Effects of Partial Organic Substitution for Chemical Fertilizer on Antibiotic Residues in Peri-Urban Agricultural Soil in China
Abstract
:1. Introduction
2. Results
2.1. Effects of Organic Substitution on Soil Properties
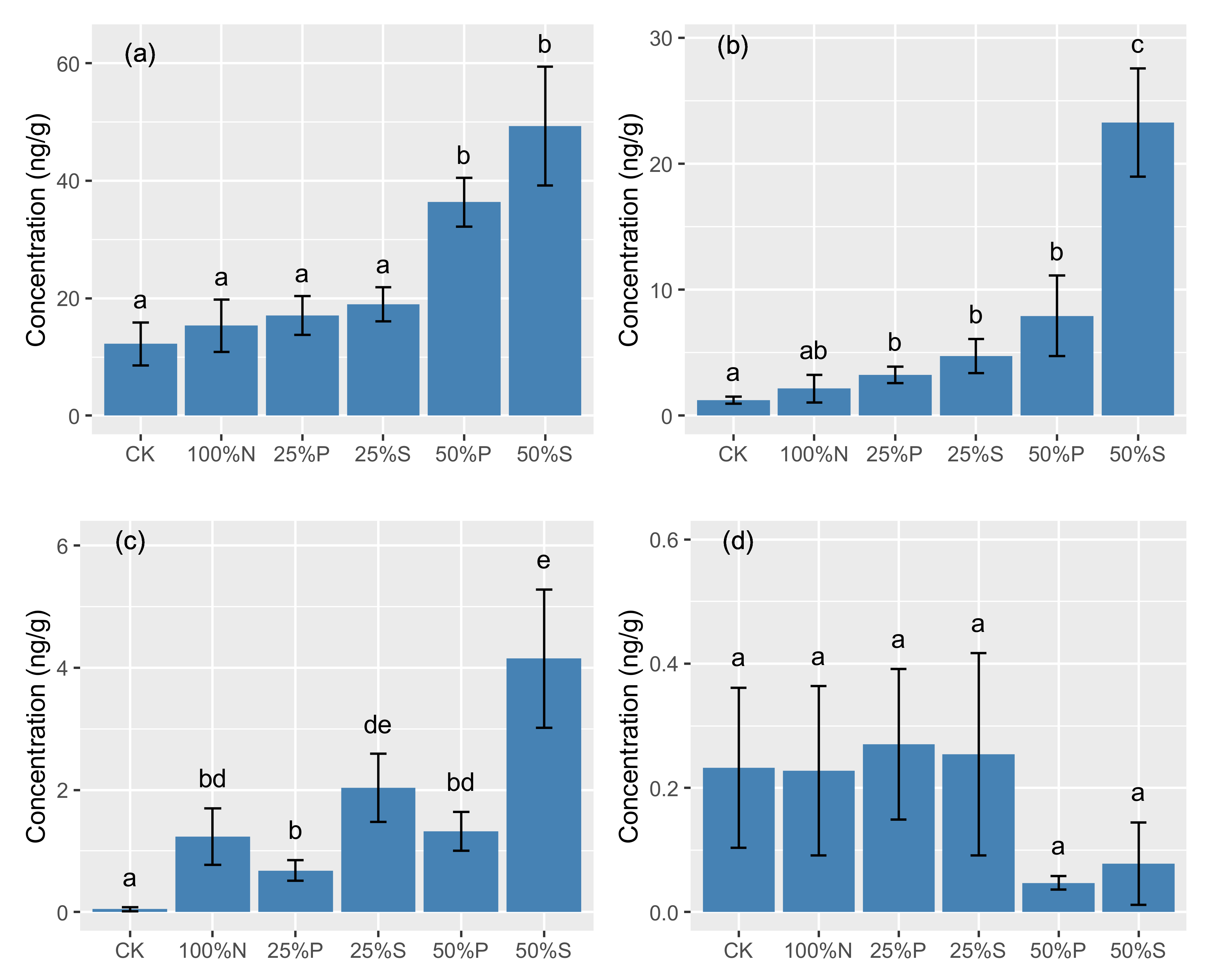
2.2. Effects of Organic Substitution on Antibiotic Residues
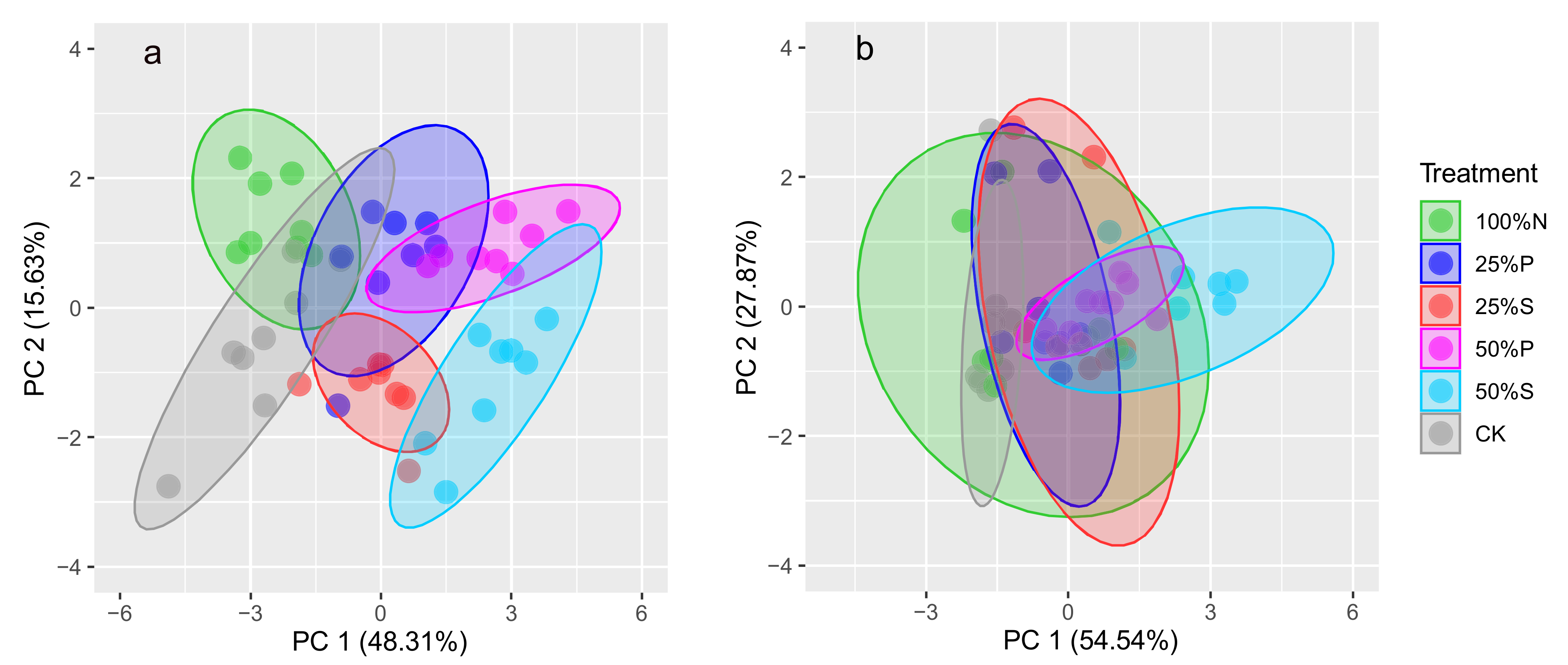
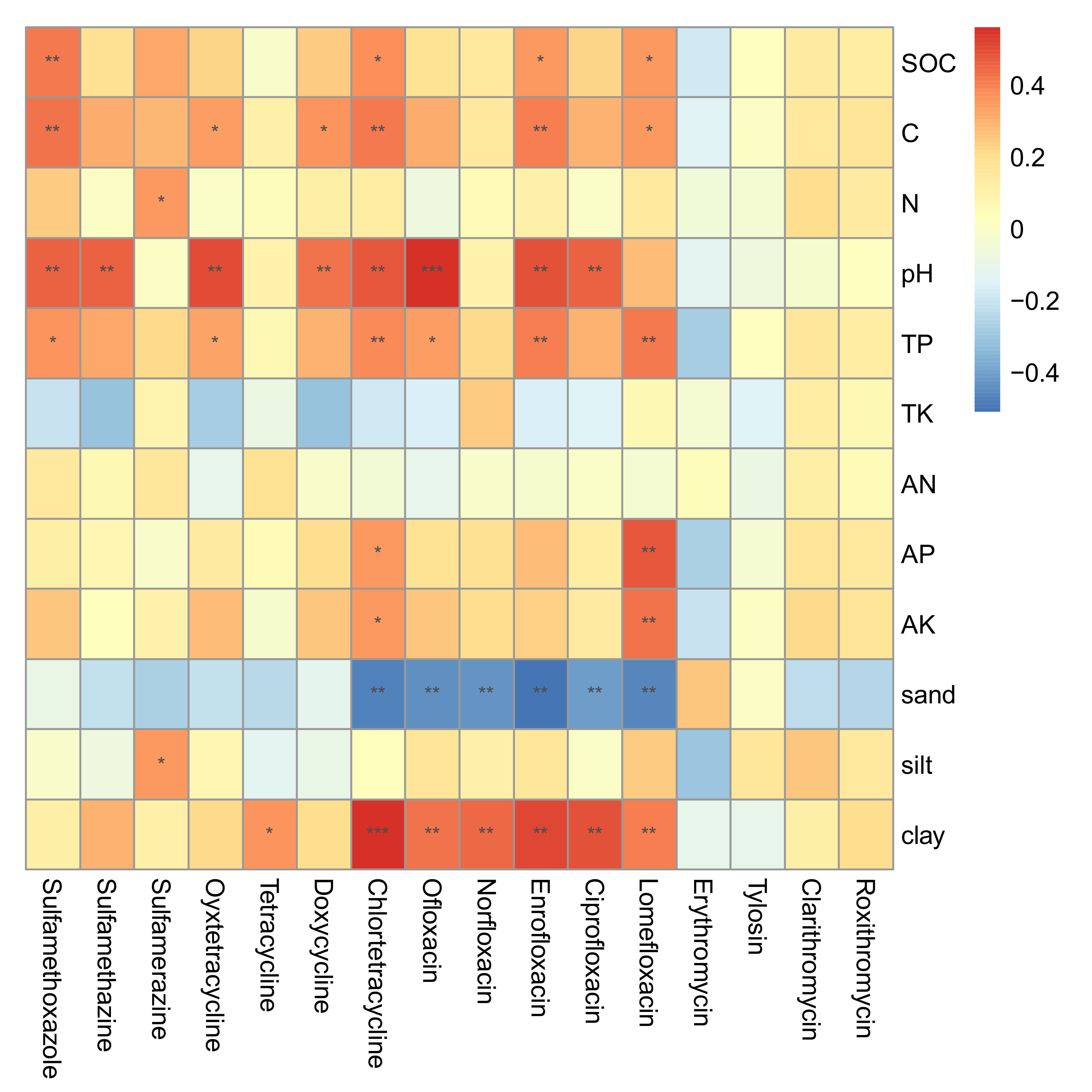
2.3. Correlations between Soil Properties and Antibiotic Residues
3. Discussion
3.1. Organic Substitution Increases Antibiotic Concentration in Soil
3.2. The Influences of Soil Properties on Antibiotic Residues
3.3. Implications
4. Materials and Methods
4.1. Site Description
4.2. Experimental Design and Field Management
4.3. Analysis of Antibiotics
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.E.; Lee, Y.B. Veterinary antibiotics (VAs) contamination as a global agro-ecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, 3463–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [PubMed]
- Bu, Q.W.; Wang, B.; Huang, J.; Liu, K.; Deng, S.B.; Wang, Y.J.; Yu, G. Estimating the use of antibiotics for humans across China. Chemosphere 2016, 144, 1384–1390. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar]
- Christou, A.; Aguera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schroder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Qiao, M.; Wang, F.H.; Zhu, Y.G. Use of commercial organic fertilizer increases the abundance of antibiotic resistance genes and antibiotics in soil. Environ. Sci. Pollut. Res. 2017, 24, 701–710. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, D.; Hong, B.; Wang, H.T.; Li, G.; Ma, Y.B.; Tang, Y.T.; Chen, Q.L. Long-term application of organic fertilization causes the accumulation of antibiotic resistome in earthworm gut microbiota. Environ. Int. 2019, 124, 145–152. [Google Scholar]
