Hierarchical Thin Film Architectures for Enhanced Sensor Performance: Liquid Crystal-Mediated Electrochemical Synthesis of Nanostructured Imprinted Polymer Films for the Selective Recognition of Bupivacaine
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals

2.2. Instrumentation and Protocols
2.2.1. Electrochemistry and Quartz Crystal Microbalance
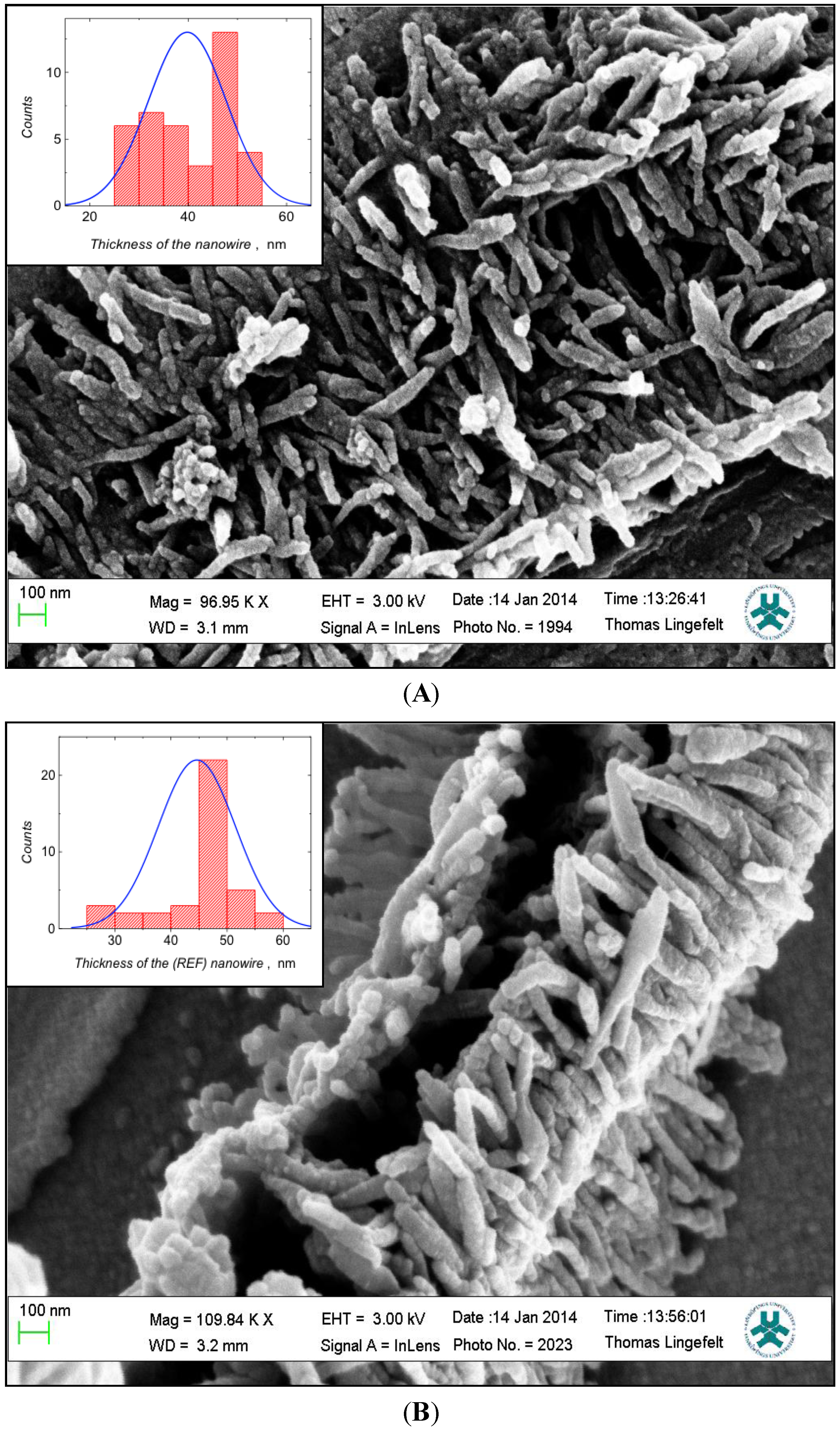
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. UV-Visible Spectroscopy
2.2.4. IR Spectroscopy
2.2.5. Flow Injection Analysis
2.3. Sensor Fabrication and Characterization
3. Results and Discussion
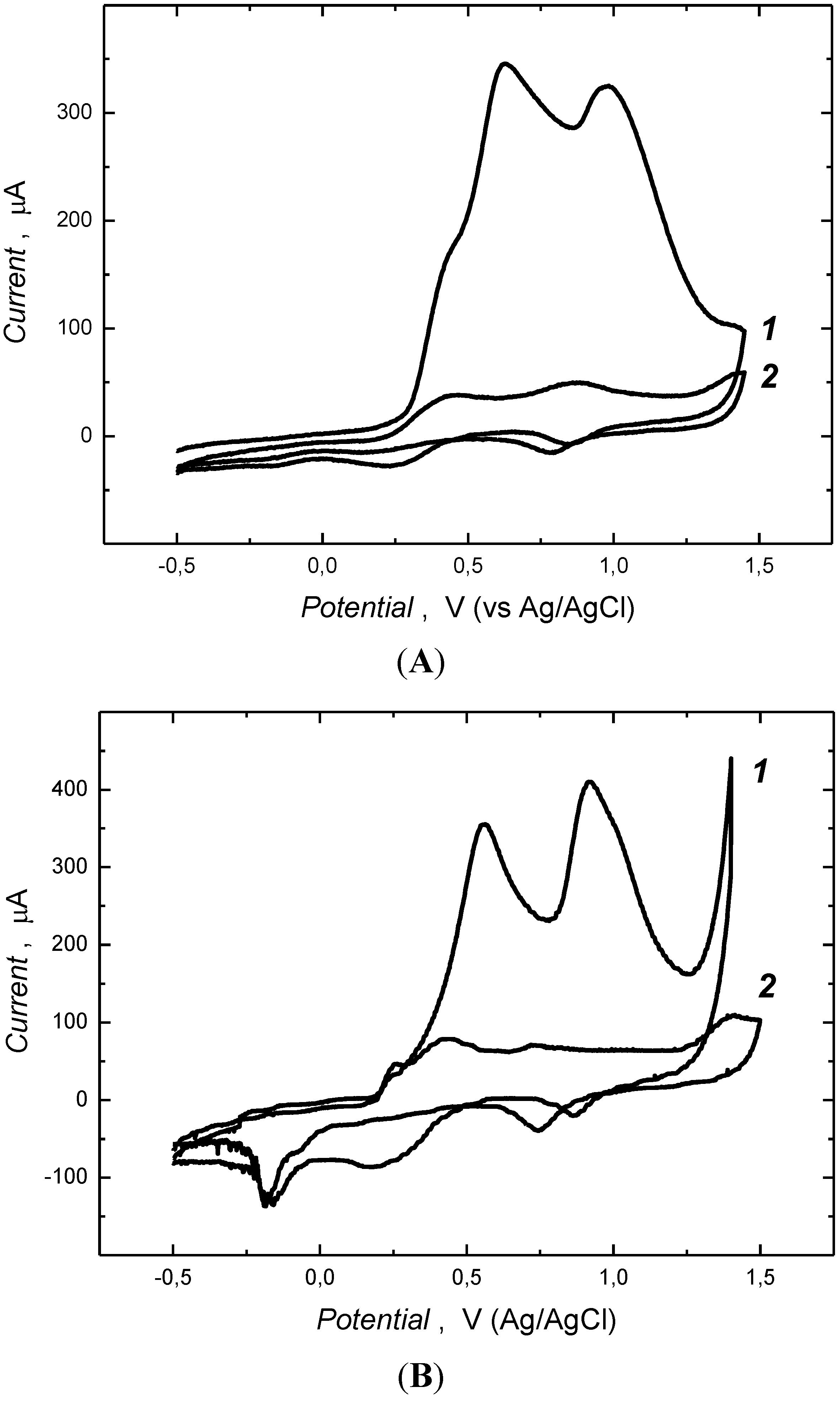
3.1. Electrochemical Preparation of MIP Films

3.2. Characterization of Polymer Films
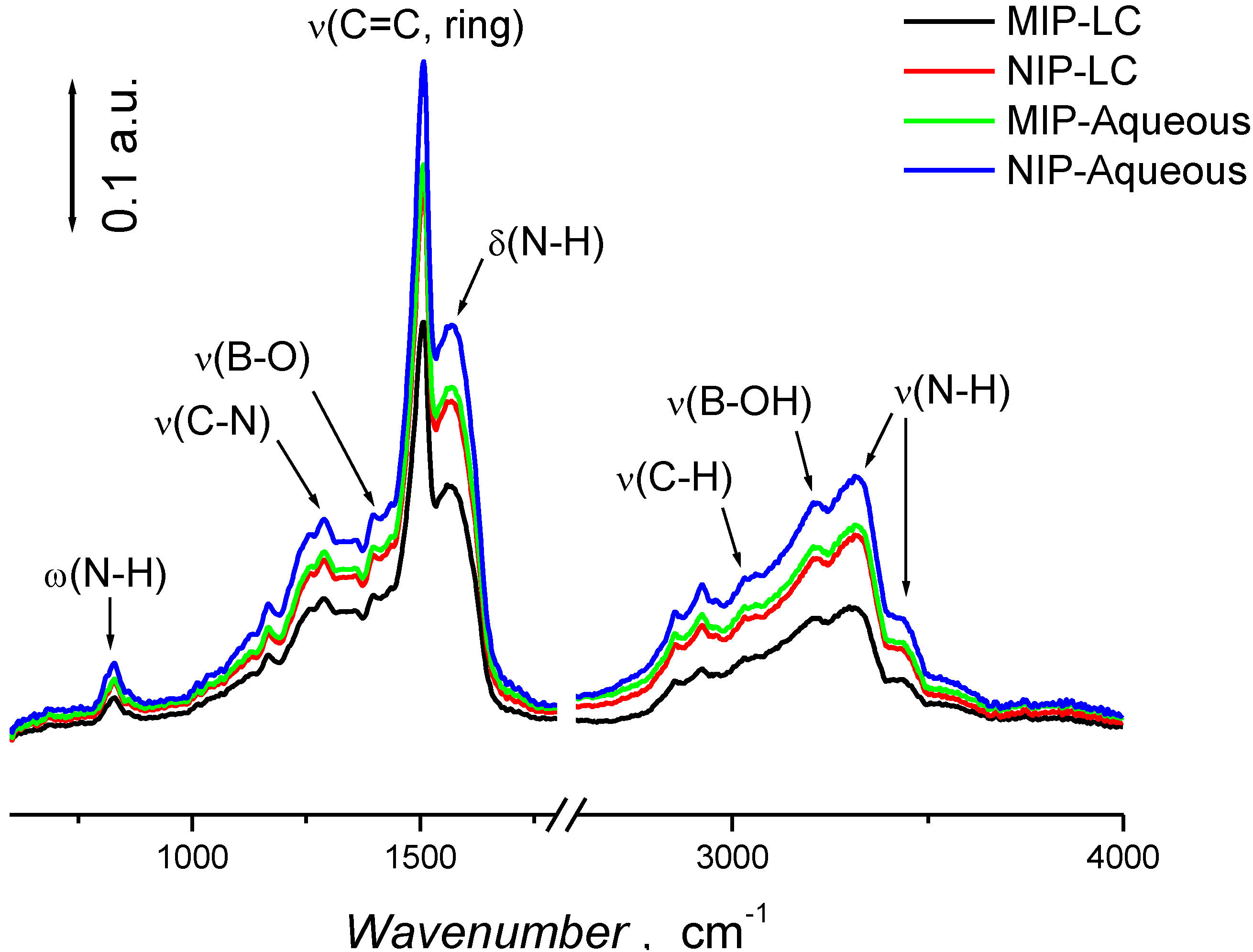
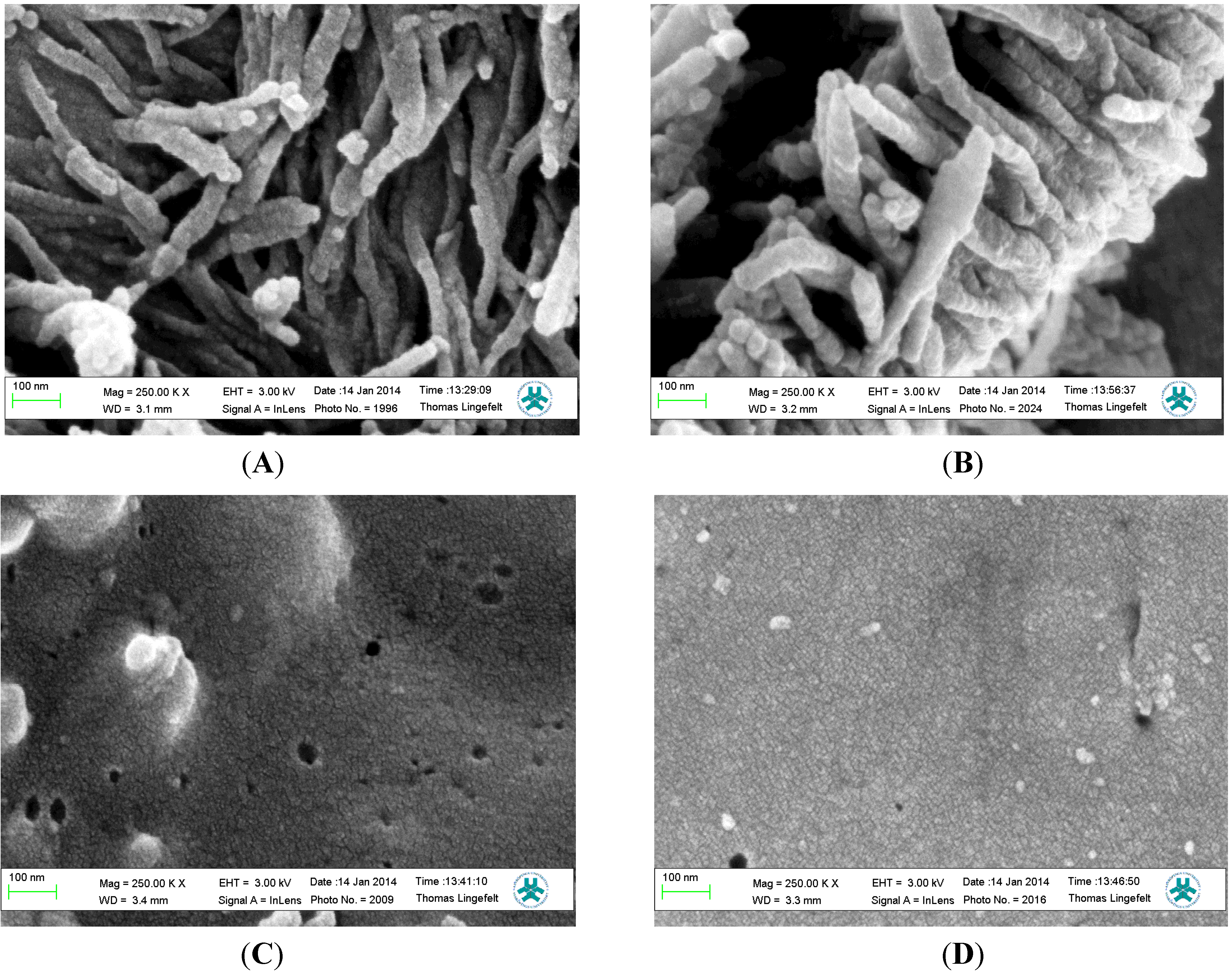
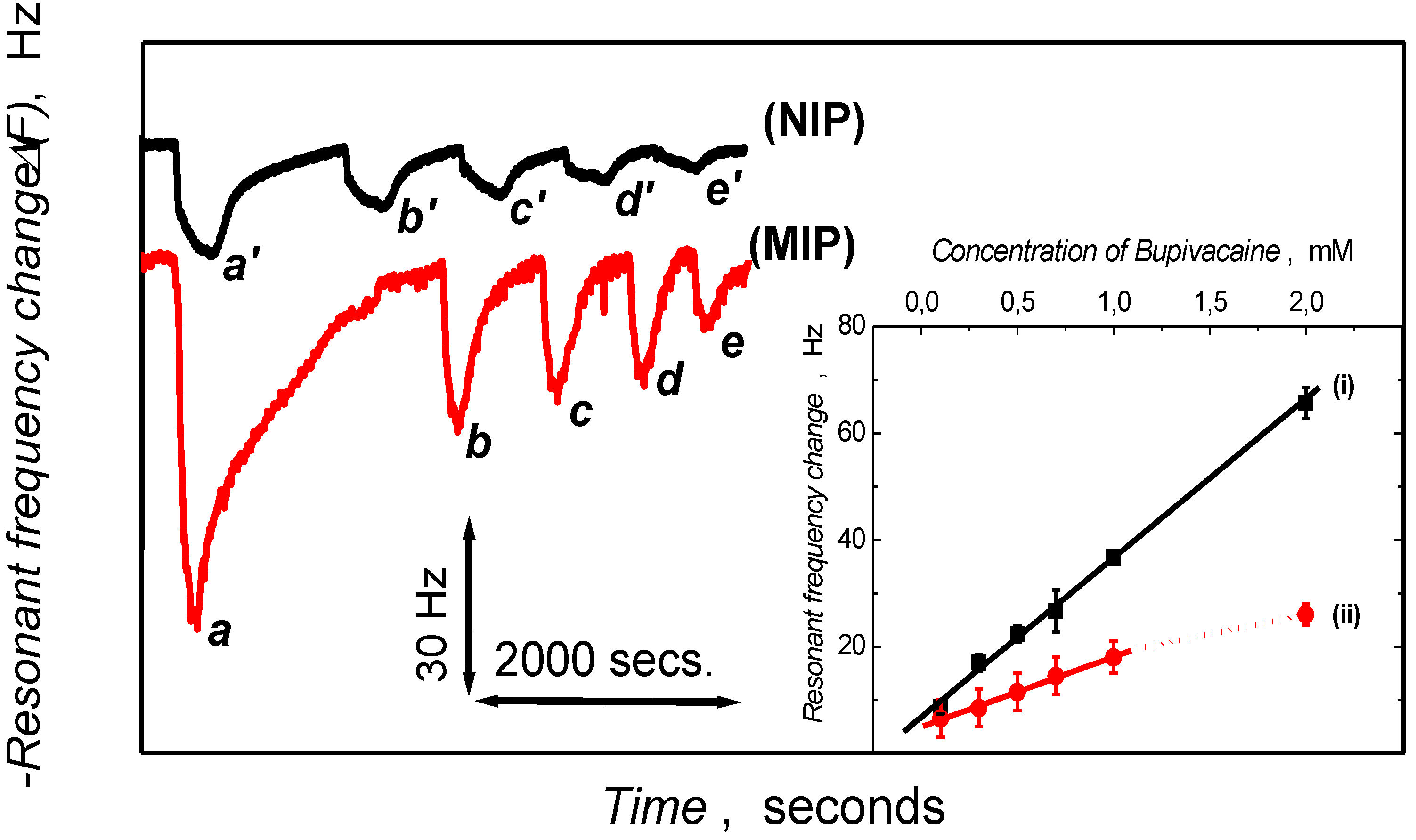
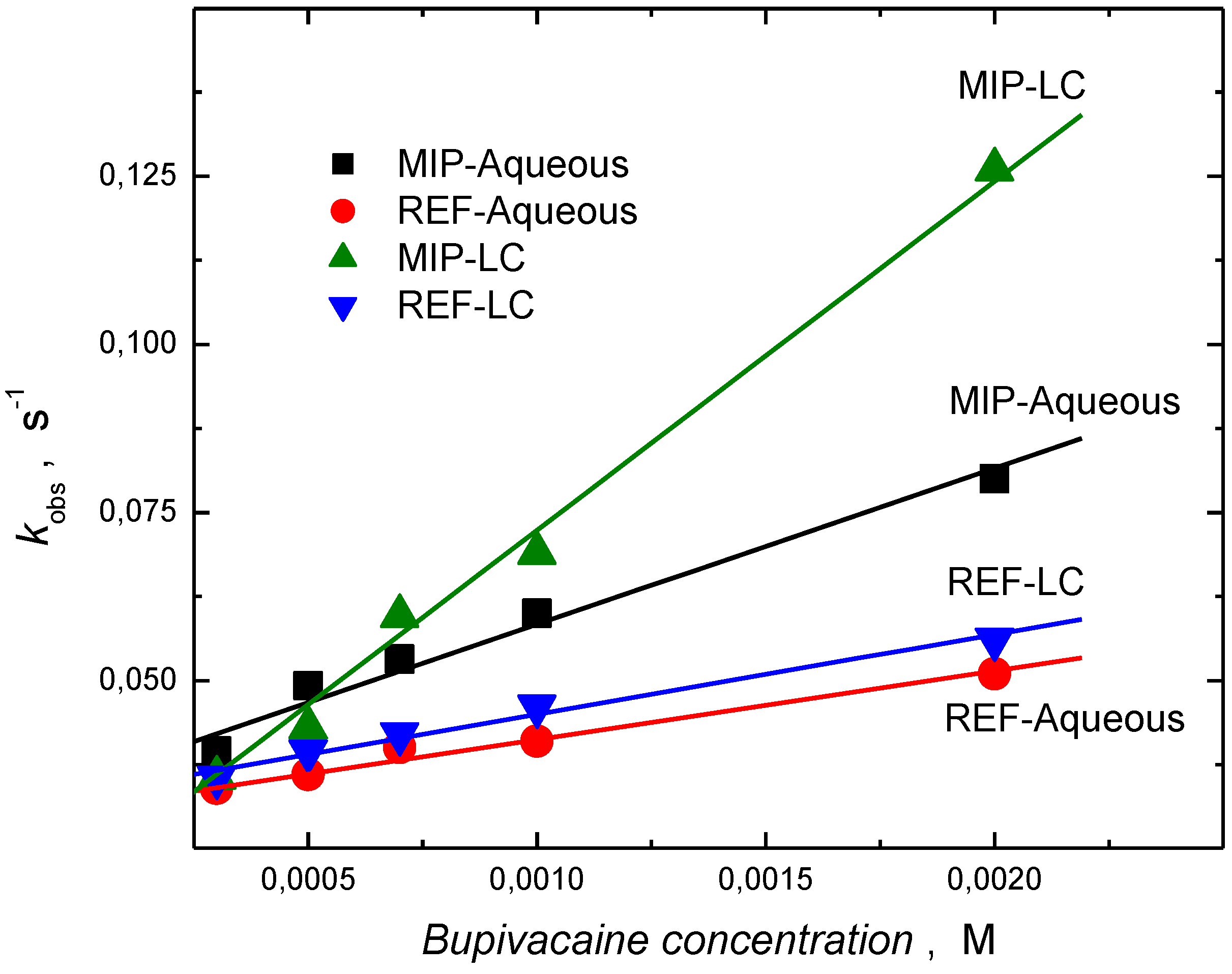
3.3. Piezoelectric Microgravimetric (QCM) Determination of Bupivacaine

| Polymer film (preparation medium) | Relative sensitivity (Hz/mM)a | Correlation coefficient | Ks (M-1) a |
|---|---|---|---|
| MIP (H2O) | 29.92 ± 4.15 | 0.998 | 660.56 ± 17.56 |
| REF (H2O) | 13.22 ± 3.22 | 0.996 | 330.12 ± 17.12 |
| MIP (LC) | 67.64 ± 4.91 | 0.995 | 2187.61 ± 29.02 |
| REF (LC) | 17.81 ± 2.67 | 0.989 | 444.27 18.71 |
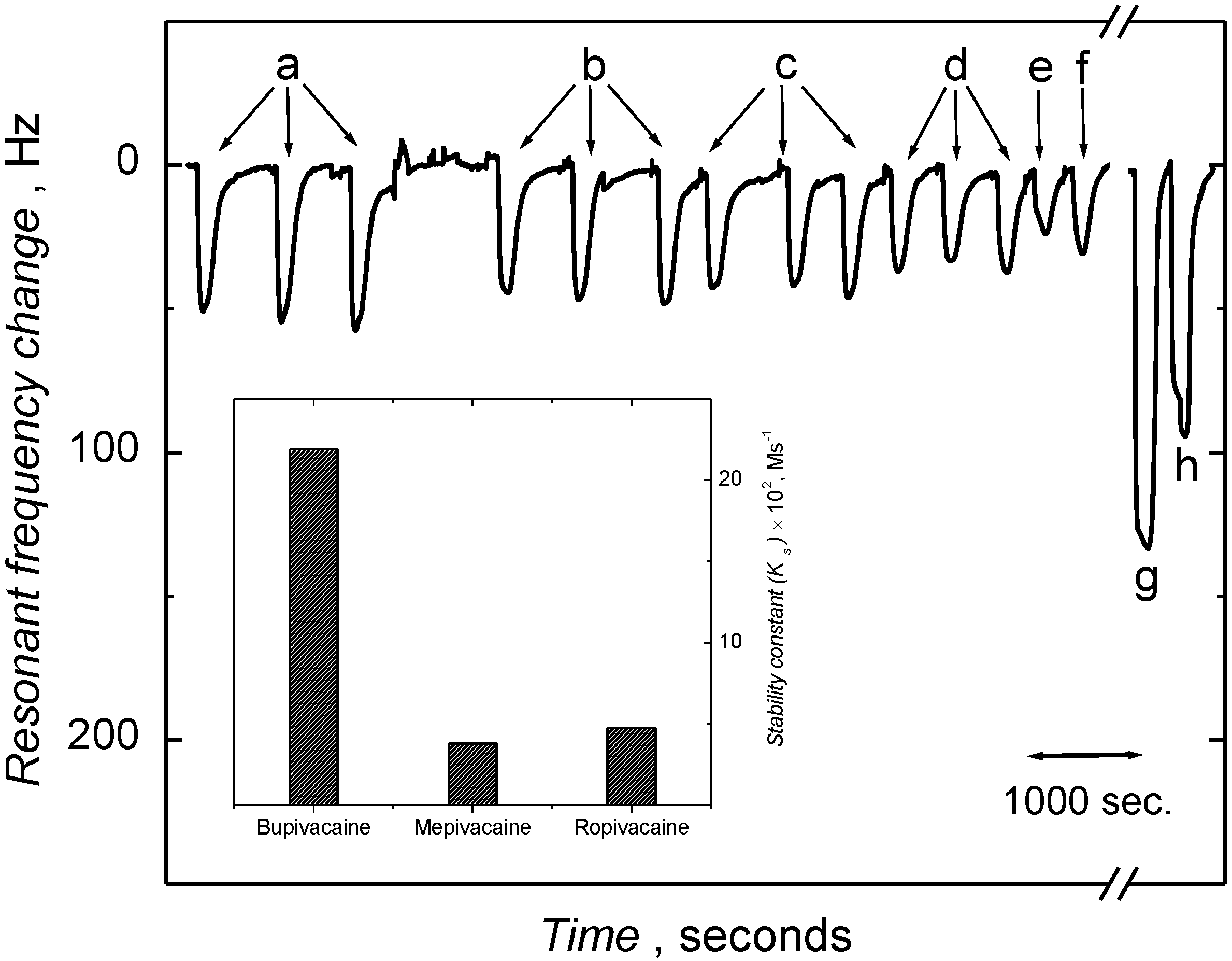
| Analyte | Relative sensitivity Hz/mM | Correlation coefficient | Interference factor, IF a |
|---|---|---|---|
| Bupivacaine (1) | 67.6 ± 4.91 | 0.995 | 1.00 |
| Mepivacaine (5) | 28.4 ± 1.51 | 0.989 | 0.17 |
| Ropivacaine (6) | 38.0 ± 5.16 | 0.990 | 0.22 |
