European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Treatment Use and Effectiveness
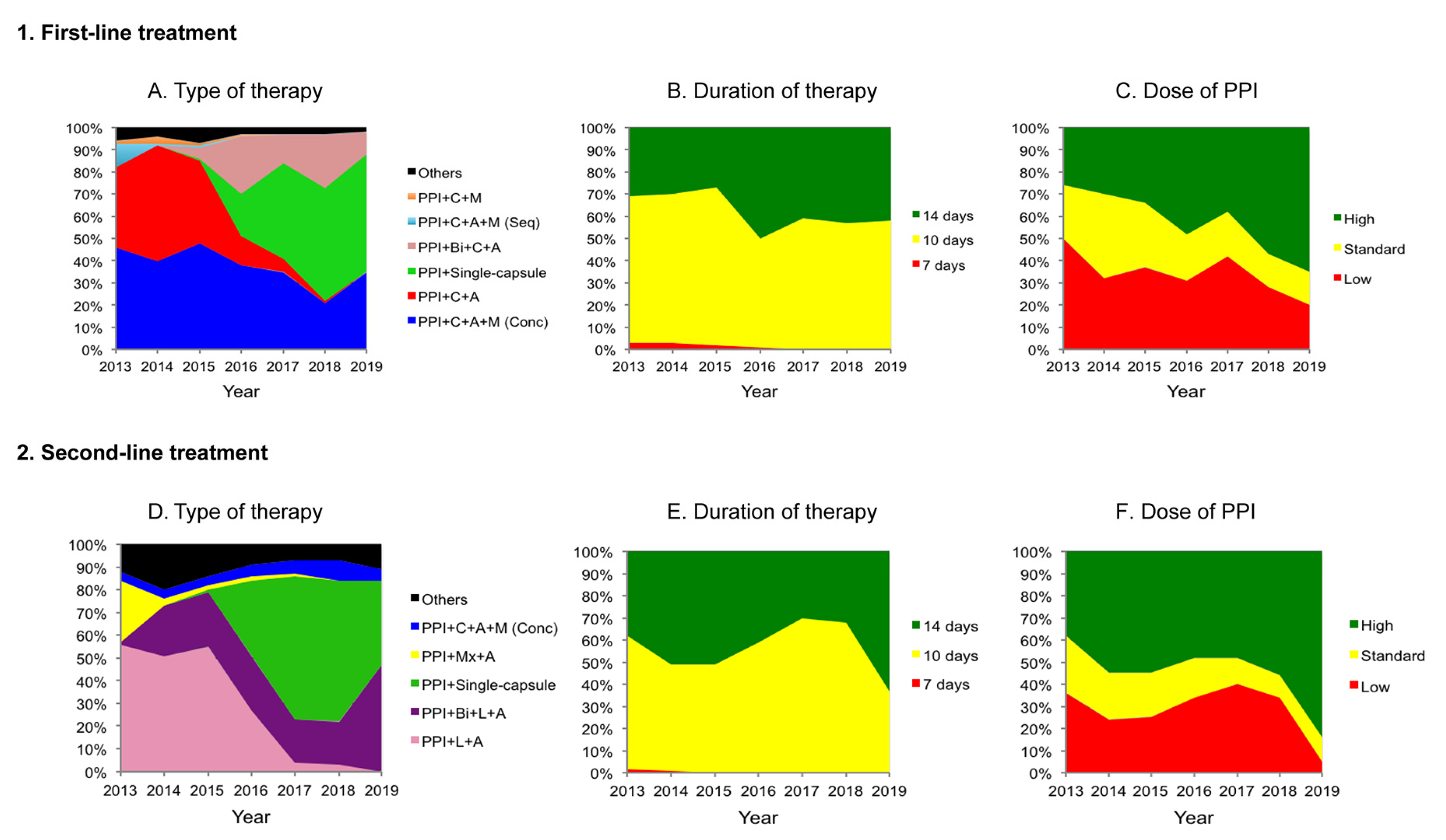
2.2.1. First-Line Treatment
2.2.2. Second-Line Treatment
2.3. Penicillin Allergic Patients
3. Discussion
4. Materials and Methods
4.1. Variables
4.2. Effectiveness Analysis
4.3. Statistical Analysis
4.4. Outcome Reporting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Pero, R.; Angrisano, T.; Brancaccio, M.; Falanga, A.; Lombardi, L.; Natale, F.; Laneri, S.; Lombardo, B.; Galdiero, S.; et al. Beta-defensins and analogs in Helicobacter pylori infections: mRNA expression levels, DNA methylation, and antibacterial activity. PLoS ONE 2019, 14, e0222295. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Yamaoka, Y. Therapy for Helicobacter pylori infection can be improved: Sequential therapy and beyond. Drugs 2008, 68, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Lee, Y.C.; Wu, M.S. Treatment of Helicobacter pylori infection and its long-term impacts on gut microbiota. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of Global Antibiotic Resistance. Prim. Care 2018, 45, 467–484. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Molina-Infante, J.; Amador, J.; Bermejo, F.; Bujanda, L.; Calvet, X.; Castro-Fernandez, M.; Cuadrado-Lavin, A.; Elizalde, J.I.; Gene, E.; et al. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol. Hepatol. 2016, 39, 697–721. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y.; Study Group Participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Gisbert, J.P. [Update on the efficacy of triple therapy for Helicobacter pylori infection and clarithromycin resistance rates in Spain (2007–2012)]. Gastroenterol. Hepatol. 2013, 36, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; McNicholl, A.G. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter 2017. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Calvet, X. Review article: The effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment. Pharmacol. Ther. 2011, 34, 1255–1268. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Calvet, X. Review article: Non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment. Pharmacol. Ther. 2011, 34, 604–617. