Activity of Two Antimicrobial Peptides against Enterococcus faecalis in a Model of Biofilm-Mediated Endodontic Infection
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic Effect of The Investigated Peptides
2.2. Activity of KP and L18R against E. faecalis Biofilm on Polystyrene Plates
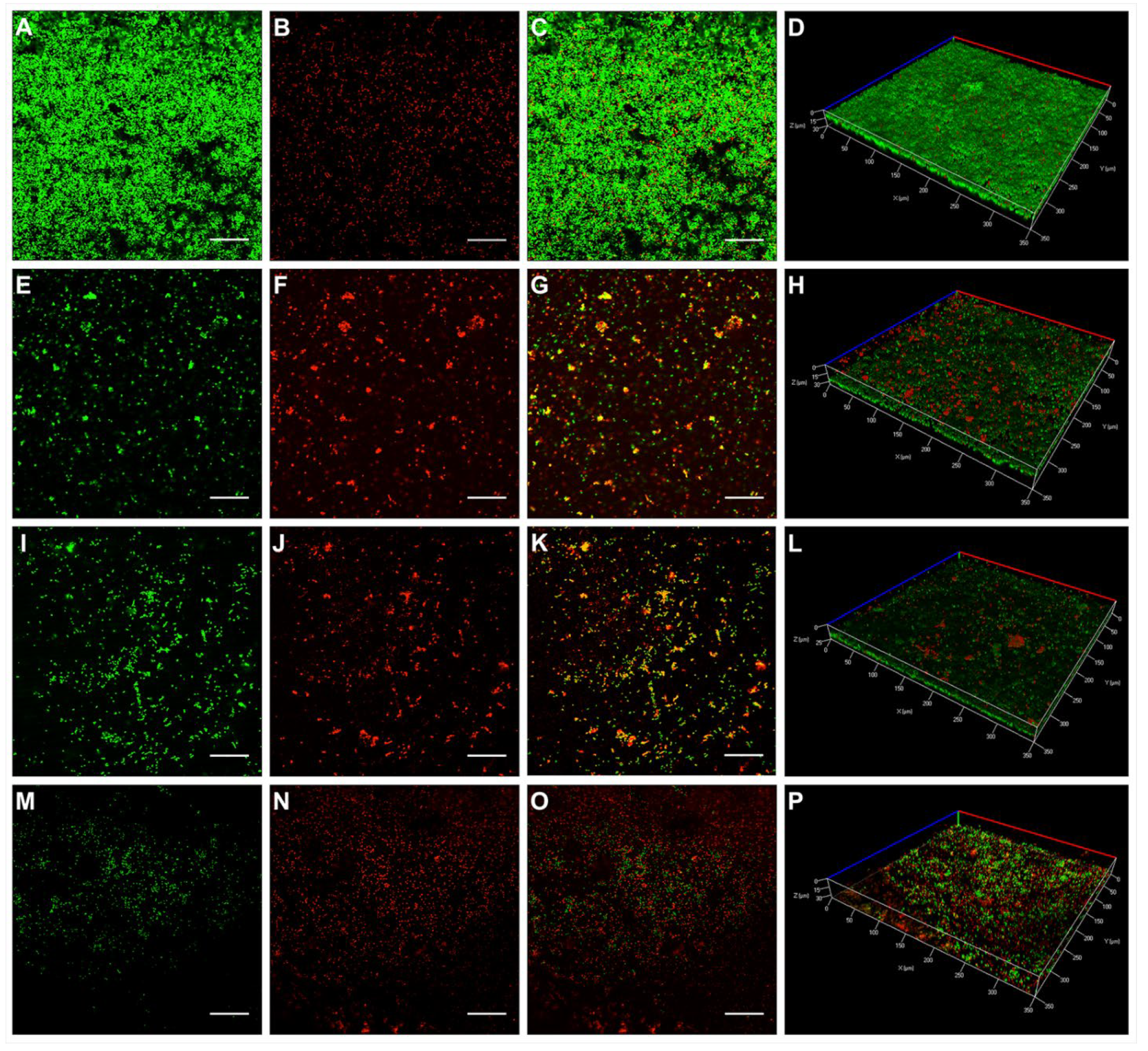
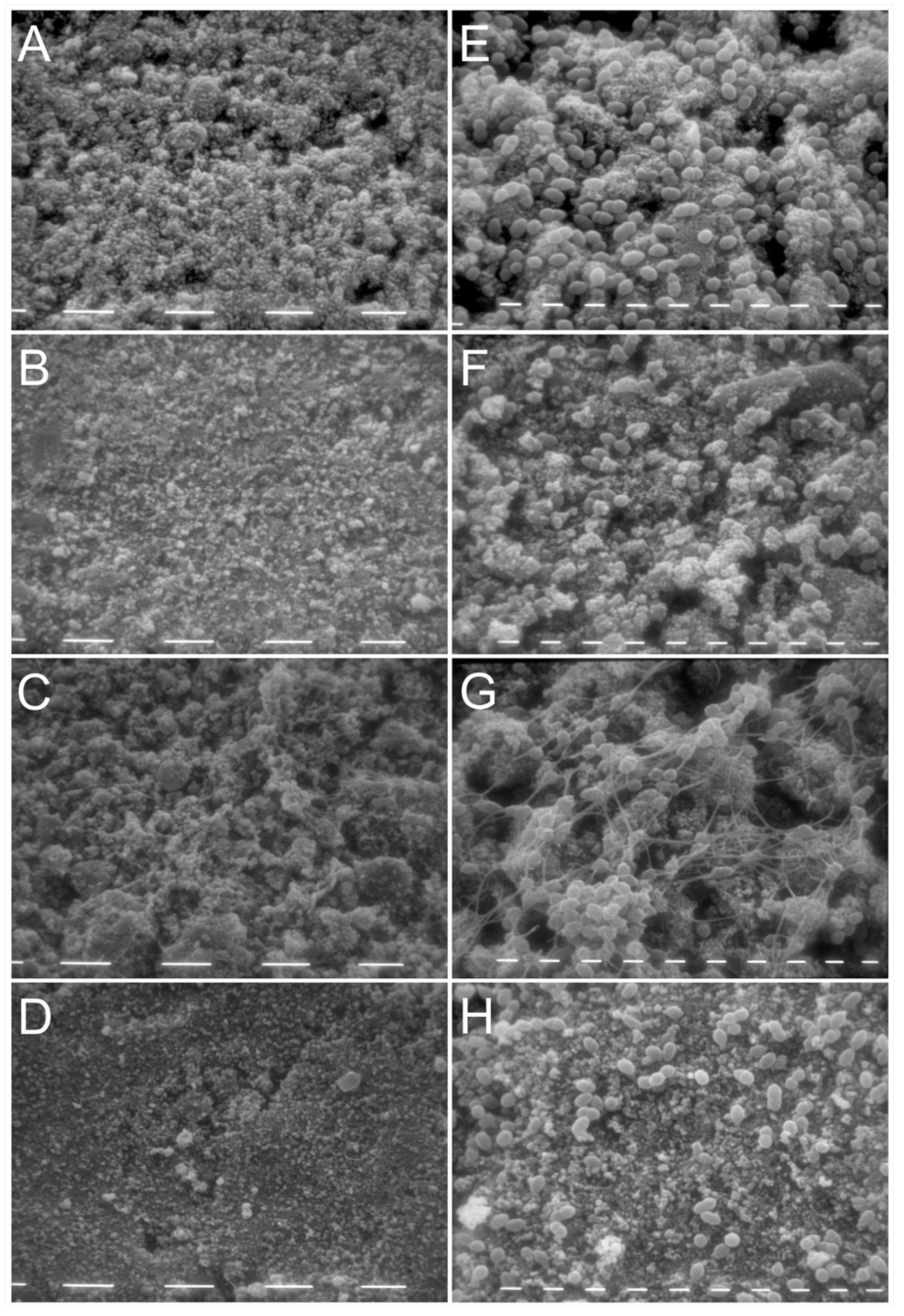
2.3. Activity of KP and L18R against E. faecalis Biofilm on Hydroxyapatite Disks
3. Discussion
4. Materials and Methods
4.1. Peptides and Bacterial Strain
4.2. Peptide Cytotoxicity Assay
4.3. Treatment of E. faecalis Biofilm Formed on Polystyrene Surfaces
4.4. Treatment of E. faecalis Biofilm Formed on Hydroxyapatite Disks
4.4.1. Confocal Laser Scanning Microscopy
4.4.2. Scanning Electron Microscopy
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berman, L.; Hargreaves, K. Cohen’s Pathways of the Pulp, 11th ed.; Elsevier: St. Louis, MO, USA, 2016. [Google Scholar]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 231–252. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, U.; Figdor, D.; Spangberg, L.; Sundqvist, G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int. Endod. J. 1991, 24, 119–125. [Google Scholar] [CrossRef]
- Shuping, G.B.; Orstavik, D.; Sigurdsson, A.; Trope, M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J. Endod. 2000, 26, 751–755. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Magalhaes, K.M.; Rocas, I.N. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J. Endod. 2007, 33, 667–672. [Google Scholar] [CrossRef]
- Vera, J.; Siqueira, J.F., Jr.; Ricucci, D.; Loghin, S.; Fernandez, N.; Flores, B.; Cruz, A.G. One- versus two-visit endodontic treatment of teeth with apical periodontitis: A histobacteriologic study. J. Endod. 2012, 38, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Pimenta, F.C.; Ito, I.Y.; Bammann, L.L. In vitro determination of direct antimicrobial effect of calcium hydroxide. J. Endod. 1998, 24, 15–17. [Google Scholar] [CrossRef]