| Method | Reported dynamic concentration range | Detection limit, LOD | Reference |
|---|---|---|---|
| Present work | 3–60 μg/mL | 30 ng/mL | - |
| HPLC | 5.0–50 μg/mL | 250 ng/mL | [58] |
| HPLC | 0.02–5.00 mg/L | 20 μg/L | [60] |
| HPLC | 0.033–3.31 μg/mL | 10 ng/mL | [67] |
| Ion-selective electrodes | 16 μM–100 mM | - | [61] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daikhin, L.; Urbakh, M. Effect of surface film structure on the quartz crystal microbalance response in liquids. Langmuir 1996, 12, 6354–6360. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, D.-F.; Liu, Q.; Qi, Z.-M.; Yang, L.; Liu, J. Wavelength-interrogated surface plasmon resonance sensor with mesoporous-silica-film-enhanced sensitivity to small molecules. Analyst 2012, 137, 4822–4828. [Google Scholar] [CrossRef]
- Hotta, K.; Yamaguchi, A.; Teramae, N. Nanoporous waveguide sensor with optimized nanoarchitectures for highly sensitive label-free biosensing. ACS Nano 2012, 6, 1541–1547. [Google Scholar]
- Berrier, A.; Offermans, P.; Cools, R.; van Megen, B.; Knoben, W.; Vecchi, G.; Rivas, J.G.; Crego-Calama, M.; Brongersma, S.H. Enhancing the gas sensitivity of surface plasmon resonance with a nanoporous silica matrix. Sens. Actuator B Chem. 2011, 160, 181–188. [Google Scholar] [CrossRef]
- Gaur, G.; Koktysh, D.S.; Weiss, S.M. Immobilization of quantum dots in nanostructured porous silicon films: Characterizations and signal amplification for dual-mode optical biosensing. Adv. Funct. Mater. 2013, 23, 3604–3614. [Google Scholar] [CrossRef]
