An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid
Abstract
:1. Introduction
2. Experimental
2.1. Apparatus
2.2. Chemicals and Reagents
2.3. Fabrication of Nanocomposite Electrode
2.4. Preparation of the MWCNT-TiO2NPs-HRP-GCE Enzyme Electrode
2.5. Electrochemical Measurements with the MWCNT-TiO2NPs-HRP-GCE
3. Results and Discussion
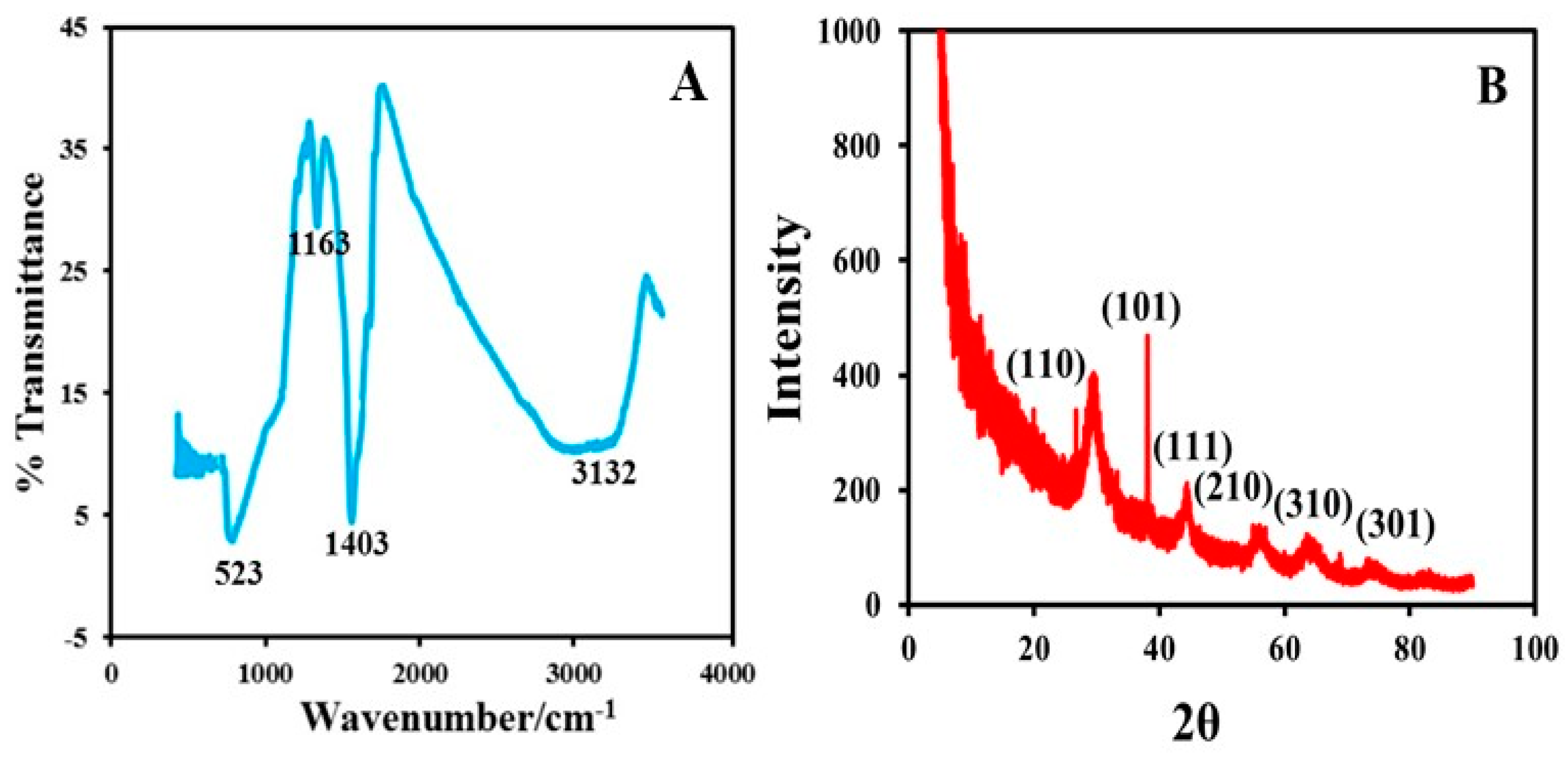
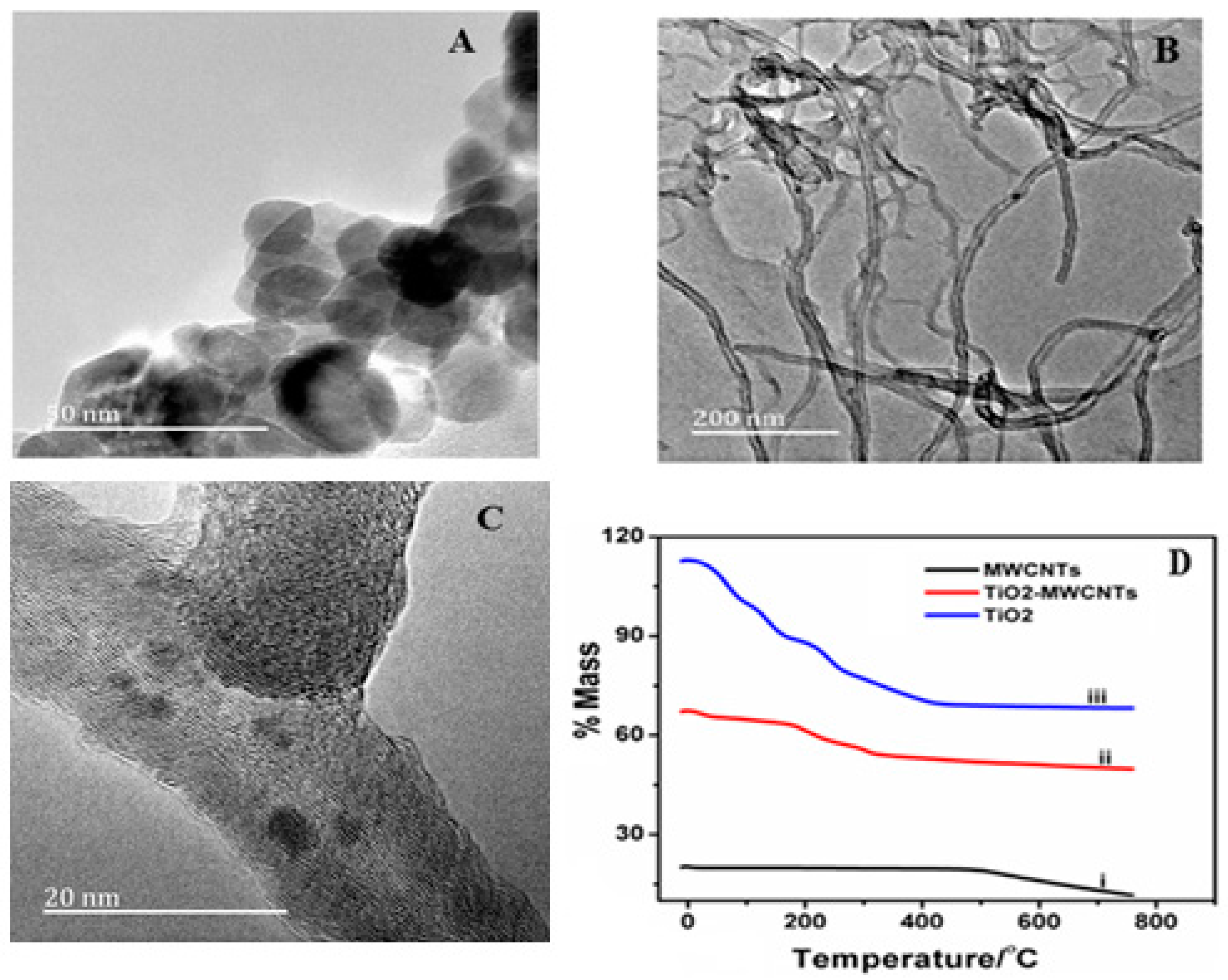
3.1. Characterization of the MWCNT-TiO2NPs-HRP-GCE
3.2. Method Optimization
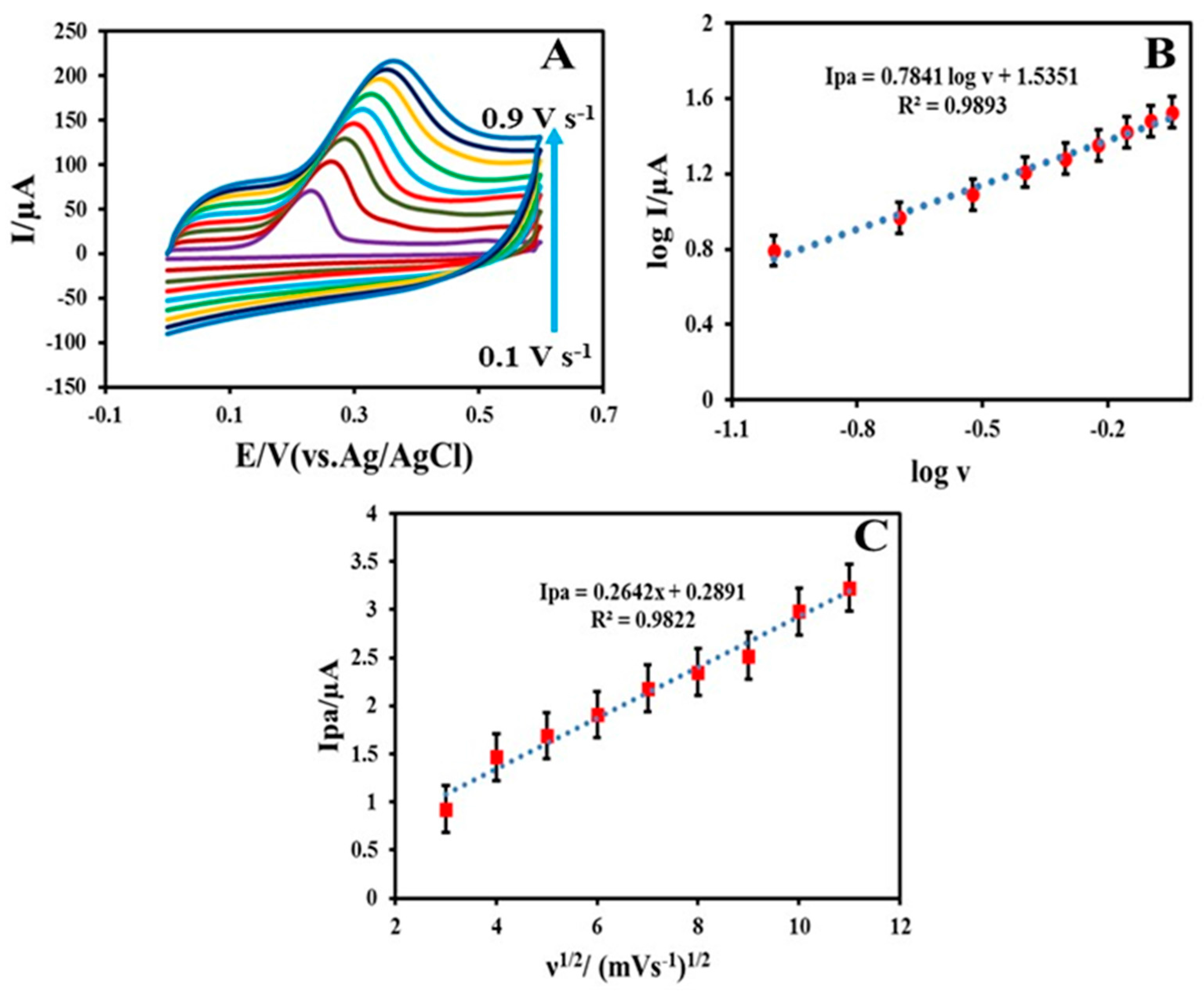
3.3. Electrochemical Behavior of INZ on the MWCNT-TiO2NPs-HRP-GCE
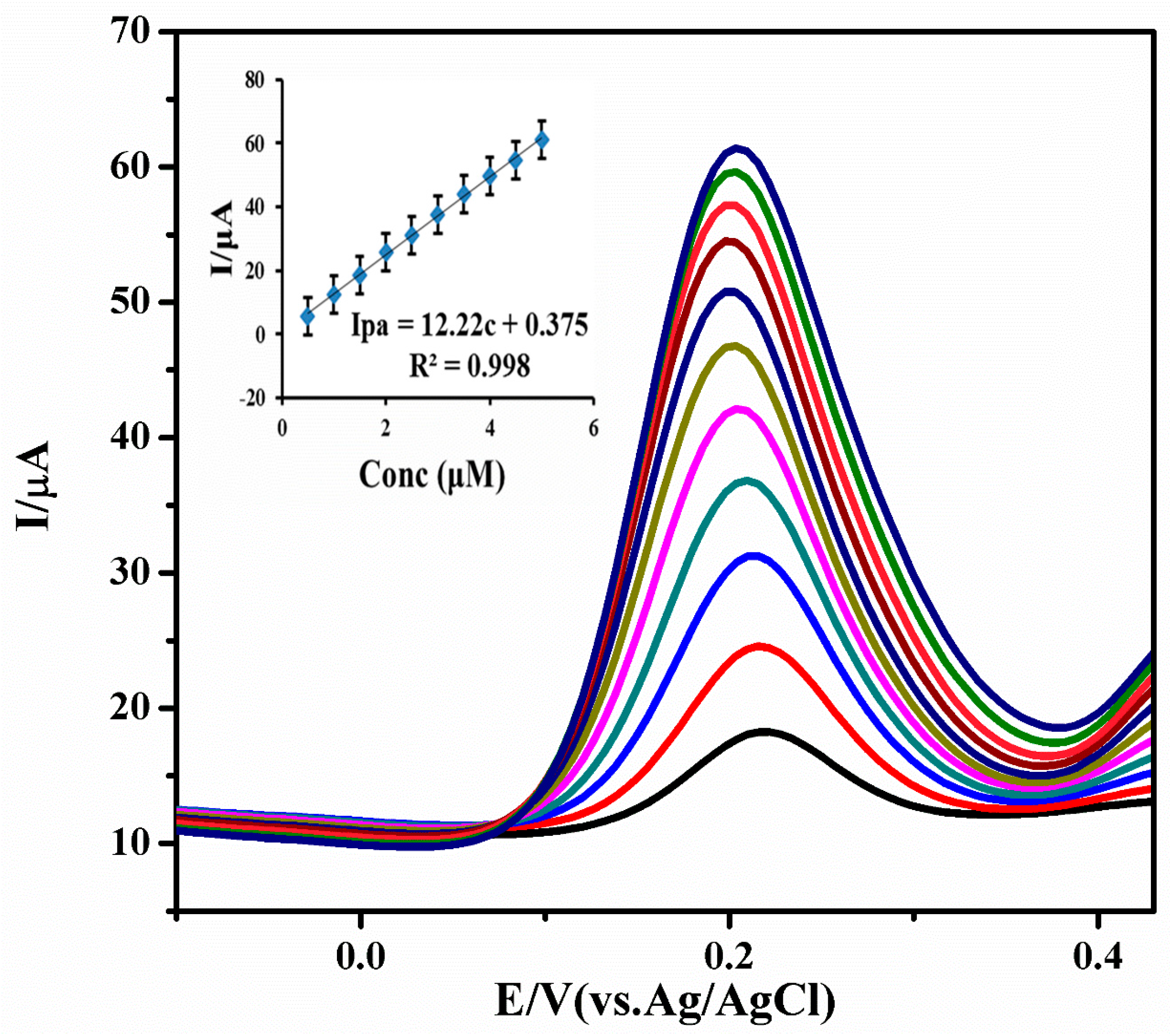
3.4. Quantitative Analysis of INZ
3.5. Repeatability and Stability
3.6. Interference Studies
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rastogi, P.K.; Ganesan, V.; Azad, U.P. Electrochemical determination of nanomolar levels of isoniazid in pharmaceutical formulation using silver nanoparticles decorated copolymer. Electrochim. Acta 2016, 188, 818–824. [Google Scholar] [CrossRef]
- Arshad, N.; Yunus, U.; Razzque, S.; Khan, M.; Saleem, S.; Mirza, B.; Rashid, N. Electrochemical and spectroscopic investigations of isoniazide and its analogs with ds.DNA at physiological pH: Evaluation of biological activities. Eur. J. Med. Chem. 2012, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Dressman, J.B.; Amidon, G.L.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Barends, D.M. International Pharmaceutical Federation, Biowaiver monographs for immediate release solid oral dosage forms: Isoniazid. J. Pharm. Sci. 2007, 96, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.F.; Cai, H.L.; Li, H.D.; Zhu, R.H.; Tan, Q.Y.; Gao, W.; Xu, P.; Liu, Y.P.; Zhang, W.Y.; Chen, Y.C.; et