Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture of PC-3 Cells and Co-Culture with T4/M13 Bacteriophages
2.2. Morphological Investigation
2.3. Wound Healing Assay
2.4. Extraction of RNA and cDNA Synthesis for qPCR Profiling
2.5. Statistical Analysis
3. Results
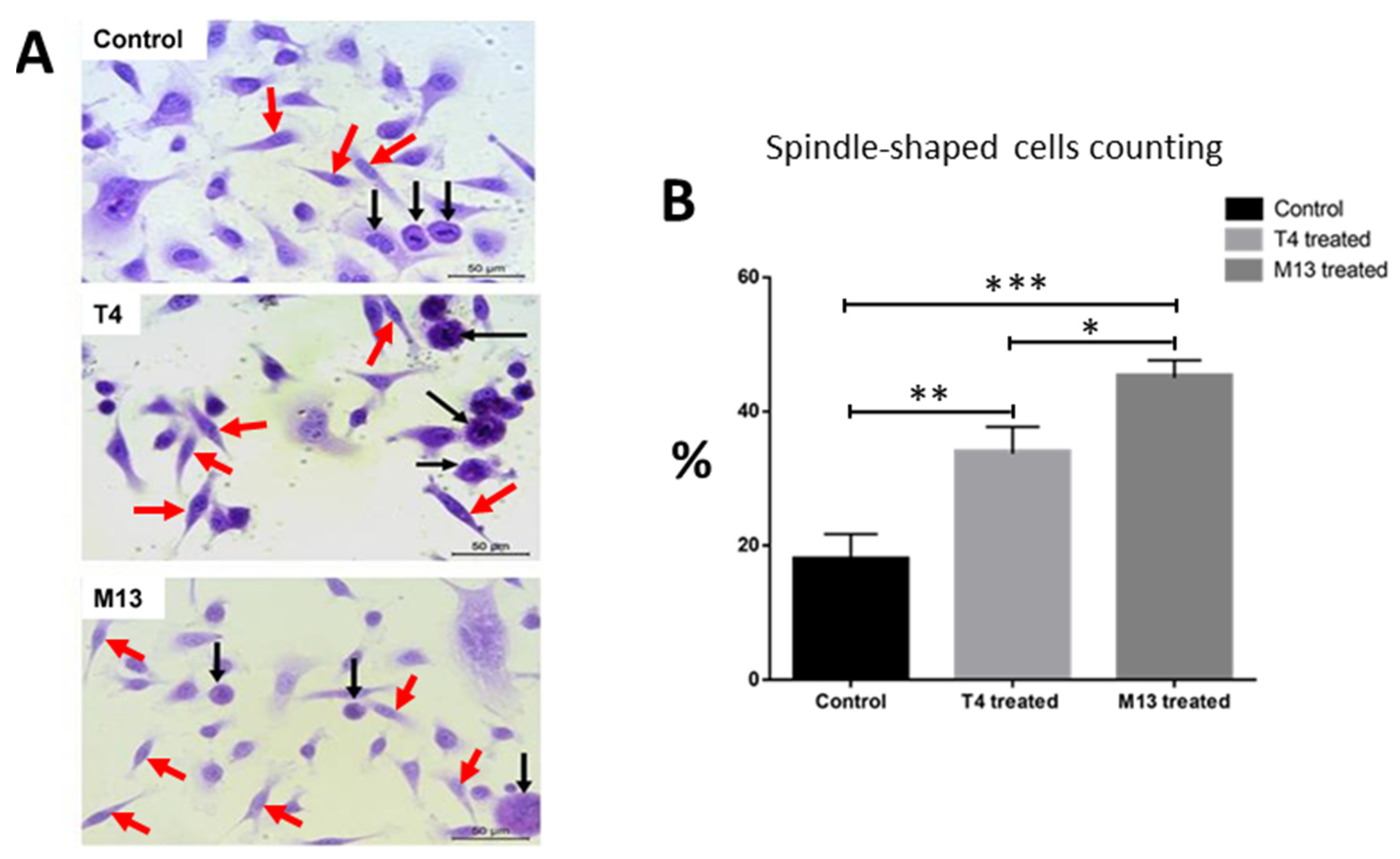
3.1. Bacteriophage-Treated PC-3 Cells Adopt a Spindle-Like Phenotype
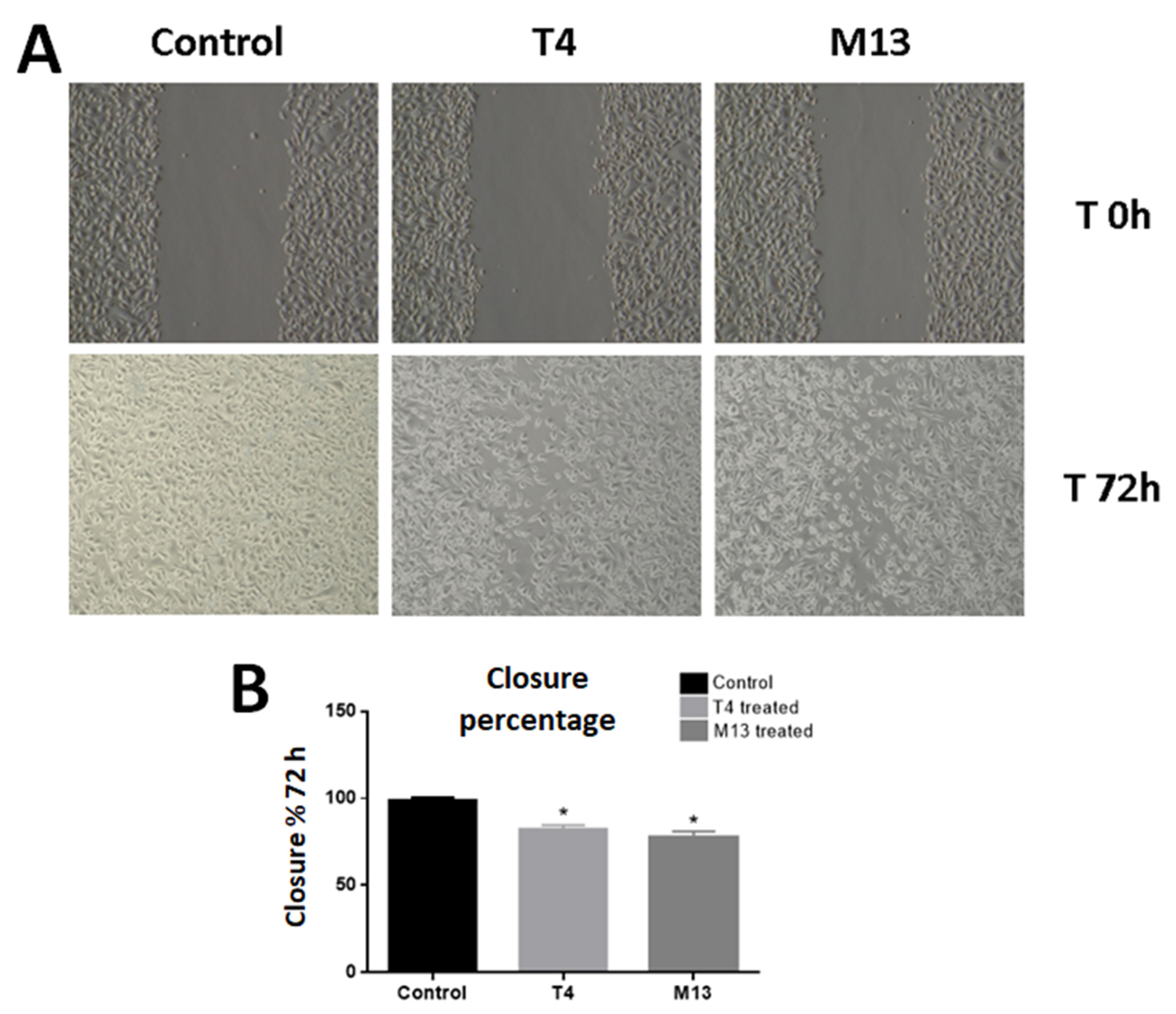
3.2. Bacteriophage-Treated PC-3 Cells Have Compromised Migration
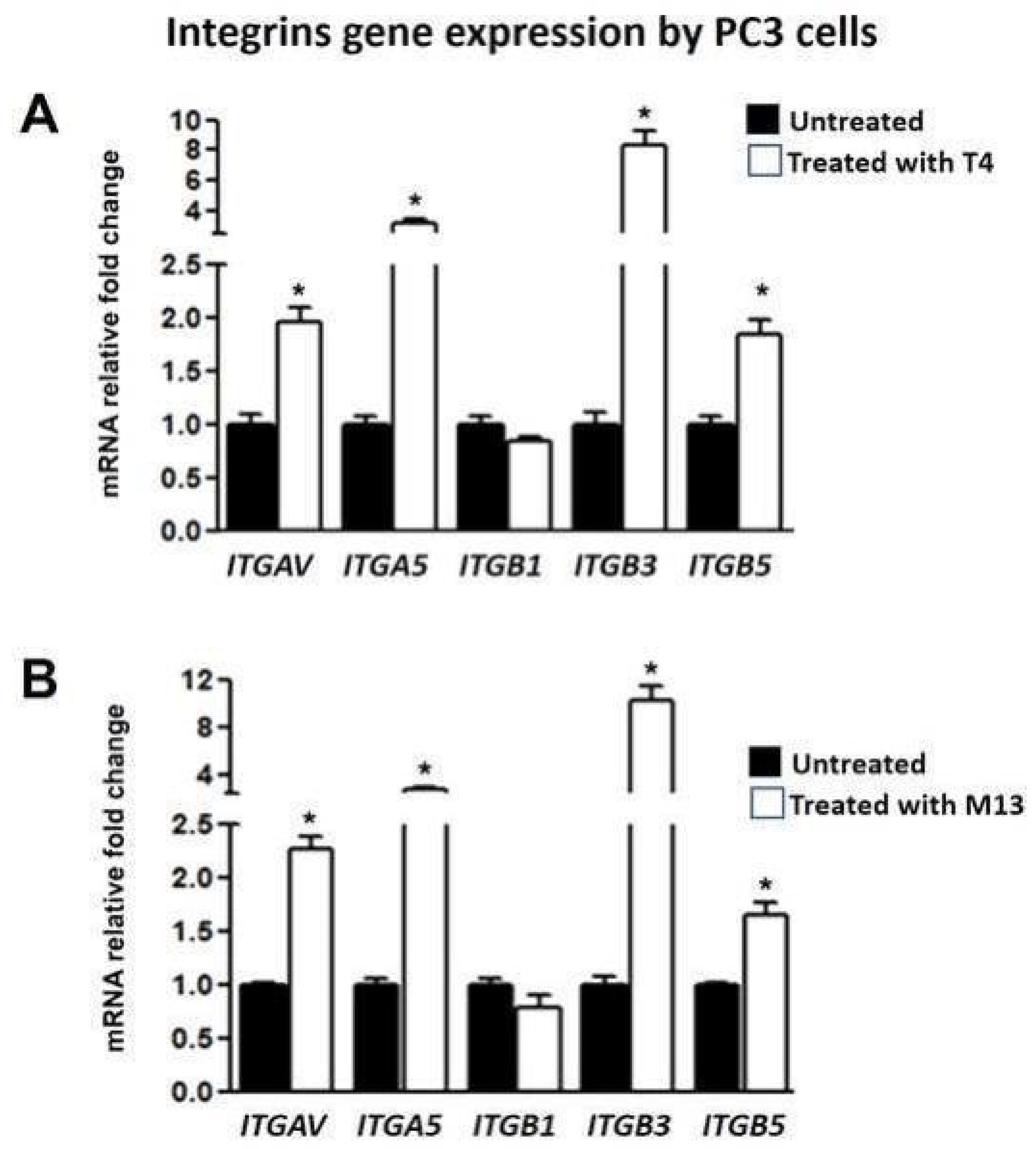
3.3. Bacteriophage-Treated PC-3 Cells Have Increased Integrin Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.H.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.C.; Holmberg, L.; et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Huang, J.; Vue, M.; Alavian, M.R.; Goel, H.L.; Altieri, D.C.; Languino, L.R.; FitzGerald, T.J. α v β 3 Integrin Mediates Radioresistance of Prostate Cancer Cells through Regulation of Survivin. Mol. Cancer Res. 2019, 17, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilsky, R.L. Personalized medicine in oncology: The future is now. Nat. Rev. Drug Discov. 2010, 9, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Huang, G.; Chen, Z.; Zhang, Y. Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy. Front. Pharmacol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef] [Green Version]
- Naci, D.; Vuori, K.; Aoudjit, F. Alpha2beta1 integrin in cancer development and chemoresistance. Semin. Cancer Biol. 2015, 35, 145–153. [Google Scholar] [CrossRef]
- Borrirukwanit, K.; Pavasant, P.; Blick, T.; Lafleur, M.A.; Thompson, E.W. High threshold of β1 integrin inhibition required to block collagen I-induced membrane type-1 matrix metalloproteinase (MT1-MMP) activation of matrix metalloproteinase 2 (MMP-2). Cancer Cell Int. 2014, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Blandin, A.-F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as Therapeutic Targets to Disrupt Hallmarks of Cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as attractive targets for cancer therapeutics. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Morozevich, G.E.; Kozlova, N.I.