Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt
Abstract
1. Introduction
2. Results
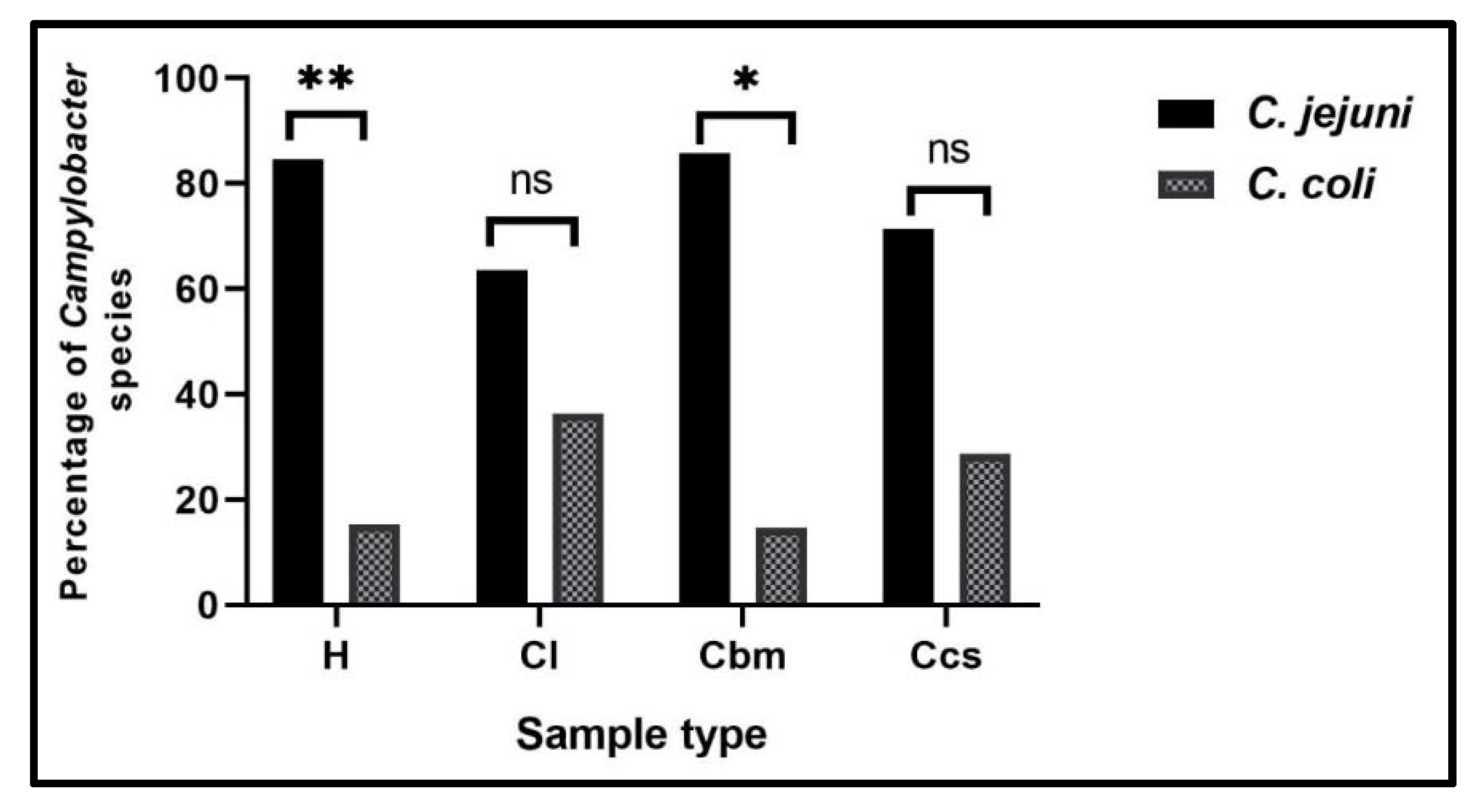
2.1. Prevalence of Campylobacter Species in Different Samples at Sharkia Governorate, Egypt
2.2. Antimicrobial Susceptibility Testing of Campylobacter Isolates
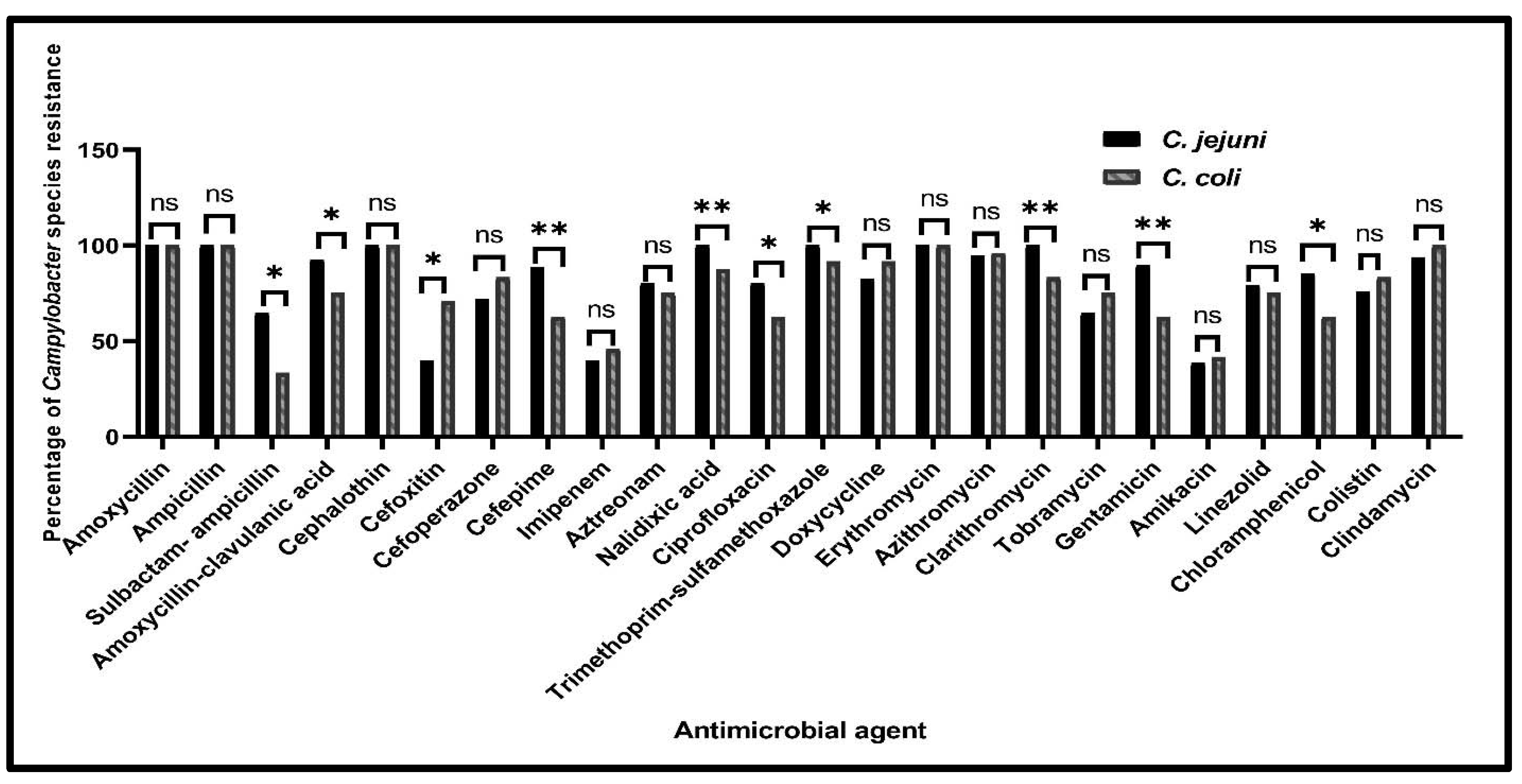
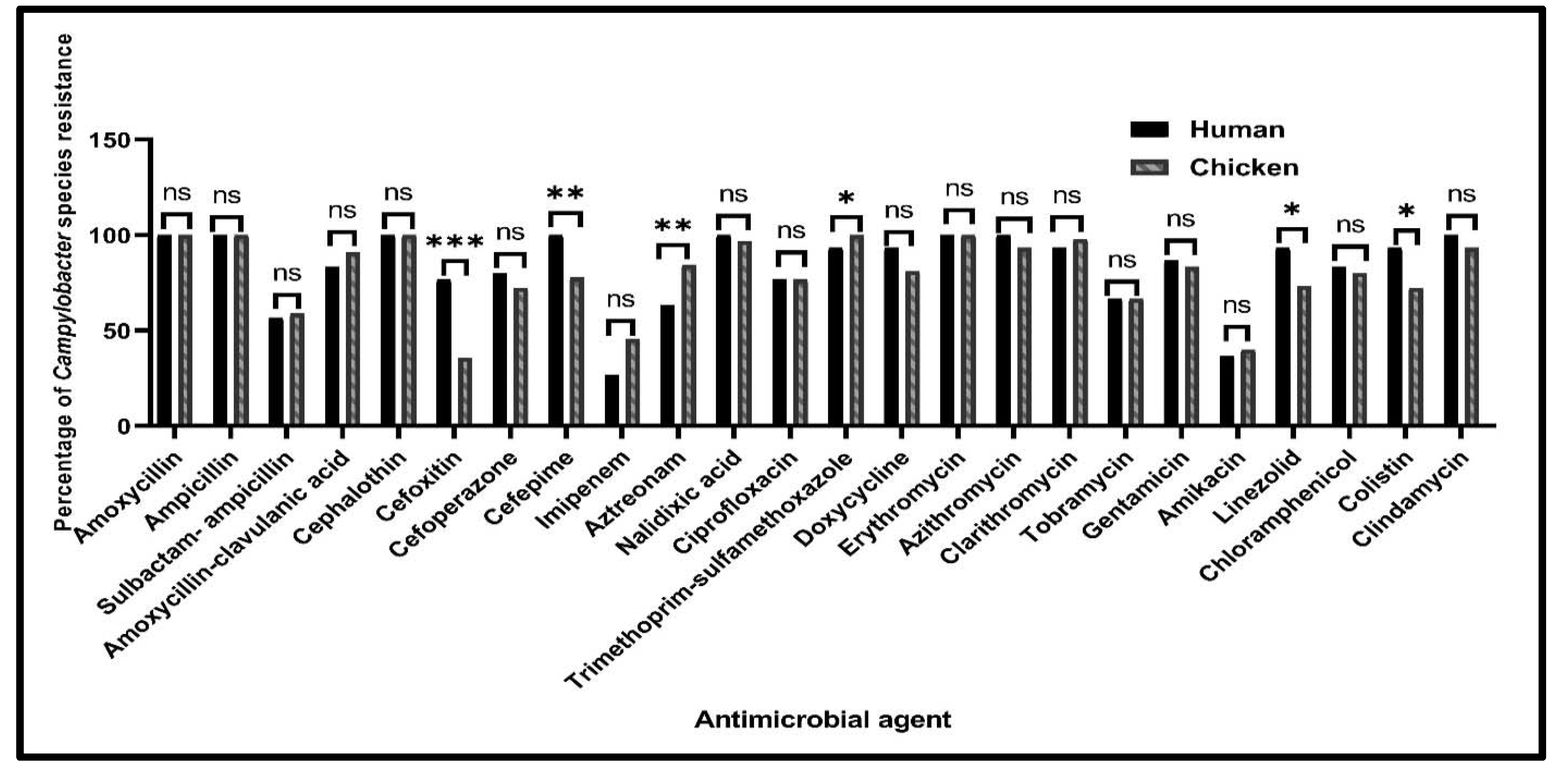
2.2.1. Antimicrobial Susceptibility Profiles of Campylobacter Species from Various Sources
2.2.2. The Minimum Inhibitory Concentrations of Ciprofloxacin against Campylobacter Isolates
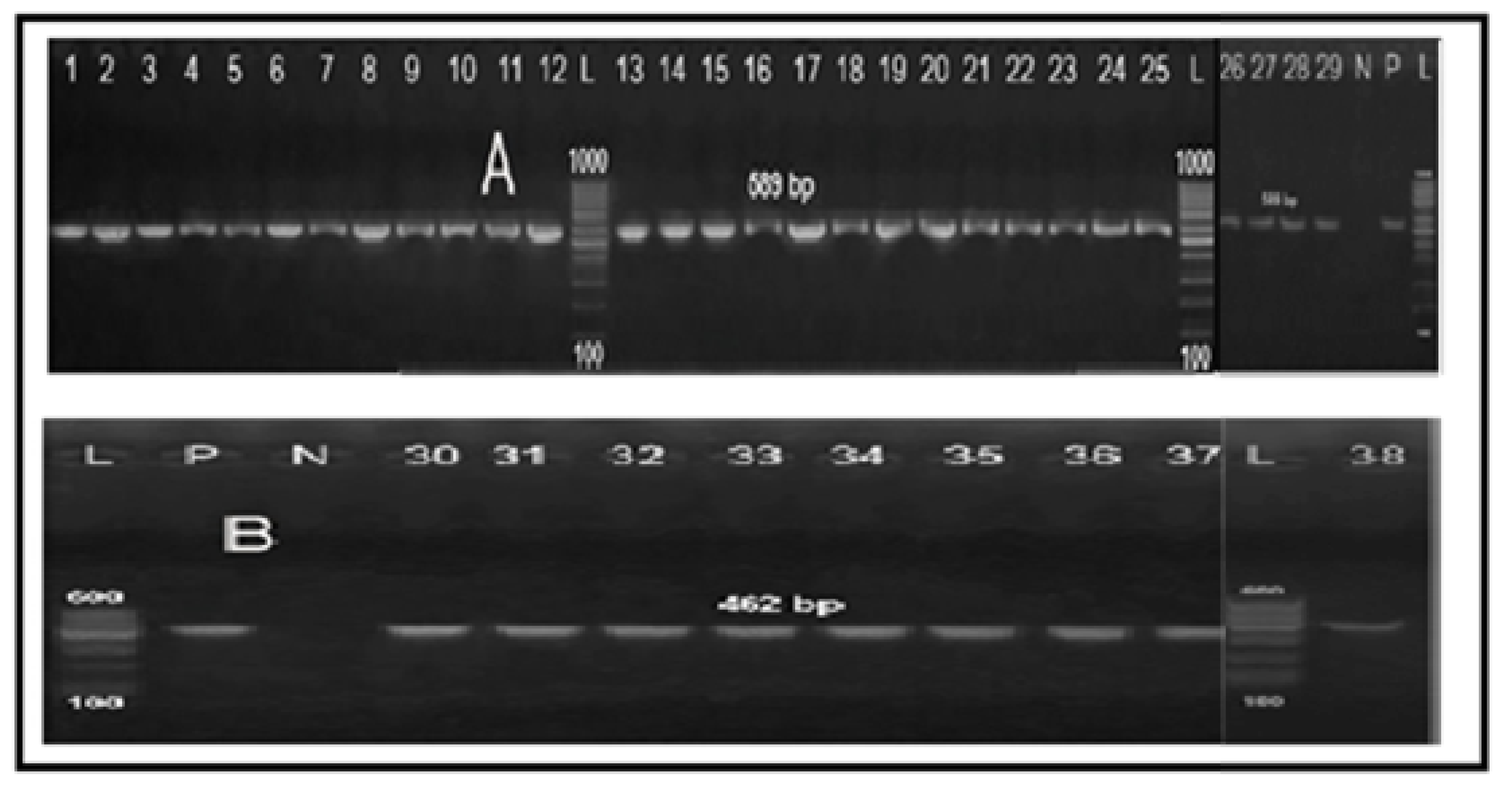
2.3. Molecular Grouping of Campylobacter Isolates from Different Sources
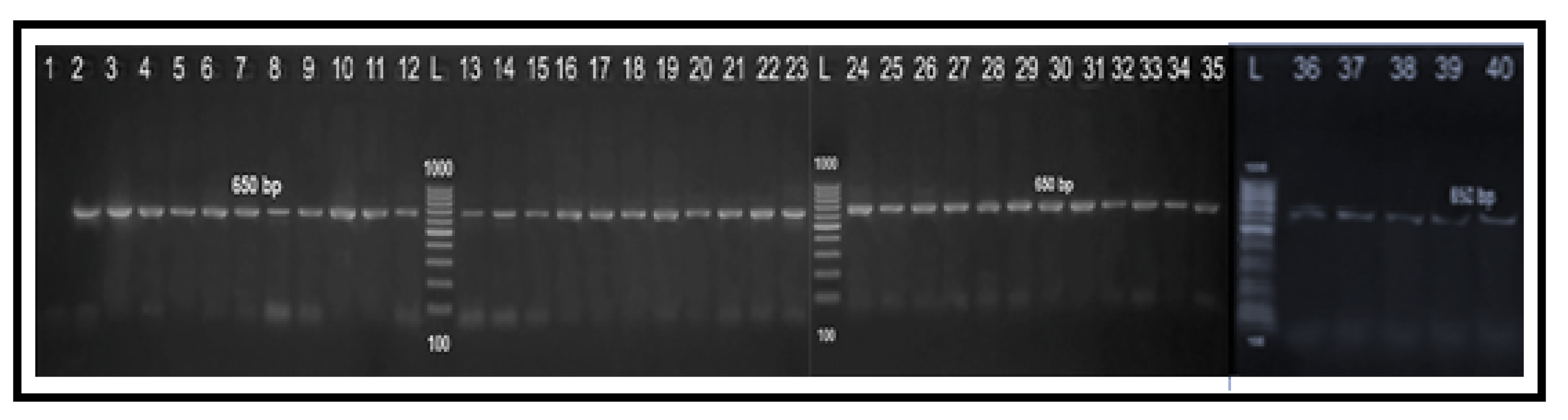
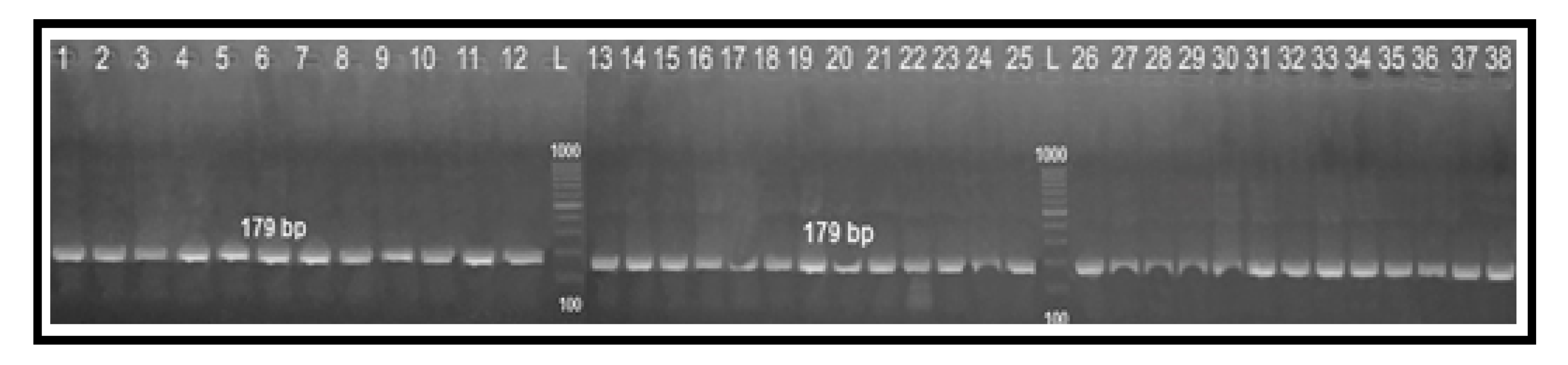
2.4. Determination of Fluoroquinolone Resistance by PCR-RFLP Technique
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Sample Collection
4.3. Isolation and Identification of Campylobacter Species
4.4. Antimicrobial Susceptibility Testing
4.4.1. Disc Diffusion Method
4.4.2. Broth Microdilution Method for Determining Ciprofloxacin Minimal Inhibitory Concentrations
4.5. Conventional PCR and PCR-RFLP Assays
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narvaez-Bravo, C.; Taboada, E.N.; Mutschall, S.K.; Aslam, M. Epidemiology of antimicrobial resistant Campylobacter spp. isolated from retail meats in Canada. Int. J. Food Microbiol. 2017, 253, 43–47. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Hamid, M.I.A.; El-Gedawy, A.A.; El-Malt, R.M.S. Campylobacter as a Major Foodborne Pathogen: A Review of Its Characteristics, Pathogenesis, Antimicrobial Resistance and Control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- Same, R.G.; Tamma, P.D. Campylobacter infections in children. Pediatr. Rev. 2018, 39, 533–541. [Google Scholar] [CrossRef]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A review of the effect of management practices on campylobacter prevalence in poultry farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Alaboudi, A.R.; Malkawi, I.M.; Osaili, T.M.; Abu-Basha, E.A.; Guitian, J. Prevalence, antibiotic resistance and genotypes of Campylobacter jejuni and Campylobacter coli isolated from chickens in Irbid governorate, Jordan. Int. J. Food Microbiol. 2020, 327, 108656. [Google Scholar] [CrossRef]
