Plasmonic Detection of Glucose in Serum Based on Biocatalytic Shape-Altering of Gold Nanostars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AuNSs–Cys–GOx Bioconjugates
2.3. Characterizations and Instrumentations
2.4. Stability of AuNSs–Cys–GOx Bioconjugates
2.5. Enzyme Activity Assays
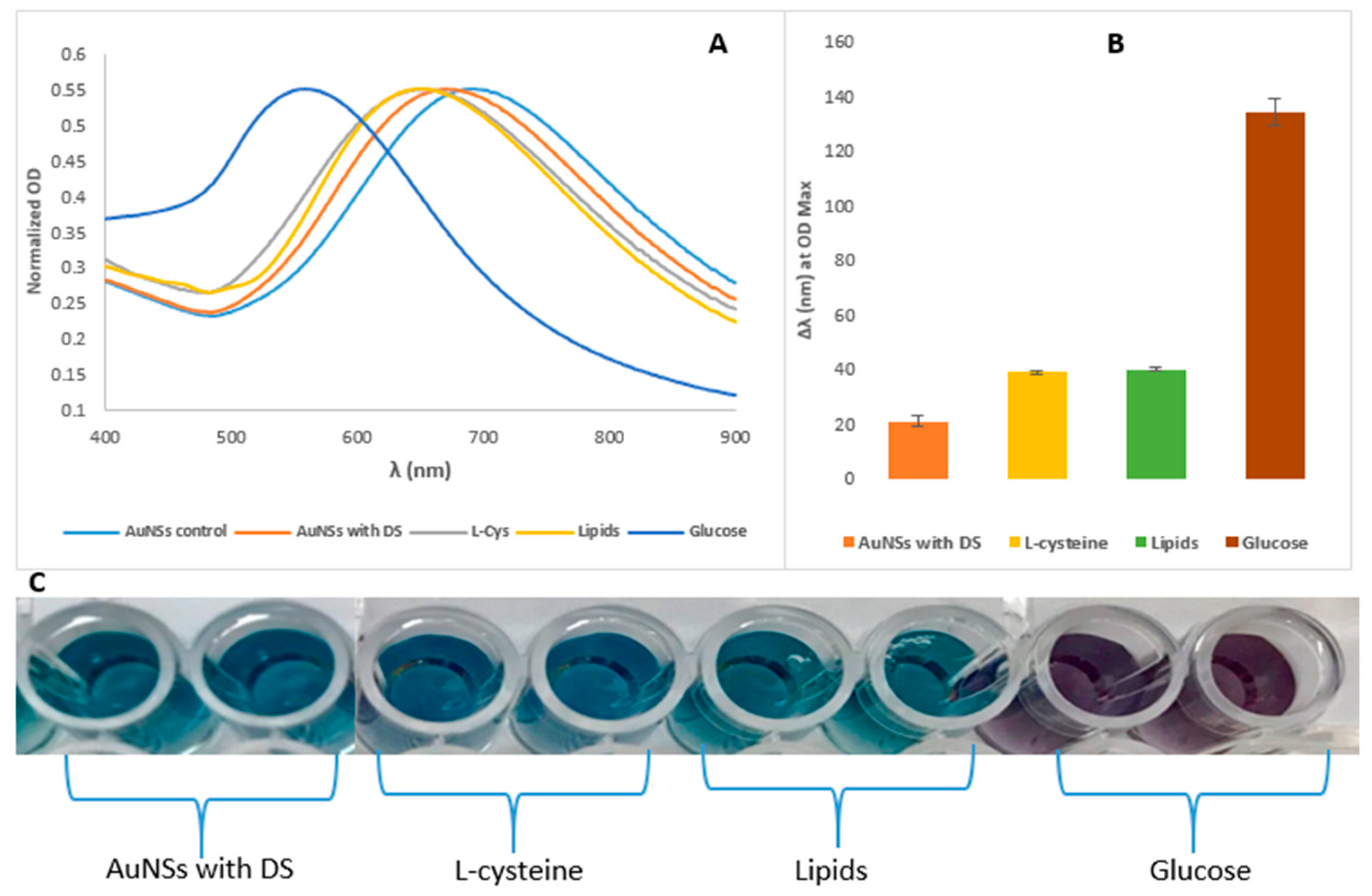
3. Results and Discussion
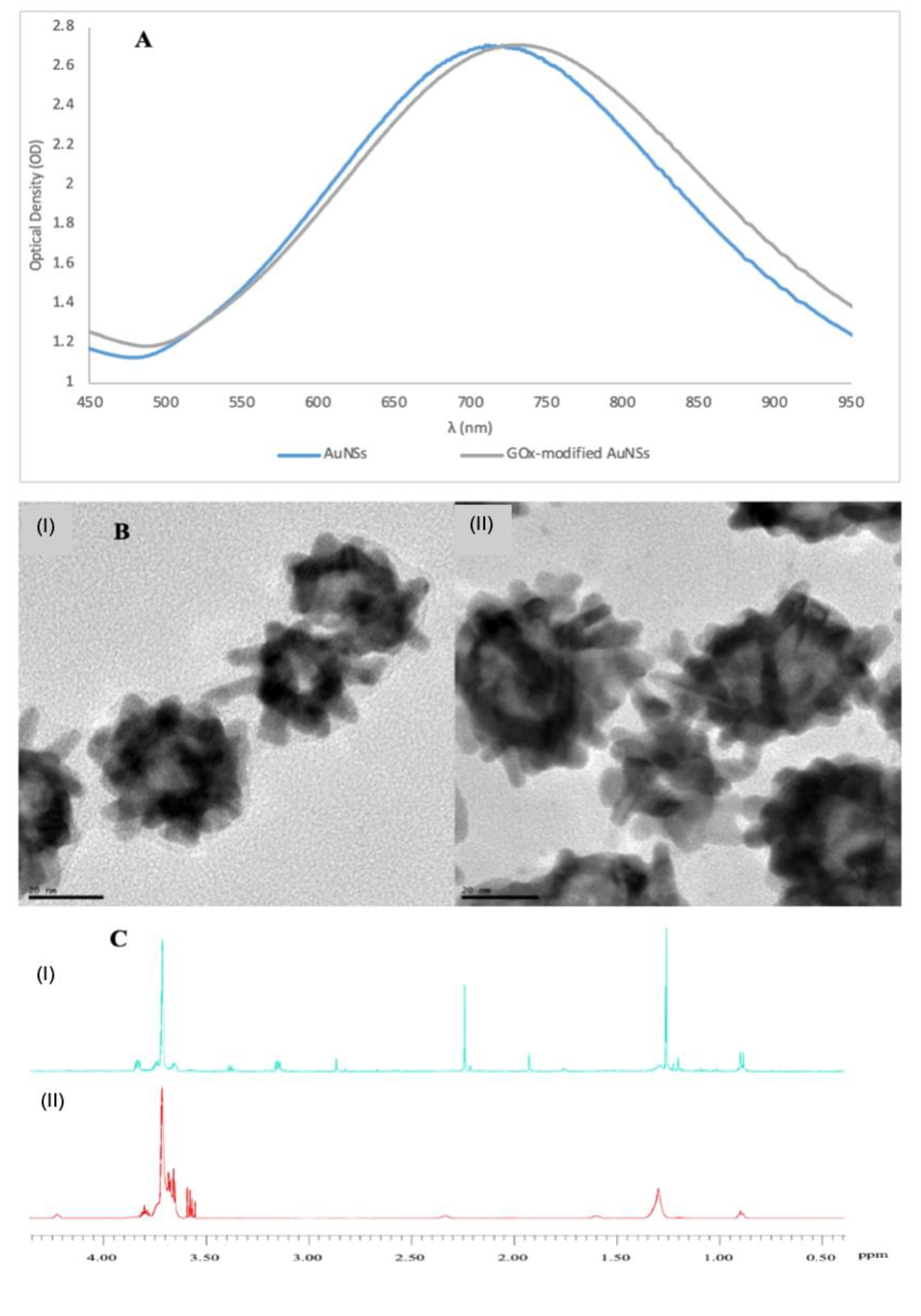
3.1. Characterisation of GOx-Modified AuNSs
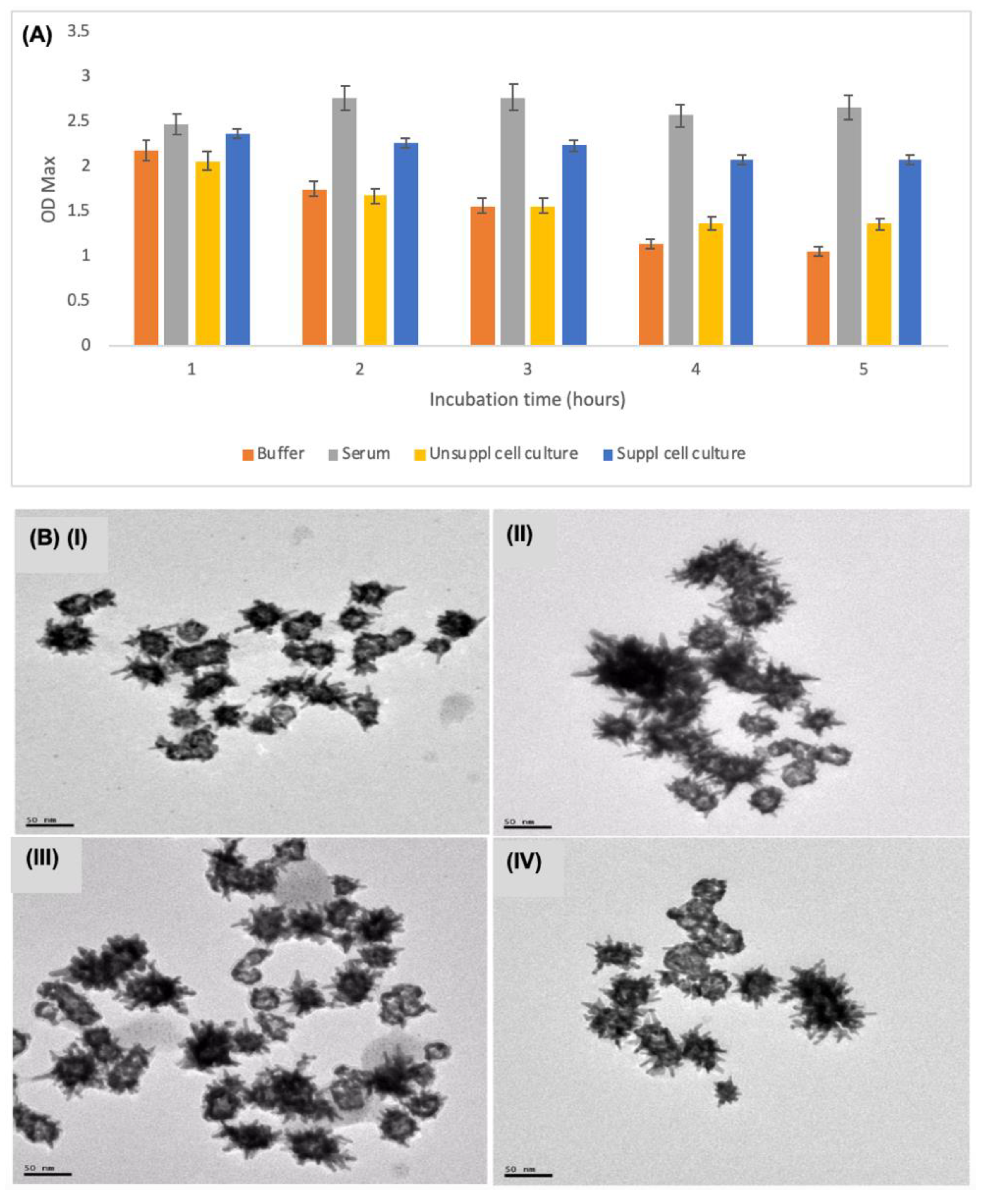
3.2. Stability and Characterisation of AuNSs–Cys–GOx Bioconjugates in Various Media
3.3. Optimisations of Plasmonic Glucose Detection Conditions in Serum
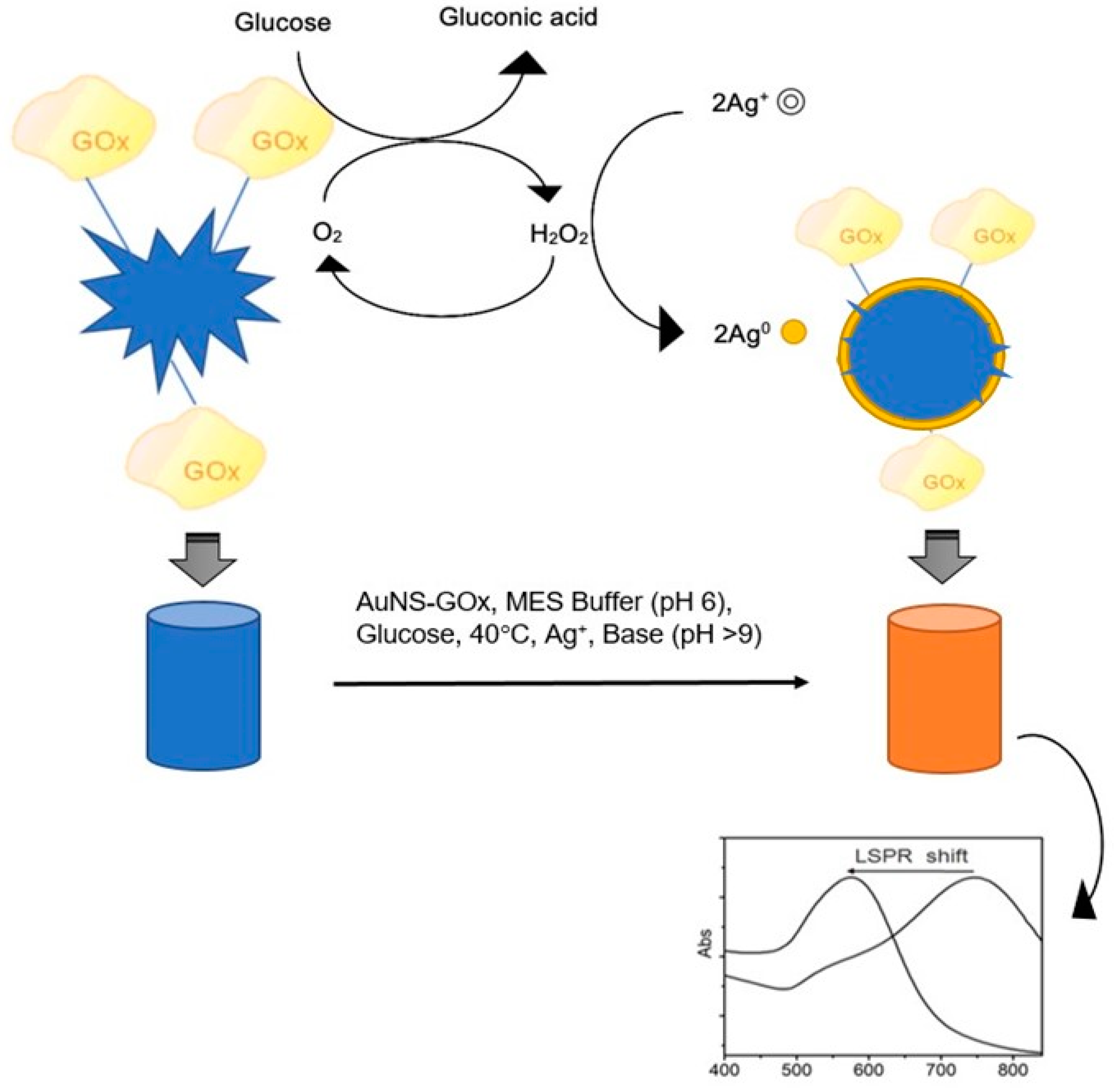
3.4. Plasmonic Glucose Detection by Means of AuNSs Shape-Altering
3.5. Analytical Performance of the Glucose Biosensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabet. Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Clark Jr, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3. [Google Scholar] [CrossRef]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. npj Flex. Electron. 2018, 2, 30. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef]
- Yi, Y.; Deng, J.; Zhang, Y.; Li, H.; Yao, S. Label-free Si quantum dots as photoluminescence probes for glucose detection. Chem. Commun. 2013, 49, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.-L.; Liu, Y.-H.; Deng, H.-H.; Hong, G.-L.