Auranofin Has Advantages over First-Line Drugs in the Treatment of Severe Streptococcus suis Infections
Abstract
1. Introduction
2. Results
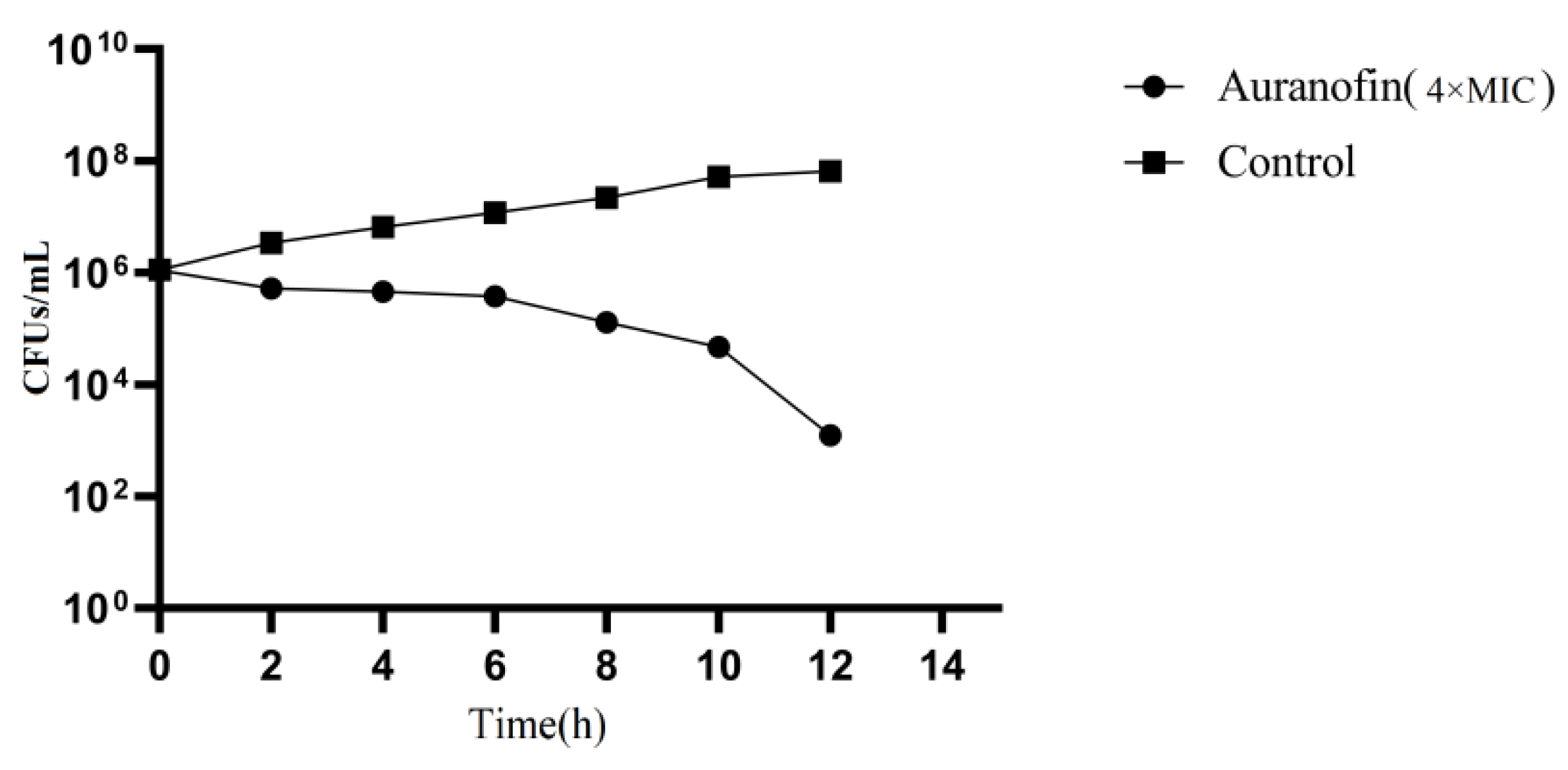
2.1. Auranofin Shows Excellent Antibacterial Activity
2.2. Safety Evaluation of Auranofin
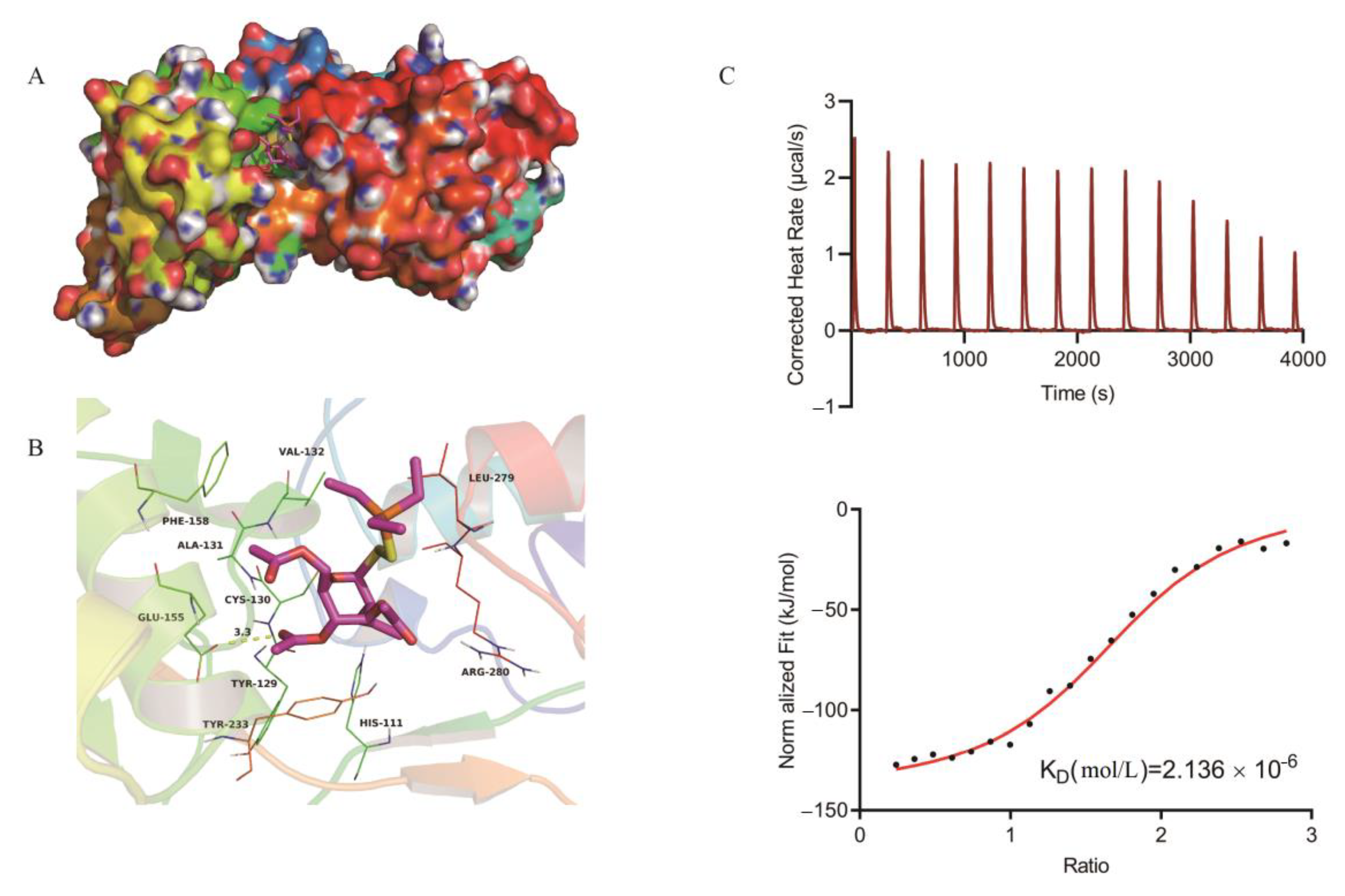
2.3. Mechanism of Auranofin Antibacterial Activity
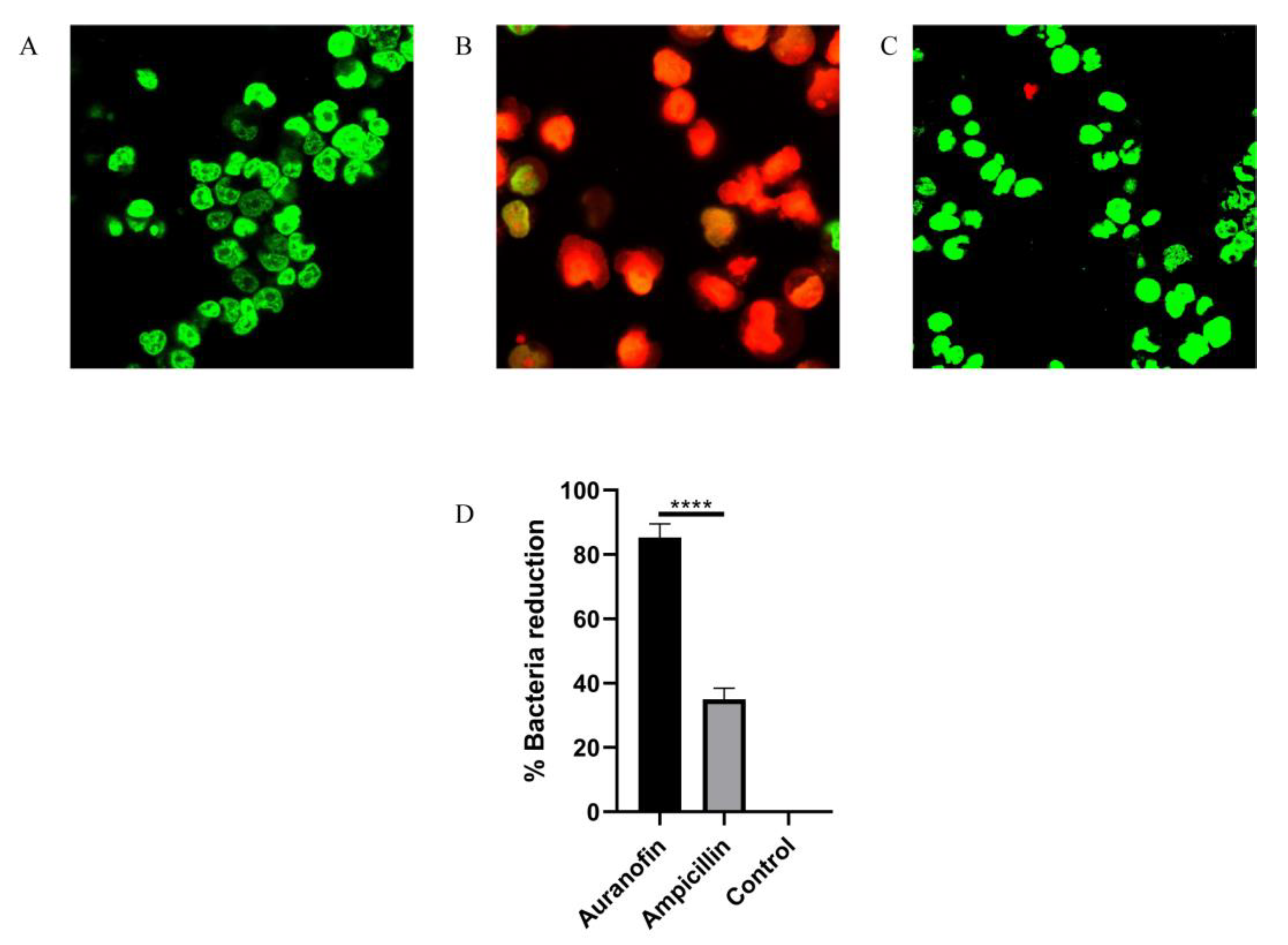
2.4. Auranofin Alleviates the SC19-Induced Injury of RAW264.7 Cells
2.5. Auranofin Treatment of SC19 Cells Results in Thiol Depletion and Compromises Their Defense against Oxidative Stress
2.6. Protective Rates of Auranofin and Ampicillin in Infected Mouse Models at Different Times
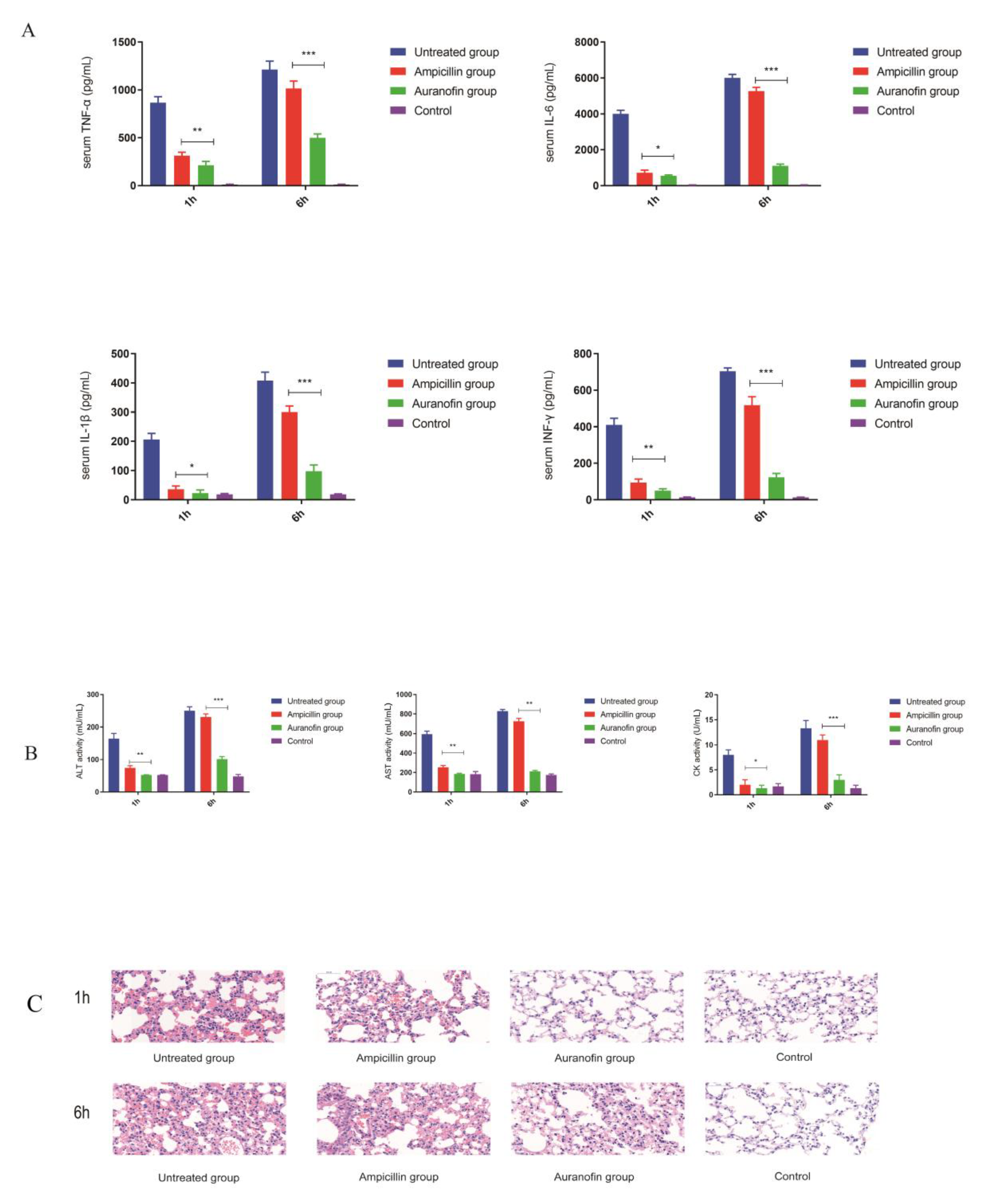
2.7. Anti-Inflammatory Effects of Auranofin Are Crucial for Improving the Rate of Protection
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Conditions, Auranofin Preparation
4.2. Assessment of the Anti-S. suis Activity of Auranofin
4.3. Time-Kill Curve
4.4. Cell Toxicity Test
4.5. In Vivo Toxicity Experiment
4.6. Production of Recombinant TrxB Protein
4.7. Homology Modeling and Molecular Docking
4.8. Auranofin and TrxB Binding Assays
4.9. Construction of TrxB-Overexpressing Strains
4.10. Thiol Depletion Assay
4.11. ROS Measurement
4.12. Cell Culture and Infection
4.13. Animal Experiments
4.14. Statistical Analysis
4.15. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segura, M. Streptococcus suis research: Progress and challenges. Pathogens 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus suis: An emerging human threat. J. Infect. Dis. 2009, 199, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Nghia, H.D.T.; Taylor, W.; Schultsz, C. Streptococcus suis: An emerging human pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Perch, B.; Kristjansen, P.; Skadhauge, K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol. Microbiol. Scand. 1968, 74, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.; Ha, N.; Huy, N.T.; Horby, P.; Nghia, H.D.; Thiem, V.D.; Zhu, X.; Hoa, N.T.; Hien, T.T.; Zamora, J.; et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 2014, 20, 1105–1114. [Google Scholar] [CrossRef]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, H.; Gottschalk, M.; Bai, X.; Lan, R.; Ji, S.; Liu, H.; Xu, J. Development of Multiplex PCR Assays for the Identification of the 33 Serotypes of Streptococcus suis. PLoS ONE 2013, 8, e72070. [Google Scholar] [CrossRef]
- Yu, H.; Jing, H.; Chen, Z.; Zheng, H.; Zhu, X.; Wang, H.; Wang, S.; Liu, L.; Zu, R.; Luo, L.; et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 2006, 12, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, C.; Feng, Y.; Yang, W.; Song, H.; Chen, Z.; Yu, H.; Pan, X.; Zhou, X.; Wang, H.; et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006, 3, e151. [Google Scholar] [CrossRef]
- Ye, C.; Zheng, H.; Zhang, J.; Jing, H.; Wang, L.; Xiong, Y.; Wang, W.; Zhou, Z.; Sun, Q.; Luo, X.; et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J. Infect. Dis. 2009, 199, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Gottschalk, M.; Gerber, P.P.; Lemire, P.; Xu, J.; Segura, M. Exacerbated type ii interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect. Immun. 