Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens
Abstract
1. Introduction
2. Results
2.1. Growth Performance
2.2. Carcass Traits
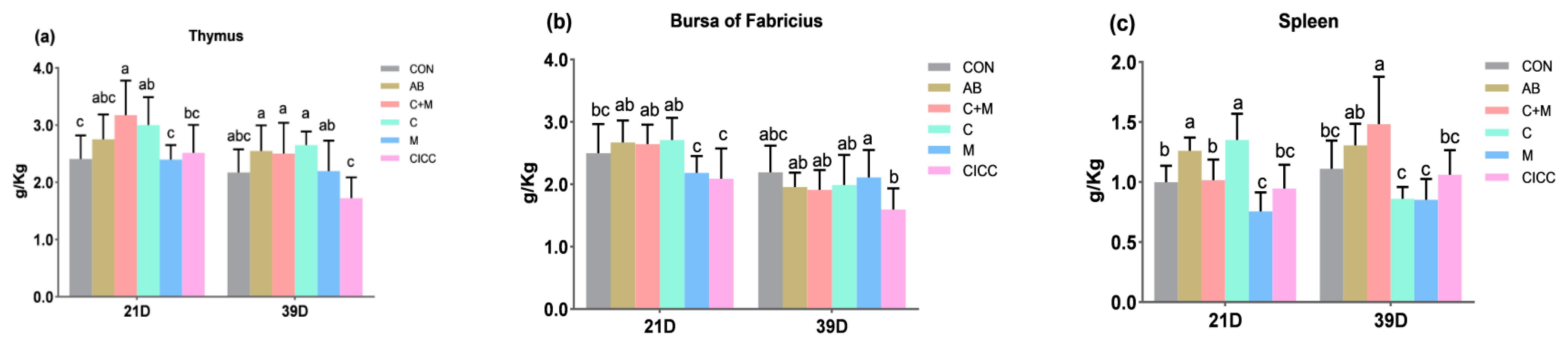
2.3. Immune Organs and Serum Immune Factors
2.4. Serum Biochemical Parameters
3. Discussion
4. Materials and Methods
4.1. Preparation of Bacterial Strain Powder
4.2. Birds, Diets, and Management
4.3. Sampling and Measurements
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kheiri, F.; Faghani, M.; Landy, N. Evaluation of thyme and ajwain as antibiotic growth promoter substitutions on growth performance, carcass characteristics and serum biochemistry in Japanese quails (Coturnix japonica). Anim. Nutr. 2018, 4, 79–83. [Google Scholar] [CrossRef]
- Gheisari, A.; Shahrvand, S.; Landy, N. Effect of ethanolic extract of propolis as an alternative to antibiotics as a growth promoter on broiler performance, serum biochemistry and immune responses. Vet. World 2017, 10, 249–254. [Google Scholar] [CrossRef]
- Henning, S.; Marianne, S. Resistence to antibiotics in the normal flora of animals. Vet. Res. 2001, 32, 227–241. [Google Scholar] [CrossRef]
- Andremont, A. Consequences of antibiotic therapy to the intestinal ecosystem. Ann. Fr. Anesth. Réanim. 2000, 19, 395–402. [Google Scholar] [CrossRef]
- Barton, M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar] [CrossRef]
- Roth, N.; Kasbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S58–S61; discussion S144–S151. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Rodjan, P.; Soisuwan, K.; Thongprajukaew, K.; Theapparat, Y.; Khongthong, S.; Jeenkeawpieam, J.; Salaeharae, T. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, e931–e940. [Google Scholar] [CrossRef]
- Bai, K.; Huang, Q.; Zhang, J.; He, J.; Zhang, L.; Wang, T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017, 96, 74–82. [Google Scholar] [CrossRef]
- Rajput, I.R.; Li, L.Y.; Xin, X.; Wu, B.B.; Juan, Z.L.; Cui, Z.W.; Yu, D.Y.; Li, W.F. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 2013, 92, 956–965. [Google Scholar] [CrossRef]
- Král, M.; Angelovičová, M.; Mrázová, Ľ. Application of Probiotics in Poultry Production. Anim. Sci. Biotechnol. 2012, 45, 55–57. Available online: https://core.ac.uk/download/pdf/25520903.pdf (accessed on 30 January 2021).
