Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis
Abstract
:1. Introduction
2. Results
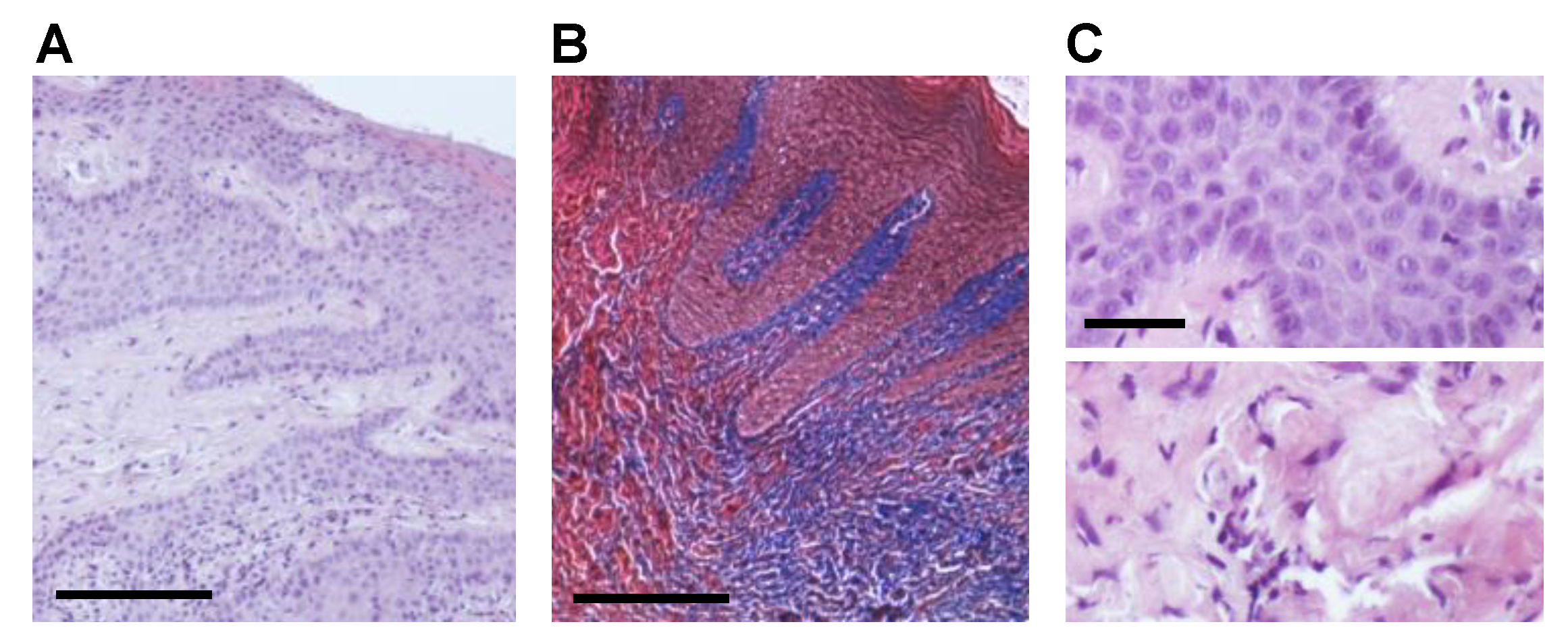
2.1. CHX Post-Surgical Mouth Rinse Increases Fibrotic Marker Expression and Myofibroblast Differentiation
2.2. CHX Influences the Expression of Key Genes Involved in Early Wound Healing
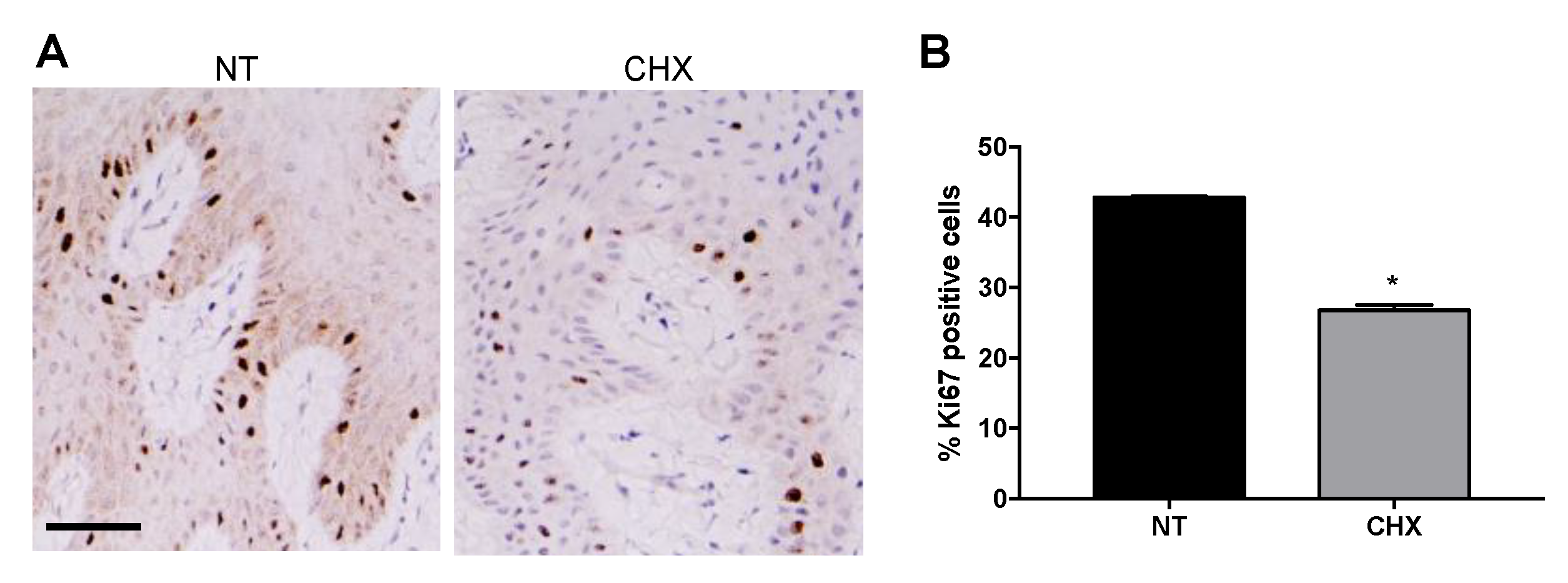
2.3. CHX Increases the Expression of Apoptotic Markers and Reduces the Proliferative Ability of Gingival Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statements
4.2. Study Design and Patient Selection
4.3. Surgical Procedures and Collection of Human Gingival Tissue Samples
4.4. Histological Analysis
4.5. Immunohistochemistry
4.6. Quantitative Real-Time PCR (qRT-PCR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hämmerle, C.H.; Giannobile, W.V.; Working Group 1 of the European Workshop on Periodontology. Biology of soft tissue wound healing and regeneration—Consensus Report of Group 1 of the 10th European Workshop on Periodontology. J. Clin. Periodontol. 2014, 41, S1–S5. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Newman, M.G.; Anderson, L.; Matoska, W.; Otomo-Corgel, J.; Saltini, C. Clinical enhancement of post-periodontal surgical therapy by a 0.12% chlorhexidine gluconate mouthrinse. J. Periodontol. 1989, 60, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Sanz, M.; Nachnani, S.; Saltini, C.; Anderson, L. Effect of 0.12% chlorhexidine on bacterial recolonization following periodontal surgery. J. Periodontol. 1989, 60, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Loe, H.; Schiott, C.R. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J. Periodontal Res. 1970, 5, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.M.; Jensen, S.B.; Schiott, C.R.; Loe, H. The effect of topical application of chlorhexidine on the bacterial colonization of the teeth and gingiva. J. Periodontal Res. 1970, 5, 96–101. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J. Comparison of plaque accumulation after topical application and mouth rinsing with chlorhexidine gluconate. J. Clin. Periodontol. 1983, 10, 69–71. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Schiott, C.R.; Loe, H.; Jensen, S.B.; Kilian, M.; Davies, R.M.; Glavind, K. The effect of chlorhexidine mouthrinses on the human oral flora. J. Periodontal Res. 1970, 5, 84–89. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Flötra, L.; Gjermo, P.; Rölla, G.; Waerhaug, J. Side effects of chlorhexidine mouth washes. Eur. J. Oral Sci. 1971, 79, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Houri-Haddad, Y.; Halabi, A.; Soskolne, W.A. Inflammatory response to chlorhexidine, minocycline HCl and doxycycline HCl in an in vivo mouse model. J. Clin. Periodontol. 2008, 35, 783–788. [Google Scholar] [CrossRef]
- Chatzigiannidou, I.; Teughels, W.; Van De Wiele, T.; Boon, N. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. NPJ Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Kenney, E.B.; Saxe, S.R.; Bowles, R.D. Effect of chlorhexidine on human polymorphonuclear leucocytes. Arch. Oral Biol. 1972, 17, 1633–1636. [Google Scholar] [CrossRef]