2013, 81, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, J.; Yu, J.; Xu, Z.; Liu, L.; Song, Y.; Sun, X.; Zhang, A.; Jin, M. HP1330 contributes to Streptococcus suis virulence by inducing toll-like receptor 2- and ERK1/2-dependent pro-inflammatory responses and influencing in vivo S. suis loads. Front. Immunol. 2017, 8, 869. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Holden, M.T.; Hauser, H.; Sanders, M.; Ngo, T.H.; Cherevach, I.; Cronin, A.; Goodhead, I.; Mungall, K.; Quail, M.A.; Price, C.; et al. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS ONE 2009, 15, e6072. [Google Scholar] [CrossRef]
- May, H.C.; Yu, J.J.; Guentzel, M.N.; Chambers, J.P.; Cap, A.P.; Arulanandam, B.P. Repurposing Auranofin, Ebselen, and PX-12 as Antimicrobial Agents Targeting the Thioredoxin System. Front. Microbiol. 2018, 5, 336. [Google Scholar] [CrossRef]
- Debnath, A.; Parsonage, D.; Andrade, R.M.; He, C.; Cobo, E.R.; Hirata, K.; Chen, S.; García-Rivera, G.; Orozco, E.; Martínez, M.B.; et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012, 18, 956–960. [Google Scholar] [CrossRef]
- Hokai, Y.; Jurkowicz, B.; Fernández-Gallardo, J.; Zakirkhodjaev, N.; Sanaú, M.; Muth, T.R.; Contel, M. Auranofin and related heterometallic gold(I)-thiolates as potent inhibitors of methicillin-resistant Staphylococcus aureus bacterial strains. J. Inorg. Biochem. 2014, 138, 81–88. [Google Scholar] [CrossRef]
- AbdelKhalek, A.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int. J. Antimicrob. Agents 2019, 53, 54–62. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; RajaMuthiah, R.; Souza, A.C.; Eatemadpour, S.; Rossoni, R.D.; Santos, D.A.; Junqueira, J.C.; Rice, L.B.; Mylonakis, E. Inhibition of bacterial and fungal pathogens by the orphaned drug auranofin. Future Med. Chem. 2016, 8, 117–132. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Seitz, M.; Schroten, H.; Schwerk, C. Biological activities of suilysin: Role in Streptococcus suis pathogenesis. Future Microbiol. 2016, 11, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Kollef, M.H.; Nathwani, D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S378–S385. [Google Scholar] [CrossRef]
- Seleem, M.N.; Jain, N.; Pothayee, N.; Ranjan, A.; Riffle, J.S.; Sriranganathan, N. Targeting Brucella melitensis with polymeric nanoparticles containing streptomycin and doxycycline. FEMS Microbiol. Lett. 2009, 294, 24–31. [Google Scholar] [CrossRef]
- Mishra, S.; Imlay, J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch. Biochem. Biophys. 2012, 525, 145–160. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 inflammasome-triggered cytokine storm contributes to streptococcal toxic shock-like syndrome (STSLS). PLoS Pathog. 2019, 15, e1007795. [Google Scholar] [CrossRef]
- ME, S.-A.; Spooner, C.; Belseck, E.; Shea, B. Auranofin versus placebo in rheumatoid arthritis. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef]
- Yang, G.; Lee, S.J.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Zouboulis, C.C.; Han, S.H.; Ma, K.H.; Jang, J.K.; Lee, J.Y. Repurposing Auranofin, an Anti-Rheumatic Gold Compound, to Treat Acne Vulgaris by Targeting the NLRP3 Inflammasome. Biomol. Ther. 2020, 28, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, H.; Kim, M.Y.; Ji, S.Y.; Kim, S.Y.; Lee, H.; Kim, G.Y.; Park, C.; Keum, Y.S.; Hong, S.H.; Cheong, J.; et al. Auranofin Attenuates Non-Alcoholic Fatty Liver Disease by Suppressing Lipid Accumulation and NLRP3 Inflammasome-Mediated Hepatic Inflammation In Vivo and In Vitro. Antioxidants 2020, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wan, Y.; Tao, Z.; Chen, H.; Zhou, R. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet. Microbiol. 2013, 162, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Osaki, M.; Sekizaki, T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 2001, 46, 140–148. [Google Scholar] [CrossRef]
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem-Resistant, Non-Carbapenemase-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2017, 62, e01970-17. [Google Scholar] [CrossRef]
- Starhof, C.; Winge, K.; Heegaard, N.H.H.; Skogstrand, K.; Friis, S.; Hejl, A. Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J. Neuroinflamm. 2018, 15, 305. [Google Scholar] [CrossRef]
- Oleg, T.; Arthur, J.O. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Huang, Y.; Wiradharma, N.; Xu, K.; Ji, Z.; Bi, S.; Li, L.; Yang, Y.Y.; Fan, W. Cationic amphiphilic alpha-helical peptides for the treatment of carbapenem-resistant Acinetobacter baumannii infection. Biomaterials 2012, 33, 8841–8847. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive oxygen species play a dominant role in all pathways of rapid quinolone-mediated killing. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zong, B.; Wang, X.; Zhu, Y.; Hu, L.; Li, P.; Zhang, A.; Chen, H.; Liu, M.; Tan, C. Fisetin Lowers Streptococcus suis serotype 2 Pathogenicity in Mice by Inhibiting the Hemolytic Activity of Suilysin. Front. Microbiol. 2018, 9, 1723. [Google Scholar] [CrossRef] [PubMed]



