Resurgence of Chloramphenicol Resistance in Methicillin-Resistant Staphylococcus aureus Due to the Acquisition of a Variant Florfenicol Exporter (fexAv)-Mediated Chloramphenicol Resistance in Kuwait Hospitals
Abstract
1. Introduction
2. Results
2.1. Molecular Typing of Chloramphenicol MRSA Isolates
2.2. Antibiotic Resistance Phenotypes and Genotypes
2.3. Determination of Susceptibility to Florfenicol
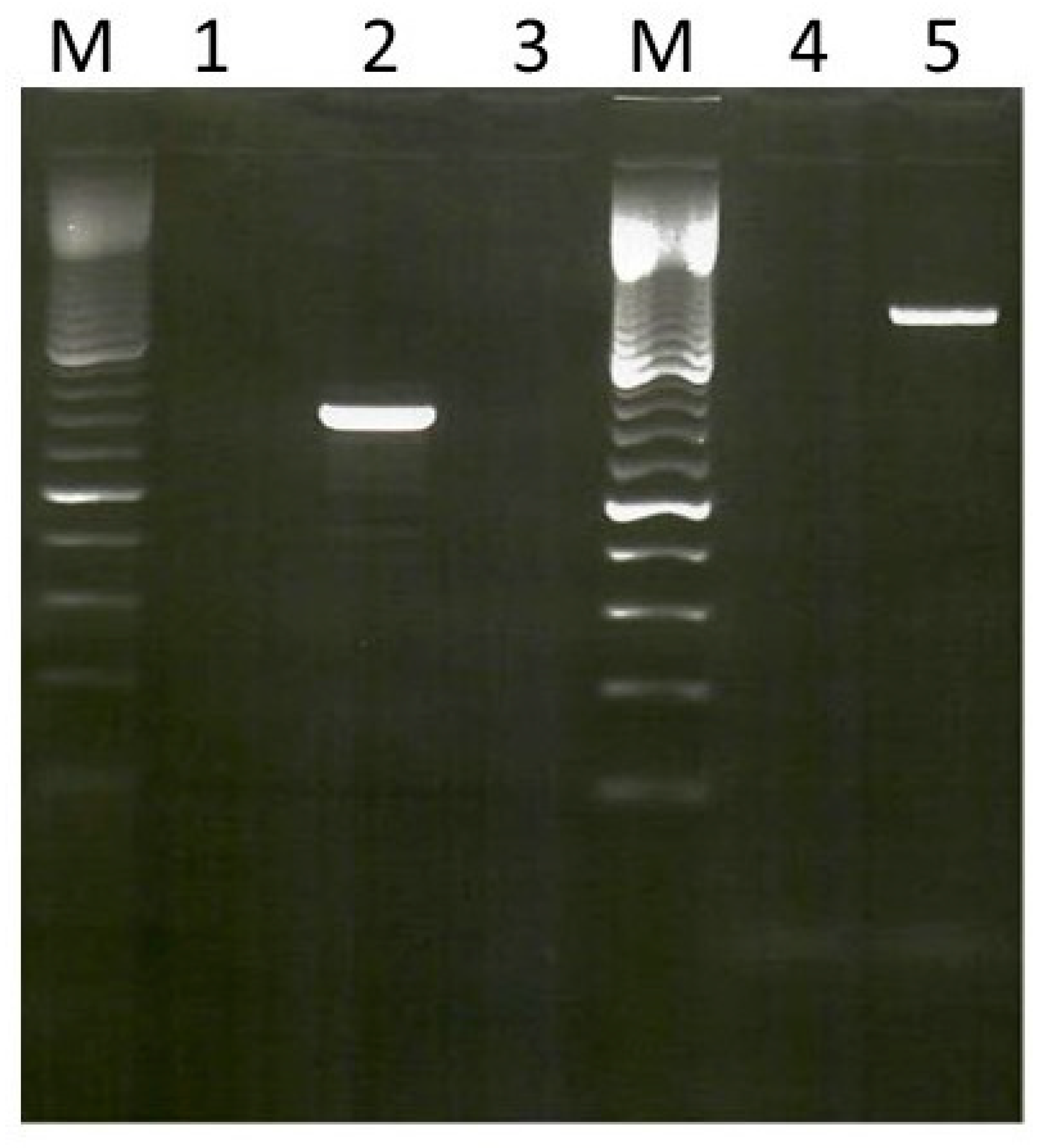
2.4. Amplification of Chloramphenicol and Florfenicol Resistance Genes
2.5. DNA Sequencing of fexA
2.6. Virulence Genes of Chloramphenicol Resistant Isolates
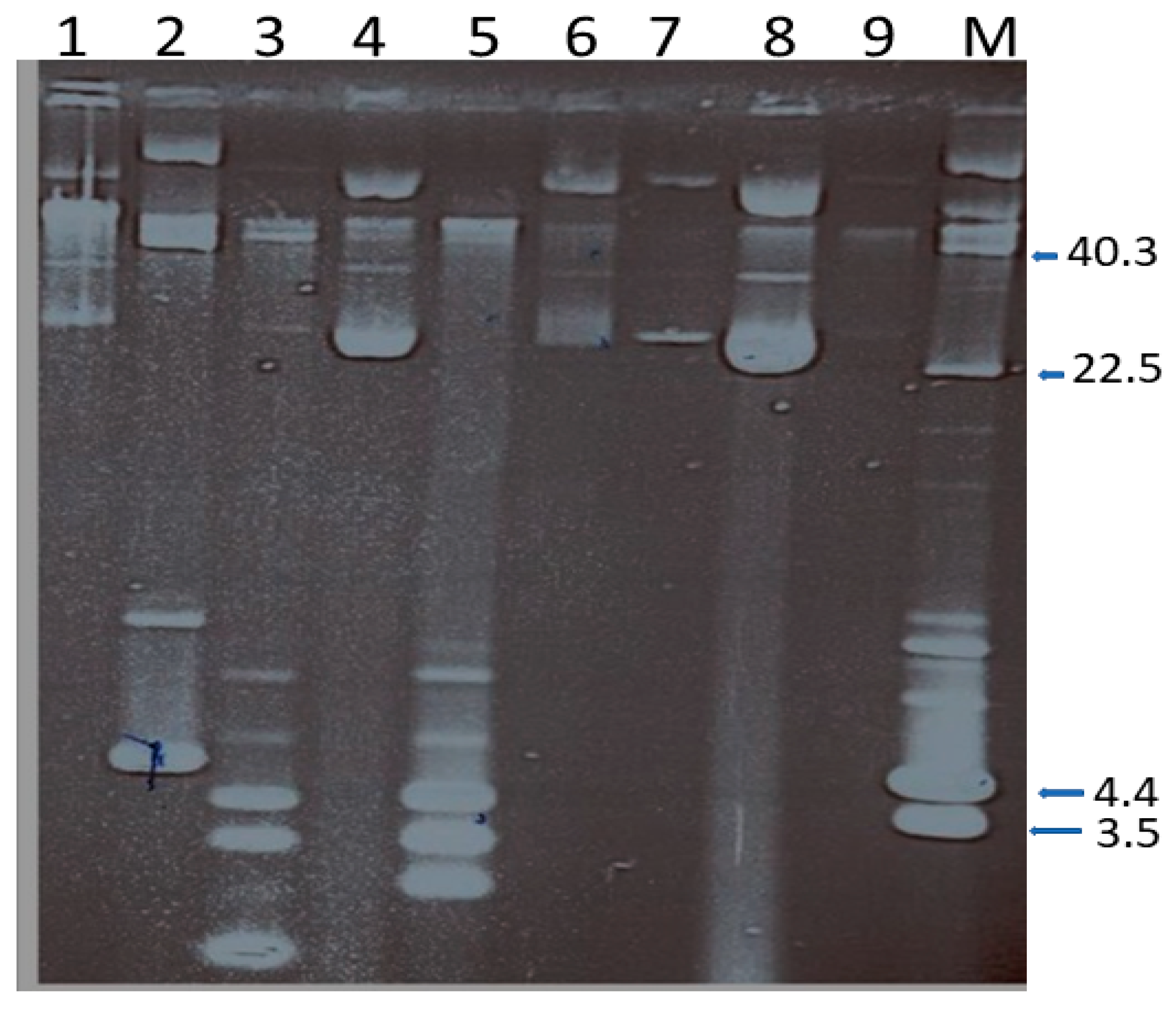
2.7. Genetic Location of Chloramphenicol/Florfenicol Resistance
2.7.1. Curing of Resistance and Plasmids
2.7.2. Transfer of Resistance and Plasmids
2.8. Amplification of Plasmid Borne Chloramphenicol Resistance Genes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antibiotic Susceptibility Testing
Determination of the Minimum Inhibitory Concentration (MIC)
4.3. Molecular Typing of Isolates
4.3.1. DNA Isolation for Amplification
4.3.2. Amplification of Chloramphenicol Resistance Genes
4.3.3. DNA Sequencing of fexA
4.3.4. Spa Typing
4.3.5. DNA Microarray
4.3.6. Multilocus Sequencing Typing (MLST)
4.4. Genetic Location of Chloramphenicol Resistance Determinants
4.4.1. Plasmid Analysis
4.4.2. Mixed Culture and Conjugation
4.4.3. Mobilisation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrlich, J.; Bartz, Q.R.; Smith, R.M.; Joslyn, D.A.; Burkholder, P.R. Chloromycetin, a New Antibiotic from a Soil Actinomycete. Science 1947, 106, 417. [Google Scholar] [CrossRef]
- Scholar, E. Chloramphenicol. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D., Eds.; Elsevier: Boston, MA, USA; Amsterdam, The Netherlands, 2007; pp. 1–7. ISBN 9780080552323. [Google Scholar]
- Britannica, The Editors of Encyclopaedia. “Chloramphenicol”. Encyclopedia Britannica. 24 December 2019. Available online: https://www.britannica.com/science/chloramphenicol (accessed on 13 July 2021).
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Dinos, G.P.; Athanassopoulos, C.M.; Missiri, D.A.; Giannopoulou, P.C.; Vlachogiannis, I.A.; Papadopoulos, G.E.; Papaioannou, D.; Kalpaxis, D.L. Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Svetlov, M.S.; Plessa, E.; Chen, C.