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H.; Chen, W. Fluorescent hydrogen peroxide sensor based on cupric oxide nanoparticles and its application for glucose and l-lactate detection. Biosens. Bioelectron. 2014, 61, 374–378. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Zhang, B.; Sun, D.; Fu, C.; Xu, W.; Xu, S. Glucose oxidase probe as a surface-enhanced Raman scattering sensor for glucose. Anal. Bioanal. Chem. 2016, 408, 7513–7520. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y. Surface-enhanced raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- Radhakumary, C.; Sreenivasan, K. Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef]
- Xianyu, Y.; Jiang, X. Nanoscale materials and approaches for optical glucose assays. Curr. Opin. Chem. Eng. 2014, 4, 144–151. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. 2010, 122, 4910–4914. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Lu, C.-H.; Willner, I. Cysteine-mediated aggregation of Au nanoparticles: the development of a H2O2 sensor and oxidase-based biosensors. ACS Nano 2013, 7, 7278–7286. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Gkogkou, D.; Schreiber, B.; Shaykhutdinov, T.; Ly, H.K.; Kuhlmann, U.; Gernert, U.; Facsko, S.; Hildebrandt, P.; Esser, N.; Hinrichs, K. Polarization-and wavelength-dependent surface-enhanced Raman spectroscopy using optically anisotropic rippled substrates for sensing. ACS Sens. 2016, 1, 318–323. [Google Scholar] [CrossRef]