- Niu, X.; Li, Y.; Tang, J.; Hu, Y.; Zhao, H.; Lan, M. Electrochemical sensing interfaces with tunable porosity for nonenzymatic glucose detection: A Cu foam case. Biosens. Bioelectron. 2014, 51, 22–28. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef]
- Anraku, Y.; Takahashi, Y.; Kitano, H.; Hakari, M. Recognition of sugars on surface-bound cap-shaped gold particles modified with a polymer brush. Colloids Surf. B 2007, 57, 61–68. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Lee, H.-H.; Liedberg, B.; Aastrup, T.; Nicholls, I.A. Protein-resistant hyperbranched polyethyleneimine brush surfaces. J. Colloid Interface Sci. 2013, 396, 307–315. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Olsson, G.D.; Karlsson, B.C.G.; Shoravi, S.; Wiklander, J.G.; Nicholls, I.A. Mechanisms underlying molecularly imprinted polymer molecular memory and the role of crosslinker: Resolving debate on the nature of template recognition in phenylalanine anilide imprinted polymers. J. Mol. Recognit. 2012, 25, 69–73. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Cywinski, P.J.; Moro, A.J.; Mohr, G.J.; Kutner, W. Chemosensors based on molecularly imprinted polymers. Mol. Imprint. 2012, 325, 165–265. [Google Scholar]
- Pietrzyk, A.; Suriyanarayanan, S.; Kutner, W.; Chitta, R.; D’Souza, F. Selective histamine piezoelectric chemosensor using a recognition film of the molecularly imprinted polymer of bis(bithiophene) derivatives. Anal. Chem. 2009, 81, 2633–2643. [Google Scholar] [CrossRef]
- Azevedo, S.D.; Lakshmi, D.; Chianella, I.; Whitcombe, M.J.; Karim, K.; Ivanova-Mitseva, P.K.; Subrahmanyam, S.; Piletsky, S.A. Molecularly imprinted polymer-hybrid electrochemical sensor for the detection of beta-estradiol. Ind. Eng. Chem. Res. 2013, 52, 13917–13923. [Google Scholar] [CrossRef]