-W.; Bougas, A.; Krokidis, M.G.; Dinos, G.P.; Polikanov, Y.S. High-resolution crystal structures of ribosome-bound chloramphenicol and erythromycin provide the ultimate basis for their competition. RNA 2019, 25, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, E.D.; Sicher, S. Mode of Action of Chloramphenicol. Nature 1952, 170, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Kehrenberg, C.; Schwarz, S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Lyon, B.R.; Skurray, R. Antimicrobial resistance of Staphylococcus aureus: Genetic basis. Microbiol. Rev. 1987, 51, 88–134. [Google Scholar] [CrossRef]
- Udo, E.E.; Al-Sweih, N.; Mokaddas, E.; Johny, M.; Dhar, R.; Gomaa, H.H.; Al-Obaid, I.; Rotimi, V.O. Antibacterial resistance and their genetic location in MRSA isolated in Kuwait hospitals, 1994–2004. BMC Infect. Dis. 2006, 6, 168. [Google Scholar] [CrossRef][Green Version]
- Udo, E.E.; Al-Sweih, N.; Dhar, R.; Dimitrov, T.S.; Mokaddas, E.M.; Johny, M.; Al-Obaid, I.A.; Gomaa, H.H.; Mobasher, L.; Rotimi, V.O.; et al. Surveillance of Antibacterial Resistance in Staphylococcus aureus Isolated in Kuwaiti Hospitals. Med. Princ. Pract. 2008, 17, 71–75. [Google Scholar] [CrossRef]
- Udo, E.E.; Boswihi, S.S. Antibiotic Resistance Trends in Methicillin-Resistant Staphylococcus aureus Isolated in Kuwait Hospitals: 2011–2015. Med. Princ. Pract. 2017, 26, 485–490. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Udo, E.E.; Al-Sweih, N. Shifts in the Clonal Distribution of Methicillin-Resistant Staphylococcus aureus in Kuwait Hospitals: 1992–2010. PLoS ONE 2016, 11, e0162744. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility testing for Bacteria Isolated from Animals, 5th ed.; Clinical and Laboratory Standards Institute Standard VET01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Müller, A.; Seinige, D.; Jansen, W.; Klein, G.; Ehricht, R.; Monecke, S.; Kehrenberg, C. Variety of Antimicrobial Resistances and Virulence Factors in Staphylococcus aureus Isolates from Meat Products Legally and Illegally Introduced to Germany. PLoS ONE 2016, 11, e0167864. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W. Decreased Antimicrobial Resistance Following Changes in Antibiotic Use. Surg. Infect. 2000, 1, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Morosini, M.I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef]