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Murshed, M.A.; Abudabos, A.M. Effects of the dietary inclusion of a probiotic, a prebiotic or their combinations on the growth performance of broiler chickens. Braz. J. Poult. Sci. 2015, 17, 99–103. [Google Scholar] [CrossRef]
- Korenblum, E.; der Weid, I.; Santos, A.L.; Rosado, A.S.; Sebastian, G.V.; Coutinho, C.M.; Magalhaes, F.C.; Paiva, M.M.; Seldin, L. Production of antimicrobial substances by Bacillus subtilis LFE-1, B. firmus HO-1 and B. licheniformis T6-5 isolated from an oil reservoir in Brazil. J. Appl. Microbiol. 2005, 98, 667–675. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y.; et al. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Fish. Sci. 2020, 13, 862–906. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Nguyen, D.V.; Tran, M.T.; Nguyen, L.T.; Nguyen, A.H.; Phan, T.N. Isolation and characterization of Bacillus subtilis CH16 strain from chicken gastrointestinal tracts for use as a feed supplement to promote weight gain in broilers. Lett. Appl. Microbiol. 2015, 60, 580–588. [Google Scholar] [CrossRef]
- Molnar, A.K.; Podmaniczky, B.; Kurti, P.; Tenk, I.; Glavits, R.; Virag, G.; Szabo, Z. Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br. Poult. Sci. 2011, 52, 658–665. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Q. Effect of probiotic on growth performance and digestive enzyme activity of Arbor Acres broilers. Res. Vet. Sci. 2010, 89, 163–167. [Google Scholar] [CrossRef]
- Clements, L.D.; Miller, B.S.; Streips, U.N. Comparative growth analysis of the facultative anaerobes Bacillus subtilis, Bacillus licheniformis, and Escherichia coli. Syst. Appl. Microbiol. 2002, 25, 284–286. [Google Scholar] [CrossRef]
- Nakano, M.M.; Dailly, Y.P.; Zuber, P.; Clark, D.P. Characterization of anaerobic fermentative growth of Bacillus subtilis: Identification of fermentation end products and genes required for growth. J. Bacteriol. 1997, 179, 6749–6755. [Google Scholar] [CrossRef]
- Hartig, E.; Hartmann, A.; Schatzle, M.; Albertini, A.M.; Jahn, D. The Bacillus subtilis nrdEF genes, encoding a class Ib ribonucleotide reductase, are essential for aerobic and anaerobic growth. Appl. Environ. Microbiol. 2006, 72, 5260–5265. [Google Scholar] [CrossRef][Green Version]
- Al-Fataftah, A.-R.; Abdelqader, A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014, 198, 279–285. [Google Scholar] [CrossRef]
- Abdelqader, A.; Abuajamieh, M.; Hayajneh, F.; Al-Fataftah, A.R. Probiotic bacteria maintain normal growth mechanisms of heat stressed broiler chickens. J. Therm. Biol. 2020, 92, 102654. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alhouri, H.A.A.; Alhidary, I.A.; Nassan, M.A.; Swelum, A.A. Ameliorative effect of Bacillus subtilis, Saccharomyces boulardii, oregano, and calcium montmorillonite on growth, intestinal histology, and blood metabolites on Salmonella-infected broiler chicken. Environ. Sci. Pollut. Res. Int. 2019, 26, 16274–16278. [Google Scholar] [CrossRef]