- Yonzon, C.R.; Haynes, C.L.; Zhang, X.; Walsh, J.T.; Van Duyne, R.P. A glucose biosensor based on surface-enhanced Raman scattering: improved partition layer, temporal stability, reversibility, and resistance to serum protein interference. Anal. Chem. 2004, 76, 78–85. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; Van Duyne, R.P. Toward a glucose biosensor based on surface-enhanced Raman scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Li, J.; Ju, H. A plasmonic colorimetric strategy for biosensing through enzyme guided growth of silver nanoparticles on gold nanostars. Biosens. Bioelectron. 2016, 78, 267–273. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.K.; Raphey, V.R.; Nivitha, K.P.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, in press. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Di, J. Colorimetric detection of glucose based on gold nanoparticles coupled with silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.; Van Nguyen, T.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huynh, C.D. A label-free colorimetric sensor based on silver nanoparticles directed to hydrogen peroxide and glucose. Arab. J. Chem. 2018, 11, 1134–1143. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; De La Rica, R.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willner, I.; Baron, R.; Willner, B. Growing metal nanoparticles by enzymes. Adv. Mater. 2006, 18, 1109–1120. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Rong, P.; Yang, J.; Wang, W.; Liu, D. A high-throughput colorimetric assay for glucose detection based on glucose oxidase-catalyzed enlargement of gold nanoparticles. Nanoscale 2015, 7, 15584–15588. [Google Scholar] [CrossRef] [PubMed]