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Romano, M.; Fernandez-Bermejo, M.; Federico, A.; Gravina, A.G.; Pozzati, L.; Garcia-Abadia, E.; Vinagre-Rodriguez, G.; Martinez-Alcala, C.; Hernandez-Alonso, M.; et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013, 145, 121–128. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Marin, A.C.; Molina-Infante, J.; Castro, M.; Barrio, J.; Ducons, J.; Calvet, X.; de la Coba, C.; Montoro, M.; Bory, F.; et al. Randomised clinical trial comparing sequential and concomitant therapies for Helicobacter pylori eradication in routine clinical practice. Gut 2014, 63, 244–249. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Bordin, D.S.; Lucendo, A.; Fadeenko, G.; Fernandez, M.C.; Voynovan, I.; Zakharova, N.V.; Sarsenbaeva, A.S.; Bujanda, L.; Perez-Aisa, A.; et al. Combination of Bismuth and Standard Triple Therapy Eradicates Helicobacter pylori Infection in More than 90% of Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 89–98. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Q.; Liang, X.; Liu, W.; Xiao, S.; Graham, D.Y.; Lu, H. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015, 64, 1715–1720. [Google Scholar] [CrossRef]
- Graham, D.Y.; Dore, M.P.; Lu, H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev. Anti-Infect. Ther. 2018, 16, 679–687. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.C.; Celinski, K.; Giguere, M.; Riviere, M.; Megraud, F.; Pylera Study, G. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Nyssen, O.P.; McNicholl, A.G.; Gisbert, J.P. Meta-analysis of three-in-one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter 2019, 24, e12570. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Hunt, R.H. Treatment after failure: The problem of “non-responders”. Gut 1999, 45 (Suppl. 1), I40–I44. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Lucendo, A.J.; Angueira, T.; Rodriguez-Tellez, M.; Perez-Aisa, A.; Balboa, A.; Barrio, J.; Martin-Noguerol, E.; Gomez-Rodriguez, B.J.; Botargues-Bote, J.M.; et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: The OPTRICON study. Aliment. Pharmacol. Ther. 2015, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lu, H.; Dore, M.P. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019, 24, e12554. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lee, S.Y. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol. Clin. N. Am. 2015, 44, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.C.; Nyssen, O.P.; McNicholl, A.G.; Gisbert, J.P. Efficacy and Safety of Quinolone-Containing Rescue Therapies After the Failure of Non-Bismuth Quadruple Treatments for Helicobacter pylori Eradication: Systematic Review and Meta-Analysis. Drugs 2017, 77, 765–776. [Google Scholar] [CrossRef]
