A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
2.2. Recombinant Protein Preparation
2.3. Disc Preparation and Electrochemical Measurements
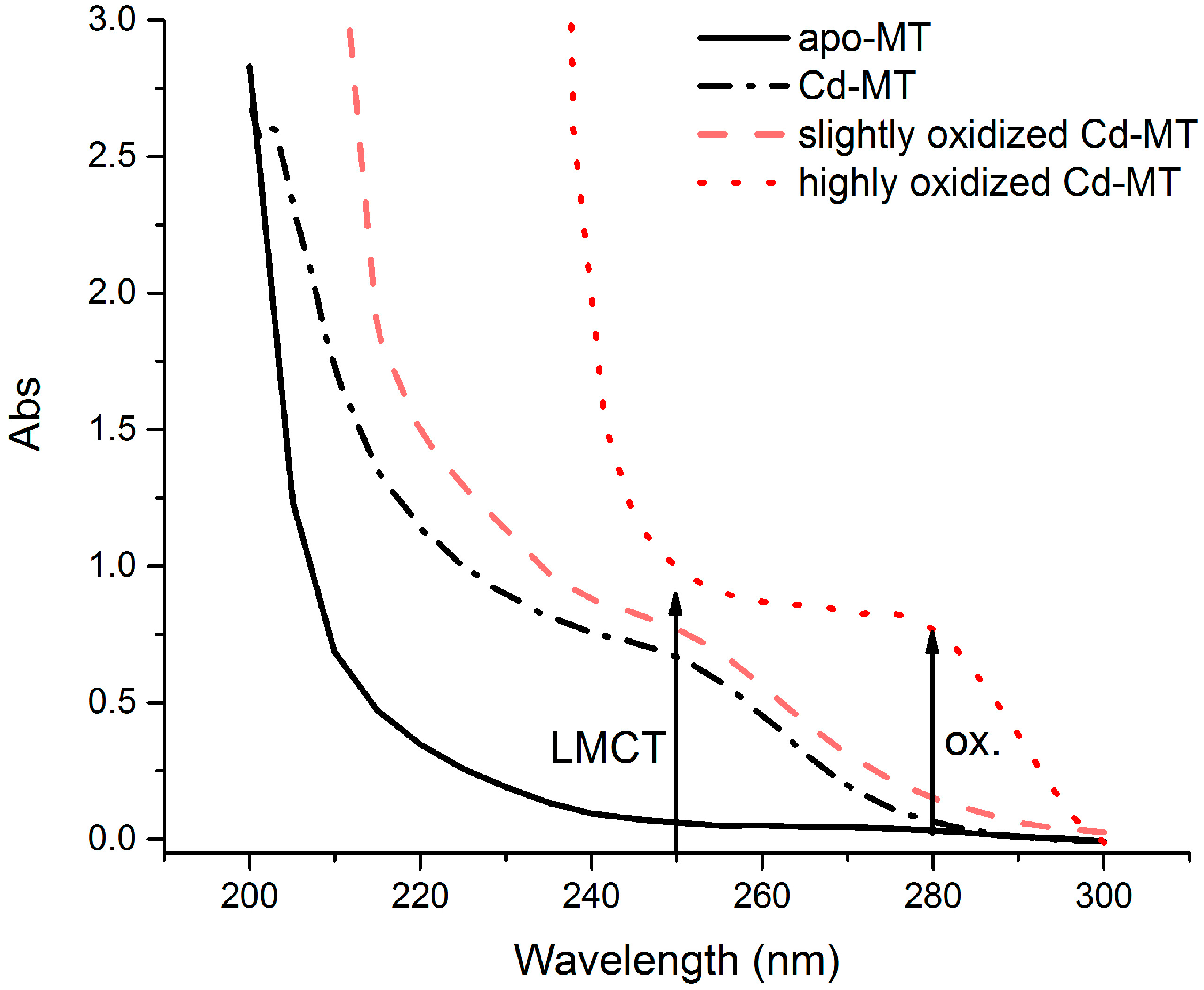
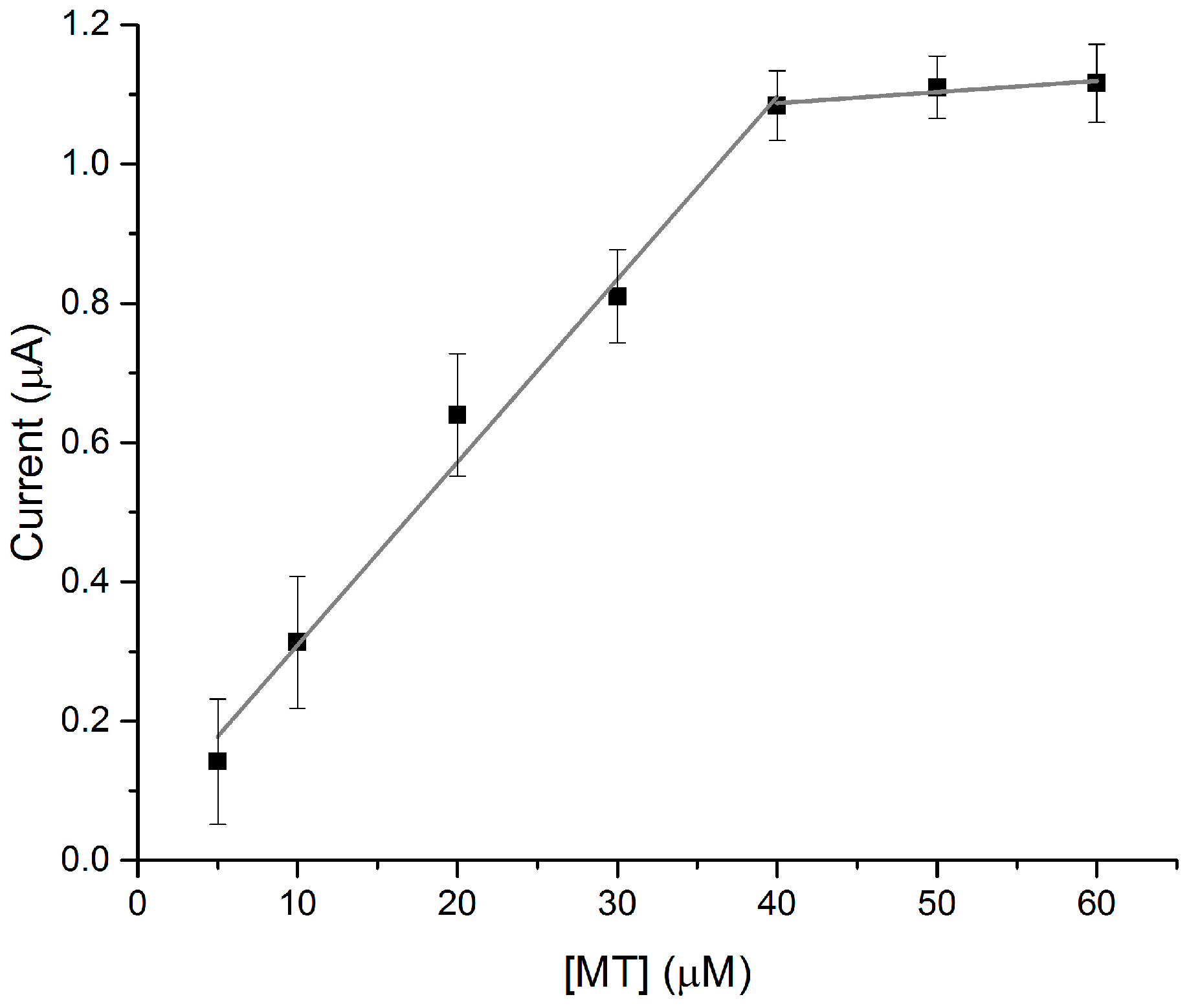
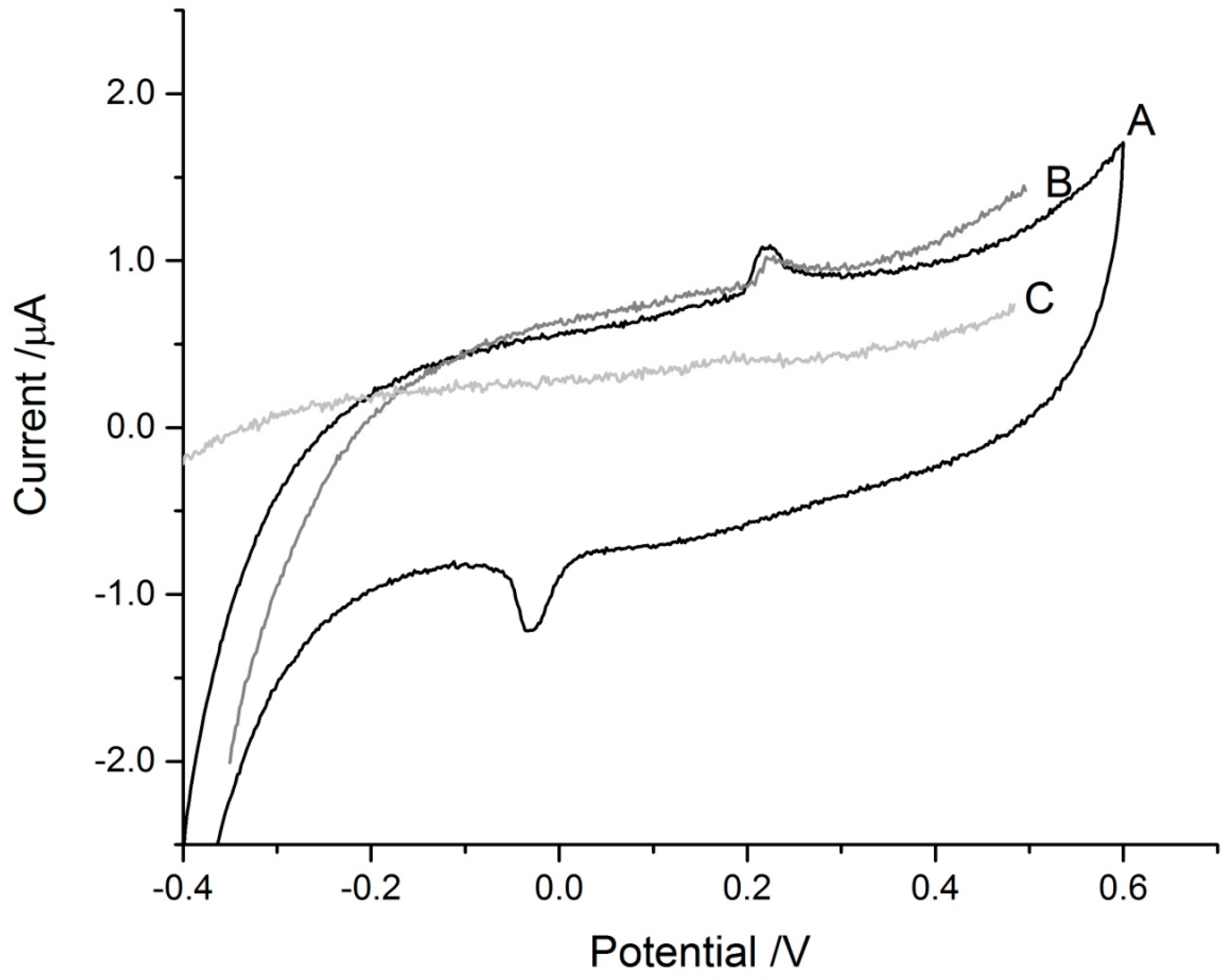
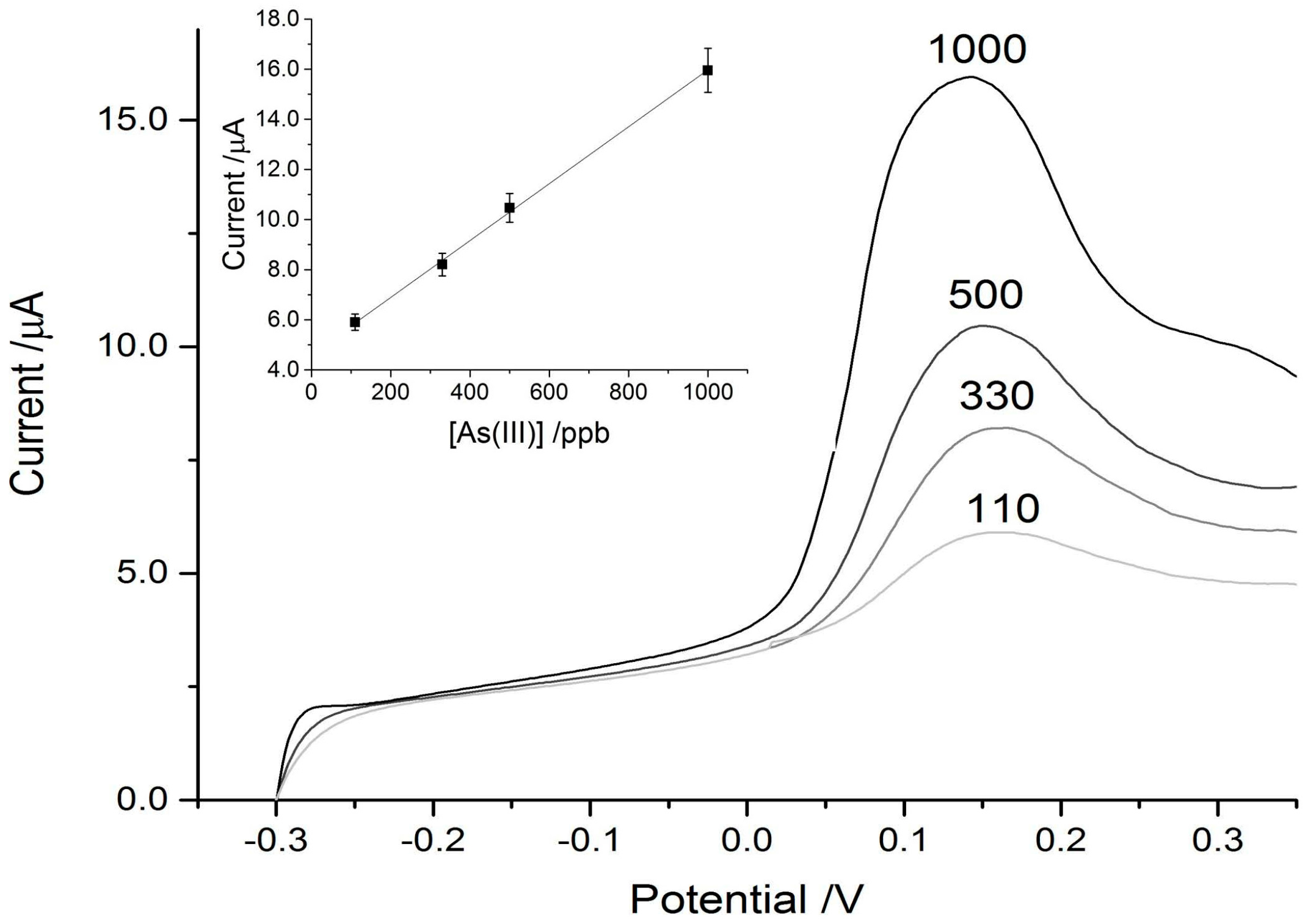
3. Results and Discussion
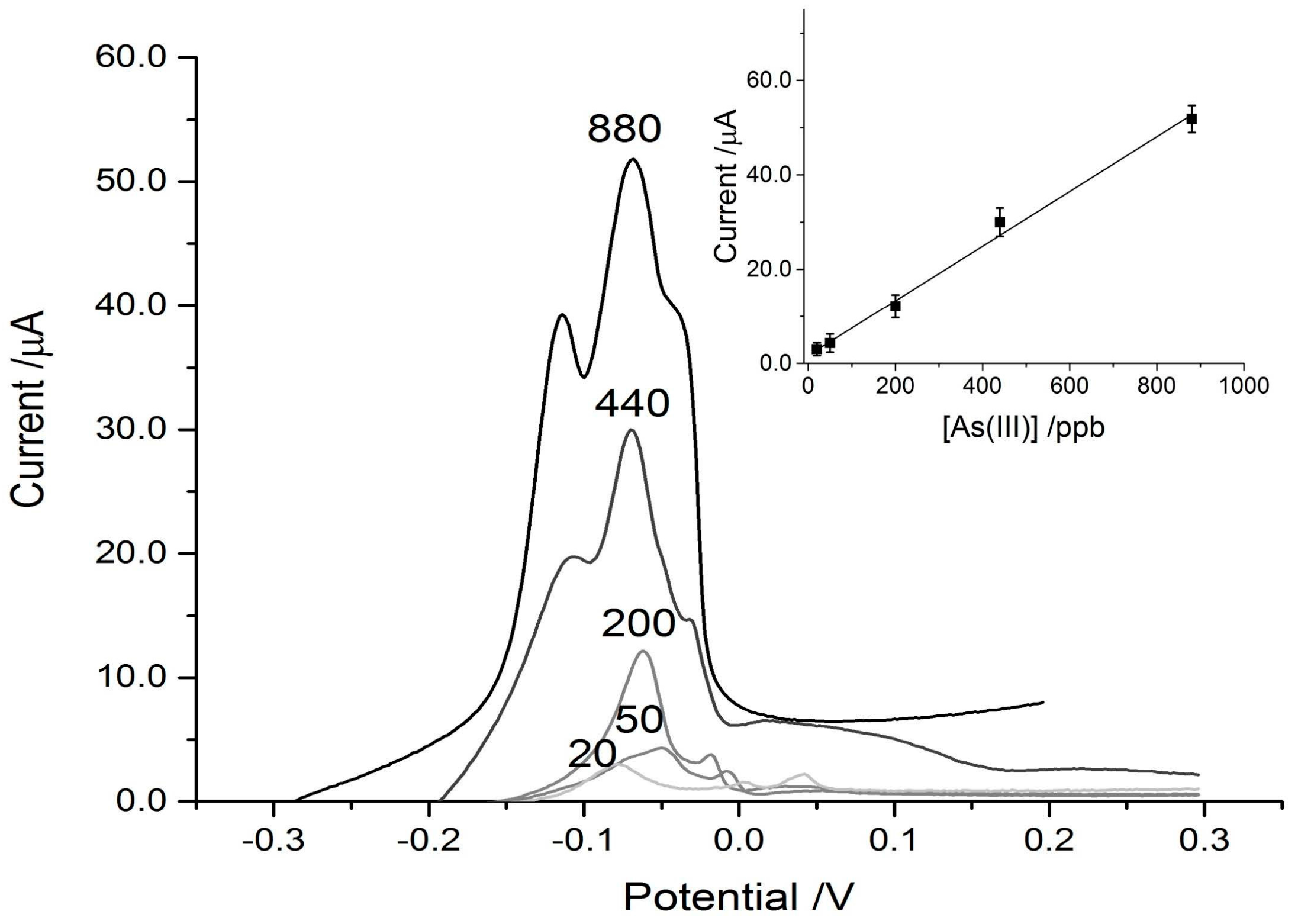
3.1. Arsenic Detection Using ASV
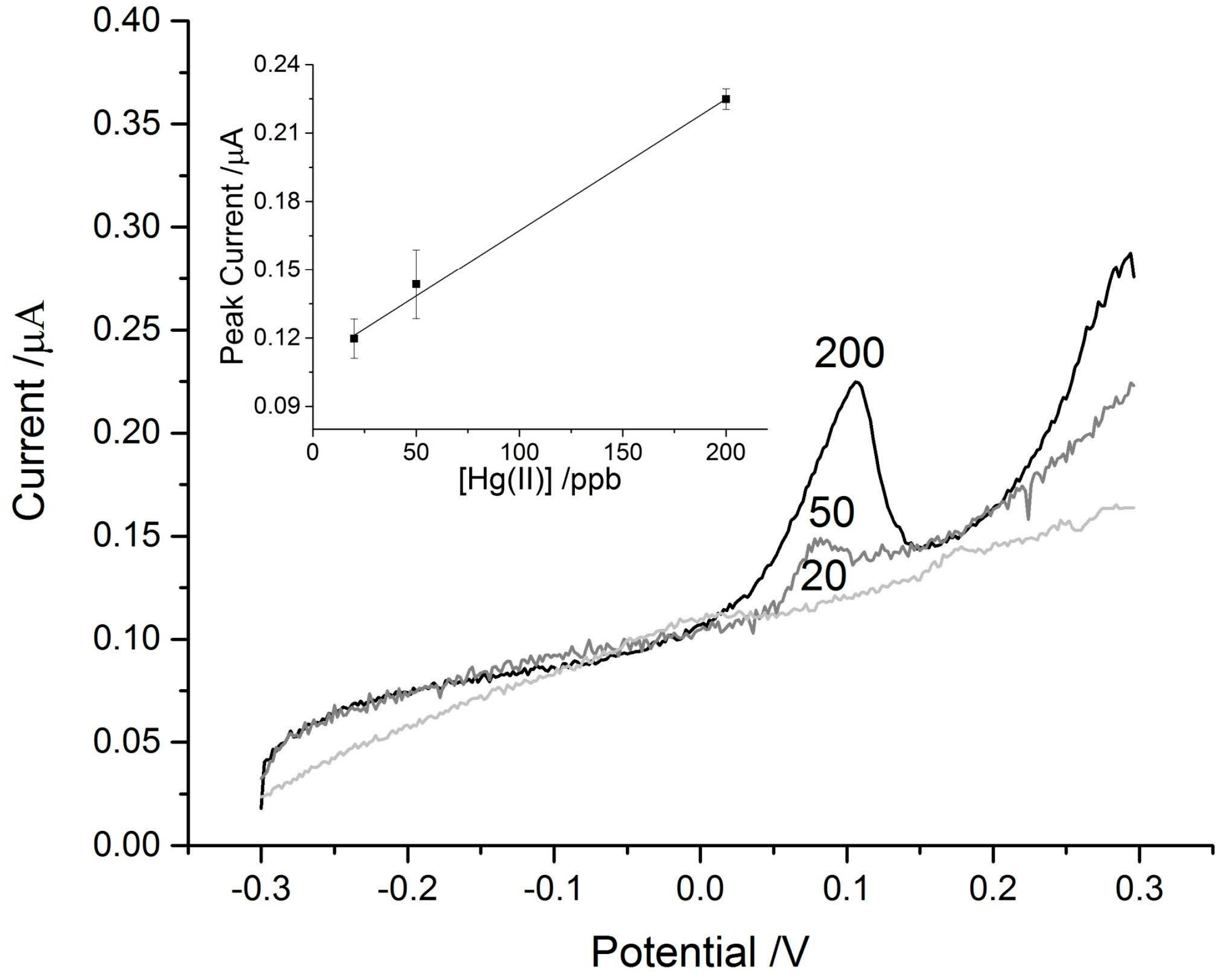
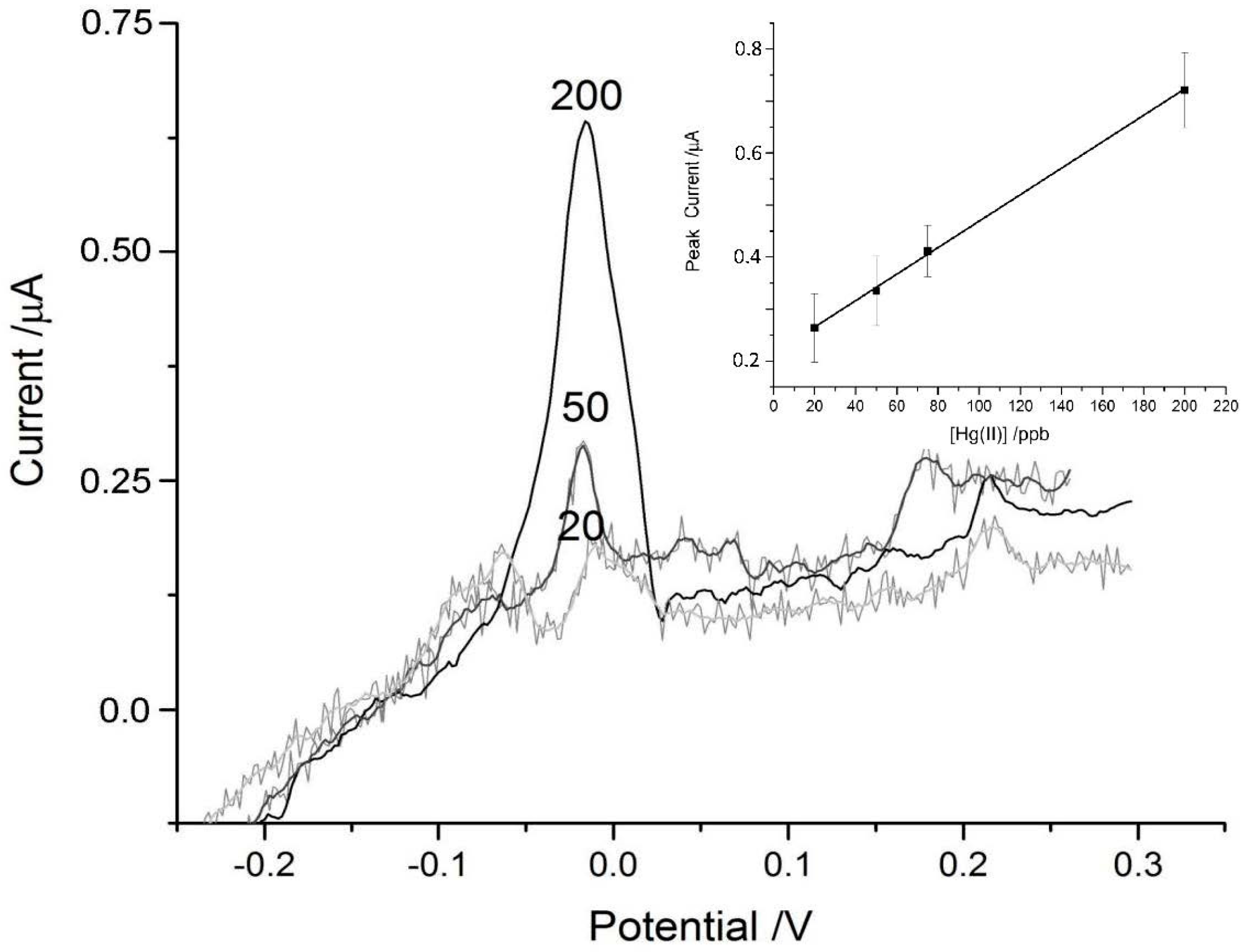
3.2. Mercury Detection Using ASV
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Phan, K.; Phan, S.; Heng, S.; Huoy, L.; Kim, K.-W. Assessing Arsenic Intake from Groundwater and Rice by Residents in Prey Veng Province, Cambodia. Environ. Pollut. 2014, 185, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; LoIacono, N.J.; Kline, J.; Factor-Litvak, P.; van Geen, A.; Mey, J.L.; Levy, D.; Abramson, R.; Schwartz, A. A Cross-Sectional Study of Well Water Arsenic and Child IQ in Maine Schoolchildren. Environ. Health 2014, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miyahara, S. A Case History; Minamata Mercury Pollution in Japan–from Loss of Human Lives to Decontamination. Water Sci. Technol. 1991, 23, 283–290. [Google Scholar]
- Wang, Q.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and Remediation for Mercury Contamination in Aquatic Systems—A Literature Review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Loux, N.T. An Assessment of Mercury-Species-Dependent Binding with Natural Organic Carbon. Chem. Speciat. Bioavailab. 1998, 10, 127–136. [Google Scholar] [CrossRef]
- Lu, W.; Stillman, M.J. Mercury-Thiolate Clusters in Metallothionein. Analysis of Circular Dichroism Spectra of Complexes Formed Between. Alpha.-Metallothionein, Apometallothionein, Zinc Metallothionein, and Cadmium Metallothionein and Mercury (2+). J. Am. Chem. Soc. 1993, 115, 3291–3299. [Google Scholar] [CrossRef]