- Zayats, M.; Baron, R.; Popov, I.; Willner, I. Biocatalytic growth of Au nanoparticles: from mechanistic aspects to biosensors design. Nano Lett. 2005, 5, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jackman, J.A.; Yang, H.-H.; Chen, P.; Cho, N.-J.; Kim, D.-H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.M.; Tel-Vered, R.; Yehezkeli, O.; Cheglakov, Z.; Willner, I. Biocatalytic Growth of Au Nanoparticles Immobilized on Glucose Oxidase Enhances the Ferrocene-Mediated Bioelectrocatalytic Oxidation of Glucose. Adv. Mater. 2008, 20, 2365–2370. [Google Scholar] [CrossRef]
- Xianyu, Y.; Wang, Z.; Jiang, X. A plasmonic nanosensor for immunoassay via enzyme-triggered click chemistry. ACS Nano 2014, 8, 12741–12747. [Google Scholar] [CrossRef]
- Tang, L.; Li, J. Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef]
- Langer, J.; Novikov, S.M.; Liz-Marzán, L.M. Sensing using plasmonic nanostructures and nanoparticles. Nanotechnology 2015, 26, 322001. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Najeeb, J.; Ali, M.A.; Aslam, M.F.; Raza, A. Biosensors: their fundamentals, designs, types and most recent impactful applications: A review. J. Biosens. Bioelectron. 2017, 8. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lai, G.; Fu, L.; Zhang, H.; Yu, A. Enzymatically catalytic deposition of gold nanoparticles by glucose oxidase-functionalized gold nanoprobe for ultrasensitive electrochemical immunoassay. Biosens. Bioelectron. 