al. Simultaneous determination of isoniazid, rifampicin, levofloxacin in mouse tissues and plasma by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, W.; Tianyan, Y.; Fenglei, L.; Wang, E.; Dong, S. Detection of Hydrazine, Methyl hydrazine, and Isoniazid by Capillary Electrophoresis with a Palladium-Modified Micro disk Array Electrode. Anal. Chem. 1996, 68, 3350–3353. [Google Scholar] [CrossRef] [PubMed]
- Rui Lapa, A.S.; Jose Lima, L.F.C.; Joao Santos, L.M. Fluorimetric determination of isoniazid by oxidation with cerium(IV) in a multi commutated flow system. Anal. Chem. 2000, 419, 17–23. [Google Scholar]
- El-Brashy, A.M.; El-Ashry, S.M. Colorimetric and titrimetric assay of isoniazid. J. Pharm. Biomed. Anal. 1992, 10, 421–426. [Google Scholar] [CrossRef]
- Verma, K.K.; Palod, S. The Titrimetric Determination of 4-Pyridine Carboxylic Acid Hydrazide (Isoniazid) in Drug Formulations with Thallium (III). Anal. Lett. 1985, 18, 11–19. [Google Scholar] [CrossRef]
- Haghighi, B.; Bozorgzadeh, S. Flow injection chemiluminescence determination of isoniazid using luminol and silver nanoparticles. Microchem. J. 2010, 95, 192–197. [Google Scholar] [CrossRef]
- Miloglu, F.D.; Oznuluer, T.; Ozdurak, B.; Miloglu, E. Design and optimization of a new voltammetric method for determination of Isoniazid by using PEDOT modified gold electrode in pharmaceuticals. Iran. J. Pharm. 2016, 15, 65–73. [Google Scholar]