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Berman, A.E. Integrin α5β1 simultaneously controls EGFR-dependent proliferation and Akt-dependent pro-survival signaling in epidermoid carcinoma cells. Aging 2012, 4, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Speicher, T.; Siegenthaler, B.; Bogorad, R.L.; Ruppert, R.; Petzold, T.; Padrissa-Altes, S.; Bachofner, M.; Anderson, D.G.; Koteliansky, V.; Fässler, R.; et al. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 2014, 5, 3862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janouskova, H.; Maglott, A.; Leger, D.Y.; Bossert, C.; Noulet, F.; Guerin, E.; Guenot, D.; Pinel, S.; Chastagner, P.; Plenat, F.; et al. Integrin α5β1 Plays a Critical Role in Resistance to Temozolomide by Interfering with the p53 Pathway in High-Grade Glioma. Cancer Res. 2012, 72, 3463–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silginer, M.; Weller, M.; Ziegler, U.; Roth, P. Integrin inhibition promotes atypical anoikis in glioma cells. Cell Death Dis. 2014, 5, e1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguin, L.; Kato, S.; Franovic, A.; Camargo, M.F.; Lesperance, J.; Elliott, K.C.; Yebra, M.; Mielgo, A.; Lowy, A.M.; Husain, H.; et al. An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014, 16, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morello, V.; Cabodi, S.; Sigismund, S.; Camacho-Leal, M.P.; Repetto, D.; Volante, M.; Papotti, M.; Turco, E.; Defilippi, P. β1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene 2011, 30, 4087–4096. [Google Scholar] [CrossRef] [Green Version]
- Kanda, R.; Kawahara, A.; Watari, K.; Murakami, Y.; Sonoda, K.; Maeda, M.; Fujita, H.; Kage, M.; Uramoto, H.; Costa, C.; et al. Erlotinib Resistance in Lung Cancer Cells Mediated by Integrin β1/Src/Akt-Driven Bypass Signaling. Cancer Res. 2013, 73, 6243–6253. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-J. Silencing profilin-1 inhibits gastric cancer progression via integrin β1/focal adhesion kinase pathway modulation. World J. Gastroenterol. 2015, 21, 2323. [Google Scholar] [CrossRef]
- Hu, C.; Ni, Z.; Li, B.; Yong, X.; Yang, X.; Zhang, J.; Zhang, D.; Qin, Y.; Jie, M.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xiao, Y.-F.; Tang, B.; Wu, Y.-Y.; Hu, C.-J.; Xie, R.; Yang, X.; Yu, S.-T.; Dong, H.; Zhao, X.-Y.; et al. hTERT mediates gastric cancer metastasis partially through the indirect targeting of ITGB1 by microRNA-29a. Sci. Rep. 2016, 6, 21955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-F.; Alshareef, A.; Wu, C.; Li, S.; Jiao, J.