- Tai, W.C.; Chiu, C.H.; Liang, C.M.; Chang, K.C.; Kuo, C.M.; Chiu, Y.C.; Wu, K.L.; Hu, M.L.; Chou, Y.P.; Chiou, S.S.; et al. Ten-Day versus 14-Day Levofloxacin-Containing Triple Therapy for Second-Line Anti-Helicobacter pylori Eradication in Taiwan. Gastroenterol. Res. Pract. 2013, 2013, 932478. [Google Scholar] [CrossRef]
- Kang, K.K.; Lee, D.H.; Oh, D.H.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N.; Jung, H.C. Helicobacter pylori eradication with moxifloxacin-containing therapy following failed first-line therapies in South Korea. World J. Gastroenterol. 2014, 20, 6932–6938. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Romano, M.; Molina-Infante, J.; Lucendo, A.J.; Medina, E.; Modolell, I.; Rodriguez-Tellez, M.; Gomez, B.; Barrio, J.; Perona, M.; et al. Two-week, high-dose proton pump inhibitor, moxifloxacin triple Helicobacter pylori therapy after failure of standard triple or non-bismuth quadruple treatments. Dig. Liver Dis. 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, Q.; Liang, X.; Zhang, W.; Sun, Q.; Liu, W.; Xiao, S.; Graham, D.Y.; Lu, H. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 2013, 18, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Romano, M.; Gravina, A.G.; Solis-Munoz, P.; Bermejo, F.; Molina-Infante, J.; Castro-Fernandez, M.; Ortuno, J.; Lucendo, A.J.; Herranz, M.; et al. Helicobacter pylori second-line rescue therapy with levofloxacin- and bismuth-containing quadruple therapy, after failure of standard triple or non-bismuth quadruple treatments. Aliment. Pharmacol. Ther. 2015, 41, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Barrio, J.; Modolell, I.; Molina-Infante, J.; Aisa, A.P.; Castro-Fernandez, M.; Rodrigo, L.; Cosme, A.; Gisbert, J.L.; Fernandez-Bermejo, M.; et al. Helicobacter pylori first-line and rescue treatments in the presence of penicillin allergy. Dig. Dis. Sci. 2015, 60, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Perez-Aisa, A.; Tepes, B.; Rodrigo-Saez, L.; Romero, P.M.; Lucendo, A.; Castro-Fernandez, M.; Phull, P.; Barrio, J.; Bujanda, L.; et al. Helicobacter pylori first-line and rescue treatments in patients allergic to penicillin: Experience from the European Registry on H pylori management (Hp-EuReg). Helicobacter 2020, 25, e12686. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; O’Morain, C.A.; Megraud, F.; Gisbert, J.P.; As Scientific Committee of the Hp-Eureg on Behalf of the National, C. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019, 24, e12630. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- McNicholl, A.G.; Gisbert, J.P. Research to the N-Power: The Strengths of Networked Clinical Collaboration in Spain. Am. J. Gastroenterol. 2017, 112, 1761–1764. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Glatt, S.; Fuhr, U.; Klotz, U.; Meineke, I.; Seufferlein, T.; Brockmoller, J. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur. J. Clin. Pharmacol. 2009, 65, 19–31. [Google Scholar] [CrossRef]
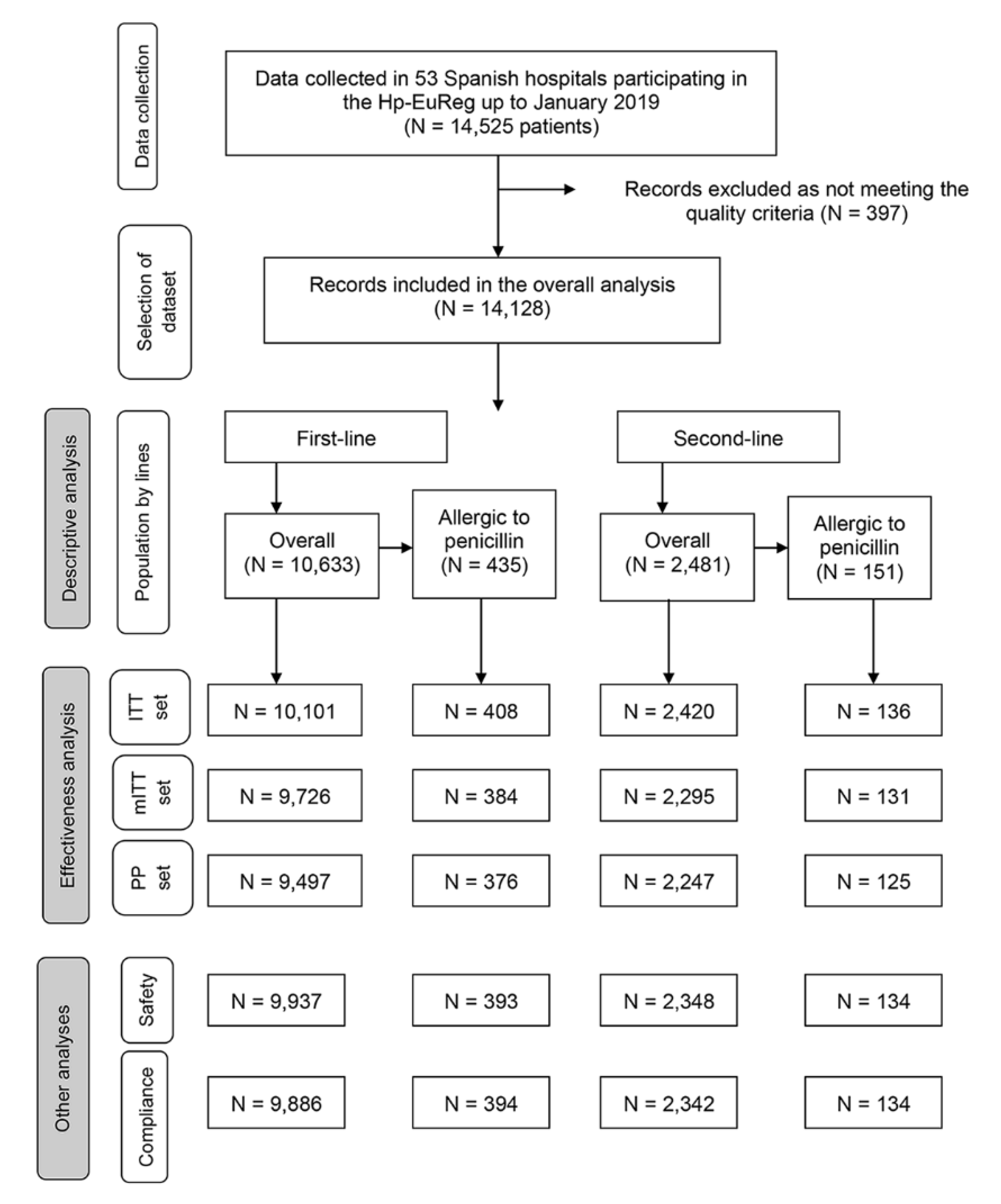
| Variables | Overall N (%) | 1st Line N (%) | 2nd Line N (%) | |
|---|---|---|---|---|
| 14,128 | 10,633 | 2481 | ||
| Gender | Female | 8795 (62) | 6477 (61) | 1632 (66) |
| Male | 5320 (38) | 4146 (39) | 847 (34) | |
| Age, mean (standard deviation) | 50 ± 15 | 51 ± 14.7 | 50 ± 14.5 | |
| Penicillin allergy | Presence | 644 (4.6) | 435 (4.1) | 151 (6.1) |
| Indication | Dyspepsia | 9152 (65) | 6823 (64) | 1656 (67) |
| Ulcer disease | 2103 (15) | 1554 (15) | 393 (16) | |
| Others | 2868 (20) | 2251 (21) | 432 (17) | |
| Diagnostic tests | Required endoscopy | 8180 (58) | 6672 (63) | 1061 (43) |
| Culture | Performed | NA | 366 (3.4) | NA |
| No resistance | 199 (54) | |||
| Clarithromycin R | 52 (14) | |||
| Metronidazole R | 93 (25) | |||
| Clarithromycin and metronidazole R | 18 (4.9) | |||
| Levofloxacin R | 63 (17) | |||
| Treatment length | 7 days | 182 (1.3) | 160 (1.5) | 17 (0.7) |
| 10 days | 8615 (61) | 6670 (63) | 1382 (56) | |
| 14 days | 5273 (37) | 3764 (35) | 1074 (43) | |
| Others | 58 (0.4) | 39 (0.4) | 8 (0.3) | |
| Proton pump inhibitor dose | Low | 5110 (37) | 4063 (39) | 751 (31) |
| Standard | 3290 (24) | 2605 (25) | 458 (19) | |
| High | 5496 (39) | 3834 (36) | 1239 (50) | |
| Compliance | No (<90% drug intake) | 415 (2.9) | 304 (3) | 69 (2.8) |
| Yes (≥90% drug intake) | 13,159 (93) | 9932 (93) | 2302 (92.8) | |
| Unknown | 554 (3.9) | 397 (4) | 110 (4.4) | |