- Patel, M.; Waites, K.B.; Hoesley, C.J.; Stamm, A.M.; Canupp, K.C.; Moser, S.A. Emergence of USA300 MRSA in a tertiary medical centre: Implications for epidemiological studies. J. Hosp. Infect. 2008, 68, 208–213. [Google Scholar] [CrossRef]
- Glaser, P.; Martins-Simões, P.; Villain, A.; Barbier, M.; Tristan, A.; Bouchier, C.; Ma, L.; Bes, M.; Laurent, F.; Guillemot, D.; et al. Demography and Intercontinental Spread of the USA300 Community-Acquired Methicillin-Resistant Staphylococcus aureus Lineage. mBio 2016, 7, e02183-15. [Google Scholar] [CrossRef]
- Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef]
- Udo, E.E.; Al-Sweih, N. Dominance of community-associated methicillin-resistant Staphylococcus aureus clones in a maternity hospital. PLoS ONE 2017, 12, e0179563. [Google Scholar] [CrossRef]
- Alfouzan, W.; Udo, E.E.; Modhaffer, A.; Alosaimi, A. Molecular Characterization of Methicillin- Resistant Staphylococcus aureus in a Tertiary Care hospital in Kuwait. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A Field Guide to Pandemic, Epidemic and Sporadic Clones of Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Coombs, G.W.; Monecke, S.; Pearson, J.C.; Tan, H.L.; Chew, Y.K.; Wilson, L.; Ehricht, R.; O’Brien, F.G.; Christiansen, K.J. Evolution and diversity of community-associated methicillin-resistant Staphylococcus aureus in a geographical region. BMC Microbiol. 2011, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Broderick, D.; Brennan, G.; Drew, R.; O’Connell, B. Epidemiological typing of methicillin resistant Staphylococcus aureus recovered from patients attending a maternity hospital in Ireland 2014–2019. Infect. Prev. Pr. 2021, 3, 100124. [Google Scholar] [CrossRef] [PubMed]
- Oksuz, L.; Dupieux, C.; Tristan, A.; Bes, M.; Etienne, J.; Gurler, N. The High Diversity of MRSA Clones Detected in a University Hospital in Istanbul. Int. J. Med. Sci. 2013, 10, 1740–1745. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Schwarz, S. fexA, a Novel Staphylococcus lentus Gene Encoding Resistance to Florfenicol and Chloramphenicol. Antimicrob. Agents Chemother. 2004, 48, 615–618. [Google Scholar] [CrossRef]
- Schwarz, S.; Werckenthin, C.; Kehrenberg, C. Identification of a Plasmid-Borne Chloramphenicol-Florfenicol Resistance Gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 2000, 44, 2530–2533. [Google Scholar] [CrossRef]
- Cardoso, M.; Schwarz, S. Chloramphenicol resistance plasmids in Staphylococcus aureus isolated from bovine subclinical mastitis. Vet. Microbiol. 1992, 30, 223–232. [Google Scholar] [CrossRef]