- Zhu, D.; An, X.L.; Chen, Q.L.; Yang, X.R.; Christie, P.; Ke, X.; Wu, L.H.; Zhu, Y.G. Antibiotics disturb the microbiome and increase the incidence of resistance genes in the gut of a common soil collembolan. Environ. Sci. Technol. 2018, 52, 3081–3090. [Google Scholar]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Human and veterinary antibiotics induce hormesis in plants: Scientific and regulatory issues and an environmental perspective. Environ. Int. 2018, 120, 489–495. [Google Scholar] [CrossRef]
- Zielezny, Y.; Groeneweg, J.; Vereecken, H.; Tappe, W. Impact of sulfadiazine and chlorotetracycline on soil bacterial community structure and respiratory activity. Soil Biol. Biochem. 2006, 38, 2372–2380. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Q.; Zhang, T.; Ma, W.; Velthof, G.L.; Hou, Y.; Oenema, O.; Zhang, F. Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: A meta-analysis. Global Change Biol. 2020, 26, 888–900. [Google Scholar]
- Xia, L.; Lam, S.K.; Yan, X.; Chen, D. How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses, and soil carbon balance? Environ. Sci. Technol. 2017, 51, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 2019, 215, 234–240. [Google Scholar] [CrossRef]
- Gros, M.; Mas-Pla, J.; Boy-Roura, M.; Geli, I.; Domingo, F.; Petrović, M. Veterinary pharmaceuticals and antibiotics in manure and slurry and their fate in amended agricultural soils: Findings from an experimental field site (Baix Empordà, NE Catalonia). Sci. Total Environ. 2019, 654, 1337–1349. [Google Scholar] [CrossRef]
- Camotti Bastos, M.; Rheinheimer dos Santos, D.; Aubertheau, É.; de Castro Lima, J.A.M.; Le Guet, T.; Caner, L.; Mondamert, L.; Labanowski, J. Antibiotics and microbial resistance in Brazilian soils under manure application. Land Degrad. Dev. 2018, 29, 2472–2484. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shan, J.; Yang, P.; Shang, X.; Xia, Y.; Yan, X. Effects of long-term pig manure application on antibiotics, abundance of antibiotic resistance genes (ARGs), anammox and denitrification rates in paddy soils. Environ. Pollut. 2018, 240, 368–377. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, L.; Yen, H.; Sun, L.; Li, S.; Li, M.; Feng, Q.; Yang, L. Multimedia mass balance approach to characterizing the transport potential of antibiotics in soil–plant systems following manure application. J. Hazard. Mater. 2020, 393, 122363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [PubMed]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total Environ. 2014, 488, 261–267. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Qian, M.; Wu, H.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; Lin, H.; Ma, J. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xin, Z.; Zhang, Y.; Chen, J.; Yan, J.; Li, H.; Hu, H. Long-term manure application increased the levels of antibiotics and antibiotic resistance genes in a greenhouse soil. Appl. Soil Ecol. 2017, 121, 193–200. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, L.; Yang, L.; Li, S.; Sun, L.; Yu, X. Distribution, dynamics and determinants of antibiotics in soils in a peri-urban area of Yangtze River Delta, Eastern China. Chemosphere 2018, 211, 261–270. [Google Scholar] [PubMed]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Liu, J.M.; Cai, Y.Q. Occurrence, distribution and potential affecting factors of antibiotics in sewage sludge of wastewater treatment plants in China. Sci. Total Environ. 2013, 445, 306–313. [Google Scholar]
- McClellan, K.; Halden, R.U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010, 44, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef]
- Rutgersson, C.; Ebmeyer, S.; Lassen, S.B.; Karkman, A.; Fick, J.; Kristiansson, E.; Brandt, K.K.; Flach, C.-F.; Larsson, D.G.J. Long-term application of Swedish sewage sludge on farmland does not cause clear changes in the soil bacterial resistome. Environ. Int. 2020, 137, 105339. [Google Scholar] [CrossRef]
- Pulkrabová, J.; Černý, J.; Száková, J.; Švarcová, A.; Gramblička, T.; Hajšlová, J.; Balík, J.; Tlustoš, P. Is the long-term application of sewage sludge turning soil into a sink for organic pollutants?: Evidence from field studies in the Czech Republic. J. Soils Sediments 2019, 19, 2445–2458. [Google Scholar] [CrossRef]