- Akbulut, M.; Lakshmi, D.; Whitcombe, M.J.; Piletska, E.V.; Chianella, I.; Guven, O.; Piletsky, S.A. Microplates with adaptive surfaces. ACS Comb. Sci. 2011, 13, 646–652. [Google Scholar] [CrossRef]
- Pietrzyk, A.; Suriyanarayanan, S.; Kutner, W.; Maligaspe, E.; Zandler, M.E.; D’Souza, F. Molecularly imprinted poly bis(2,2'-bithienyl)methane film with built-in molecular recognition sites for a piezoelectric microgravimetry chemosensor for selective determination of dopamine. Bioelectrochemistry 2010, 80, 62–72. [Google Scholar] [CrossRef]
- Pietrzyk, A.; Kutner, W.; Chitta, R.; Zandler, M.E.; D’Souza, F.; Sannicolo, F.; Mussini, P.R. Melamine acoustic chemosensor based on molecularly imprinted polymer film. Anal. Chem. 2009, 81, 10061–10070. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Liu, Y.; Tong, H.; Xu, Y.; Liu, S. Preparation of a hollow porous molecularly imprinted polymer using tetrabromobisphenol A as a dummy template and its application as SPE sorbent for determination of bisphenol a in tap water. Talanta 2013, 117, 281–287. [Google Scholar] [CrossRef]
- Carter, S.R.; Rimmer, S. Surface molecularly imprinted polymer core-shell particles. Adv. Funct. Mater. 2004, 14, 553–561. [Google Scholar] [CrossRef]
- Diaz-Garcia, M.E.; Laino, R.B. Molecular imprinting in sol-gel materials: Recent developments and applications. Microchim. Acta 2005, 149, 19–36. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Moudrakovski, I.L.; Ozin, G.A. Molecularly imprinted mesoporous organosilica. ACS Nano 2011, 5, 2277–2287. [Google Scholar] [CrossRef]