2015, 71, 353–358. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Ladenson, J.H.; Tsai, L.-M.B.; Michael, J.; Kessler, G.; Joist, J.H. Serum versus heparinized plasma for eighteen common chemistry tests: is serum the appropriate specimen? Am. J. Clin. Pathol. 1974, 62, 545–552. [Google Scholar] [CrossRef]
- Nader, R.; Andrea, R.H.; Carl T, W. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6 ed., 6th ed.; Elservier: New York, NY, USA, 2018. [Google Scholar]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Urban, D.A.; Rodriguez-Lorenzo, L.; Balog, S.; Kinnear, C.; Rothen-Rutishauser, B.; Petri-Fink, A. Plasmonic nanoparticles and their characterization in physiological fluids. Colloids Surf. B Biointerfaces 2016, 137, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [Green Version]
- Melby, E.S.; Lohse, S.E.; Park, J.E.; Vartanian, A.M.; Putans, R.A.; Abbott, H.B.; Hamers, R.J.; Murphy, C.J.; Pedersen, J.A. Cascading Effects of Nanoparticle Coatings: Surface Functionalization Dictates the Assemblage of Complexed Proteins and Subsequent Interaction with Model Cell Membranes. ACS Nano 2017, 11, 5489–5499. [Google Scholar] [CrossRef]
- Jenkins, S.V.; Qu, H.; Mudalige, T.; Ingle, T.M.; Wang, R.; Wang, F.; Howard, P.C.; Chen, J.; Zhang, Y. Rapid determination of plasmonic nanoparticle agglomeration status in blood. Biomaterials 2015, 51, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, V.; Kinnear, C.; Rodriguez-Lorenzo, L.; Monnier, C.A.