- Tamtam, F.; van Oort, F.; Le Bot, B.; Dinh, T.; Mompelat, S.; Chevreuil, M.; Lamy, I.; Thiry, M. Assessing the fate of antibiotic contaminants in metal contaminated soils four years after cessation of long-term waste water irrigation. Sci. Total Environ. 2011, 409, 540–547. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Lanzén, A.; Mijangos, I.; Garbisu, C. The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl. Soil Ecol. 2019, 135, 73–84. [Google Scholar] [CrossRef]
- Cucina, M.; Ricci, A.; Zadra, C.; Pezzolla, D.; Tacconi, C.; Sordi, S.; Gigliotti, G. Benefits and risks of long-term recycling of pharmaceutical sewage sludge on agricultural soil. Sci. Total Environ. 2019, 695, 133762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; An, X.L.; Li, H.; Su, J.Q.; Ma, Y.B.; Zhu, Y.G. Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, C.; Deng, D.; Li, Y.; Luo, L. Factors affecting sorption behaviors of tetracycline to soils: Importance of soil organic carbon, pH and Cd contamination. Ecotoxicol. Environ. Saf. 2020, 197, 110572. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Single and simultaneous adsorption of three sulfonamides in agricultural soils: Effects of pH and organic matter content. Sci. Total Environ. 2020, 744, 140872. [Google Scholar] [CrossRef]
- Figueroa, R.A.; Leonard, A.; MacKay, A.A. Modeling tetracycline antibiotic sorption to clays. Environ. Sci. Technol. 2004, 38, 476–483. [Google Scholar] [CrossRef]
- Pils, J.R.V.; Laird, D.A. Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay−humic complexes. Environ. Sci. Technol. 2007, 41, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.M.P.; Alleoni, L.R.F.; Tornisielo, V.L.; Regitano, J.B. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasquillo, A.J.; Bruland, G.L.; MacKay, A.A.; Vasudevan, D. Sorption of ciprofloxacin and oxytetracycline zwitterions to soils and soil minerals: Influence of compound structure. Environ. Sci. Technol. 