- Suriyanarayanan, S.; Petrone, L.; Ederth, T.; Nicholls, I.A. Biotinyl moiety-selective polymer films with highly ordered macropores. Chem. Commun. 2013, 49, 5274–5276. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ramstrom, O.; Moller, P.; Sanchez, D.; Mosbach, K. A facile method for preparing molecularly imprinted polymer spheres using spherical silica templates. J. Mater. Chem. 2002, 12, 1577–1581. [Google Scholar] [CrossRef]
- Giovannoli, C.; Anfossi, L.; Biagioli, F.; Passini, C.; Baggiani, C. Solid phase extraction of penicillins from milk by using sacrificial silica beads as a support for a molecular imprint. Microchim. Acta 2013, 180, 1371–1377. [Google Scholar] [CrossRef]
- Yilmaz, E.; Haupt, K.; Mosbach, K. The use of immobilized templates—A new approach in molecular imprinting. Angew. Chem. Int. Ed. 2000, 39, 2115–2118. [Google Scholar] [CrossRef]
- Ahir, S.V.; Petrov, P.G.; Terentjev, E.M. Rheology at the phase transition boundary: 2. Hexagonal phase of Triton X-100 surfactant solution. Langmuir 2002, 18, 9140–9148. [Google Scholar] [CrossRef]
- Liu, S.Q.; Bakovic, L.; Chen, A.C. Specific binding of glycoproteins with poly(aniline boronic acid) thin film. J. Electroanal. Chem. 2006, 591, 210–216. [Google Scholar] [CrossRef]
- Shoji, E.; Freund, M.S. Potentiometric saccharide detection based on the pKa changes of poly(aniline boronic acid). J. Am. Chem. Soc. 2002, 124, 12486–12493. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Tan, Y.; Hu, J. Biosensor for the determination of sorbitol based on molecularly imprinted electro synthesized polymers. Biosens. Bioelectron. 