- Schwarz, S.; Werckenthin, C.; Pinter, L.; Kent, L.E.; Noble, W.C. Chloramphenicol resistance in Staphylococcus intermedius from a single veterinary centre: Evidence for plasmid and chromosomal location of the resistance genes. Vet. Microbiol. 1995, 43, 151–159. [Google Scholar] [CrossRef]
- Kehrennberg, C.; Schwarz, S. Florfenicol-chloramphenicol exporter gene, fexA is part of the novel transposon Tn558. Antimicrob. Agents Chemother. 2005, 49, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Belas, A.; Centeno, M.; Van Duijkeren, E.; Pomba, C. First description of fexA-positive meticillin-resistant Staphylococcus aureus ST398 from calves in Portugal. J. Glob. Antimicrob. Resist. 2014, 2, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; O’Donoghue, M.; Guardabassi, L.; Moodley, A.; Boost, M. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Pig Carcasses in Hong Kong. Zoonoses Public Health 2012, 59, 416–423. [Google Scholar] [CrossRef]
- Li, J.; Jiang, N.; Ke, Y.; Feßler, A.T.; Wang, Y.; Schwarz, S.; Wu, C. Characterization of pig-associated methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 2017, 201, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Asanin, J.; Misic, D.; Aksentijevic, K.; Tambur, Z.; Rakonjac, B.; Kovacevic, I.; Spergser, J.; Loncaric, I. Genetic Profiling and Comparison of Human and Animal Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Serbia. Antibiotics 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanz, E.; Kadlec, K.; Feβler, A.T.; Zarazaga, M.; Torre, C.; Schwarz, S. A novel FexA variant from a canine Staphylococcus pseudintermedius isolate that does not confer florfenicol resistance. Antimicrob. Agents Chemother. 2013, 57, 5763–5766. [Google Scholar] [CrossRef] [PubMed]
- Alseqely, M.; Newton-Foot, M.; Khalil, A.; El-Nakeeb, M.; Whitelaw, A.; Abouelfetouh, A. Association between fluoroquinolone resistance and MRSA genotype in Alexandria, Egypt. Sci. Rep. 2021, 11, 4253. [Google Scholar] [CrossRef] [PubMed]
- El-Ashker, M.; Gwida, M.; Monecke, S.; El-Gohary, F.; Ehricht, R.; Elsayed, M.; Akinduti, P.; El-Fateh, M.; Maurischat, S. Antimicrobial resistance pattern and virulence profile of S. aureus isolated from household cattle and buffalo with mastitis in Egypt. Vet. Microbiol. 2020, 240, 108535. [Google Scholar] [CrossRef] [PubMed]