| Effectiveness | Adverse Events | Compliance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ITT | mITT | PP | |||||||
| N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | |
| 1st line | 10,101 (83.5) | 83–84 | 9726 (88) | 88–89 | 9497 (89) | 88–89 | 9937 (25) | 25–26 | 9886 (97) | 96–97 |
| PPI + C + A + M (Conc) | 3996 (86) | 85–87 | 3880 (90) | 89–91 | 3781 (90) | 89–91 | 3963 (28) | 26–29 | 3942 (97) | 96–97 |
| PPI + C + A | 2712 (78) | 76–80 | 2544 (83) | 82–85 | 2498 (84) | 82–85 | 2617 (15) | 13–16 | 2598 (98) | 97–98 |
| PPI + Single–capsule | 1574 (88) | 86–89 | 1540 (95) | 94–96 | 1514 (96) | 95–97 | 1566 (25) | 23–27 | 1562 (97) | 96–98 |
| PPI + Bi + C + A | 1034 (88) | 86–90 | 1015 (91) | 89–93 | 1002 (91) | 89–93 | 1019 (40) | 37–44 | 1021 (98) | 98–99 |
| PPI + C + A + M (Seq) | 230 (79) | 73–84 | 222 (81.5) | 76–86 | 192 (84) | 79–89 | 230 (49) | 42–55 | 222 (86.5) | 81–91 |
| PPI + C + M | 124 (59) | 50–68 | 113 (65) | 55–73 | 112 (65) | 65–74 | 119 (16) | 10–24 | 118 (97.5) | 93–99 |
| 2nd line | 2420 (79) | 77–80 | 2295 (84) | 82–85 | 2247 (84) | 82–86 | 2348 (28) | 27–30 | 2342 (97) | 96–98 |
| PPI + L + A | 944 (74) | 71–77 | 893 (78.5) | 76–81 | 881 (79) | 76–82 | 919 (26) | 23–29 | 908 (99) | 98–99 |
| PPI + Bi + L + A | 463 (86) | 83–89 | 451 (89) | 86–92 | 435 (90) | 87–93 | 454 (33) | 28–37 | 459 (95) | 93–97 |
| PPI + Single–capsule | 443 (80) | 76–84 | 409 (88) | 85–91 | 398 (89) | 85–92 | 422 (31) | 27–36 | 420 (96) | 94–98 |
| PPI + Mx + A | 135 (87) | 80–92 | 129 (91) | 84–95 | 129 (91) | 84–95 | 134 (19) | 13–27 | 133 (99) | 96–100 |
| PPI + C + A + M (Conc) | 120 (74) | 65–82 | 110 (82) | 73–89 | 109 (82) | 73–88 | 112 (24) | 17–33 | 112 (98) | 94–100 |
| Variables | Overall | PPI + C + A + M (Conc) | PPI + C + A | PPI + Single-Capsule | PPI + Bi + C + A | PPI + C + A + M (Seq) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | ||
| Gender | [R: Female] | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Male | 1.22 (1.07–1.40) | 0.004 | 1.39 (1.11–1.75) | 0.004 | 1.26 (1.00–1.59) | 0.050 | 0.83 (0.51–1.34) | 0.442 | 1.10 (0.79–1.74) | 0.690 | 1.85 (0.84–4.04) | 0.125 | |
| Age (years) | [R: 18–30] | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 31–50 | 1.15 (0.92–1.44) | 0.232 | 1.32 (0.92–1.90) | 0.136 | 1.13 (0.77–1.65) | 0.530 | 0.73 (0.24–2.18) | 0.568 | 0.86 (0.38–1.95) | 0.723 | 0.33 (0.07–1.57) | 0.163 | |