2004, 19, 1513–1519. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Wang, E.K.; Yang, X.R. Capacitive detection of glucose using molecularly imprinted polymers. Biosens. Bioelectron. 2001, 16, 179–185. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Abd El-Rehim, S.S.; El-Deeb, M.M.; Kamal, S.M.; Azooz, R.E. Electropolymerization of p-phenylenediamine on Pt-electrode from aqueous acidic solution: Kinetics, mechanism, electrochemical studies, and characterization of the polymer obtained. J. Appl. Polym. Sci. 2010, 117, 943–952. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Ei-Rabiey, M.M.; El-Rehim, S.S.A.; Azooz, R.E. Electropolymerization kinetics of a binary mixture of pyrrole and o-aminobenzoic acid and characterization of the obtained polymer films. J. Appl. Polym. Sci. 2008, 109, 1643–1653. [Google Scholar] [CrossRef]
- Mackay, R.A.; Texter, J. Electrochemistry in colloids and dispersions. J. Chem. Educ. 1993, 70. [Google Scholar] [CrossRef]
- Raoof, J.-B.; Ojani, R.; Hosseini, S.R. Electrochemical fabrication of novel Pt/poly (m-toluidine)/Triton X-100 composite catalyst at the surface of carbon nanotube paste electrode and its application for methanol oxidation. Int. J. Hydrogen Energy 2011, 36, 52–63. [Google Scholar] [CrossRef]
- Girija, T.C.; Sangaranarayanan, M.V. Polyaniline-based nickel electrodes for electrochemical supercapacitors—Influence of Triton X-100. J. Power Sources 2006, 159, 1519–1526. [Google Scholar] [CrossRef]