| Strain ID | Phenotypic Properties | Source | Auranofin (mg/L) |
|---|---|---|---|
| SC19 | Resistant to CLI, TET and LEV | China (Hu Bei) | 0.25 |
| S. suis (160413) | Resistant to CLI, TET, AMP and LEV | China (Hu Bei) | 0.125 |
| S. suis (16042) | Resistant to CLI, TET, AMP and LEV | China (Hu Nan) | 0.25 |
| S. suis (16091) | Resistant to TET, AMP and LEV and STX | China (Hu Bei) | 0.0625 |
| S. suis (16095) | Resistant to CLI, TET, AMP and STX | China (Guang Zhou) | 0.125 |
| S. suis (16072) | Resistant to CLI, TET, AMP, LEV and STX | China (Hu Bei) | 0.125 |
| S. suis (18051) | Resistant to CLI, TET, AMP and LEV | China (Hu Bei) | 0.125 |
| S. suis (180515) | Resistant to TET, AMP and LEV | China (Hu Bei) | 0.0625 |
| S. suis (170612) | Resistant to CLI, TET, AMP and STX | China (Hu Bei) | 0.125 |
| S. suis (170601) | Resistant to CLI, TET, AMP and LEV | China (Hu Bei) | 0.25 |
| S. suis (170603) | Resistant to TET, AMP, LEV and STX | China (Zhe Jiang) | 0.125 |
| S. pneumoniae (16035) | MDRSP | China (Shan Dong) | 0.0625 |
| S. pneumoniae (16076) | MDRSP | China (Shan Dong) | 0.125 |
| S. agalactiae (160205) | Beta-hemolytic, Serogroup: Group B | China (Shan Dong) | 0.125 |
| S. agalactiae (160503) | Beta-hemolytic, Serogroup: Group B | China (Shan Dong) | 0.0625 |
| S. aureus (160206) | VRSA | China (Shan Dong) | 0.125 |
| S. aureus (160408) | VRSA | China (Shan Dong) | 0.125 |
| pSET2-TrxB/SC19 | overexpressed strain | 1 |
| Treatment a | ALT (U/L) b | AST (U/L) b | Creatinine (μmol/L) | Urea Nitrogen (mmol/L) |
|---|---|---|---|---|
| Control | 42.52 ± 1.12 | 87.91 ± 1.78 | 44.12 ± 0.892 | 11.32 ± 1.68 |