- Ma, H.; Su, Y.; Ma, L.; Ma, L.; Li, P.; Du, X.; Gölz, G.; Wang, S.; Lu, X. Prevalence and characterization of Campylobacter jejuni isolated from retail chicken in Tianjin, China. J. Food Prot. 2017, 80, 1032–1040. [Google Scholar] [CrossRef]
- Endtz, H.P. Campylobacter Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 507–511. [Google Scholar]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Malt, R.M.S.; El-Gedawy, A.A.; Khalifa, E.; Elnahriry, S.S.; Abd El-Hamid, M.I. Prevalence, Antimicrobial Susceptibility, Virulence and Genotyping of Campylobacter jejuni with a Special Reference to the Anti-Virulence Potential of Eugenol and Beta-Resorcylic Acid on Some Multi-Drug Resistant Isolates in Egypt. Animals 2021, 1, 3. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef]
- Abd El-Tawab, A.A.; Ammar, A.M.; Ahmed, H.A.; Hofy, F.I.E.; Hefny, A.A. Fluoroquinolone resistance and gyrA mutations in Campylobacter jejuni and Campylobacter coli isolated from chicken in Egypt. J. Glob. Antimicrob. Resist. 2018, 13, 22–23. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Antimicrobial resistance mechanisms among campylobacter. Biomed. Res. Int. 2013, 2013, 340605. [Google Scholar] [CrossRef]
- Said, M.M.; El-Mohamady, H.; El-Beih, F.M.; Rockabrand, D.M.; Ismail, T.F.; Monteville, M.R.; Ahmed, S.F.; Klena, J.D.; Salama, M.S. Detection of gyrA Mutation Among Clinical Isolates of Campylobacter jejuni Isolated in Egypt by MAMA-PCR. J. Infect. Dev. Ctries. 2010, 4, 546–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sierra-Arguello, Y.M.; Furian, T.Q.; Perdoncini, G.; Moraes, H.L.S.; Salle, C.T.P.; Rodrigues, L.B.; dos Santos, L.R.; Gomes, M.J.P.; Nascimento, V.P.D. Fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli from poultry and human samples assessed by PCR-restriction fragment length polymorphism assay. PLoS ONE 2018, 13, e0199974. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Sierra-Arguello, Y.M.; Perdoncini, G.; Morgan, R.B.; Salle, C.T.P.; Moraes, H.L.S.; Gomes, M.J.P.; Nascimento, V.P.D. Fluoroquinolone and macrolide resistance in Campylobacter jejuni isolated from broiler slaughterhouses in southern Brazil. Avian Pathol. 2016, 45, 66–72. [Google Scholar] [CrossRef]
- Ghaly, M.; Shaheen, A.; Bouhy, A.; Bendary, M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020, 202, 1–10. [Google Scholar] [CrossRef]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55. [Google Scholar] [PubMed]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aurues recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Ghaith, D.M.; Zafer, M.M.; Said, H.M.; Elanwary, S.; Elsaban, S.; Al-Agamy, M.H.; Bohol, M.F.F.; Bendary, M.M.; Al-Qahtani, A.; Al-Ahdal, M.N. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M.I.A. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.M.; Attia, A.M.; Marwa, I.; Abd El-Hamid, M.I.; El-Shorbagy, I.M.; Shaimaa, A.; Abd El-Kader, S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016, 62, 7–15. [Google Scholar] [PubMed]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.A.; Elsohaby, I.; Ghaith, D.M.; Alshareef, W.A. What is behind phylogenetic analysis of hospital-, communityand livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [PubMed]
- Marwa, I. Abd El-Hamid; Bendary, M.M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell. Mol. Biol. 2015, 61, 106–117. [Google Scholar]
- Wieczorek, K.; Denis, E.; Osek, J. Comparative analysis of antimicrobial resistance and genetic diversity of Campylobacter from broilers slaughtered in Poland. Int. J. Food Microbiol. 2015, 210, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.N.; Abdel Aziz, S.A.A. The prevalence of Campylobacter species in broiler flocks and their environment: Assessing the efficiency of chitosan/zinc oxide nanocomposite for adopting control strategy. Environ. Sci. Pollut. Res. 2019, 26, 30177–30187. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.; Sayed, Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic Diversity of Campylobacter jejuni Isolated from Avian and Human Sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Tawab, A.; El Hofy, F.; Ammar, A.; Ahmed, H.; Hefny, A. Bacteriological and Molecular Identification of Campylobacter Species in Chickens and Humans, at Zagazig City, Egypt. Benha Vet. Med. J. 2015, 28, 17–26. [Google Scholar] [CrossRef][Green Version]
- Abd El-Aziz, N.K.; Ammar, A.M.; Hamdy, M.M.; Gobouri, A.A.; Azab, E.; Sewid, A.H. First Report of aacC5-aadA7Δ4 Gene Cassette Array and Phage Tail Tape Measure Protein on Class 1 Integrons of Campylobacter Species Isolated from Animal and Human Sources in Egypt. Animals 2020, 10, 2067. [Google Scholar] [CrossRef]
- Gharbi, M.; Béjaoui, A.; Ben Hamda, C.; Jouini, A.; Ghedira, K.; Zrelli, C.; Hamrouni, S.; Aouadhi, C.; Bessoussa, G.; Ghram, A.; et al. Prevalence and antibiotic resistance patterns of Campylobacter spp. isolated from broiler chickens in the North of Tunisia. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Cha, S.Y.; Yoon, R.H.; Kang, M.; Roh, J.H.; Seo, H.S.; Lee, J.A.; Jang, H.K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control. 2016, 62, 63–68. [Google Scholar] [CrossRef]
- Chatur, Y.A.; Brahmbhatt, M.N.; Modi, S.; Nayak, J.B. Fluoroquinolone resistance and detection of topoisomerase gene mutation in Campylobacter jejuni isolated from animal and human sources. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 773–783. [Google Scholar]
- Samad, A.; Abbas, F.; Ahmed, Z.; Akbar, A.; Naeem, M.; Sadiq, M.B.; Ali, I.; Bugti, F.S.; Achakzai, S.K. Prevalence, antimicrobial susceptibility, and virulence of Campylobacter jejuni isolated from chicken meat. J. Food Saf. 2019, 39, e12600. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Occurrence, Virulence and Antimicrobial Resistance-Associated Markers in Campylobacter Species Isolated from Retail Fresh Milk and Water Samples in Two District Municipalities in the Eastern Cape Province, South Africa. Antibiotics 2020, 9, 426. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Naenaeey, E.Y.; Kandeel, T.M.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Sayed, M.E.; Ali Aisha, R.; Abdallah, H.M.; Marwa, I.A.; El-mowalid, G.A. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 101–108. [Google Scholar] [CrossRef]
- Hegazy, W.A.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms 2020, 8, 1285. [Google Scholar] [CrossRef]
- ISO10272-1. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Campylobacter spp. Part. 1: Detection Method; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI Guideline M45; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Dahiya, S.; Sayal, P. Evaluation of multiple antibiotic resistance (MAR) index and Doxycycline susceptibility of Acinetobacter species among inpatients. Indian J. Microbiol. Res. 2016, 3, 299–304. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Song, D.-G.; Cha, K.H.; Yoon, K.-Y.; Mboera, L.E.G.; Matee, M.I.; Mutangana, D.; Amachawadi, R.G.; Komba, E.V.G.; Pan, C.-H. Susceptibility of Campylobacter Strains to Selected Natural Products and Frontline Antibiotics. Antibiotics 2020, 9, 790. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Catania, S.; Bottinelli, M.; Fincato, A.; Gastaldelli, M.; Barberio, A.; Gobbo, F.; Vicenzoni, G. Evaluation of Minimum Inhibitory Concentrations for 154 Mycoplasma synoviae isolates from Italy collected during 2012–2017. PLoS ONE 2019, 14, e0224903. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Lee, Y. Comparison of Three Different Methods for Campylobacter Isolation from Porcine Intestines. J. Microbiol. Biotechnol. 2009, 19, 647–650. [Google Scholar] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
| Sample Type (Symbol, No.) | Total No. of Campylobacter * Isolates (%) | No. of Campylobacter spp. (%) * | |
|---|---|---|---|
| C. jejuni | C. coli | ||
| Human stool swabs (H, 35) | 30 (85.7) | 25 (71.4) | 5 (14.3) |
| Broiler chicken samples (C, 175) | 90 (51.4) | 71 (40.6) | 19 (10.9) |
| Cloacal swabs (Ccs, 35) | 31 (88.6) | 24 (68.6) | 7 (20) |