- Kupila, E.L.; Kankare, J. Electropolymerization of pyrrole—Effects of pH and anions on the conductivity and growth-kinetics of polypyrrole. Synth. Met. 1993, 55, 1402–1405. [Google Scholar] [CrossRef]
- Boogaerts, J.G.; Lafont, N.D.; Carlino, S.; Noel, E.; Raynal, P.; Goffinet, G.; Legros, F.J. Biodistribution of liposome-associated bupivacaine after extradural administration to rabbits. Br. J. Anaesth. 1995, 75, 319–325. [Google Scholar] [CrossRef]
- Corciova, A. Spectrophotometric method for determination of bupivacaine hydrochloride in pharmaceutical preparations. Eur. Chem. Bull. 2013, 2, 554–557. [Google Scholar]
- Siva Rama Krishna, V.; Bhat, N.; Amrutur, B.; Chakrapani, K.; Sampath, S. Detection of Glycated Hemoglobin Using 3-Aminophenylboronic Acid Modified Graphene Oxide. In Proceedings of 2011 IEEE/NIH Life Science Systems and Applications Workshop (LiSSA), Bethesda, MD, USA, 7–8 April 2011; pp. 1–4.
- Zhang, Y.-P.; Lee, S.-H.; Reddy, K.R.; Gopalan, A.I.; Lee, K.-P. Synthesis and characterization of core-shell SiO2 nanoparticles/poly(3-aminophenylboronic acid) composites. J. Appl. Polym. Sci. 2007, 104, 2743–2750. [Google Scholar] [CrossRef]
- Pham, Q.L.; Haldorai, Y.; van Hoa, N.; Tuma, D.; Shim, J.-J. Facile synthesis of poly(p-phenylenediamine)/MWCNT nanocomposites and characterization for investigation of structural effects of carbon nanotubes. Bull. Mater. Sci. 2011, 34, 37–43. [Google Scholar] [CrossRef]
- Li, X.G.; Huang, M.R.; Chen, R.F.; Jin, Y.; Yang, Y.L. Preparation and characterization of poly(p-phenylenediamine-co-xylidine). J. Appl. Polym. Sci. 2001, 81, 3107–3116. [Google Scholar] [CrossRef]