- Janin, Y.L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, N.S.K.; Kesavan, S.; John, S.A. Monitoring isoniazid level in human fluids in the presence of theophylline using gold platinum core shell nanoparticles modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 230, 157–166. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Asadian, E. Simultaneous voltammetric determination of ascorbic acid, acetaminophen and isoniazid using thionine immobilized multi-walled carbon nanotube modified carbon paste electrode. Electrochim. Acta 2010, 55, 666–672. [Google Scholar] [CrossRef]
- Szlosarczyk, M.; Piech, R.; Bator, B.P.; Maslanka, A.; Opoka1, W.; Krzek, J. Voltammetric determination of isoniazid using cyclic renewable mercury film silver based electrode. Pharm. Anal. Acta 2012, 3, 1–5. [Google Scholar]
- Regis, L.; Ferraz, B.; Roberto, F.; Leite, F.; Malagutti, A.R. Simultaneous determination of ethionamide and pyrazinamide using poly (l-cysteine) film-modified glassy carbon electrode. Talanta 2016, 154, 197–207. [Google Scholar]
- Shahrokhian, S.; Amiri, M. Multi-walled carbon nanotube paste electrode for selective voltammetric detection of isoniazid. Microchim. Acta 2006, 157, 149–158. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.Y.; Wang, H.H.; Huang, G.Q.; Li, M.M. Electrochemical sensor for Isoniazid based on the glassy carbon electrode modified with reduced graphene oxide-Au nanomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Dwivedi, A.K.; Singh, S. Estimation of ant tubercular drugs combination in pharmaceutical formulations using multivariate calibration. Anal. Chim. Acta 2005, 538, 345–353. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 96, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Trocino, S.; Donato, A.; Latino, M.; Donato, N.; Leonardi, S.G.; Neri, G. Pt-TiO2/MWCNTs Hybrid Composites for Monitoring Low Hydrogen Concentrations in Air. Sensors 2012, 12, 12361–12373. [Google Scholar] [CrossRef]