| Breast muscles (Cbm, 35) | 28 (80) | 24 (68.6) | 4 (11.4) |
| Liver (Cl, 35) | 31 (88.6) | 23 (65.7) | 8 (22.9) |
| Chicken franks (Cf, 35) | 0 | 0 | 0 |
| Chicken luncheon meats (Cln, 35) | 0 | 0 | 0 |
| Total (210) | 120 (57.1) | 96 (45.7) | 24 (11.4) |
| Antimicrobial Class | Antimicrobial Agent | No. of C. jejuni Isolates (%) (n = 96) | No. of C. coli Isolates (%) (n = 24) | Total No. of Campylobacter Isolates (%) (n = 120) | ||
|---|---|---|---|---|---|---|
| Human (25) | Chicken (71) | Human (5) | Chicken (19) | |||
| Beta-lactams | Amoxycillin | 25 (100) | 71 (100) | 5 (100) | 19 (100) | 120 (100) |
| Ampicillin | 25 (100) | 71 (100) | 5 (100) | 19 (100) | 120 (100) | |
| Sulbactam-ampicillin | 15 (60) | 47 (66.2) | 2 (40) | 6 (31.6) | 70 (58.3) | |
| Amoxycillin-clavulanic acid | 23 (92) | 66 (93) | 2 (40) | 16 (84.2) | 107 (89.2) | |
| Cephalothin | 25 (100) | 71 (100) | 5 (100) | 19 (100) | 120 (100) | |
| Cefoxitin | 18 (72) | 20 (28.2) | 5 (100) | 12 (63.2) | 55 (45.8) | |
| Cefoperazone | 19 (76) | 50 (70.4) | 5 (100) | 15 (78.9) | 89 (74.2) | |
| Cefepime | 25 (100) | 60 (84.5) | 5 (100) | 10 (52.6) | 100 (83.3) | |
| Imipenem | 6 (24) | 32 (45.1) | 2 (40) | 9 (47.4) | 49 (40.8) | |
| Aztreonam | 14 (56) | 63 (88.7) | 5 (100) | 13 (68.4) | 95 (79.2) | |
| Quinolones | Nalidixic acid | 25 (100) | 71 (100) | 5 (100) | 16 (84.2) | 117 (97.5) |
| Ciprofloxacin | 21 (84) | 56 (78.9) | 2 (40) | 13 (68.4) | 92 (76.7) | |
| Sulfonamides | Trimethoprim-sulfamethoxazole | 25 (100) | 71 (100) | 3 (60) | 19 (100) | 118 (98.3) |
| Tetracyclines | Doxycycline | 25 (100) | 54 (76.1) | 3 (60) | 19 (100) | 101 (84.2) |
| Macrolides | Erythromycin | 25 (100) | 71 (100) | 5 (100) | 19 (100) | 120 (100) |
| Azithromycin | 25 (100) | 66 (93) | 5 (100) | 18 (94.7) | 114 (95) | |
| Clarithromycin | 25 (100) | 71 (100) | 3 (60) | 17 (89.5) | 116 (96.7) | |
| Aminoglycosides | Tobramycin | 15 (60) | 47 (66.2) | 5 (100) | 13 (68.4) | 80 (66.7) |
| Gentamicin | 23 (92) | 63 (88.7) | 3 (60) | 12 (63.2) | 101 (84.2) | |