- Guo, M.; Li, M.; Zhang, C.; Zhang, X.; Wu, Y. Dietary administration of the Bacillus Subtilis enhances immune responses and disease resistance in chickens. Front. Microbiol. 2020, 11, 1768. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Rogiewicz, A.; Mogielnicka, M.; Rutkowski, A.; Jones, R.O.; Slominski, B.A. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult. Sci. 2014, 93, 1745–1753. [Google Scholar] [CrossRef]
- Stefanello, C.; Vieira, S.L.; Rios, H.V.; Simões, C.T.; Ferzola, P.H.; Sorbara, J.O.B.; Cowieson, A.J. Effects of energy, α-amylase, and β-xylanase on growth performance of broiler chickens. Anim. Feed Sci. Technol. 2017, 225, 205–212. [Google Scholar] [CrossRef]
- Xie, J.H.; Zhang, R.J.; Shang, C.J.; Guo, Y.Q. Isolation and characterization of a bacteriocin produced by an isolated Bacillus subtilis LFB112 that exhibits antimicrobial activity against domestic animal pathogens. Afr. J. Biotechnol. 2009, 8, 5611–5619. [Google Scholar] [CrossRef]
- Cai, J.; Liu, F.; Liao, X.; Zhang, R. Complete genome sequence of Bacillus amyloliquefaciens LFB112 isolated from Chinese herbs, a strain of a broad inhibitory spectrum against domestic animal pathogens. J. Biotechnol. 2014, 175, 63–64. [Google Scholar] [CrossRef]
- Wei, X.; Liao, X.; Cai, J.; Zheng, Z.; Zhang, L.; Shang, T.; Fu, Y.; Hu, C.; Ma, L.; Zhang, R. Effects of Bacillus amyloliquefaciens LFB112 in the diet on growth of broilers and on the quality and fatty acid composition of broiler meat. Anim. Prod. Sci. 2017, 57, 1899–1905. [Google Scholar] [CrossRef]
- Onderci, M.; Sahin, N.; Cikim, G.; Aydin, A.; Ozercan, I.; Ozkose, E.; Ekinci, S.; Hayirli, A.; Sahin, K. β-Glucanase-producing bacterial culture improves performance and nutrient utilization and alters gut morphology of broilers fed a barley-based diet. Anim. Feed Sci. Technol. 2008, 146, 87–97. [Google Scholar] [CrossRef]
- Bansal, G.R.; Singh, V.P.; Sachan, N. Effect of probiotic supplementation on the performance of broilers. Asian J. Anim. Sci. 2011, 5, 277–284. [Google Scholar] [CrossRef]
- Tellez, G.; Higgins, S.E.; Donoghue, A.M.; Hargis, B.M. Digestive physiology and the role of microorganisms. J. Appl. Poult. Res. 2006, 15, 136–144. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010, 89, 58–67. [Google Scholar] [CrossRef]
- Hooge, D.M.; Ishimaru, H.; Sims, M.D. Influence of dietary Bacillus Subtilis c-3102 spores on live performance of broiler chickens in four controlled pen trials. J. Appl. Poult. Res. 2004, 13, 222–228. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cho, J.H.; Kim, I.H. Effects of Bacillus subtilis UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest. Sci. 2013, 155, 343–347. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhou, T.X.; Ao, X.; Kim, I.H. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize-soybean meal based diets. Livest. Sci. 2012, 150, 419–424. [Google Scholar] [CrossRef]