- Stillman, M.J.; Thomas, D.; Trevithick, C.; Guo, X.; Siu, M. Circular Dichroism, Kinetic and Mass Spectrometric Studies of Copper (I) and Mercury (II) Binding to Metallothionein. J. Inorg. Biochem. 2000, 79, 11–19. [Google Scholar] [CrossRef]
- Chen, R.W.; Ganther, H.E.; Hoekstra, W.G. Studies on the Binding of Methylmercury by Thionein. Biochem. Biophys. Res. Commun. 1973, 51, 383–390. [Google Scholar] [CrossRef]
- Piotrowski, J.K.; Trojanowska, B.; Wiśniewska-Knypl, J.M.; Bolanowska, W. Mercury Binding in the Kidney and Liver of Rats Repeatedly Exposed to Mercuric Chloride: Induction of Metallothionein by Mercury and Cadmium. Toxicol. Appl. Pharmacol. 1974, 27, 11–19. [Google Scholar] [CrossRef]
- Irvine, G.W.; Duncan, K.E.R.; Gullons, M.; Stillman, M.J. Metalation Kinetics of the Human A-Metallothionein 1a Fragment Is Dependent on the Fluxional Structure of the Apo-Protein. Chemistry 2015, 21, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chen, L.; Russell, D.H. Metal-Induced Conformational Changes of Human Metallothionein-2a: A Combined Theoretical and Experimental Study of Metal-Free and Partially Metalated Intermediates. J. Am. Chem. Soc. 2014, 136, 9499–9508. [Google Scholar] [CrossRef] [PubMed]
- Polya, D.A.; Gault, A.G.; Bourne, N.J.; Lythgoe, P.R.; Cooke, D.A. Coupled Hplc-Icp-Ms Analysis Indicates Highly Hazardous Concentrations of Dissolved Arsenic Species in Cambodian Groundwaters. Plasma Source Mass Spectrom. 2003, 288, 127–140. [Google Scholar]
