.22 | 96.93 |
| E15+ES25 | (1.85 ± 0.10) × 105 | 0.99 | 92.65 |
| E15+ES50 | (1.65 ± 0.07) × 105 | 1.04 | 93.77 |
| Treatments (μg/mL) | CFU/mL (Mean ± SD) | Log Reduction | Avg % Inhibition |
|---|---|---|---|
| E0+ES0 | (1.60 ± 0.07) × 105 | 0 | 0 |
| E10 | (1.18 ± 0.05) × 105 | 0.13 | 27.16 |
| E15 | (8.30 ± 0.65) × 104 | 0.29 | 49.91 |
| ES100 | (7.75 ± 0.62) × 104 | 0.31 | 53.33 |
| ES150 | (9.20 ± 0.25) × 104 | 0.24 | 43.99 |
| E15+ES50 | (4.40 ± 0.33) × 104 | 0.56 | 75.20 |
| E15+ES100 | (4.85 ± 0.23) × 104 | 0.52 | 72.33 |
| E15+ES150 | (1.35 ± 0.03) × 104 | 1.07 | 94.84 |
| E15+ES200 | (3.75 ± 0.11) × 104 | 0.63 | 79.38 |
| Treatments (μg/mL) | CFU/mL (Mean ± SD) | Log Reduction | Avg % Inhibition |
|---|---|---|---|
| E0+ES0 | (3.40 ± 0.36) × 106 | 0 | 0 |
| E10 | (1.41 ± 0.09) × 106 | 0.38 | 61.14 |
| E15 | (1.53 ± 0.12) × 106 | 0.35 | 57.47 |
| ES25 | (2.00 ± 0.12) × 106 | 0.23 | 42.99 |
| ES50 | (1.74 ± 0.14) × 106 | 0.29 | 51.10 |
| E10+ES200 | (1.39 ± 0.05) × 106 | 0.39 | 64.65 |
| E15+ES25 | (1.48 ± 0.15) × 105 | 0.36 | 59.12 |
| E15+ES50 | (8.25 ± 0.85) × 105 | 0.61 | 83.00 |
| E15+ES100 | (4.45 ± 0.36) × 105 | 0.88 | 95.10 |
| E15+ES150 | (1.44 ± 0.07) × 106 | 0.37 | 63.02 |
| Treatments (μg/mL) | CFU/mL (Mean ± SD) | Log Reduction | Avg % Inhibition |
|---|---|---|---|
| TE0+ES0 | (4.41 ± 0.34) × 106 | 0 | 0 |
| TE15 | (2.29 ± 0.18) × 106 | 0.29 | 52.65 |
| TE30 | (2.00 ± 0.16) × 106 | 0.34 | 59.38 |
| ES50 | (3.18 ± 0.27) × 106 | 0.14 | 30.14 |
| ES150 | (1.59 ± 0.11) × 106 | 0.44 | 69.40 |
| ES200 | (2.01 ± 0.15) × 106 | 0.34 | 59.07 |
| TE15+ES50 | (2.20 ± 0.34) × 106 | 0.30 | 57.37 |
| TE15+ES100 | (2.03 ± 0.20) × 106 | 0.34 | 61.73 |
| TE15+ES150 | (9.45 ± 0.37) × 105 | 0.67 | 89.88 |
| TE15+ES200 | (7.75 ± 0.62) × 105 | 0.75 | 94.35 |
| TE15+ES250 | (1.58 ± 0.04) × 106 | 0.45 | 73.53 |
| Treatments (μg/mL) | CFU/mL (Mean ± SD) | Log Reduction | Avg % Inhibition |
|---|---|---|---|
| TE0+ES0 | (4.72 ± 0.15) × 106 | 0 | 0 |
| TE15 | (2.10 ± 0.35) × 106 | 0.35 | 55.52 |
| TE30 | (2.03 ± 0.16) × 106 | 0.37 | 56.95 |
| ES50 | (3.15 ± 0.29) × 106 | 0.18 | 33.36 |
| ES100 | (2.35 ± 0.50) × 106 | 0.30 | 50.20 |
| TE15+ES100 | (1.34 ± 0.23) × 106 | 0.55 | 71.69 |
| TE15+ES200 | (4.78 ± 0.64) × 105 | 0.99 | 89.87 |
| TE15+ES250 | (2.19 ± 0.25) × 105 | 1.33 | 95.36 |
| TE15+ES500 | (1.71 ± 0.01) × 106 | 0.44 | 63.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, S.; Lee, L.H.; Chu, T. Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics 2021, 10, 102. https://doi.org/10.3390/antibiotics10020102
Shinde S, Lee LH, Chu T. Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics. 2021; 10(2):102. https://doi.org/10.3390/antibiotics10020102
Chicago/Turabian StyleShinde, Shrameeta, Lee H. Lee, and Tinchun Chu. 2021. "Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics" Antibiotics 10, no. 2: 102. https://doi.org/10.3390/antibiotics10020102
APA StyleShinde, S., Lee, L. H., & Chu, T. (2021). Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics, 10(2), 102. https://doi.org/10.3390/antibiotics10020102