- Ahirwal, G.K.; Mitra, C.K. Direct Electrochemistry of Horseradish Peroxidase-Gold Nanoparticles Conjugate. Sensors 2009, 9, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Strushkevicha, N.; MacKenziea, F.; Cherkesovab, T.; Grabovecb, I.; Usanovb, S.; Park, H.-W. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. USA 2011, 108, 10139–10143. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.Y.; Lin, Y.C.; Tsai, R.Y.; Chen, H.C.; Liu, Y.C. A hydrogen peroxide sensor based on a horseradish peroxidase/polyaniline/carboxy-functionalized multiwalled carbon nanotube modified gold electrode. Electrochim. Acta 2011, 56, 9488–9495. [Google Scholar] [CrossRef]
- Azad, U.P.; Ganesan, V. Efficient electrocatalytic oxidation and selective determination of isoniazid by Fe32+ exchanged Nafion modified electrode. J. Solid State Electrochem. 2012, 16, 2907–2911. [Google Scholar] [CrossRef]
- Zeynali, K.A.; Shabangoli, Y.; Nejati, K. Electrochemical synthesis of Fe/Al-layered double hydroxide on a glassy carbon electrode: Application for electrocatalytic reduction of isoniazid. J. Iran. Chem. Soc. 2015, 13, 29–36. [Google Scholar] [CrossRef]
- You, T.; Niu, L.; Gui, J.Y.; Dong, S.; Wang, E. Detection of hydrazine, methyl hydrazine and isoniazid by capillary electrophoresis with a 4-pyridyl hydroquinone self-assembled micro disk platinum electrode. J. Pharm. Biomed. Anal. 1999, 19, 231–237. [Google Scholar] [CrossRef]