- El-Ashker, M.; Gwida, M.; Tomaso, H.; Monecke, S.; Ehricht, R.; El-Gohary, F.; Hotzel, H. Staphylococci in cattle and buffaloes with mastitis in Dakahlia Governorate, Egypt. J. Dairy Sci. 2015, 98, 7450–7459. [Google Scholar] [CrossRef]
- Antonelli, A.; D’Andrea, M.M.; Brenciani, A.; Galeotti, C.L.; Morroni, G.; Pollini, S.; Varaldo, P.E.; Rossolini, G.M. Characterization of poxtA, a novel phenicol–oxazolidinone–tetracycline resistance gene from an MRSA of clinical origin. J. Antimicrob. Chemother. 2018, 73, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, 12th ed.; MO2-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Udo, E.E.; Mokadas, E.M.; Al-Haddad, A.; Mathew, B.; Jacob, L.E.; Sanyal, S.C. Rapid detection of methicillin resistance in staphylococci using a slide latex agglutination kit. Int. J. Antimicrob. Agents 2000, 15, 19–24. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Udo, E.E.; Monecke, S.; Mathew, B.; Noronha, B.; Verghese, T.; Tappa, S.B. Emerging variants of methicillin-resistant Staphylococcus aureus genotypes in Kuwait hospitals. PLoS ONE 2018, 13, e0195933. [Google Scholar] [CrossRef]
- Kim, T.J.; Na, Y.R.; Lee, J.I. Investigations into the Basis of Chloramphenicol and Tetracycline Resistance in Staphylococcus intermedius Isolates from Cases of Pyoderma in Dogs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 119–124. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.; Witte, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of Methicillin-Resistant Staphylococcus aureus in a University Hospital Setting by Using Novel Software for spa Repeat Determination and Database Management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Slickers, P.; Ehricht, R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008, 53, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Langvik, M.; Yang, A.; Turner, B.; Rico, A.; Jankowski, S.; Theelen, J.; Pradhan, A.; Nutter, R. Fast, Accurate, and Automated Workflow for Multi Locus Sequence Typing of Staphylococcus aureus using the Applied Biosystems Genetic Analyzers and SeqScape® Software. In Proceedings of the 16th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Nice, France, 1–4 April 2006; p. 533. [Google Scholar]