- Duan, G.; Liu, W.; Chen, X.; Hu, Y.; Zhu, Y. Association of Arsenic with Nutrient Elements in Rice Plants. Metallomics 2013, 5, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Sampson, M.; Le, C.; Irvine, K.; Gerads, R.; Smith, L.; Parr, T.; Irvine, K.; Murphy, T.; Vermette, S. Arsenic Bioaccumulation in an Arsenic Rich Area of Cambodia. Water Resour. Dev. Southeast Asia 2010, 1, 57–88. [Google Scholar]
- Berg, M.; Stengel, C.; Trang, P.T.K.; Viet, P.H.; Sampson, M.L.; Leng, M.; Samreth, S.; Fredericks, D. Magnitude of Arsenic Pollution in the Mekong and Red River Deltas—Cambodia and Vietnam. Sci. Total Environ. 2007, 372, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.L.; Bostick, B.; Chiew, H.; Hagan, J.M.; Shantz, A. Arsenicosis in Cambodia: Case Studies and Policy Response. Appl. Geochem. 2008, 23, 2977–2986. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of Drinking-Water by Arsenic in Bangladesh: A Public Health Emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar] [PubMed]
- Mitra, A.K.; Bose, B.K.; Kabir, H.; Das, B.K.; Hussain, M. Arsenic-Related Health Problems among Hospital Patients in Southern Bangladesh. J. Health Popul. Nutr. 2002, 20, 198–204. [Google Scholar] [PubMed]
- Babkina, S.S.; Ulakhovich, N.A. Complexing of Heavy Metals with DNA and New Bioaffinity Method of Their Determination Based on Amperometric DNA-Based Biosensor. Anal. Chem. 2005, 77, 5678–5685. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Singh, M. Biosensors for Heavy Metals. BioMetals 2005, 18, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, J.M.; Trojanowska, B.; Piotrowski, J.; Jakubowski, M. Binding of Mercury in the Rat Kidney by Metallothionein. Toxicol. Appl. Pharmacol. 1970, 16, 754–763. [Google Scholar] [CrossRef]
- Waalkes, M.P.; Harvey, M.J.; Klaassen, C.D. Relative in Vitro Affinity of Hepatic Metallothionein for Metals. Toxicol. Lett. 1984, 20, 33–39. [Google Scholar] [CrossRef]