- Gomes, B.P.; Ferraz, C.C.; Garrido, F.D.; Rosalen, P.L.; Zaia, A.A.; Teixeira, F.B.; de Souza-Filho, F.J. Microbial susceptibility to calcium hydroxide pastes and their vehicles. J. Endod. 2002, 28, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Hume, W.R. Diffusion of hydrogen ion and hydroxyl ion from various sources through dentine. Int. Endod. J. 1988, 21, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Davies, J.K.; Sundqvist, G.; Figdor, D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int. Endod. J. 2002, 35, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N. Distinctive features of the microbiota associated with different forms of apical periodontitis. J. Oral Microbiol. 2009, 1, 2009. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F., Jr. Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J. Endod. 2010, 36, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, J.-H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Nakajo, K.; Komori, R.; Ishikawa, S.; Ueno, T.; Suzuki, Y.; Iwami, Y.; Takahashi, N. Resistance to acidic and alkaline environments in the endodontic pathogen Enterococcus faecalis. Oral Microbiol. Immunol. 2006, 21, 283–288. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, S.M.F.; de Padua, G.M.; Sousa, M.; Freire, M.S.; Franco, O.L.; Rezende, T.M.B. Antimicrobial peptide-based treatment for endodontic infections--biotechnological innovation in endodontics. Biotechnol. Adv. 2015, 33, 203–213. [Google Scholar] [CrossRef]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Boto, A.; Perez de la Lastra, J.M.; Gonzalez, C.C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, B.; Augusto, M.T.; Felicio, M.R.; Hollmann, A.; Franco, O.L.; Goncalves, S.; Santos, N.C. Designing improved active peptides for therapeutic approaches against infectious diseases. Biotechnol. Adv. 2018, 36, 415–429. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Hale, J.D.F.; Hancock, R.E.W. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti-Infect. Ther. 2007, 5, 951–959. [Google Scholar] [CrossRef]