2008, 42, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Sonne, C.; Ok, Y.S.; Dietz, R.; Alstrup, A.K.O. Pig slurry needs modifications to be a sustainable fertilizer in crop production. Environ. Res. 2019, 178, 108718. [Google Scholar] [CrossRef]
- Liang, Y.; Pei, M.; Wang, D.; Cao, S.; Xiao, X.; Sun, B. Improvement of soil ecosystem multifunctionality by dissipating manure-induced antibiotics and resistance genes. Environ. Sci. Technol. 2017, 51, 4988–4998. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar-mediated sorption of antibiotics in pig manure. J. Hazard. Mater. 2019, 364, 663–670. [Google Scholar] [CrossRef]
- Jiao, W.; Du, R.; Ye, M.; Sun, M.; Feng, Y.; Wan, J.; Zhao, Y.; Zhang, Z.; Huang, D.; Du, D.; et al. ‘Agricultural Waste to Treasure’—Biochar and eggshell to impede soil antibiotics/antibiotic resistant bacteria (genes) from accumulating in Solanum tuberosum L. Environ. Pollut. 2018, 242, 2088–2095. [Google Scholar] [CrossRef]
- Tang, Q.; Ti, C.; Xia, L.; Xia, Y.; Wei, Z.; Yan, X. Ecosystem services of partial organic substitution for chemical fertilizer in a peri-urban zone in China. J. Clean. Prod. 2019, 224, 779–788. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, J.; Chen, K.; Yao, J.; Yu, Z.; Lin, X. Soil microbial activity measured by microcalorimetry in response to long-term fertilization regimes and available phosphorous on heat evolution. Soil Biol. Biochem. 2009, 41, 2094–2099. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, G.H.; Luan, L.L.; Liu, F. Temporal variation in soil resistance to flowing water erosion for soil incorporated with plant litters in the Loess Plateau of China. Catena 2016, 145, 239–245. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Liu, N.; He, H.; Cao, X.; Lv, C.; Zhang, K.; Dai, J. Effects of different types of microbial inoculants on available nitrogen and phosphorus, soil microbial community, and wheat growth in high-P soil. Environ. Sci. Pollut. Res. 2021, 28, 23036–23047. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolde, R. Package ‘Pheatmap’. Bioconductor. 2012, pp. 1–6. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 8 August 2021).