- Knuuttila, M.; Söderling, E. Effect of chlorhexidine on the release of lysosomal enzymes from cultured macrophages. Acta Odontol. Scand. 1981, 39, 285–289. [Google Scholar] [CrossRef]
- Helgeland, K.; Heyden, G.; Rölla, G. Effect of chlorhexidine on animal cells in vitro. Eur. J. Oral Sci. 1971, 79, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.; Kallenberger, A. Influence of chlorhexidine rinsing on the healing of oral mucosa and osseous lesions. A histomorphometric study on experimental animals. J. Clin. Periodontol. 1980, 7, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Wurzburger, B.J.; Rubin, Y.L.; Sinensky, M.C.; Blau, L. An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell Biol. Toxicol. 1995, 11, 79–88. [Google Scholar] [CrossRef]
- Mariotti, A.J.; Rumpf, D.A. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.; Celes, M.R.; De Rossi, A.; Silva, L.A.; Silva, J.S.; Rossi, M.A. Evaluation of chlorhexidine toxicity injected in the paw of mice and added to cultured L929 fibroblasts. J. Endod. 2007, 33, 715–722. [Google Scholar] [CrossRef]
- Faria, G.; Cardoso, C.R.; Larson, R.E.; Silva, J.S.; Rossi, M.A. Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: A role for endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2009, 234, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.T.; Fernandes, M.H. In vitro comparison of chlorhexidine and povidone–iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin. Oral Investig. 2007, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Huang, F.M.; Tai, K.W.; Chou, M.Y. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Fujioka-Kobayashi, M.; Schaller, B.; Pikos, M.A.; Sculean, A.; Miron, R.J. Cytotoxicity and gene expression changes of a novel homeopathic antiseptic oral rinse in comparison to chlorhexidine in gingival fibroblasts. Materials 2020, 13, 3190. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Kotwicka, M.; Urbaniak, P.; Nowak, A.; Skrzypczak-Jankun, E.; Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016, 37, 1594–1600. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Shi, Q.; Qing, Y.; Yao, Y.C.; Cao, Y.G. Cytotoxicity of modified nonequilibrium plasma with chlorhexidine digluconate on primary cultured human gingival fibroblasts. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 137–141. [Google Scholar] [CrossRef]
- Chen, L.; Arbieva, Z.H.; Guo, S.; Marucha, P.T.; Mustoe, T.A.; DiPietro, L.A. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genom. 2010, 11, 471. [Google Scholar] [CrossRef] [Green Version]