| Auranofin (2.4 mg/L) | 41.21 ± 1.23 (p > 0.05) | 87.31 ± 1.52 (p > 0.05) | 44.34 ± 0.963 (p > 0.05) | 12.52 ± 1.25 (p > 0.05) |
| Primer | Sequence (5′-3′) | Remark |
|---|---|---|
| P1 | CCCAAGCTATCCAGGCTATGACCATATTTCA(HindIII) | TrxB protein expression recombination vector |
| P2 | CCGCTCCTATTCAGCTAGTTCTGTGATGTAG(XhoI) | |
| P3 | CGCGGACTATCCAGGCTATGACCATATTTCA(BamHI) | TrxB overexpression vector |
| P4 | CCGGAACTATTCAGCTAGTTCTGTGATGTAG(EcoRI) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Lu, W.; Zhu, Y.; Wang, C.; Shi, L.; Li, X.; Wu, Z.; Wang, G.; Dong, W.; Tan, C.; et al. Auranofin Has Advantages over First-Line Drugs in the Treatment of Severe Streptococcus suis Infections. Antibiotics 2021, 10, 26. https://doi.org/10.3390/antibiotics10010026
Lu H, Lu W, Zhu Y, Wang C, Shi L, Li X, Wu Z, Wang G, Dong W, Tan C, et al. Auranofin Has Advantages over First-Line Drugs in the Treatment of Severe Streptococcus suis Infections. Antibiotics. 2021; 10(1):26. https://doi.org/10.3390/antibiotics10010026
Chicago/Turabian StyleLu, Hao, Wenjia Lu, Yongwei Zhu, Chenchen Wang, Liming Shi, Xiaodan Li, Zhaoyuan Wu, Gaoyan Wang, Wenqi Dong, Chen Tan, and et al. 2021. "Auranofin Has Advantages over First-Line Drugs in the Treatment of Severe Streptococcus suis Infections" Antibiotics 10, no. 1: 26. https://doi.org/10.3390/antibiotics10010026
APA StyleLu, H., Lu, W., Zhu, Y., Wang, C., Shi, L., Li, X., Wu, Z., Wang, G., Dong, W., Tan, C., & Liu, M. (2021). Auranofin Has Advantages over First-Line Drugs in the Treatment of Severe Streptococcus suis Infections. Antibiotics, 10(1), 26. https://doi.org/10.3390/antibiotics10010026