- Mergoni, G.; Manfredi, M.; Bertani, P.; Ciociola, T.; Conti, S.; Giovati, L. Antibacterial effects of two synthetic peptides against Enterococcus faecalis biofilms: A preliminary in vitro study. G. Ital. Endod. 2020, 34, 47–54. [Google Scholar] [CrossRef]
- Polonelli, L.; Magliani, W.; Ciociola, T.; Giovati, L.; Conti, S. From Pichia anomala killer toxin through killer antibodies to killer peptides for a comprehensive anti-infective strategy. Antonie Leeuwenhoek 2011, 99, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Magliani, W.; Conti, S.; Ciociola, T.; Giovati, L.; Zanello, P.P.; Pertinhez, T.; Spisni, A.; Polonelli, L. Killer peptide: A novel paradigm of antimicrobial, antiviral and immunomodulatory auto-delivering drugs. Future Med. Chem. 2011, 3, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Ciociola, T.; Sperinde, M.; Giovati, L.; D’Adda, T.; Galati, S.; Travassos, L.R.; Magliani, W.; Conti, S. Fungicidal activity of peptides encoded by immunoglobulin genes. Sci. Rep. 2017, 7, 10896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and assessment of anti-biofilm peptides: Steps toward clinical application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Giovati, L.; Santinoli, C.; Mangia, C.; Vismarra, A.; Belletti, S.; D’Adda, T.; Fumarola, C.; Ciociola, T.; Bacci, C.; Magliani, W.; et al. Novel activity of a synthetic decapeptide against Toxoplasma gondii tachyzoites. Front. Microbiol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlinger, C.; Dartevelle, P.; Zaet, A.; Kurashige, Y.; Haïkel, Y.; Metz-Boutigue, M.