- Wealleans, A.L.; Sirukhi, M.; Egorov, I.A. Performance, Gut morphology and microbiology effects of a Bacillus probiotic, avilamycin and their combination in mixed grain broiler diets. Br. Poult. Sci. 2017, 58, 523–529. [Google Scholar] [CrossRef]
- Khajeh Bami, M.; Afsharmanesh, M.; Ebrahimnejad, H. Effect of dietary Bacillus coagulans and different forms of zinc on performance, intestinal microbiota, carcass and meat quality of broiler chickens. Probiotics Antimicrob. Proteins 2020, 12, 461–472. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Q.; Yang, C.; Yu, Y.; Fu, Z.; Wang, H.; Fan, X.; Yue, M.; Xu, Y. Effects of Clostridium butyricum- and Bacillus spp.-based potential probiotics on the growth performance, intestinal morphology, immune responses, and caecal microbiota in broilers. Antibiotics 2021, 10, 624. [Google Scholar] [CrossRef]
- Willis, W.L.; Reid, L. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult. Sci. 2008, 87, 606–611. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014, 93, 2054–2059. [Google Scholar] [CrossRef]
- Lee, K.; Lillehoj, H.S.; Siragusa, G.R. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 2010, 47, 106–114. [Google Scholar] [CrossRef]
- Flores, C.A.; Duong, T.; Augspurger, N.; Lee, J.T. Efficacy of Bacillus subtilis administered as a direct-fed microorganism in comparison to an antibiotic growth promoter and in diets with low and high DDGS inclusion levels in broiler chickens. J. Appl. Poult. Res. 2019, 28, 902–911. [Google Scholar] [CrossRef]
- Falaki, M.; Shargh, M.S.; Dastar, B.; Zrehdaran, S. Effects of different levels of probiotic and prebiotic on performance and carcass characteristics of broiler chickens. J. Anim. Vet. Adv. 2010, 9, 2390–2395. [Google Scholar] [CrossRef][Green Version]
- Salehizadeh, M.; Modarressi, M.H.; Mousavi, S.N.; Ebrahimi, M.T. Effects of probiotic lactic acid bacteria on growth performance, carcass characteristics, hematological indices, humoral immunity, and IGF-I gene expression in broiler chicken. Trop Anim. Health Prod. 2019, 51, 2279–2286. [Google Scholar] [CrossRef]
- Pelicano, E.R.L.; de Souza, P.; de Souza, H.B.A.; Oba, A.; Norkus, E.A.; Kodawara, L.M.; de Lima, T.M.A. Effect of different probiotics on broiler carcass and meat quality. Braz. J. Poult. Sci. 2003, 5, 207–214. [Google Scholar] [CrossRef]
- Sarangi, N.R.; Babu, L.K.; Kumar, A.; Pradhan, C.R.; Pati, P.K.; Mishra, J.P. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet. World 2016, 9, 313–319. [Google Scholar] [CrossRef]
- Santoso, U.; Tanaka, K.; Ohtani, S. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Br. J. Nutr. 1995, 74, 523–529. [Google Scholar] [CrossRef]
- Weis, J.; Hrnčár, C.; Pál, G.; Baračska, B.; Bujko, J.; Malíková, L. Effect of probiotic strain Enterococcus faecium M74 supplementation on the carcass parameters of different hybrid combination chickens. Sci. Pap. Anim. Sci. Biotech. 2011, 44, 149–152. Available online: https://www.scinapse.io/papers/1790402455 (accessed on 30 January 2021).