- Vescarelli, E.; Pilloni, A.; Dominici, F.; Pontecorvi, P.; Angeloni, A.; Polimeni, A.; Ceccarelli, S.; Marchese, C. Autophagy activation is required for myofibroblast differentiation during healing of oral mucosa. J. Clin. Periodontol. 2017, 44, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Ceccarelli, S.; Gerini, G.; Vescarelli, E.; Marini, L.; Marchese, C.; Pilloni, A. Gene expression profiles of oral soft tissue-derived fibroblast from healing wounds: Correlation with clinical outcome, autophagy activation and fibrotic markers expression. J. Clin. Periodontol. 2021, 48, 705–720. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontol. 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Wake, N.; Asahi, Y.; Noiri, Y.; Hayashi, M.; Motooka, D.; Nakamura, S.; Gotoh, K.; Miura, J.; Machi, H.; Iida, T.; et al. Temporal dynamics of bacterial microbiota in the human oral cavity determined using an in situ model of dental biofilms. NPJ Biofilms Microbiomes 2016, 2, 16018. [Google Scholar] [CrossRef]
- Kejner, A.E.; Burch, M.B.; Sweeny, L.; Rosenthal, E.L. Bone morphogenetic protein 6 expression in oral cavity squamous cell cancer is associated with bone invasion. Laryngoscope 2013, 123, 3061–3065. [Google Scholar] [CrossRef] [Green Version]
- Allred, D.C.; Bustamante, M.A.; Daniel, C.O.; Gaskill, H.V.; Cruz, A.B., Jr. Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Arch. Surg. 1990, 125, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.A.; Marini, L.; Pilloni, A.; Sahrmann, P. Early wound healing outcomes after regenerative periodontal surgery with enamel matrix derivatives or guided tissue regeneration: A systematic review. BMC Oral Health 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, T.; Koerber, A.; Jacobsen, F.; Dissemond, J.; Steinau, H.-U.; Gatermann, S.; Al-Benna, S.; Kesting, M.; Seipp, H.-M.; Steinstraesser, L. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J. Surg. Res. 2010, 164, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Bar-Or, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma 2009, 66, 82–91. [Google Scholar] [CrossRef]
- Coelho, A.S.; Laranjo, M.; Gonçalves, A.C.; Paula, A.; Paulo, S.; Abrantes, A.M.; Caramelo, F.; Ferreira, M.M.; Silva, M.J.; Carrilho, E.; et al. Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblasts. Odontology 2020, 108, 260–270. [Google Scholar] [CrossRef]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Francis, M.; DiPietro, L.A. Differential apoptosis in mucosal and dermal wound healing. Adv. Wound Care 2014, 3, 751–761. [Google Scholar] [CrossRef]
- Laplante, P.; Sirois, I.; Raymond, M.-A.; Kokta, V.; Béliveau, A.; Prat, A.; Pshezhetsky, A.V.; Hébert, M.-J. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ. 2010, 17, 291–303. [Google Scholar] [CrossRef]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. In Vitro 2008, 22, 308–317. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Cregan, S.P.; Maclaurin, J.G.; Craig, C.G.; Robertson, G.S.; Nicholson, D.W.; Park, D.; Slack, R. Bax-dependent caspase-3 activation is a key determinant in p53-induced apoptosis in neurons. J. Neurosci. 1999, 19, 7860–7869. [Google Scholar] [CrossRef]