- Funk, A.E.; FDay, A.; Brady, F.O. Displacement of Zinc and Copper from Copper-Induced Metallothionein by Cadmium and by Mercury: In Vivo and Ex Vivo Studies. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1987, 86, 1–6. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Wang, Q. Chemical Interactions of Mercury Species and Some Transition and Noble Metals Towards Metallothionein (Zn 7 Mt-2) Evaluated Using Sec/Icp-Ms, Rp-Hplc/Esi-Ms and Maldi-Tof-Ms. Metallomics 2013, 5, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Stillman, M.J. (Mercury) 18-Metallothionein. J. Am. Chem. Soc. 1988, 110, 7872–7873. [Google Scholar] [CrossRef]
- Ngu, T.T.; Easton, A.; Stillman, M.J. Kinetic Analysis of Arsenic—Metalation of Human Metallothionein: Significance of the Two-Domain Structure. J. Am. Chem. Soc. 2008, 130, 17016–17028. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.W.; Stillman, M.J. Topographical Analysis of as-Induced Folding of A-Mt1a. Biochem. Biophys. Res. Commun. 2013, 441, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Freisinger, E.; Milan, V. Cadmium in Metallothioneins. In Cadmium: From Toxicity to Essentiality; Springer: Berlin, Germany, 2013; pp. 339–371. [Google Scholar]
- Jiang, G.; Gong, Z.; Li, X.-F.; Cullen, W.R.; Le, X.C. Interaction of Trivalent Arsenicals with Metallothionein. Chem. Res. Toxicol. 2003, 16, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-M.; Lin, L.-Y. Immobilization of Metallothionein as a Sensitive Biosensor Chip for the Detection of Metal Ions by Surface Plasmon Resonance. Biosens. Bioelectr. 2004, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Turkewitz, A.P.; Martín-González, A.; Gutiérrez, J.