- Agboola, A.; Omidiwura, R.; Iyayi, E. Influence of supplemental levels of probiotic on growth response, intestinal microbiota and carcass characteristics of broilers. Am. J. Exp. Agric. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Bohm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef]
- Rhayat, L.; Jacquier, V.; Brinch, K.S.; Nielsen, P.; Nelson, A.; Geraert, P.A.; Devillard, E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult. Sci. 2017, 96, 2274–2280. [Google Scholar] [CrossRef]
- Hossain, M.M.; Begum, M.; Kim, I.H. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet. Med. 2016, 60, 77–86. [Google Scholar] [CrossRef]
- Luan, S.J.; Sun, Y.B.; Wang, Y.; Sa, R.N.; Zhang, H.F. Bacillus amyloliquefaciens spray improves the growth performance, immune status, and respiratory mucosal barrier in broiler chickens. Poult. Sci. 2019, 98, 1403–1409. [Google Scholar] [CrossRef]
- Soliman, E.S.; Hamad, R.T.; Abdallah, M.S. Preventive antimicrobial action and tissue architecture ameliorations of Bacillus subtilis in challenged broilers. Vet. World 2021, 14, 523–536. [Google Scholar] [CrossRef]
- Slawinska, A.; Siwek, M.; Zylinska, J.; Bardowski, J.; Brzezinska, J.; Gulewicz, K.A.; Nowak, M.; Urbanowski, M.; Plowiec, A.; Bednarczyk, M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. 2014, 62, 277–285. [Google Scholar] [CrossRef]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Adil, M.; Ali, H.M.; Muhammad, N.; Rehman, T.; Rehman, A.; Rehman, H.F. Effects of Bacillus subtilis on performance, immune system and gut in Salmonella-challenged broilers. S. Afr. J. Anim. Sci. 2020, 50, 654–662. [Google Scholar] [CrossRef]
- Shabani, R.; Fakhraei, J.; Yarahmadi, H.M.; Seidavi, A. Effect of different sources of selenium on performance and characteristics of immune system of broiler chickens. Rev. Bras. Zootec. 2019, 48, e20180256. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Shah, M.; Rehman, H. Growth performance, immune status and organ morphometry in broilers fed Bacillus subtilis-supplemented diet. S. Afr. J. Anim. Sci. 2017, 47, 378–388. [Google Scholar] [CrossRef]
- Zhang, X.; Calvert, R.A.; Sutton, B.J.; Dore, K.A. IgY: A key isotype in antibody evolution. Biol. Rev. Camb. Philos. Soc. 2017, 92, 2144–2156. [Google Scholar] [CrossRef]
- Balan, P.; Sik-Han, K.; Moughan, P.J. Impact of oral immunoglobulins on animal health—A review. Anim. Sci. J. 2019, 90, 1099–1110. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Kim, I.H. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 2014, 93, 364–370. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Li, Z.; Qamar, H.; Mehmood, K.; Zhang, L.; Liu, J.; Zhang, H.; Li, J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Fact. 2019, 18, 112. [Google Scholar] [CrossRef]
- Wang, Y.; Heng, C.; Zhou, X.; Cao, G.; Jiang, L.; Wang, J.; Li, K.; Wang, D.; Zhan, X. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br. J. Nutr. 2021, 125, 494–507. [Google Scholar] [CrossRef]
- Salim, H.M.; Kang, H.K.; Akter, N.; Kim, D.W.; Kim, J.H.; Kim, M.J.; Na, J.C.; Jong, H.B.; Choi, H.C.; Suh, O.S.; et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult. Sci. 2013, 92, 2084–2090. [Google Scholar] [CrossRef]