| Amikacin | 8 (32) | 29 (40.8) | 3 (60) | 7 (36.8) | 47 (39.2) | |
| Oxazolidones | Linezolid | 25 (100) | 51 (71.8) | 3 (60) | 15 (78.9) | 94 (78.3) |
| Phenicols | Chloramphenicol | 23 (92) | 59 (83.1) | 2 (40) | 13 (68.4) | 97 (80.8) |
| Polypeptides | Colistin | 23 (92) | 50 (70.4) | 5 (100) | 15 (78.9) | 93 (77.5) |
| Lincosamide | Clindamycin | 25 (100) | 65 (91.5) | 5 (100) | 19 (100) | 114 (95) |
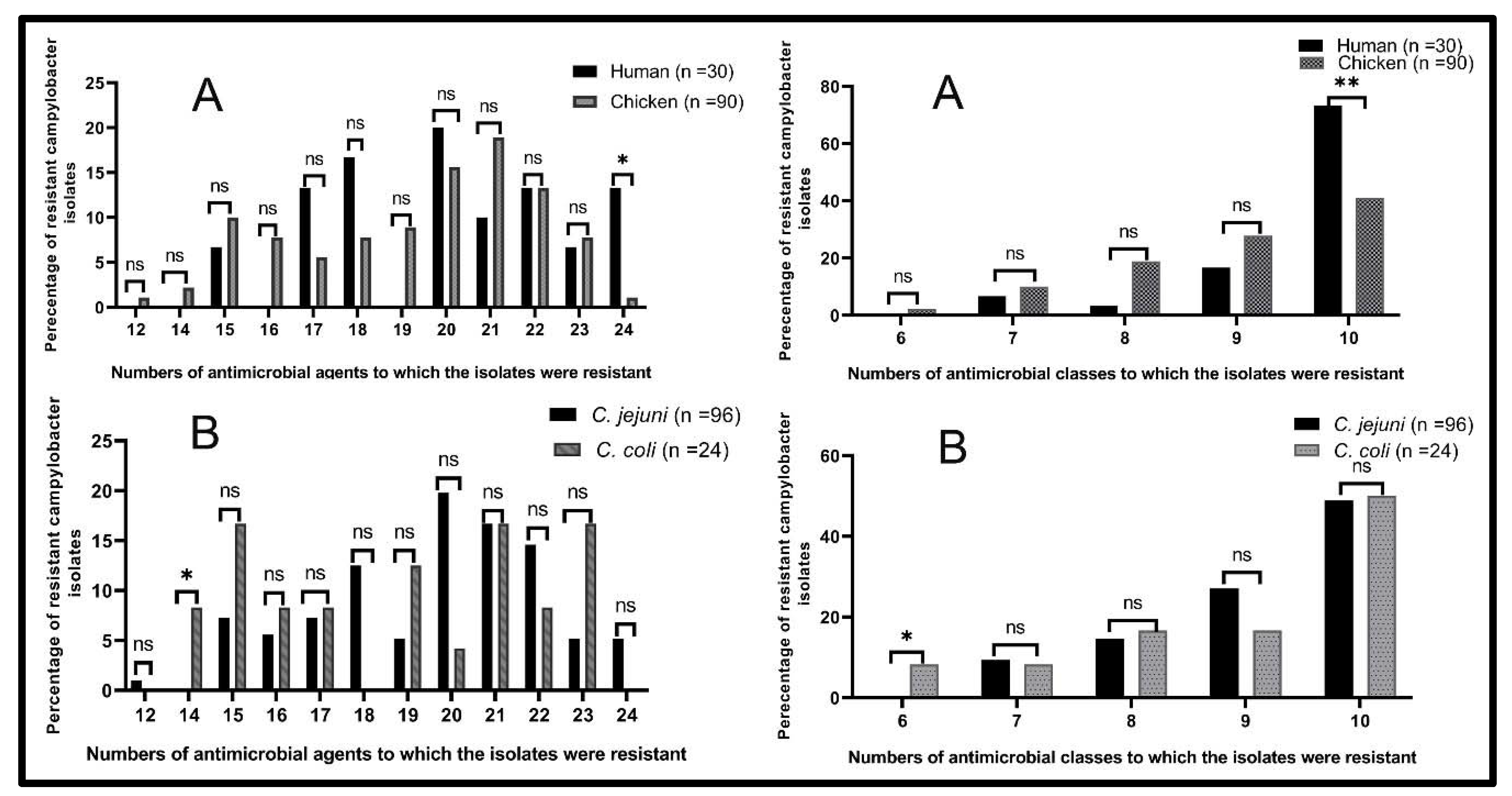
| MAR Index | No. of Antimicrobials to Which the Isolates Were Resistant | No. of AMC | No. of Resistant Campylobacter Isolates from Different Sources (%) | Total (120) | Character of Resistant Strains | |||
|---|---|---|---|---|---|---|---|---|
| Human (30) | Chicken (90) | |||||||
| C. jejuni (25) | C. coli (5) | C. jejuni (71) | C. coli (19) | |||||
| 0.50 | 12 | 7 | - | - | 1 (1.4) | - | 1 (0.8) | MDR |
| 0.58 | 14 | 6 | - | - | - | 2 (10.5) | 2 (1.7) | |
| 0.63 | 15 | 7 | - | 2 (40) | 5 (7.1) | - | 7 (5.8) | |
| 8 | - | - | 2 (2.8) | 2 (10.5) | 4 (3.3) | |||
| 0.67 | 16 | 8 | - | - | 5 (7) | - | 5 (4.2) | |
| 10 | - | - | - | 2 (10.5) | 2 (1.7) | |||
| 0.71 | 17 | 7 | - | - | 3 (4.2) | - | 3 (2.5) | |
| 8 | - | 1 (20) | - | - | 1 (0.8) | |||
| 9 | - | - | 1 (1.4) | 1 (5.3) | 2 (1.7) | |||
| 10 | 3 (12) | - | - | - | 3 (2.5) | |||
| 0.75 | 18 | 8 | - | - | 2 (2.8) | - | 2 (1.7) | |
| 9 | 2 (8) | - | 3 (4.2) | - | 5 (4.2) | |||
| 10 | 3 (12) | - | 2 (2.8) | - | 5 (4.2) | |||
| 0.79 | 19 | 8 | - | - | 1 (1.4) | 1 (5.3) | 2 (1.7) | |
| 9 | - | - | 1 (1.4) | 1 (5.3) | 2 (1.7) | |||
| 10 | - | - | 3 (4.2) | 1 (5.3) | 4 (3.3) | |||
| 0.83 | 20 | 9 | 2 (8) | - | 4 (5.6) | - | 6 (5) | |
| 10 | 4 (16) | - | 9 (12.7) | 1 (5.3) | 14 (11.7) | |||
| 0.88 | 21 | 9 | - | - | 13 (18.3) | 1 (5.3) | 14 (11.7) | |
| 10 | 3 (12) | - | - | 3 (15.8) | 6 (5) | |||
| 0.92 | 22 | 8 | - | - | 4 (5.6) | - | 4 (3.3) | XDR |
| 10 | 4 (16) | - | 6 (8.5) | 2 (10.5) | 12 (10) | |||
| 0.96 | 23 | 9 | - | 1 (20) | - | - | 1 (0.8) | |
| 10 | - | 1 (20) | 5 (7) | 2 (10.5) | 8 (6.7) | |||
| 1 | 24 | 10 | 4 (16) | - | 1 (1.4) | - | 5 (4.2) | PDR |