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. In vitro dosimetry of agglomerates. Nanoscale 2014, 6, 7325–7331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bicer, E.M.; Morgan, A.B.; Pfeffer, P.E.; Monopoli, M.; Dawson, K.A.; Eriksson, J.; Edwards, K.; Lynham, S.; Arno, M. Enrichment of immunoregulatory proteins in the biomolecular corona of nanoparticles within human respiratory tract lining fluid. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical− chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Phiri, M.M.; Mulder, D.W.; Vorster, B.C. Seedless gold nanostars with seed-like advantages for biosensing applications. R. Soc. Open Sci. 2019, 6, 181971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phiri, M.M.; Mulder, D.W.; Mason, S.; Vorster, B.C. Facile immobilisation of glucose oxidase onto gold nanostars with enhanced binding affinity and optimal function. R. Soc. Open Sci. 2019, 6. [Google Scholar] [CrossRef]
- Venter, L.; Mienie, L.J.; van Rensburg, P.J.J.; Mason, S.; Vosloo, A.; Lindeque, J.Z. The cross-tissue metabolic response of abalone (Haliotis midae) to functional hypoxia. Biol. Open 2018, 7, bio031070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellinger, J.J.; Chylla, R.A.; Ulrich, E.L.; Markley, J.L. Databases and software for NMR-based metabolomics. Curr. Metab. 2013, 1, 28–40. [Google Scholar]