-H.; Marban, C. A new combination with D-cateslytin to eradicate root canal pathogens. Int. J. Pept. Res. Ther. 2019, 25, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Tsutsui, T.W.; Kobayashi, T.; Ohno, M.; Higo, Y.; Inaba, T.; Tsutsui, T. Sensitivity of human dental pulp cells to eighteen chemical agents used for endodontic treatments in dentistry. Odontology 2013, 101, 43–51. [Google Scholar] [CrossRef]
- Pertinhez, T.A.; Conti, S.; Ferrari, E.; Magliani, W.; Spisni, A.; Polonelli, L. Reversible Self-Assembly: A Key Feature for a New Class of Autodelivering Therapeutic Peptides. Mol. Pharm. 2009, 6, 1036–1039. [Google Scholar] [CrossRef]
- Bystrom, A.; Claesson, R.; Sundqvist, G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod. Dent. Traumatol. 1985, 1, 170–175. [Google Scholar] [CrossRef]
- Orstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef]


| Concentration (µg/mL) | Cell Viability (%) after Treatment with | |
|---|---|---|
| KP | L18R | |
| 400 | 104 ± 17 | 127 ± 24 |
| 200 | 121 ± 15 | 99 ± 20 |
| 100 | 145 ± 34 | 102 ± 0 |
| 50 | 120 ± 39 | 120 ± 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergoni, G.; Manfredi, M.; Bertani, P.; Ciociola, T.; Conti, S.; Giovati, L. Activity of Two Antimicrobial Peptides against Enterococcus faecalis in a Model of Biofilm-Mediated Endodontic Infection. Antibiotics 2021, 10, 1220. https://doi.org/10.3390/antibiotics10101220
Mergoni G, Manfredi M, Bertani P, Ciociola T, Conti S, Giovati L. Activity of Two Antimicrobial Peptides against Enterococcus faecalis in a Model of Biofilm-Mediated Endodontic Infection. Antibiotics. 2021; 10(10):1220. https://doi.org/10.3390/antibiotics10101220
Chicago/Turabian StyleMergoni, Giovanni, Maddalena Manfredi, Pio Bertani, Tecla Ciociola, Stefania Conti, and Laura Giovati. 2021. "Activity of Two Antimicrobial Peptides against Enterococcus faecalis in a Model of Biofilm-Mediated Endodontic Infection" Antibiotics 10, no. 10: 1220. https://doi.org/10.3390/antibiotics10101220