- Chen, W.C.; Unnikrishnan, B.; Chen, S.M. Electrochemical oxidation and amperometric determination of Isoniazid at functionalized multiwalled carbon nanotube modified electrode. Int. J. Electrochem. Sci. 2012, 7, 9138–9149. [Google Scholar]
- Devadas, B.; Cheemalapati, S.; Chen, S.M.; Ajmal Ali, M.; Al-Hemaid, F.M.A. Highly sensing graphene oxide/poly-arginine-modified electrode for the simultaneous electrochemical determination of buspirone, isoniazid and pyrazinamide drugs. Ionics 2015, 21, 547–555. [Google Scholar] [CrossRef]
- Bergamini, M.F.; Santos, D.P.; Zanoni, M.V. Determination of isoniazid in human urine using screen-printed carbon electrode modified with poly-L-histidine. Bioelectrochemistry 2010, 77, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.Y.; Hu, X.Y. Determination of Isoniazid Using a Gold Electrode by Differential Pulse Voltammetry. Anal. Lett. 2005, 38, 1405–1414. [Google Scholar]
- Absalan, G.; Akhond, M.; Soleimani, M.; Ershadifar, H. Efficient electrocatalytic oxidation and determination of isoniazid on carbon ionic liquid electrode modified with electrodeposited palladium nanoparticles. J. Electroanal. Chem. 2016, 761, 1–7. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Baradie, K.Y.; Tawfik, A. Electrochemical behaviour of the anti-tuberculosis drug isoniazid and its square-wave adsorptive stripping voltammetric estimation in bulk form, tablets and biological fluids at a mercury electrode. J. Pharm. Biomed. Anal. 2003, 33, 673–685. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Palanisamy, S.; Chen, S.M. Electrochemical determination of Isoniazid at electrochemically reduced graphene oxide modified electrode. Int. J. Electrochem. Sci. 2013, 8, 3953–3962. [Google Scholar]