- Li, D.; He, Q.; Cui, Y.; Duan, L.; Li, J. Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability. Biochem. Biophys. Res. Commun. 2007, 355, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Leff, D.V.; Brandt, L.; Heath, J.R. Synthesis and characterization of hydrophobic, organically-soluble gold nanocrystals functionalized with primary amines. Langmuir 1996, 12, 4723–4730. [Google Scholar] [CrossRef]
- Patil, V.; Malvankar, R.; Sastry, M. Role of particle size in individual and competitive diffusion of carboxylic acid derivatized colloidal gold particles in thermally evaporated fatty amine films. Langmuir 1999, 15, 8197–8206. [Google Scholar] [CrossRef]
- Aryal, S.; Remant, B.; Dharmaraj, N.; Bhattarai, N.; Kim, C.H.; Kim, H.Y. Spectroscopic identification of SAu interaction in cysteine capped gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 160–163. [Google Scholar] [CrossRef]
- Hirsch, V.; Kinnear, C.; Moniatte, M.; Rothen-Rutishauser, B.; Clift, M.J.; Fink, A. Surface charge of polymer coated SPIONs influences the serum protein adsorption, colloidal stability and subsequent cell interaction in vitro. Nanoscale 2013, 5, 3723–3732. [Google Scholar] [CrossRef]
- Park, Y.I.; Im, H.; Weissleder, R.; Lee, H. Nanostar clustering improves the sensitivity of plasmonic assays. Bioconjug. Chem. 2015, 26, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
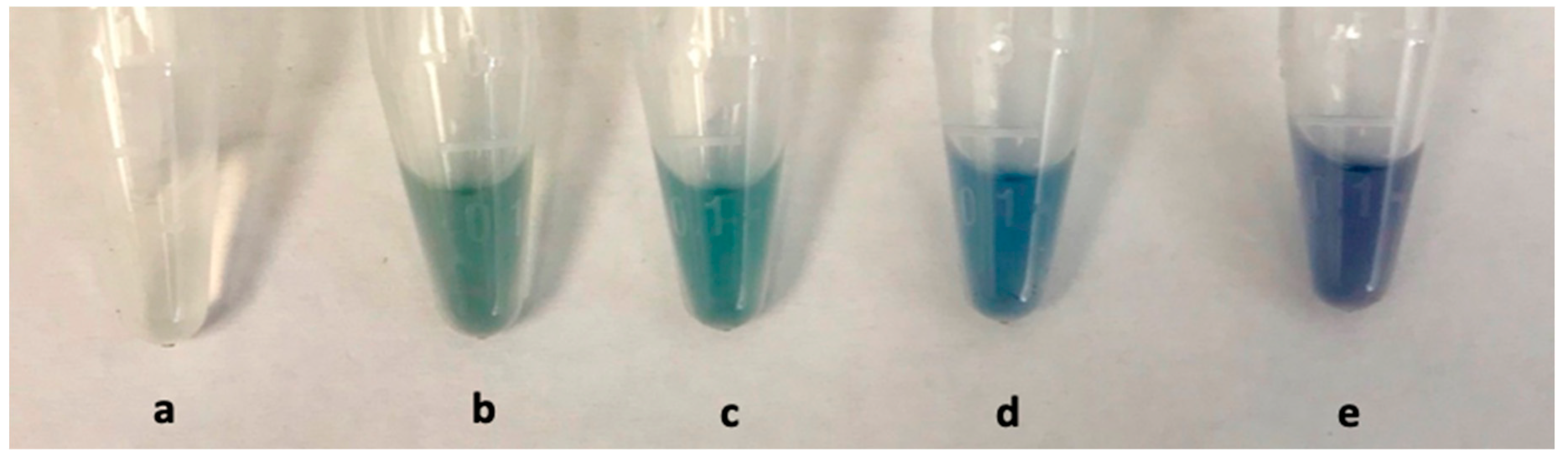
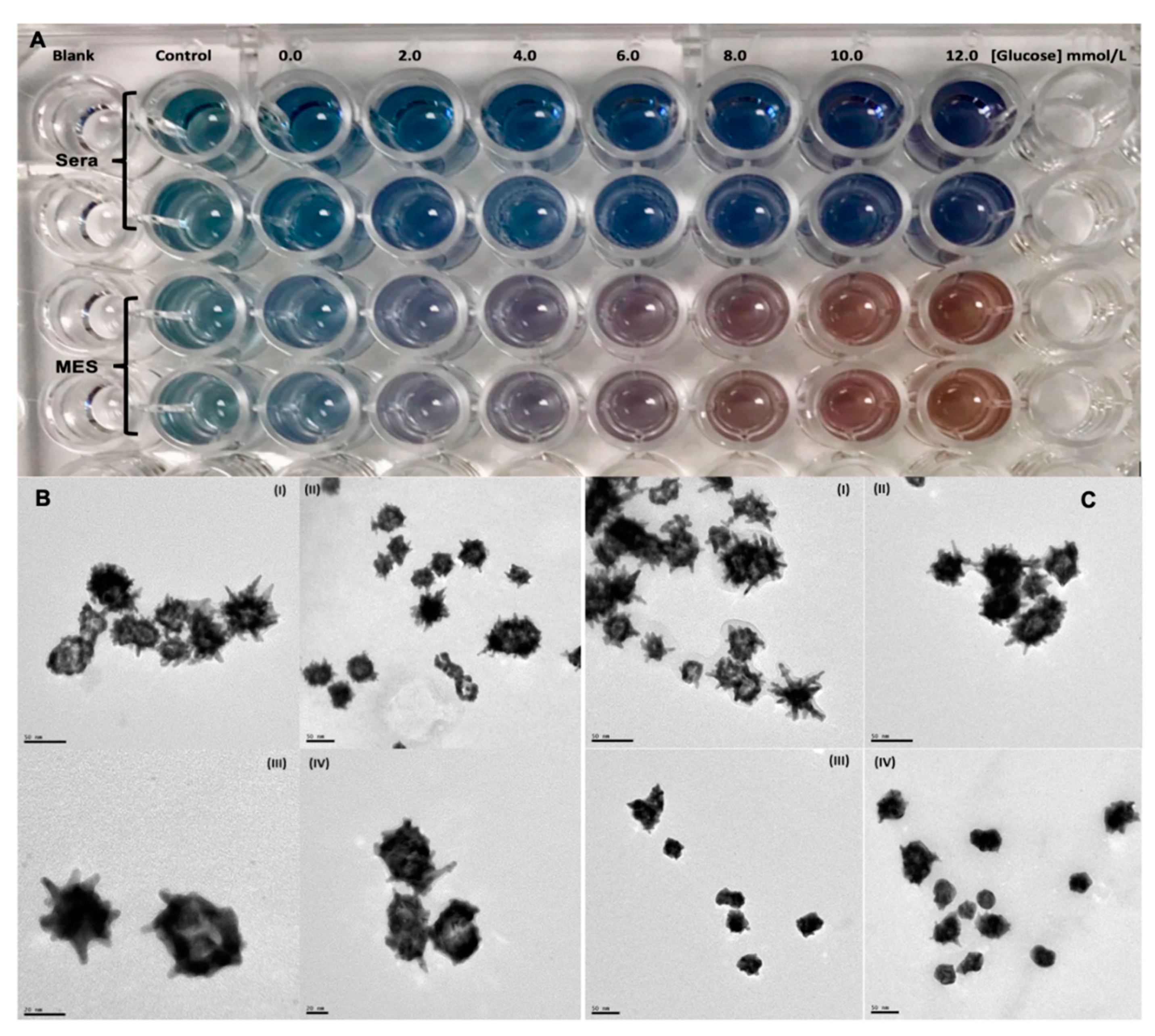

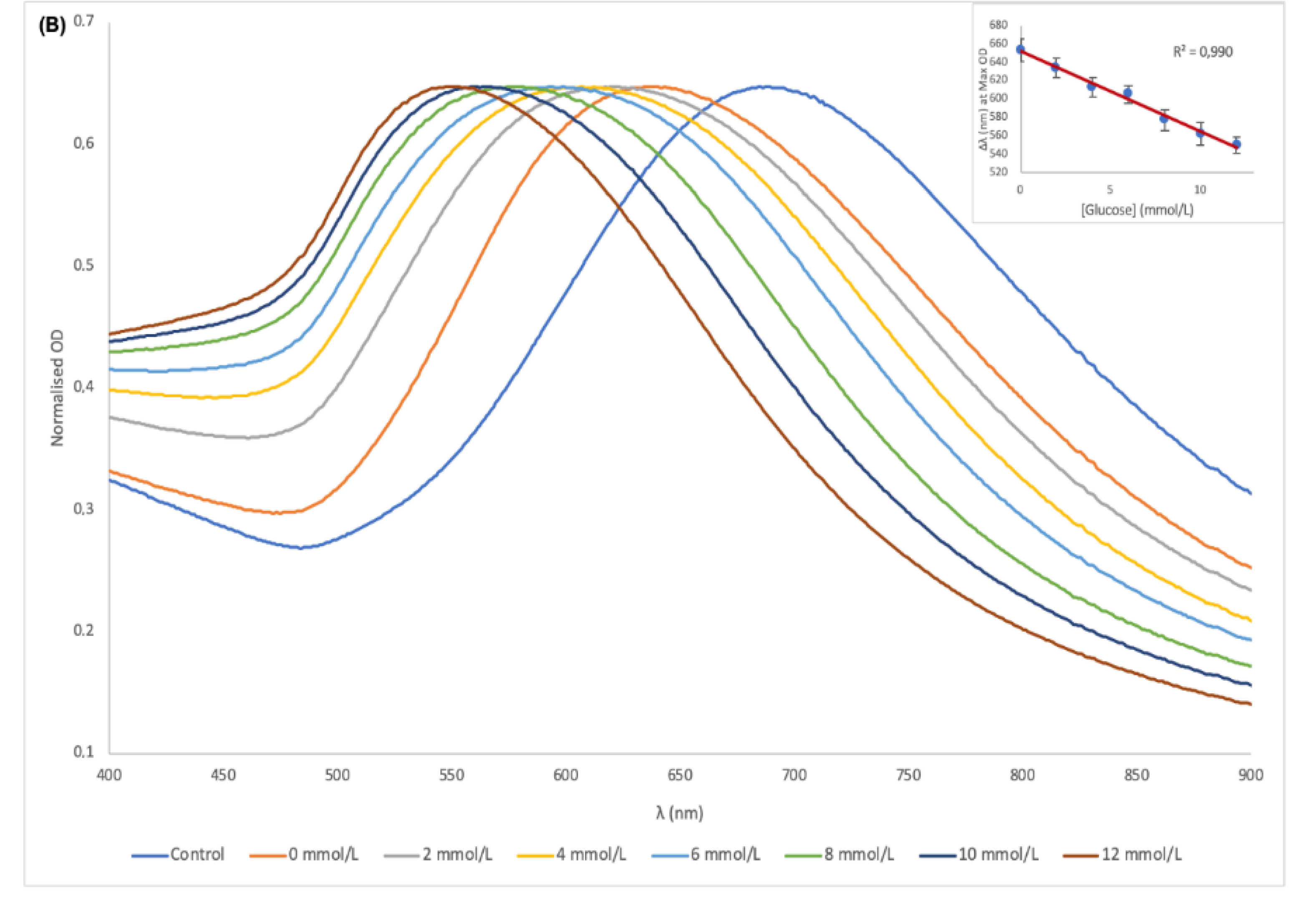
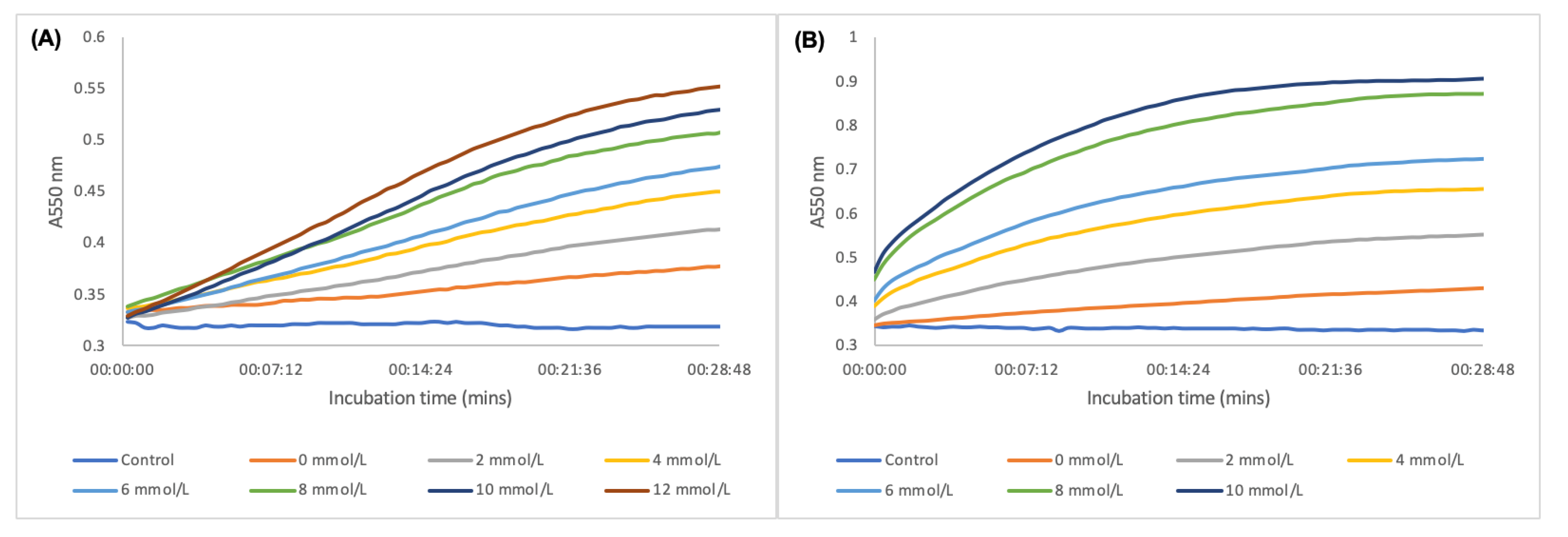