- R Core Team R: A Language and Environment for Statistical Computing, Version 3.6.1 [Computer Program]; R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project.org/(accessed on 8 August 2021).
| Soil Properties | CK | 100%N | 25%P | 25%S | 50%P | 50%S |
|---|---|---|---|---|---|---|
| Clay (%) | 21.38 ± 2.20 a | 25.08 ± 2.18 b | 25.41 ± 1.95 b | 25.00 ± 2.11 b | 30.74 ± 2.36 c | 29.29 ± 3.36 c |
| Silt (%) | 37.50 ± 2.61 a | 35.56 ± 3.08 a | 36.80 ± 1.86 a | 36.20 ± 1.91 a | 37.52 ± 2.11 a | 37.89 ± 2.17 a |
| Sand (%) | 41.12 ± 2.59 a | 39.37 ± 3.16 ab | 37.79 ± 2.50 b | 38.81 ± 1.06 b | 31.74 ± 3.70 c | 32.82 ± 4.74 c |
| pH | 4.90 ± 0.27 a | 4.44 ± 0.16 b | 5.12 ± 0.23 a | 6.65 ± 0.12 c | 5.87 ± 0.09 d | 7.02 ± 0.19 e |
| C (g/kg) | 24.39 ± 2.89 a | 26.17 ± 2.55 ab | 32.71 ± 3.73 b | 30.68 ± 5.01 b | 35.79 ± 4.88 b | 36.85 ± 2.74 b |
| N (g/kg) | 3.11 ± 0.39 a | 3.54 ± 0.34 ab | 3.92 ± 0.56 b | 3.41 ± 0.47 b | 4.04 ± 0.48 b | 3.61 ± 0.29 b |
| TP (g/kg) | 0.93 ± 0.18 a | 0.88 ± 0.07 a | 1.44 ± 0.25 b | 1.40 ± 0.22 bc | 1.69 ± 0.22 d | 1.70 ± 0.14 cd |
| TK (g/kg) | 19.87 ± 0.61 a | 20.01 ± 0.51 ab | 20.16 ± 0.54 b | 19.86 ± 0.93 b | 20.37 ± 0.49 b | 19.56 ± 0.71 b |
| OC (%) | 2.38 ± 0.31 a | 2.51 ± 0.23 ab | 3.07 ± 0.43 b | 2.89 ± 0.41 bc | 3.48 ± 0.46 c | 3.32 ± 0.32 c |
| AN (g/kg) | 0.25 ± 0.03 a | 0.35 ± 0.04 b | 0.31 ± 0.04 d | 0.26 ± 0.03 ac | 0.30 ± 0.04 cd | 0.29 ± 0.04 cd |
| AP (mg/kg) | 118.30 ± 24.06 a | 126.86 ± 11.67 a | 208.30 ± 25.16 b | 159.68 ± 11.58 c | 267.13 ± 27.67 c | 190.63 ± 15.63 d |
| AK (mg/kg) | 407.40 ± 32.36 ab | 390.99 ± 24.20 a | 433.73 ± 26.00 a | 402.92 ± 22.03 b | 486.32 ± 17.13 b | 457.13 ± 28.82 c |