| 51–70 | 1.08 (0.86–1.35) | 0.514 | 1.23 (0.86–1.77) | 0.265 | 1.10 (0.75–1.61) | 0.615 | 0.72 (0.24–2.12) | 0.547 | 0.82 (0.37–1.85) | 0.634 | 0.32 (0.07–1.62) | 0.170 | |
| ≥71 | 1.18 (0.87–1.59) | 0.289 | 1.29 (0.78–2.12) | 0.327 | 1.26 (0.76–2.08) | 0.369 | 0.70 (0.20–2.47) | 0.575 | 0.65 (0.24–1.79) | 0.406 | 0.23 (0.03–1.53) | 0.128 | |
| Presence of ulcer | [R: No] | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 1.23 (1.01–1.49) | 0.042 | 1.15 (0.83–1.60) | 0.389 | 1.48 (1.07–2.04) | 0.019 | 1.02 (0.50–2.10) | 0.950 | 3.12 (0.96–10.2) | 0.059 | 0.93 (0.36–2.41) | 0.875 | |
| Length (days) | [R: 7] | 1 | NA | 1 | NA † | NA | NA ‡ | ||||||
| 10 | 4.46 (3.20–6.23) | 0.000 | 1 | 2.78 (1.92–4.04) | 0.000 | 1 | |||||||
| 14 | 4.11 (2.88–5.87) | 0.000 | 1.28 (0.98–1.66) | 0.068 | 2.46 (1.60–3.77) | 0.000 | 0.71 (0.06–8.54) | 0.790 | |||||
| PPI dose of OE | [R: Low] | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Standard | 1.42 (1.21–1.66) | 0.000 | 0.98 (0.75–1.29) | 0.888 | 2.14 (1.67–2.74) | 0.000 | 1.50 (0.84–2.69) | 0.168 | 6.69 (1.35–33.3) | 0.020 | 1.99 (0.24–16.4) | 0.521 | |
| High | 2.05 (1.72–2.44) | 0.000 | 1.59 (1.24–2.03) | 0.000 | 3.54 (2.49–5.03) | 0.000 | 1.97 (1.08–3.59) | 0.027 | 2.26 (0.63–8.08) | 0.211 | 0.56 (0.22–1.49) | 0.244 | |
| Compliance | [R: <90% DI] | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| ≥90% DI | 4.07 (3.04–5.45) | 0.000 | 3.41 (2.14–5.43) | 0.000 | 7.12 (3.69–13.7) | 0.000 | 16 (6.99–36.6) | 0.000 | 1.78 (0.37–8.45) | 0.469 | 2.98 (1.28–6.94) | 0.011 | |
| Variables | Overall | PPI + L + A | PPI + Bi + L + A | PPI + Single-Capsule | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | OR (95% CI) | p-Values | ||
| Gender | [R: Female] | 1 | 1 | 1 | 1 | ||||
| Male | 1.39 (1.07–1.81) | 0.014 | 1.03 (0.70–1.50) | 0.898 | 2.83 (1.33–6.05) | 0.007 | 0.96 (0.47–1.93) | 0.898 | |
| Age | [R: 18–30] | 1 | 1 | 1 | 1 | ||||
| 31–50 | 0.60 (0.36–0.99) | 0.045 | 0.81 (0.39–1.70) | 0.582 | 0.64 (0.17–2.34) | 0.506 | 0.33 (0.07–1.52) | 0.156 | |
| 51–70 | 0.44 (0.27–0.73) | 0.001 | 0.47 (0.23–0.97) | 0.041 | 0.36 (0.10–1.29) | 0.118 | 0.38 (0.08–1.73) | 0.211 | |
| ≥71 | 0.37 (0.20–0.69) | 0.002 | 0.43 (0.18–1.03) | 0.059 | 0.24 (0.05–1.09) | 0.064 | 0.73 (0.09–5.82) | 0.766 | |
| Presence of ulcer | [R: No] | 1 | 1 | 1 | 1 | ||||