- Yisa, T.; Ibrahim, O.; Tsadu, S.; Yakubu, U. Effect of probiotics (Lactobacillus acidophilus and Bifidobacterium bifidum) as immune stimulant on hybrid catfish Heteroclarias. Br. Microbiol. Res. J. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Awais, M.M.; Jamal, M.A.; Akhtar, M.; Hameed, M.R.; Anwar, M.I.; Ullah, M.I. Immunomodulatory and ameliorative effects of Lactobacillus and Saccharomyces based probiotics on pathological effects of eimeriasis in broilers. Microb. Pathog. 2019, 126, 101–108. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food. Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Ifrah, M.E.; Perelman, B.; Finger, A.; Uni, Z. The role of the bursa of Fabricius in the immune response to vaccinal antigens and the development of immune tolerance in chicks (Gallus domesticus) vaccinated at a very young age. Poult. Sci. 2017, 96, 51–57. [Google Scholar] [CrossRef]
- Shackih, E.V.; Korolkova-Subbotkina, D.E.; Galiev, D.M. The influence of biologically active additives on the morpho-biochemical parameters of the blood of broiler chickens. Agrar. Bull. Ural. 2021, 207, 93–98. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2016, 45, 538–542. [Google Scholar] [CrossRef]
- Hussein, E.O.S.; Ahmed, S.H.; Abudabos, A.M.; Aljumaah, M.R.; Alkhlulaifi, M.M.; Nassan, M.A.; Suliman, G.M.; Naiel, M.A.E.; Swelum, A.A. Effect of antibiotic, Phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with clostridium perfringens. Animals 2020, 10, 507. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Selim, D.A.; Basuony, H.A.; Sabic, E.M.; Saleh, A.A.; Ebeid, T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health. Prod. 2020, 52, 671–680. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Devi, R.S.; Srikumar, R.; Manikandan, S.; Thangaraj, R. Protective effect of triphala on cold stress-induced behavioral and biochemical abnormalities in rats. Yakugaku Zasshi 2007, 127, 1863–1867. [Google Scholar] [CrossRef]
- Grisoni, M.L.; Uzu, G.; Larbier, M.; Geraert, P.A. Effect of dietary lysine level on lipogenesis in broilers. Reprod. Nutr. Dev. 1991, 31, 683–690. [Google Scholar] [CrossRef]
- Abaza, I.M.; Shehata, M.A.; Shoieb, M.S.; Hassan, I.I. Evaluation of some natural feed additive in growing chicks diets. Int. J. Poult. Sci. 2008, 7, 872–879. [Google Scholar] [CrossRef]
- Chiang, S.H.; Hsieh, W.M. Effect of direct-fed microorganisms on broiler growth performance and litter ammonia level. Asian-Australas. J. Anim. Sci. 1995, 8, 159–162. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, J.S.; Lee, S.I.; Kim, I.H. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens. Poult. Sci. 2016, 95, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.R.; Ismail, W.; Heintz, D.; Schaeffer, C.; Van Dorsselaer, A.; Fuchs, G. Study of anoxic and oxic cholesterol metabolism by Sterolibacterium denitrificans. J. Bacteriol. 2008, 190, 905–914. [Google Scholar] [CrossRef]
- Safalaoh, A. Body weight gain, dressing percentage, abdominal fat and serum cholesterol of broilers supplemented with a microbial preparation. Afr. J. Food Agric. Nutr. Dev. 2006, 6. [Google Scholar] [CrossRef][Green Version]