-W.; Cao, H.-H.; Lai, R.; Xu, L.-Y.; Li, E.-M. Loss of miR-200b promotes invasion via activating the Kindlin-2/integrin β1/AKT pathway in esophageal squamous cell carcinoma: An E-cadherin-independent mechanism. Oncotarget 2015, 6, 28949–28960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Kang, Y. Targeting tumor–stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Pontes-Júnior, J.; Reis, S.T.; de Oliveira, L.C.N.; Sant’Anna, A.C.; Dall’Oglio, M.F.; Antunes, A.A.; Ribeiro-Filho, L.A.; Carvalho, P.A.; Cury, J.; Srougi, M.; et al. Association between integrin expression and prognosis in localized prostate cancer. Prostate 2010, 70, 1189–1195. [Google Scholar] [CrossRef]
- Zheng, D.Q.; Woodard, A.S.; Fornaro, M.; Tallini, G.; Languino, L.R. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999, 59, 1655–1664. [Google Scholar]
- Vellon, L.; Menendez, J.A.; Liu, H.; Lupu, R. Up-regulation of αVβ3 integrin expression is a novel molecular response to chemotherapy-induced cell damage in a heregulin-dependent manner. Differentiation 2007, 75, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, F.; Ray, A.; Dontenwill, M. Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers 2013, 5, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Lucie, S.; Elisabeth, G.; Stéphanie, F.; Guy, S.; Amandine, H.; Corinne, A.-R.; Didier, B.; Catherine, S.; Alexeï, G.; Pascal, D.; et al. Clustering and Internalization of Integrin αvβ3 With a Tetrameric RGD-synthetic Peptide. Mol. Ther. 2009, 17, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.-W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience 2021, 24, 102287. [Google Scholar] [CrossRef]
- Warner, C.M.; Barker, N.; Lee, S.-W.; Perkins, E.J. M13 bacteriophage production for large-scale applications. Bioprocess Biosyst. Eng. 2014, 37, 2067–2072. [Google Scholar] [CrossRef]
- Keen, E.C. A century of phage research: Bacteriophages and the shaping of modern biology. BioEssays 2015, 37, 6–9. [Google Scholar] [CrossRef]
