Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Model Development
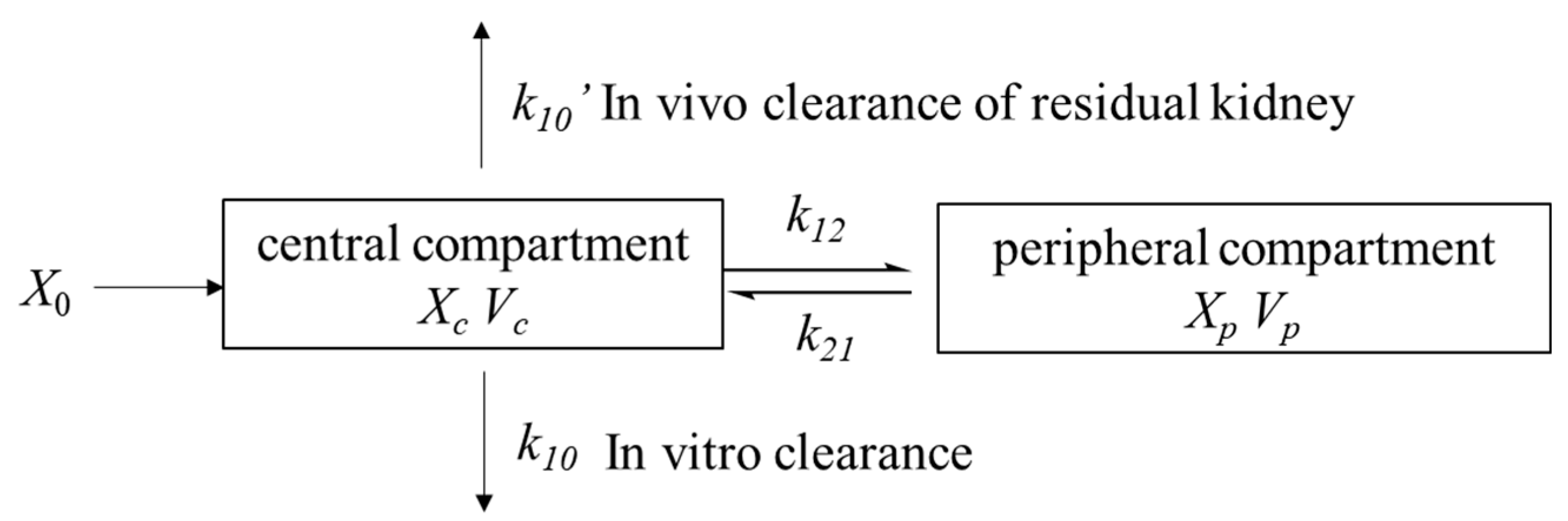
2.2.1. Basic Model
2.2.2. Covariate Model
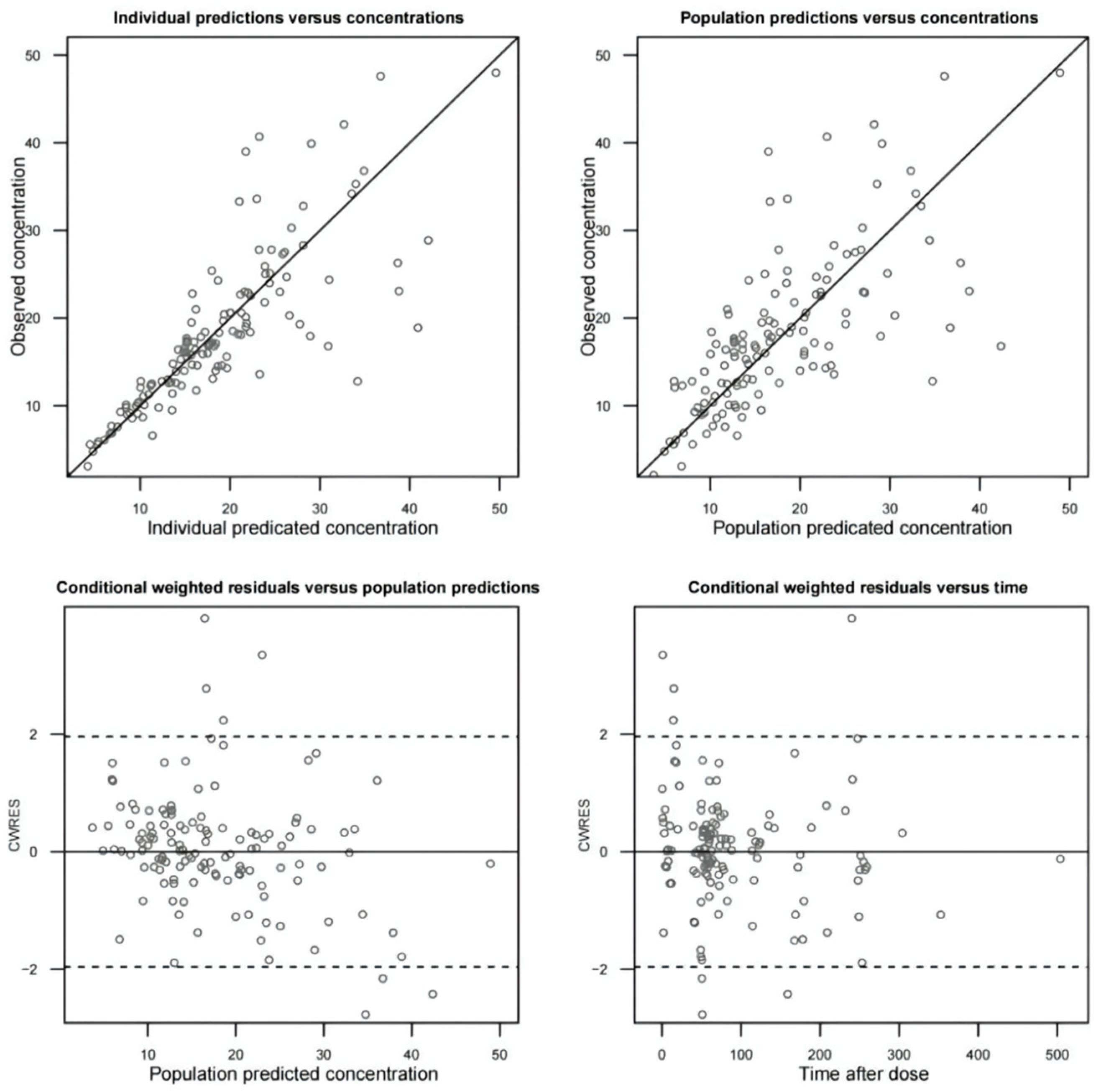
2.2.3. Model Evaluation
2.3. Population PK/PD Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Sampling and Analytical Assay
4.3. Data Collection
4.4. Statistical Analysis
4.5. Population PK Model Development
4.5.1. Structural Model
4.5.2. Statistical Model
4.5.3. Covariate Model
4.5.4. Model Evaluation
4.6. Population PK/PD Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Zimmerman, J.J. Pediatric sepsis from start to finish. Pediatr. Crit. Care Med. 2015, 16, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Lee, A.; Choi, G.; Bellomo, R.; Kanji, S.; Mudaliar, M.Y.; Peake, S.L.; Stephens, D.; Taccone, F.S.; et al. The Effect of Renal Replacement Therapy and Antibiotic Dose on Antibiotic Concentrations in Critically Ill Patients: Data from the Multinational Sampling Antibiotics in Renal Replacement Therapy Study. Clin. Infect. Dis. 2021, 72, 1369–1378. [Google Scholar] [CrossRef]
- Udy, A.A.; Covajes, C.; Taccone, F.S.; Jacobs, F.; Vincent, J.L.; Lipman, J.; Roberts, J.A. Can population pharmacokinetic modelling guide vancomycin dosing during continuous renal replacement therapy in critically ill patients? Int. J. Antimicrob. Agents 2013, 41, 564–568. [Google Scholar] [CrossRef]
- Wahby, K.A.; Cunmuljaj, L.; Mouabbi, K.; Almadrahi, Z.; Wilpula, L. Evaluation of dosing strategies and trough concentrations of vancomycin in patients undergoing continuous venovenous hemofiltration. Pharmacotherapy 2021, 41, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Walther, S.; Leone, M.; Barie, P.S.; Rello, J.; Lipman, J.; Marshall, J.C.; Anzueto, A.; Sakr, Y.; Pickkers, P.; et al. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: Results from the EPIC II study. Int. J. Antimicrob. Agents 2011, 38, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Robinson, J.O. Risk factors and outcomes of methicillin-resistant Staphylococcus aureus bacteraemia in critically ill patients: A case control study. Anaesth. Intensive Care 2009, 37, 457–463. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A history. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef]