- Cheemalapati, S.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M. Enhanced electrocatalytic oxidation of isoniazid at electrochemically modified rhodium electrode for biological and pharmaceutical analysis. Colloids Surf. B 2014, 121, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Azad, U.P.; Prajapati, N.; Ganesan, V. Selective determination of isoniazid using bentonite clay modified electrodes. Bioelectrochemistry 2015, 101, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.N.; Wang, H.L.; Li, Z.D.; Huang, Z.Q.; Qi, H.P.; Jiang, W.F. Fabrication of the novel core-shell MCM-41@mTiO2 composite microspheres with large specific surface area for enhanced photo catalytic degradation of di nitro butyl phenol (DNBP). Appl. Surf. Sci. 2016, 372, 108–115. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Ravichandran, K. Synthesis and characterization of TiO2 quantum dots for photo catalytic application. J. Saudi Chem. Soc. 2015, 19, 589–594. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase Characterization of TiO2 Powder by XRD and TEM. Kasetsart J. (Nat. Sci.) 2008, 42, 357–361. [Google Scholar]


| Electrode | Technique | Detection Limits/(μM) | Linear Range/(μM) | Buffer and pH | References |
|---|---|---|---|---|---|
| Nf/Fe/GCE a | CV | 13.00 | 50–20,000 | Na2SO4 (9) | [29] |
| LDH/GCE b | DPV | 4.00 | 4.9–770 | BR (9) | [30] |
| 4-pyridyl hydroquinone SAM/platinum electrode c | CV | 20.0 | - | PBS (7.2) | [31] |
| poly-L-histidine/SPE d | DPV | 0.50 | - | PBS (7) | [32] |
| Gold electrode e | DPV | 0.09 | - | NaOH (13.6) | [33] |
| PdNP/CPE f | CV | 0.47 | - | PBS (7) | [27] |
| Hanging mercury drop electrode g | SWADCS | 1.18 | - | BR (5.5) | [34] |