- Cao, B.; Yang, M.; Mao, C. Phage as a Genetically Modifiable Supramacromolecule in Chemistry, Materials and Medicine. Acc. Chem. Res. 2016, 49, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Bose, M.; Mukherjee, P. Role of microbiome in modulating immune responses in cancer. Mediat. Inflamm. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Sanmukh, S.G.; dos Santos, N.J.; Barquilha, C.N.; Cucielo, M.S.; de Carvalho, M.; dos Reis, P.P.; Delella, F.K.; Carvalho, H.F.; Felisbino, S.L. Bacteriophages M13 and T4 Increase the Expression of Anchorage-Dependent Survival Pathway Genes and Down Regulate Androgen Receptor Expression in LNCaP Prostate Cell Line. Viruses 2021, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Kantoch, M.; Mordaski, M. Binding of bacterial viruses by tumor cells in vitro. Postepy Hig. Med. Dosw. 1958, 12, 191–192. [Google Scholar] [PubMed]
- Rajotte, D.; Arap, W.; Hagedorn, M.; Koivunen, E.; Pasqualini, R.; Ruoslahti, E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Investig. 1998, 102, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scodeller, P.; Asciutto, E.K. Targeting Tumors Using Peptides. Molecules 2020, 25, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleiko, K.; Põšnograjeva, K.; Haugas, M.; Paiste, P.; Tobi, A.; Kurm, K.; Riekstina, U.; Teesalu, T. In vivo phage display: Identification of organ-specific peptides using deep sequencing and differential profiling across tissues. Nucleic Acids Res. 2021, 49, e38. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, A.; Nisticò, N.; Mimmi, S.; Maisano, D.; Vecchio, E.; Fiume, G.; Iaccino, E.; Quinto, I. Phage-Displayed Peptides for Targeting Tyrosine Kinase Membrane Receptors in Cancer Therapy. Viruses 2021, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Millikan, R.E.; Christianson, D.R.; Cardó-Vila, M.; Driessen, W.H.P.; Giordano, R.J.; Hajitou, A.; Hoang, A.G.; Wen, S.; Barnhart, K.F.; et al. Targeting the interleukin-11 receptor α in metastatic prostate cancer: A first-in-man study. Cancer 2015, 121, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Staquicini, F.I.; Ferrara, F.; Staquicini, D.I.; Sharma, G.; Tarleton, C.A.; Nguyen, H.; Naranjo, L.A.; Sidman, R.L.; Arap, W.; et al. Selection of phage-displayed accessible recombinant targeted antibodies (SPARTA): Methodology and applications. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- DePorter, S.M.; McNaughton, B.R. Engineered M13 Bacteriophage Nanocarriers for Intracellular Delivery of Exogenous Proteins to Human Prostate Cancer Cells. Bioconjug. Chem. 2014, 25, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.Y.; Jin, H.