-C. Whole-Cell Biosensors for Detection of Heavy Metal Ions in Environmental Samples Based on Metallothionein Promoters from Tetrahymena Thermophila. Microb. Biotechnol. 2011, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin Electrochemical Biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar] [CrossRef]
- Hu, S.; Ye, B.; Yi, X.; Cao, Z.; Wu, D.; Shen, C.; Wang, J. Dumbbell-Shaped Metallothionein-Templated Silver Nanoclusters with Applications in Cell Imaging and Hg2+ Sensing. Talanta 2016, 155, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Trnkova, L.; Krizkova, S.; Adam, V.; Hubalek, J.; Kizek, R. Immobilization of Metallothionein to Carbon Paste Electrode Surface Via Anti-Mt Antibodies and Its Use for Biosensing of Silver. Biosens. Bioelectr. 2011, 26, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Huska, D.; Beklova, M.; Hubalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Protein-Based Electrochemical Biosensor for Detection of Silver (I) Ions. Environ. Toxicol. Chem. 2010, 29, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Huang, Z.; Watt, I.; Kille, P.; Stillman, M.J. Characterization of the Conformational Changes in Recombinant Human Metallothioneins Using Esi-Ms and Molecular Modeling. Can. J. Chem. 2007, 85, 898–912. [Google Scholar] [CrossRef]
- Sekar, N.C.; Ge, L.; Shaegh, S.A.M.; Ng, S.H.; Tan, S.N. A Mediated Turnip Tissue Paper-Based Amperometric Hydrogen Peroxide Biosensor. Sens. Actuators B Chem. 2015, 210, 336–342. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Choudhuri, S.; McKim, J.M., Jr.; Lehman-McKeeman, L.D.; Kershaw, W.C. In Vitro and in Vivo Studies on the Degradation of Metallothionein. Environ. Health Perspect. 1994, 102 (Suppl. 3), 141. [Google Scholar] [CrossRef]