| Isolates Source | Campylobacter Species | No. of Campylobacter Isolates Showing MIC Values of Ciprofloxacin (µg/mL) * | MIC50 | MIC90 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | ||||
| Chicken (25) | C. jejuni (18) | 2 | 5 | 5 | 1 | 2 | 2 | 1 | 16 | 128 |
| C. coli (7) | 2 | 3 | 2 | - | - | - | - | 8 | 16 | |
| Human (13) | C. jejuni (11) | 2 | 2 | 3 | - | - | - | 4 | 16 | 256 |
| C. coli (2) | - | - | 1 | - | 1 | - | - | 16 | 64 | |
| Total (38) | 6 | 10 | 11 | 1 | 3 | 2 | 5 | 16 | 256 | |
| Specificity (Target Gene) | Primer Sequence (5′–3′) | Amplified Product (bp) | Reference |
|---|---|---|---|
| Genus Campylobacter (23S rRNA) | F: TATACCGGTAAGGAGTGCTGGAG | 650 | [49] |
| R: ATCAATTAACCTTCGAGCACCG | |||
| Campylobacter coli (ceuE) | F: AATTGA AAATTG CTCCAACTATG | 462 | [50] |
| R: TGATTT TATTATTTGTAGCAGCG | |||
| Campylobacter jejuni (mapA) | F: CTATTTTATTTTTGAGTGCTTGTG | 589 | [50] |
| R: GCTTTATTTGCCATTTGTTTTATTA | |||
| PCR-RFLP (gyrA) | F: AAATCAGCCCTATAGTGGGTGCTGTTATAGGTCGTTAT C ACCCACACATGGAGGT | 179 | [17] |
| R: TCAGTATAACGCATCGCAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics 2021, 10, 1342. https://doi.org/10.3390/antibiotics10111342
Ammar AM, Abd El-Hamid MI, El-Malt RMS, Azab DS, Albogami S, Al-Sanea MM, Soliman WE, Ghoneim MM, Bendary MM. Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics. 2021; 10(11):1342. https://doi.org/10.3390/antibiotics10111342
Chicago/Turabian StyleAmmar, Ahmed M., Marwa I. Abd El-Hamid, Rania M. S. El-Malt, Doaa S. Azab, Sarah Albogami, Mohammad M. Al-Sanea, Wafaa E. Soliman, Mohammed M. Ghoneim, and Mahmoud M. Bendary. 2021. "Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt" Antibiotics 10, no. 11: 1342. https://doi.org/10.3390/antibiotics10111342
APA StyleAmmar, A. M., Abd El-Hamid, M. I., El-Malt, R. M. S., Azab, D. S., Albogami, S., Al-Sanea, M. M., Soliman, W. E., Ghoneim, M. M., & Bendary, M. M. (2021). Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics, 10(11), 1342. https://doi.org/10.3390/antibiotics10111342