- Nichani, K.; Li, J.; Suzuki, M.; Houston, J.P. Evaluation of caspase-3 activity during apoptosis with fluorescence lifetime-based cytometry measurements and phasor analyses. Cytom. Part A 2020, 97, 1265–1275. [Google Scholar] [CrossRef]
- Cregan, S.P.; Dawson, V.L.; Slack, R.S. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 2004, 23, 2785–2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Müllauer, F.; Böck, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmar, B.; El-Gibaly, A.M.; Scheuer, C.; Strik, M.W.; Bruch, H.P.; Menger, M.D. Acceleration of cutaneous wound healing by transient p53 inhibition. Lab. Investig. 2002, 82, 1063–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Rognoni, E.; Gomez, C.; Pisco, A.O.; Rawlins, E.L.; Simons, B.D.; Watt, F.M.; Driskell, R.R. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 2016, 143, 2522–2535. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.P.; Lee, C.J.; Subeq, Y.M.; Hsu, B.G. A model of chlorhexidine digluconate-induced peritoneal fibrosis in rats. Tzu Chi Med. J. 2012, 24, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- Marini, L.; Rojas, M.A.; Sahrmann, P.; Aghazada, R.; Pilloni, A. Early Wound Healing Score: A system to evaluate the early healing of periodontal soft tissue wounds. J. Periodontal Implant. Sci. 2018, 48, 274–283. [Google Scholar] [CrossRef]
- Marini, L.; Sahrmann, P.; Rojas, M.A.; Cavalcanti, C.; Pompa, G.; Papi, P.; Pilloni, A. Early Wound Healing Score (EHS): An intra- and inter-examiner reliability study. Dent. J. 2019, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Tipton, D.A.; Braxton, S.D.; Dabbous, M.K. Effects of a Bleaching Agent on Human Gingival Fibroblasts. J. Periodontol. 1995, 66, 7–13. [Google Scholar] [CrossRef] [PubMed]



| Patient | IHC Score a | |||||
|---|---|---|---|---|---|---|
| αSMA | Col1a1 | Vimentin | ||||
| NT | CHX | NT | CHX | NT | CHX | |
| 1 | 0 | 1 | 2 | 3 | 1 | 1 |
| 2 | 0 | 1 | 1 | 2 | 1 | 1 |
| 3 | 0 | 1 | 1 | 3 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilloni, A.; Ceccarelli, S.; Bosco, D.; Gerini, G.; Marchese, C.; Marini, L.; Rojas, M.A. Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics 2021, 10, 1192. https://doi.org/10.3390/antibiotics10101192
Pilloni A, Ceccarelli S, Bosco D, Gerini G, Marchese C, Marini L, Rojas MA. Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics. 2021; 10(10):1192. https://doi.org/10.3390/antibiotics10101192
Chicago/Turabian StylePilloni, Andrea, Simona Ceccarelli, Daniela Bosco, Giulia Gerini, Cinzia Marchese, Lorenzo Marini, and Mariana A. Rojas. 2021. "Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis" Antibiotics 10, no. 10: 1192. https://doi.org/10.3390/antibiotics10101192
APA StylePilloni, A., Ceccarelli, S., Bosco, D., Gerini, G., Marchese, C., Marini, L., & Rojas, M. A. (2021). Effect of Chlorhexidine Digluconate in Early Wound Healing of Human Gingival Tissues. A Histological, Immunohistochemical and Biomolecular Analysis. Antibiotics, 10(10), 1192. https://doi.org/10.3390/antibiotics10101192