| Antibiotics | CK | 100%N | 25%P | 25%S | 50%P | 50%S | |
|---|---|---|---|---|---|---|---|
| SAs | 0.04 ± 0.09 | 1.23 ± 1.22 | 0.68 ± 0.44 | 2.03 ± 1.47 | 1.32 ± 0.83 | 4.15 ± 2.99 | |
| SMX | 0.03 ± 0.06 | 0.16 ± 0.24 | 0.22 ± 0.52 | 0.47 ± 0.19 | 0.17 ± 0.67 | 0.71 ± 0.11 | |
| SMZ | n.d. | 1.05 ± 1.27 | 0.45 ± 1.74 | 1.55 ± 0.39 | 1.13 ± 3.23 | 3.42 ± 0.80 | |
| SMR | 0.02 ± 0.04 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.03 ± 0.03 | 0.02 ± 0.06 | |
| TCs | 12.2 ± 9.75 | 15.3 ± 11.7 | 17.1 ± 8.70 | 18.9 ± 7.73 | 36.3 ± 10.9 | 49.3 ± 26.8 | |
| OTC | 0.26 ± 0.56 | 0.5 ± 1.00 | 1.07 ± 1.80 | 0.97 ± 0.96 | 2.1 ± 8.59 | 10.92 ± 1.43 | |
| TC | 1.12 ± 1.28 | 3.51 ± 3.63 | 3.55 ± 3.57 | 2.88 ± 5.60 | 3.43 ± 2.46 | 3.71 ± 0.67 | |
| DOX | 1.43 ± 0.98 | 1.98 ± 2.06 | 3.85 ± 2.92 | 2.83 ± 3.54 | 5.04 ± 10.9 | 10.93 ± 2.48 | |
| CTC | 9.4 ± 7.90 | 9.36 ± 9.00 | 8.64 ± 4.45 | 12.29 ± 2.49 | 25.78 ± 11.5 | 23.75 ± 8.81 | |
| QNs | 1.23 ± 0.76 | 2.15 ± 2.92 | 3.25 ± 1.75 | 4.73 ± 3.61 | 7.92 ± 8.44 | 23.2 ± 11.3 | |
| OFL | 0.22 ± 0.21 | 0.18 ± 0.40 | 0.5 ± 2.09 | 1.91 ± 0.32 | 2.5 ± 5.54 | 9.24 ± 4.53 | |
| NOR | 0.21 ± 0.27 | 0.28 ± 0.30 | 0.29 ± 0.25 | 0.19 ± 0.37 | 0.45 ± 0.30 | 0.48 ± 0.39 | |
| ENR | 0.27 ± 0.20 | 0.39 ± 0.33 | 1.19 ± 0.88 | 1.2 ± 0.59 | 2.39 ± 4.91 | 6.28 ± 1.93 | |
| CIP | 0.33 ± 0.38 | 1.28 ± 2.71 | 0.62 ± 1.17 | 1.3 ± 0.83 | 1.8 ± 3.67 | 6.34 ± 2.19 | |
| LOM | 0.21 ± 0.39 | 0.02 ± 0.04 | 0.65 ± 0.18 | 0.13 ± 0.67 | 0.79 ± 0.39 | 0.95 ± 0.87 | |
| MLs | 0.23 ± 0.34 | 0.22 ± 0.36 | 0.26 ± 0.32 | 0.25 ± 0.43 | 0.04 ± 0.02 | 0.07 ± 0.17 | |
| ERY | 0.15 ± 0.34 | 0.22 ± 0.36 | 0.17 ± 0.40 | 0.23 ± 0.29 | n.d. | n.d. | |
| TYL | 0.04 ± 0.05 | n.d. | 0.02 ± 0.03 | 0.02 ± 0.03 | n.d. | 0.02 ± 0.01 | |
| CLA | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 | n.d. | 0.02 ± 0.04 | 0.02 ± 0.01 | |
| ROX | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.05 ± 0.01 | 0.01 ± 0.07 | 0.02 ± 0.08 | 0.04 ± 0.02 | |