- Udo, E.E.; Jacob, L.E. Conjugative transfer of high-level mupirocin resistance and the mobilization of non-conjugative plasmids in Staphylococcus aureus. Microb. Drug Resist. 1998, 4, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.E.; Sarkhoo, E. The dissemination of ST80-SCCmec-IV community-associated methicillin resistant Staphylococcus aureus clone in Kuwait hospitals. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 31. [Google Scholar] [CrossRef]

| S/N | Strain Description | ST | Spa Type | # | ArcC | AroE | GlpF | GmK | ptA | tpi | YqiL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CC5-MRSA-VI+SCCfus | 627 | t688 | 43 | 1 | 4 | 1 | 4 | 1 | 1 | 10 |
| 2 | CC5-MRSA-VI+SCCfus | 627 | t450 | 1 | 1 | 4 | 1 | 4 | 1 | 1 | 10 |
| 3 | CC5-MRSA-VI+SCCfus | 627 | t954 | 1 | 1 | 4 | 1 | 4 | 1 | 1 | 10 |
| 4 | CC5.MRSA-V | 5 | t688 | 6 | 1 | 4 | 1 | 4 | 2 | 1 | 3 |
| 5 | CC8-MRSA-III | 239 | t037 | 2 | 2 | 3 | 1 | 1 | 4 | 4 | 3 |
| 6 | CC8-MRSA-III | 239 | t860 | 1 | 2 | 3 | 1 | 1 | 4 | 4 | 3 |
| S/N | MRSA Clones | # | Antibiotic Resistance | Resistance Genes |
|---|---|---|---|---|
| 1 | ST5-V-t688 (WA MRSA 11/34) | 5 | Em, Clin, Tet, Cip | fexA, erm(C), tet(K), tet(M) |
| 2 | ST5-V-t688 (WA MRSA 81/85) | 1 | Em, Clin, Tet | fexA, erm(C), tet(K) |
| 3 | ST627-VI-t450 (MRSA-VI +SCCfus) | 1 | Tet, Tp, Fd | fexA, fusC, dfrS1, tet(M) |
| 4 | ST627-VI-t688 (MRSA-VI +SCCfus) | 43 | Tet, Tp, Fd | fexA, fusC, dfrS1, tet(M) |
| 5 | ST627-VI-t954 (MRSA-VI +SCCfus) | 1 | Tet, Tp, Fd | fexA, fusC, dfrS1, tet(M) |
| 6 | ST239-III-t037 (Vienna/Brazilian) | 2 | Gm, Km, Em, Clin, Tet, Tp, Fd | cat, aacA-aphD, aphA3, tet(K), tet(M), erm(A) |
| 7 | ST239-III-t860 (Vienna/Brazilian) | 1 | Gm, Km, Em, Clin, Tet, Tp, Fd, Mup | cat, aacA-aphD, aadD, aphA3, tet(M), erm(A), mupA |
| S/N | MRSA Strain | Resistance Profile | Plasmid Content, kb | Resistance Lost | Plasmids Lost, kb |
|---|---|---|---|---|---|
| 1 | 13973 (ST5-V-t688) | Cm, Em, Clin, Tet | 28.0, 2.8 | Em | 2.8 |
| 2 | 14071 (ST5-V-t688) | Cm, Em, Clin, Tet | c.40.0, 28.0 | None | None |
| 3 | 14098 (ST627-VI-t688) | Cm, Tet, Tp, Fd | 28.0 | None | None |
| 4 | 14284 (ST239-III-t037) | Cm, Gm, Km, Em, Clin, Cip, Fd, Mup | 40.0, 4.4 | Cm, Gm, Km, Mup | 4.4, 40.0 |
| 5 | 14299 (ST239-III-037) | Cm, Gm, Km, Em, Clin, Tet, Fd | 40.0, 3.5, 2.8, <2.0 | Cm | 3.5 |
| 6 | 14314 (ST5-V-t688) | Cm, Tet, Tp, Fd | 28.0 | None | None |
| 7 | 14387 (ST239-III-t860) | Cm, Gm, Km, Em, Clin, Tet, Fd | 28.0, 3.5, 2.8, 2.0 | Cm, Em, Clin | 3.5, 2.8 |
| 8 | 14434 (ST627-VI-t688) | Cm, Tet, Tp, Fd | 28.0 | None | None |