- Fukushima, M.; Nakano, M. The effect of a probiotic on faecal and liver lipid classes in rats. Br. J. Nutr. 1995, 73, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Peebles, E.D.; Burnham, M.R.; Walzem, R.L.; Branton, S.L.; Gerard, P.D. Effects of fasting on serum lipids and lipoprotein profiles in the egg-laying hen (Gallus domesticus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 305–311. [Google Scholar] [CrossRef]
- DeRodas, B.Z.; Gilliland, S.E.; Maxwell, C.V. Hypocholesterolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. J. Dairy Sci. 1996, 79, 2121–2128. [Google Scholar] [CrossRef]
- Hussein, E.; Selim, S. Efficacy of yeast and multi-strain probiotic alone or in combination on growth performance, carcass traits, blood biochemical constituents, and meat quality of broiler chickens. Livest. Sci. 2018, 216, 153–159. [Google Scholar] [CrossRef]
- Saleh, A.A.; Shukry, M.; Farrag, F.; Soliman, M.M.; Abdel-Moneim, A.M.E. Effect of feeding wet feed or wet feed fermented by Bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chickens. Animals 2021, 11, 83. [Google Scholar] [CrossRef]
- Aggrey, S.E.; Rekaya, R. Dissection of Koch’s residual feed intake: Implications for selection. Poult. Sci. 2013, 92, 2600–2605. [Google Scholar] [CrossRef] [PubMed]

| Item | Treatments 2 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | AB | C + M | C | M | CICC | |||
| Initial BW (g) | 47.67 | 47.35 | 47.68 | 47.55 | 47.40 | 47.23 | 0.040 | 0.901 |
| 1 to 21 days | ||||||||
| ADG (g/day) | 23.94 c | 23.53b c | 25.93 ab | 24.67 ab | 26.00 a | 25.36 a | 0.138 | 0.028 |
| ADFI (g/day) | 37.82 | 36.43 | 37.97 | 37.74 | 39.78 | 40.32 | 0.251 | 0.052 |
| FCR | 1.58 ab | 1.55 ab | 1.58 ab | 1.53 b | 1.57 ab | 1.59 a | 0.006 | 0.014 |
| 22 to 39 days | ||||||||
| ADG (g/day) | 60.61 c | 62.27 bc | 66.89 ab | 68.90 a | 67.09 ab | 61.53 c | 0.940 | 0.003 |
| ADFI (g/day) | 106.07 b | 110.88 ab | 113.71 a | 118.87 a | 116.76 a | 106.08 b | 1.540 | 0.006 |
| FCR | 1.75 a | 1.78 a | 1.70 b | 1.73 ab | 1.74 ab | 1.73 ab | 0.014 | 0.020 |
| 1 to 39 days | ||||||||
| ADG (g/day) | 40.33 b | 43.41 b | 45.72 a | 45.65 a | 44.89 a | 41.73 b | 0.462 | <0.001 |
| ADFI (g/day) | 66.43 bc | 70.83 b | 73.23 ab | 75.31 a | 73.70 ab | 68.25 b | 0.905 | 0.020 |
| FCR | 1.68 a | 1.63 ab | 1.60 b | 1.63 a | 1.64 a | 1.64 a | 0.011 | 0.011 |
| Final BW (g) | 1620.17 b | 1662.37 b | 1830.58 a | 1821.21 a | 1798.21 a | 1675.04 b | 18.901 | <0.001 |
| Total gain weight (g) | 1572.37 b | 1614.77 b | 1783.28 a | 1773.31 a | 1750.61 a | 1627.44 b | 15.182 | <0.001 |
| Item | Treatment 3 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | AB | C + M | C | M | CICC | |||
| Carcass yield | 88.23 b | 89.35 ab | 91.77 a | 91.86 a | 90.32 ab | 89.25 ab | 0.354 | 0.006 |
| Semi-eviscerated rate | 80.16 b | 81.03 b | 86.21 a | 83.18 ab | 81.69 ab | 81.22 b | 0.526 | 0.005 |
| Eviscerated rate | 68.30 b | 68.90 b | 73.99 a | 69.24 b | 69.15 b | 68.69 b | 0.471 | 0.001 |
| Thigh muscle yield | 12.97 ab | 13.61 ab | 14.67 a | 14.20 ab | 13.92 ab | 12.68 b | 0.192 | 0.015 |
| Breast muscle yield | 14.37 b | 14.81 ab | 16.41 a | 15.48 ab | 15.44 ab | 13.85 b | 0.227 | 0.010 |
| Abdominal fat | 1.72 a | 1.37 bc | 1.21 bc | 1.34 bc | 1.38 ab | 1.48 c | 0.050 | 0.012 |