| GO/GCE h | LSV | 0.17 | 2–70 | PBS (7) | [35] |
| F-MWCNT/GCE i | CV | 0.27 | 1–70 | AB (4) | [36] |
| Rh/GCE j | CV | 13.00 | 70–130 | PBS (7) | [37] |
| Bentonite clay/GCE k | LSV | 0.80 | - | Na2SO4 (13.5) | [38] |
| MWCNT-TiO2NPs-HRP-GCE | DPV | 0.03 | 0.5–5 | PBS (7) | Present work |
| Interferents | Concentration/(µM) | Signal Change (%) |
|---|---|---|
| Ascorbic acid | 250 | 4.03 |
| Uric acid | 250 | 1.24 |
| Glucose | 500 | 0.34 |
| Fe3+ | 500 | 0.81 |
| Al3+ | 500 | 1.27 |
| Cl− | 500 | 0.67 |
| Na+ | 500 | 2.01 |
| K+ | 500 | 1.35 |
| Declared Amount Tablet (mg) | Found (mg) | Recovery (%) | Relative Standard Deviation (RSD) (%) | Added (mg) | Found (mg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Sample 1 (100 mg) | 99.2 | 99.2 | 1.69 | 20 | 19.3 | 96.5 | 0.32 |
| Sample 2 (100 mg) | 98.9 | 98.9 | 1.98. | 10 | 9.5 | 95.0 | 0.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokkareddy, R.; Bhajanthri, N.K.; Redhi, G.G. An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid. Biosensors 2017, 7, 21. https://doi.org/10.3390/bios7020021
Chokkareddy R, Bhajanthri NK, Redhi GG. An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid. Biosensors. 2017; 7(2):21. https://doi.org/10.3390/bios7020021
Chicago/Turabian StyleChokkareddy, Rajasekhar, Natesh Kumar Bhajanthri, and Gan G. Redhi. 2017. "An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid" Biosensors 7, no. 2: 21. https://doi.org/10.3390/bios7020021
APA StyleChokkareddy, R., Bhajanthri, N. K., & Redhi, G. G. (2017). An Enzyme-Induced Novel Biosensor for the Sensitive Electrochemical Determination of Isoniazid. Biosensors, 7(2), 21. https://doi.org/10.3390/bios7020021