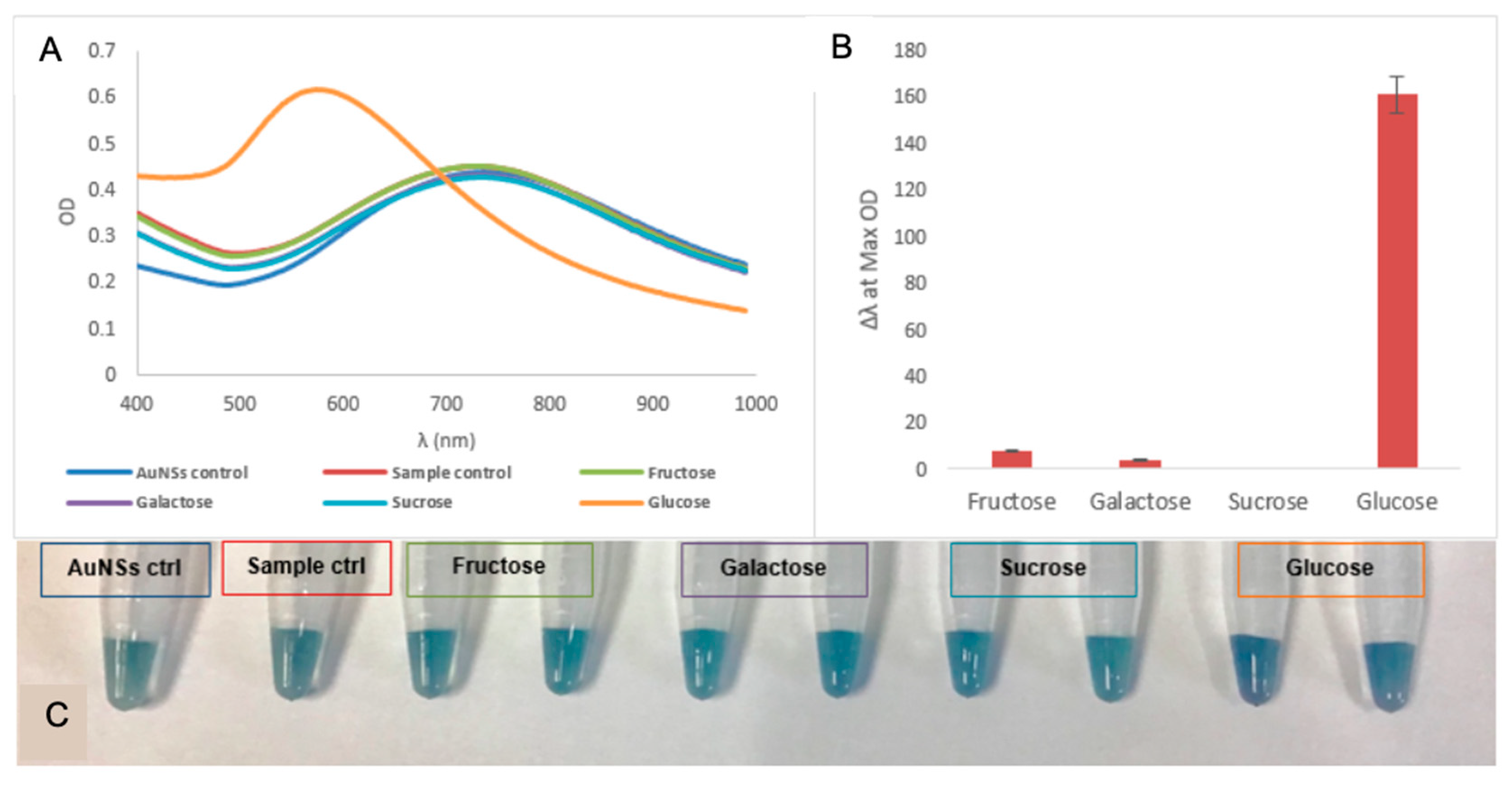
| Glucose | AuNSs (Control) | 0 mmol/L | 0.06 mmol/L | 0.12 mmol/L |
|---|---|---|---|---|
| Δd (nm) in serum | 57.49 ± 6.24 | 54.28 ± 6.79 | 54.15 ± 11.42 | 56.13 ± 9.08 |
| Δd (nm) in MES | 50.88 ± 8.23 | 50.31 ± 6.67 | 48.62 ± 3.57 | 50.14 ± 80.34 |
| Concentration (mmol/L) | λ (nm) at OD Max | Concentration Determined (mmol/L) | Recovery (%) |
|---|---|---|---|
| 0.04 | 630 | 0.039 ± 0.004 | 97 |
| 630 | |||
| 633 | |||
| 0.08 | 612 | 0.082 ± 0.006 | 102 |
| 616 | |||
| 612 | |||
| 0.12 | 598 | 0.119 | 99 |
| 598 | |||
| 598 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiri, M.M.; Mulder, D.W.; Vorster, B.C. Plasmonic Detection of Glucose in Serum Based on Biocatalytic Shape-Altering of Gold Nanostars. Biosensors 2019, 9, 83. https://doi.org/10.3390/bios9030083
Phiri MM, Mulder DW, Vorster BC. Plasmonic Detection of Glucose in Serum Based on Biocatalytic Shape-Altering of Gold Nanostars. Biosensors. 2019; 9(3):83. https://doi.org/10.3390/bios9030083
Chicago/Turabian StylePhiri, Masauso Moses, Danielle Wingrove Mulder, and Barend Christiaan Vorster. 2019. "Plasmonic Detection of Glucose in Serum Based on Biocatalytic Shape-Altering of Gold Nanostars" Biosensors 9, no. 3: 83. https://doi.org/10.3390/bios9030083
APA StylePhiri, M. M., Mulder, D. W., & Vorster, B. C. (2019). Plasmonic Detection of Glucose in Serum Based on Biocatalytic Shape-Altering of Gold Nanostars. Biosensors, 9(3), 83. https://doi.org/10.3390/bios9030083