- Macias, W.L.; Mueller, B.A.; Scarim, S.K. Vancomycin pharmacokinetics in acute renal failure: Preservation of nonrenal clearance. Clin. Pharmacol. Ther. 1991, 50, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Chaijamorn, W.; Jitsurong, A.; Wiwattanawongsa, K.; Wanakamanee, U.; Dandecha, P. Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Int. J. Antimicrob. Agents 2011, 38, 152–156. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; De Waele, J.J.; Dimopoulos, G.; Koulenti, D.; Martin, C.; Montravers, P.; Rello, J.; Rhodes, A.; Starr, T.; Wallis, S.C.; et al. DALI: Defining Antibiotic Levels in Intensive care unit patients: A multi-centre point of prevalence study to determine whether contemporary antibiotic dosing for critically ill patients is therapeutic. BMC Infect. Dis. 2012, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.R.; Forrest, A.; Meagher, A.K.; Birmingham, M.C.; Schentag, J.J. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 2003, 42, 1411–1423. [Google Scholar] [CrossRef]
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- De Paepe, P.; Belpaire, F.M.; Buylaert, W.A. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin. Pharmacokinet. 2002, 41, 1135–1151. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar]
- Li, L.; Li, X.; Xia, Y.; Chu, Y.; Zhong, H.; Li, J.; Liang, P.; Bu, Y.; Zhao, R.; Liao, Y.; et al. Recommendation of Antimicrobial Dosing Optimization during Continuous Renal Replacement Therapy. Front. Pharmacol. 2020, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, F.; Sang, L.; Li, P.; Lv, B.; Tan, L.; Liu, X.; Chen, W. Pharmacokinetics of and maintenance dose recommendations for vancomycin in severe pneumonia patients undergoing continuous venovenous hemofiltration with the combination of predilution and postdilution. Eur. J. Clin. Pharmacol. 2020, 76, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Trotman, R.L.; Williamson, J.C.; Shoemaker, D.M.; Salzer, W.L. Antibiotic Dosing in Critically Ill Adult Patients Receiving Continuous Renal Replacement Therapy. Clin. Infect. Dis. 2005, 41, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.; Andresen, M.; Downey, P.; Gai, M.N.; Regueira, T.; Bórquez, T.; Lipman, J.; Roberts, J.A. Population pharmacokinetics and dose simulation of vancomycin in critically ill patients during high-volume haemofiltration. Int. J. Antimicrob. Agents 2014, 44, 163–167. [Google Scholar] [CrossRef]
- Covajes, C.; Scolletta, S.; Penaccini, L.; Ocampos-Martinez, E.; Abdelhadii, A.; Beumier, M.; Jacobs, F.; de Backer, D.; Vincent, J.L.; Taccone, F.S. Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int. J. Antimicrob. Agents 2013, 41, 261–266. [Google Scholar] [CrossRef]