| Total | 13.73 ± 9.62 | 18.96 ± 12.6 | 21.31 ± 8.28 | 25.99 ± 7.41 | 45.65 ± 15.7 | 76.83 ± 35.0 | |
| Antibiotics | Pig Manure | Sewage Sludge | ||
|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |
| Oxytetracycline | 1216.02 | 658.40 | 222.67 | 89.61 |
| Chlortetracycline | 19,820.02 | 7898.04 | 235.70 | 39.81 |
| Tetracycline | 3966.35 | 1758.68 | 82.65 | 50.11 |
| Doxycycline | 1348.94 | 747.90 | 1396.94 | 614.97 |
| Norfloxacin | 73.12 | 88.41 | 170.90 | 58.34 |
| Ofloxacin | 96.61 | 103.22 | 174.28 | 89.52 |
| Ciprofloxacin | 59.15 | 77.45 | 4610.81 | 2209.96 |
| Lomefloxacin | 0.50 | 0.97 | 0.33 | 0.42 |
| Enrofloxacin | 69.79 | 57.69 | 37.47 | 23.05 |
| Sulfamethoxazole | 202.33 | 212.70 | 29.79 | 18.64 |
| Sulfamethazine | 6069.81 | 6381.04 | 12,907.08 | 4840.82 |
| Sulfamerazine | 198.32 | 87.93 | 518.50 | 252.29 |
| Erythromycin | 2.10 | 6.31 | 0.11 | 0.18 |
| Tylosin | 29.61 | 30.70 | 56.20 | 32.93 |
| Clarithromycin | 0.32 | 0.55 | 2.76 | 2.02 |
| Roxithromycin | 0.08 | 0.14 | 4.95 | 3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Li, W.; Xu, W. Effects of Partial Organic Substitution for Chemical Fertilizer on Antibiotic Residues in Peri-Urban Agricultural Soil in China. Antibiotics 2021, 10, 1173. https://doi.org/10.3390/antibiotics10101173
Dong B, Li W, Xu W. Effects of Partial Organic Substitution for Chemical Fertilizer on Antibiotic Residues in Peri-Urban Agricultural Soil in China. Antibiotics. 2021; 10(10):1173. https://doi.org/10.3390/antibiotics10101173
Chicago/Turabian StyleDong, Baocheng, Wei Li, and Wenyong Xu. 2021. "Effects of Partial Organic Substitution for Chemical Fertilizer on Antibiotic Residues in Peri-Urban Agricultural Soil in China" Antibiotics 10, no. 10: 1173. https://doi.org/10.3390/antibiotics10101173
APA StyleDong, B., Li, W., & Xu, W. (2021). Effects of Partial Organic Substitution for Chemical Fertilizer on Antibiotic Residues in Peri-Urban Agricultural Soil in China. Antibiotics, 10(10), 1173. https://doi.org/10.3390/antibiotics10101173