| Item | Treatment 1 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | AB | C + M | C | M | CICC | |||
| 21 d | ||||||||
| Glucose (mmol/L) | 10.67 ab | 10.98 ab | 11.31 a | 11.21 a | 11.01 ab | 9.45 b | 0.175 | 0.011 |
| Total cholesterol (mmol/L) | 3.34 ab | 4.12 a | 3.31 b | 3.49 ab | 3.05 b | 3.35 ab | 0.090 | 0.008 |
| Triglyceride (mmol/L) | 0.32 b | 0.39 b | 0.53 a | 0.50 a | 0.49 a | 0.41 b | 0.015 | <0.001 |
| Total protein (g/L) | 25.50 b | 23.12 c | 26.23 a | 27.32 a | 25.73 ab | 25.57 b | 0.367 | 0.002 |
| Albumin (g/L) | 12.00 | 10.65 | 10.83 | 11.70 | 11.15 | 10.98 | 0.181 | 0.221 |
| Urea (mmol/L) | 0.53 ab | 0.59 a | 0.44 b | 0.49 ab | 0.44 b | 0.51 ab | 0.017 | 0.049 |
| Creatinine (μmol/L) | 9.05 b | 11.92 a | 10.33 b | 9.6 b | 8.78 b | 9.53 b | 0.266 | 0.003 |
| 39 d | ||||||||
| Glucose (mmol/L) | 10.50 | 11.09 | 12.06 | 11.13 | 10.82 | 10.77 | 0.161 | 0.079 |
| Total cholesterol (mmol/L) | 3.57 | 2.95 | 3.22 | 3.02 | 3.07 | 3.76 | 0.108 | 0.176 |
| Triglyceride (mmol/L) | 0.37 b | 0.45 b | 0.52 a | 0.56 a | 0.43 b | 0.42 b | 0.016 | 0.004 |
| Total protein (g/L) | 31.85 | 27.15 | 32.63 | 32.85 | 34.00 | 34.48 | 0.840 | 0.117 |
| Albumin (g/L) | 10.38 b | 9.48 b | 11.92 a | 11.15 a | 11.38 a | 11.20 a | 0.170 | 0.003 |
| Urea (mmol/L) | 0.44 b | 0.59 a | 0.49 ab | 0.53 ab | 0.41 b | 0.42 b | 0.018 | 0.017 |
| Creatinine (μmol/L) | 9.75 bc | 11.43 ab | 10.23 ab | 11.75 a | 8.28 c | 9.62 bc | 0.305 | 0.005 |
| Item | Starter (1–21 Days) | Finisher (22–39 Days) |
|---|---|---|
| Ingredients (%) | ||
| Corn | 54.70 | 56.90 |
| Soybean meal | 34.70 | 32.40 |
| Dicalcium phosphate | 1.50 | 1.40 |
| Limestone | 1.20 | 1.20 |
| Soybean oil | 0.90 | 1.10 |
| Wheat | 5.00 | 5.00 |
| Chicken bone meal | 0.00 | 2.00 |
| Premix 1 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels 2 | ||
| ME (kcal/kg) | 3100.00 | 3200.00 |
| Dry matter (%) | 87.35 | 87.70 |
| Crude protein (%) | 22.00 | 20.50 |
| Crude fiber (%) | 3.40 | 3.50 |
| Lysine (%) | 1.40 | 1.27 |
| Methionine + cystine (%) | 0.98 | 0.93 |
| Threonine (%) | 0.95 | 0.84 |
| Calcium (%) | 0.93 | 0.90 |
| Total phosphorus (%) | 0.69 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmat, M.; Cheng, J.; Abbas, Z.; Cheng, Q.; Fan, Z.; Ahmad, B.; Hou, M.; Osman, G.; Guo, H.; Wang, J.; et al. Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics 2021, 10, 1427. https://doi.org/10.3390/antibiotics10111427
Ahmat M, Cheng J, Abbas Z, Cheng Q, Fan Z, Ahmad B, Hou M, Osman G, Guo H, Wang J, et al. Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics. 2021; 10(11):1427. https://doi.org/10.3390/antibiotics10111427
Chicago/Turabian StyleAhmat, Marhaba, Junhao Cheng, Zaheer Abbas, Qiang Cheng, Zhen Fan, Baseer Ahmad, Min Hou, Ghenijan Osman, Henan Guo, Junyong Wang, and et al. 2021. "Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens" Antibiotics 10, no. 11: 1427. https://doi.org/10.3390/antibiotics10111427
APA StyleAhmat, M., Cheng, J., Abbas, Z., Cheng, Q., Fan, Z., Ahmad, B., Hou, M., Osman, G., Guo, H., Wang, J., & Zhang, R. (2021). Effects of Bacillus amyloliquefaciens LFB112 on Growth Performance, Carcass Traits, Immune, and Serum Biochemical Response in Broiler Chickens. Antibiotics, 10(11), 1427. https://doi.org/10.3390/antibiotics10111427