- RENAL Study Investigators. Renal replacement therapy for acute kidney injury in Australian and New Zealand intensive care units: A practice survey. Crit. Care Resusc. 2008, 10, 225–230. [Google Scholar]
- He, N.; Su, S.; Ye, Z.; Du, G.; He, B.; Li, D.; Liu, Y.; Yang, K.; Zhang, X.; Zhang, Y.; et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin. Infect. Dis. 2020, 71, S363–S371. [Google Scholar] [CrossRef]
- David, N.J.; Henry, F.C.; George, M.E.; Michael, S.S.; Andrew, T.P. Antibiotic dose adjustment of adults with repaired renal function. In The Sanford Guide to Antimicrobial Therapy 2018 (Chinese Version), 48th ed.; Jeb, C.S., Ed.; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2018; p. 230. [Google Scholar]
- Zhao, S.; He, N.; Zhang, Y.; Wang, C.; Zhai, S.; Zhang, C. Population Pharmacokinetic Modeling and Dose Optimization of Vancomycin in Chinese Patients with Augmented Renal Clearance. Antibiot. Basel 2021, 10, 1238. [Google Scholar] [CrossRef]
- McConeghy, K.W.; Liao, S.; Clark, D.; Worboys, P.; Barriere, S.L.; Rodvold, K.A. Variability in telavancin cross-reactivity among vancomycin immunoassays. Antimicrob. Agents Chemother. 2014, 58, 7093–7097. [Google Scholar] [CrossRef][Green Version]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Lin, X.B.; Li, Z.W.; Yan, M.; Zhang, B.K.; Liang, W.; Wang, F.; Xu, P.; Xiang, D.X.; Xie, X.B.; Yu, S.J.; et al. Population pharmacokinetics of voriconazole and CYP2C19 polymorphisms for optimizing dosing regimens in renal transplant recipients. Br. J. Clin. Pharmacol. 2018, 84, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, T.; Liu, Y.; Wang, J.; Hu, K.; Bao, F.; Zhang, C. Model-based Voriconazole Dose Optimization in Chinese Adult Patients with Hematologic Malignancies. Clin. Ther. 2019, 41, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, S.; Sun, J.; Cai, J.; Cheng, X.; Dong, H.; Wang, X.; Xing, J.; Dong, W.; Yao, H.; et al. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J. Antimicrob. Chemother. 2014, 69, 463–470. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Value |
|---|---|
| Number of patients | 11 |
| Number of samples | 131 |
| Age, median [IQR] (range), y | 63 [30.5] (20–83) |
| Body weight, median [IQR] (range), kg | 70 [22.5] (52–90) |
| Urinary output, median [IQR] (range), mL | 300 [436.8] (0–3340) |