| Yes | 1.07 (0.74–1.54) | 0.729 | 1.04 (0.63–1.73) | 0.869 | 1.97 (0.44–8.76) | 0.374 | 0.79 (0.32–1.94) | 0.600 | |
| Previous C | [R: No] | 1 | 1 | 1 | NA † | ||||
| Yes | 0.63 (0.33–1.21) | 0.167 | 0.82 (0.16–4.12) | 0.809 | 0.21 (0.03–1.79) | 0.155 | |||
| Length | [R: 10] | 1 | 1 | 1 | |||||
| 14 | 1.51 (1.11–2.05) | 0.009 | 3.88 (2.24–6.71) | 0.000 | 3.12 (0.35–27.6) | 0.307 | 0.64 (0.07–5.93) | 0.692 | |
| PPI dose of OE | [R: Low] | 1 | 1 | 1 | 1 | ||||
| Standard | 1.21 (0.88–1.65) | 0.241 | 1.20 (0.81–1.79) | 0.360 | 2.50 (0.40–15.5) | 0.325 | 1.45 (0.56–3.77) | 0.443 | |
| High | 1.88 (1.37–2.59) | 0.000 | 1.66 (0.99–2.77) | 0.055 | 3.24 (1.09–9.59) | 0.034 | 1.43 (0.72–2.87) | 0.310 | |
| Compliance | [R: <90% DI] | 1 | 1 | 1 | 1 | ||||
| ≥90% DI | 3.43 (1.71–6.88) | 0.001 | 5.47 (1.27–23.5) | 0.023 | 3.01 (0.91–9.99) | 0.071 | 4.46 (1.02–19.5) | 0.047 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldas, M.; Pérez-Aisa, Á.; Castro-Fernández, M.; Bujanda, L.; Lucendo, A.J.; Rodrigo, L.; Huguet, J.M.; Pérez-Lasala, J.; Molina-Infante, J.; Barrio, J.; et al. European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics 2021, 10, 13. https://doi.org/10.3390/antibiotics10010013
Caldas M, Pérez-Aisa Á, Castro-Fernández M, Bujanda L, Lucendo AJ, Rodrigo L, Huguet JM, Pérez-Lasala J, Molina-Infante J, Barrio J, et al. European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics. 2021; 10(1):13. https://doi.org/10.3390/antibiotics10010013
Chicago/Turabian StyleCaldas, María, Ángeles Pérez-Aisa, Manuel Castro-Fernández, Luis Bujanda, Alfredo J. Lucendo, Luis Rodrigo, Jose M. Huguet, Jorge Pérez-Lasala, Javier Molina-Infante, Jesús Barrio, and et al. 2021. "European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain" Antibiotics 10, no. 1: 13. https://doi.org/10.3390/antibiotics10010013
APA StyleCaldas, M., Pérez-Aisa, Á., Castro-Fernández, M., Bujanda, L., Lucendo, A. J., Rodrigo, L., Huguet, J. M., Pérez-Lasala, J., Molina-Infante, J., Barrio, J., Fernández-Salazar, L., Lanas, Á., Perona, M., Domínguez-Cajal, M., Ortuño, J., Gómez-Rodríguez, B. J., Almela, P., Botargués, J. M., Núñez, Ó., ... Gisbert, J. P., on behalf of the Hp-EuReg Investigators. (2021). European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics, 10(1), 13. https://doi.org/10.3390/antibiotics10010013