- Polya, D.A.; Berg, M.; Gault, A.G.; Takahashi, Y. Arsenic in Groundwaters of South-East Asia: With Emphasis on Cambodia and Vietnam. Appl. Geochem. 2008, 23, 2968–2976. [Google Scholar] [CrossRef]
- Kopanica, M.; Novotný, L. Determination of Traces of Arsenic (III) by Anodic Stripping Voltammetry in Solutions, Natural Waters and Biological Material. Anal. Chim. Acta 1998, 368, 211–218. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive Electrochemical Detection of Arsenic (III) Using Gold Nanoparticle Modified Carbon Nanotubes Via Anodic Stripping Voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Nekrassova, O.; Hyde, M.E.; Compton, R.G. Anodic Stripping Voltammetry of Arsenic (III) Using Gold Nanoparticle-Modified Electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Jia, X.; Han, Y.; Wang, E. Electrochemical Determination of Arsenic (III) on Mercaptoethylamine Modified Au Electrode in Neutral Media. Anal. Chim. Acta 2012, 733, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Fuku, X.; Iftikar, F.; Hess, E.; Iwuoha, E.; Baker, P. Cytochrome C Biosensor for Determination of Trace Levels of Cyanide and Arsenic Compounds. Anal. Chim. Acta 2012, 730, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Varriale, A.; Staiano, M.; Rossi, M.; D’Auria, S. High-Affinity Binding of Cadmium Ions by Mouse Metallothionein Prompting the Design of a Reversed-Displacement Protein-Based Fluorescence Biosensor for Cadmium Detection. Anal. Chem. 2007, 79, 5760–5762. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Presa, À.; Capdevila, M.; Gonzàlez-Duarte, P. Mercury (II) Binding to Metallothioneins. Eur. J. Biochem. 2004, 271, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury Pollution in Asia: A Review of the Contaminated Sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, C.-Q.; Wu, P.; Yang, Y. The Geochemical Characteristics of Mine-Waste Calcines and Runoff from the Wanshan Mercury Mine, Guizhou, China. Appl. Geochem. 2004, 19, 1735–1744. [Google Scholar] [CrossRef]