-E.; Choi, D.S.; Kobayashi, M.; Farouz, Y.; Wang, S.; Lee, S.-W. M13 Bacteriophage and Adeno-Associated Virus Hybrid for Novel Tissue Engineering Material with Gene Delivery Functions. Adv. Healthc. Mater. 2016, 5, 88–93. [Google Scholar] [CrossRef]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Górski, A.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Międzybrodzki, R.; Weber-Dąbrowska, B.; Borysowski, J.; Letkiewicz, S.; Bagińska, N.; Sfanos, K.S. Phage Therapy in Prostatitis: Recent Prospects. Front. Microbiol. 2018, 9, 1434. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; Jean, J.S.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef]
- Sanmukh, S.G.; Felisbino, S.L. Development of pipette tip gap closure migration assay (s-ARU method) for studying semi-adherent cell lines. Cytotechnology 2018, 70, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, Y.; Arnold, R.; McLaughlin, M.; Nim, S.; Joshi, R.; Ray, D.; Liu, B.; Teyra, J.; Pawson, T.; Moffat, J.; et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. USA 2014, 111, 2542–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmukh, S.G.; Alcantara, S.A.; Santos, D.; Felisbino, L. Natural bacteriophages T4 and M13 down-regulates Hsp90 gene expression in human prostate cancer cells (PC-3) representing a potential nanoparticle against cancer. Virol. Res. J. 2017, 1, 21–23. [Google Scholar]
- Dąbrowska, K.; Miernikiewicz, P.; Piotrowicz, A.; Hodyra, K.; Owczarek, B.; Lecion, D.; Kaźmierczak, Z.; Letarov, A.; Gorski, A. Immunogenicity Studies of Proteins Forming the T4 Phage Head Surface. J. Virol. 2014, 88, 12551–12557. [Google Scholar] [CrossRef] [Green Version]
- Da<monospace>̧</monospace>browska, K.; Skaradziński, G.; Jończyk, P.; Kurzȩpa, A.; Wietrzyk, J.; Owczarek, B.; Zaczek, M.; Świtała-Jeleń, K.; Boratyński, J.; Poźniak, G.; et al. The effect of bacteriophages T4 and HAP1 on in vitro melanoma migration. BMC Microbiol. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Ruoslahti, E.; Pierschbacher, M. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Ruoslahti, E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J. Cell Biol. 1995, 130, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Sakai, T.; Fassler, R.; Ruoslahti, E. Antiangiogenic proteins require plasma fibronectin or vitronectin for in vivo activity. Proc. Natl. Acad. Sci. USA 2003, 100, 11435–11438. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.H. The role of engineering approaches in analysing cancer invasion and metastasis. Nat. Rev. Cancer 2013, 13, 596–603. [Google Scholar] [CrossRef]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.; Araújo, A.; Pinto, J.; Jerónimo, C.; Henrique, R.; Bastos, M.; Carvalho, M.; de Guedes Pinho, P. GC-MS-Based Endometabolome Analysis Differentiates Prostate Cancer from Normal Prostate Cells. Metabolites 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of integrin αv facilitates proliferation and invasion of oral squamous cell carcinoma cells via MEK/ERK signaling pathway that is activated by interaction of integrin αvβ8 with type Ⅰ collagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.-J.; Guo, J.-C.; Wu, Z.-Y.; Xu, X.-E.; Wu, J.-Y.; Chen, B.; Ran, L.-Q.; Liao, L.-D.; Li, E.-M.; Xu, L.-Y. Integrin α5 promotes tumor progression and is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Hum. Pathol. 2016, 48, 69–75. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Q.; Guan, G.; Cheng, W.; Cheng, P.; Wu, A. Integrin Beta 5 Is a Prognostic Biomarker and Potential Therapeutic Target in Glioblastoma. Front. Oncol. 2019, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Bodner, K.; Melkonian, A.L.; Covert, M.W. The Enemy of My Enemy: New Insights Regarding Bacteriophage–Mammalian Cell Interactions. Trends Microbiol. 2021, 29, 528–541. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e8. [Google Scholar] [CrossRef] [Green Version]
- Møller-Olsen, C.; Ho, S.F.S.; Shukla, R.D.; Feher, T.; Sagona, A.P. Engineered K1F bacteriophages kill intracellular Escherichia coli K1 in human epithelial cells. Sci. Rep. 2018, 8, 17559. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. MBio 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [Green Version]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapała, J.; Drab, M.; Jończyk-Matysiak, E.; Lecion, D.; Kaźmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef] [Green Version]
- Øie, C.I.; Wolfson, D.L.; Yasunori, T.; Dumitriu, G.; Sørensen, K.K.; McCourt, P.A.; Ahluwalia, B.S.; Smedsrød, B. Liver sinusoidal endothelial cells contribute to the uptake and degradation of entero bacterial viruses. Sci. Rep. 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C.H. Integrins as architects of cell behavior. Mol. Biol. Cell 2016, 27, 2885–2888. [Google Scholar] [CrossRef]