| Creatinine clearance before CVVH, median [IQR] (range), mL/min | 29.1 [13.6] (11–66) |
| Albumin, median [IQR] (range), g/L | 28.6 [5.1] (26–39) |
| Ultrafiltration rate, median [IQR] (range), mL/kg/h | 33.3 [6.5] (18–39) |
| APACHE II score, median [IQR] (range) | 24 [1] (9–27) |
| SOFA score, median [IQR] (range) | 9 [3] (5–12) |
| SEX, no. (%) | |
| Female | 5 (45.5%) |
| Male | 6 (54.5%) |
| Parameters | Values (%RSE) | Bootstrap | |
|---|---|---|---|
| Median | 95%CI | ||
| CLPOP (L/h) | 1.15 (17) | 1.17 | 0.63–1.69 |
| Vc (L) | 16.9 (11) | 16.77 | 11.78–23.35 |
| Vp (L) | 25.9 (19) | 26.72 | 15.38–38.74 |
| Q (L/h) | 7.72 (18) | 7.65 | 4.60–21.42 |
| α,ALB effect on CL | 5.52 (20) | 5.00 | 1.38–7.89 |
| β,UFR effect on CL | 0.0377 (14) | 0.0378 | 0.024–0.056 |
| IIV CL | 0.0647 (28) | 0.056 | 0.0126–0.101 |
| RV (proportional) | 0.0507 (22) | 0.0466 | 0.023–0.068 |
| Points below PI (Count) | Points below PI (%) | 95% CI below (%) | Points above PI (Count) | Points above PI (%) | 95% CI above (%) | |
|---|---|---|---|---|---|---|
| 0% PI | 54 | 41.22 | 32.06–67.18 | 77 | 58.78 | 32.82–67.94 |
| 20% PI | 42 | 32.06 | 23.66–56.49 | 54 | 41.22 | 23.66–57.25 |
| 40% PI | 36 | 27.48 | 15.26–45.80 | 40 | 30.53 | 15.27–46.56 |
| 50% PI | 28 | 21.37 | 11.45–41.22 | 34 | 25.95 | 10.68–41.22 |
| 60% PI | 24 | 18.32 | 8.40–34.35 | 27 | 20.61 | 7.63–35.11 |
| 80% PI | 14 | 10.69 | 2.29–21.37 | 16 | 12.21 | 2.29–21.37 |
| 90% PI | 7 | 5.34 | 0.76–12.98 | 9 | 6.87 | 0.00–12.98 |
| 95% PI | 3 | 2.29 | 0.00–7.63 | 6 | 4.58 | 0.00–8.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, C.; Li, X.; Zhao, S.; He, N.; Zhai, S.; Ge, Q. Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis. Antibiotics 2021, 10, 1392. https://doi.org/10.3390/antibiotics10111392
Wang C, Zhang C, Li X, Zhao S, He N, Zhai S, Ge Q. Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis. Antibiotics. 2021; 10(11):1392. https://doi.org/10.3390/antibiotics10111392
Chicago/Turabian StyleWang, Chuhui, Chao Zhang, Xiaoxiao Li, Sixuan Zhao, Na He, Suodi Zhai, and Qinggang Ge. 2021. "Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis" Antibiotics 10, no. 11: 1392. https://doi.org/10.3390/antibiotics10111392
APA StyleWang, C., Zhang, C., Li, X., Zhao, S., He, N., Zhai, S., & Ge, Q. (2021). Dose Optimization of Vancomycin for Critically Ill Patients Undergoing CVVH: A Prospective Population PK/PD Analysis. Antibiotics, 10(11), 1392. https://doi.org/10.3390/antibiotics10111392