- Feng, X.; Dai, Q.; Qiu, G.; Li, G.; He, L.; Wang, D. Gold Mining Related Mercury Contamination in Tongguan, Shaanxi Province, Pr China. Appl. Geochem. 2006, 21, 1955–1968. [Google Scholar] [CrossRef]
- Bofill, R.; Orihuela, R.; Romagosa, M.; Domenech, J.; Atrian, S.; Capdevila, M. Caenorhabditis Elegans Metallothionein Isoform Specificity-Metal Binding Abilities and the Role of Histidine in Cemt1 and Cemt2. FEBS J. 2009, 276, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Mocny, C.S.; Pecoraro, V.L. De Novo Protein Design as a Methodology for Synthetic Bioinorganic Chemistry. Acc. Chem. Res. 2015, 48, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Bosscher, M.; Zhang, C.; Özçubukçu, S.; Zhang, L.; Zhang, W.; Li, C.J.; Liu, J.; Jensen, M.P.; Lai, L. A Protein Engineered to Bind Uranyl Selectively and with Femtomolar Affinity. Nat. Chem. 2014, 6, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Oxidative Metal Release from Metallothionein Via Zinc-Thiol/Disulfide Interchange. Proc. Natl. Acad. Sci. USA 1994, 91, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Minkel, D.T.; Poulsen, K.; Wielgus, S.; Shaw, C.F.; Petering, D.H. On the Sensitivity of Metallothioneins to Oxidation During Isolation. Biochem. J. 1980, 191, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kassim, R.; Ramseyer, C.; Enescu, M. Oxidation Reactivity of Zinc-Cysteine Clusters in Metallothionein. J. Biol. Inorg. Chem. 2013, 18, 333–342. [Google Scholar] [CrossRef] [PubMed]
| Experiment | Linear Regression Equation | R2 |