- Lehti, T.A.; Pajunen, M.I.; Skog, M.S.; Finne, J. Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells. Nat. Commun. 2017, 8, 1915. [Google Scholar] [CrossRef] [Green Version]
- Van Belleghem, J.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.; Bollyky, P. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foglizzo, V.; Marchiò, S. Bacteriophages as Therapeutic and Diagnostic Vehicles in Cancer. Pharmaceuticals 2021, 14, 161. [Google Scholar] [CrossRef] [PubMed]
| Genes | Sense Primer | Antisense Primer |
|---|---|---|
| ACTB | GATTCCTATGTGGGCGACGA | TGTAGAAGGTGTGGTGCCAG |
| ITGA5 | GGGTGGTGCTGTCTACCTC | GTGGAGCGCATGCCAAGATG |
| ITGAV | AGGCACCCTCCTTCTGATCC | CTTGGCATAATCTCTATTGCCTGT |
| ITGB1 | GCCAAATGGGACACGCAAGA | GTGTTGTGGGATTTGCACGG |
| ITGB3 | CTGCCGTGACGAGATTGAGT | CCTTGGGACACTCTGGCTCT |
| ITGB5 | GGGCTCTACTCAGTGGTTTCG | GGCTTCCGAAGTCCTCTTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmukh, S.G.; Santos, N.J.; Barquilha, C.N.; dos Santos, S.A.A.; Duran, B.O.S.; Delella, F.K.; Moroz, A.; Justulin, L.A.; Carvalho, H.F.; Felisbino, S.L. Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells. Antibiotics 2021, 10, 1202. https://doi.org/10.3390/antibiotics10101202
Sanmukh SG, Santos NJ, Barquilha CN, dos Santos SAA, Duran BOS, Delella FK, Moroz A, Justulin LA, Carvalho HF, Felisbino SL. Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells. Antibiotics. 2021; 10(10):1202. https://doi.org/10.3390/antibiotics10101202
Chicago/Turabian StyleSanmukh, Swapnil Ganesh, Nilton J. Santos, Caroline Nascimento Barquilha, Sérgio Alexandre Alcantara dos Santos, Bruno Oliveira Silva Duran, Flávia Karina Delella, Andrei Moroz, Luis Antonio Justulin, Hernandes F. Carvalho, and Sérgio Luis Felisbino. 2021. "Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells" Antibiotics 10, no. 10: 1202. https://doi.org/10.3390/antibiotics10101202
APA StyleSanmukh, S. G., Santos, N. J., Barquilha, C. N., dos Santos, S. A. A., Duran, B. O. S., Delella, F. K., Moroz, A., Justulin, L. A., Carvalho, H. F., & Felisbino, S. L. (2021). Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells. Antibiotics, 10(10), 1202. https://doi.org/10.3390/antibiotics10101202