| Isolates | Resistance Profile | Plasmid Content kb | * Mode of Transfer | Resistance Transferred | Plasmid Transferred, kb | Resistance Genes |
|---|---|---|---|---|---|---|
| 14284 ST239-III-t037 | Cm, Gm, Km, Em, Clin, Cip, Fd, Mup | c.40.0, 4.4 | C | Gm, Mup, Cm | c.40.0, 4.4 | cat, mupA, aacA-aphD |
| C | Gm, Mup | c.40.0 | mupA, aacA-aphD | |||
| C | Cm | 4.0 | cat | |||
| 14299 ST239-III-t037 | Cm, Gm, Km, Em, Clin, Tet, Tp, Fd | c.40, 4.4 | M | Cm | 3.5 | cat |
| 14387 ST239-III-t860 | Cm, Gm, Km, Em, Clin, Tet, Fd | 28.0, 3.5, 2.8 2.0 | M | Cm | 3.5 | cat |
| 13973 ST5-V-t688 | Cm, Em, Clin, Tet | 28.0, 2.8 | M | Em | 2.8 | erm(C) |
| 14434 ST627-VI-t688 | Cm, Tet, Tp, Fd | 28.0 | C, M | None | None | None |
| 14071 ST5-V-t688 | Cm, Em, Clin, Tet | c.40.0, 28.0 | C, M | None | None | None |
| 14098 ST627-VI-t688 | Cm, Tet, Tp, Fd | 28.0 | C, M | None | None | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udo, E.E.; Boswihi, S.S.; Mathew, B.; Noronha, B.; Verghese, T. Resurgence of Chloramphenicol Resistance in Methicillin-Resistant Staphylococcus aureus Due to the Acquisition of a Variant Florfenicol Exporter (fexAv)-Mediated Chloramphenicol Resistance in Kuwait Hospitals. Antibiotics 2021, 10, 1250. https://doi.org/10.3390/antibiotics10101250
Udo EE, Boswihi SS, Mathew B, Noronha B, Verghese T. Resurgence of Chloramphenicol Resistance in Methicillin-Resistant Staphylococcus aureus Due to the Acquisition of a Variant Florfenicol Exporter (fexAv)-Mediated Chloramphenicol Resistance in Kuwait Hospitals. Antibiotics. 2021; 10(10):1250. https://doi.org/10.3390/antibiotics10101250
Chicago/Turabian StyleUdo, Edet E., Samar S. Boswihi, Bindu Mathew, Bobby Noronha, and Tina Verghese. 2021. "Resurgence of Chloramphenicol Resistance in Methicillin-Resistant Staphylococcus aureus Due to the Acquisition of a Variant Florfenicol Exporter (fexAv)-Mediated Chloramphenicol Resistance in Kuwait Hospitals" Antibiotics 10, no. 10: 1250. https://doi.org/10.3390/antibiotics10101250
APA StyleUdo, E. E., Boswihi, S. S., Mathew, B., Noronha, B., & Verghese, T. (2021). Resurgence of Chloramphenicol Resistance in Methicillin-Resistant Staphylococcus aureus Due to the Acquisition of a Variant Florfenicol Exporter (fexAv)-Mediated Chloramphenicol Resistance in Kuwait Hospitals. Antibiotics, 10(10), 1250. https://doi.org/10.3390/antibiotics10101250