|---|---|---|
| As3+ control | y = 1.135 × 10−8(x) + 4.621 × 10−6 | 0.9977 |
| As3+ MT-discs | y = 5.999 × 10−8(x) + 1.425 × 10−6 | 0.9860 |
| Hg2+ control | y = 5.494 × 10−10(x) + 1.755 × 10−7 | 0.9843 |
| Hg2+ MT-dics | y = 2.545 × 10−9(x) + 2.132 × 10−7 | 0.9993 |
| Metal | Method/Electrode Type | Modifiers and Extra Components | Detection Limit | Reference |
|---|---|---|---|---|
| Cd, Zn, Ni | SPR | MT on carboxymethlyated chips | 1–2.25 ppm | [21] |
| Cd, Zn | HDME | TCEP | 5.6 ppb | [41] |
| Cd | Fluorescence | Zn-Chelex resin, Rh-labelled MT | 50 ppb | [36] |
| Pt (cisplatin) | HDME | TCEP | 10 ppb | [23] |
| Ag | Carbon paste | anti MT-antibodies | 0.05 ppb | [25] |
| Pd | HDME | TCEP | 10 ppb | [42] |
| As | Paper disc/SPCE | MT-adsorption | 13 ppb | This work |
| Hg | Paper disc/SPCE | MT-adsorption | 45 ppb | This work |
| Hg, Cu, Zn, Cd | Modified gold electrode | Coupling agents, bacterial MT, continuous flow set-up | 1 × 10−15 M | [43] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irvine, G.W.; Tan, S.N.; Stillman, M.J. A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury. Biosensors 2017, 7, 14. https://doi.org/10.3390/bios7010014
Irvine GW, Tan SN, Stillman MJ. A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury. Biosensors. 2017; 7(1):14. https://doi.org/10.3390/bios7010014
Chicago/Turabian StyleIrvine, Gordon W., Swee Ngin Tan, and Martin J. Stillman. 2017. "A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury" Biosensors 7, no. 1: 14. https://doi.org/10.3390/bios7010014
APA StyleIrvine, G. W., Tan, S. N., & Stillman, M. J. (2017). A Simple Metallothionein-Based Biosensor for Enhanced Detection of Arsenic and Mercury. Biosensors, 7(1), 14. https://doi.org/10.3390/bios7010014