- Brewer, S.H.; Allen, A.M.; Lappi, S.E.; Chasse, T.L.; Briggman, K.A.; Gorman, C.B.; Franzen, S. Infrared detection of a phenylboronic acid terminated alkane thiol monolayer on gold surfaces. Langmuir 2004, 20, 5512–5520. [Google Scholar]
- DrugBank: Bupivacaine (DB00297). Available online: http://www.drugbank.ca/drugs/DB00297 (accessed on 14 March 2014).
- DrugBank: M-Aminophenylboronic Acid (DB01896). Available online: http://www.drugbank.ca/drugs/DB01896 (accessed on 14 March 2014).
- O’Shannessy, D.J.; Brighma-Burke, M.; Soneson, K.K.; Hensley, P.; Brooks, I. Determination of rate and equilibrium binding constants for macromolecular interactions using surface plasmon resonance: Use of nonlinear least squares analysis methods. Anal. Biochem. 1993, 212, 457–468. [Google Scholar] [CrossRef]
- Morton, T.A.; Myszka, D.G.; Chaiken, I.M. Interpreting complex binding-kinetics from optical biosensors—A comparison of analysis by linearization, the integrated rate-equation, and numerical-integration. Anal. Biochem. 1995, 227, 176–185. [Google Scholar] [CrossRef]
- Navratilova, I.; Skladal, P.; Viklicky, V. Development of piezoelectric immunosensors for measurement of albuminuria. Talanta 2001, 55, 831–839. [Google Scholar] [CrossRef]
- Skladal, P.; Horacek, J. Kinetic studies of affinity interactions: Comparison of piezoelectric and resonant mirror-based biosensors. Anal. Lett. 1999, 32, 1519–1529. [Google Scholar] [CrossRef]
- Pribyl, J.; Hepel, M.; Skladal, P. Piezoelectric immunosensors for polychlorinated biphenyls operating in aqueous and organic phases. Sens. Actuator B Chem. 2006, 113, 900–910. [Google Scholar] [CrossRef]
- Halamek, J.; Hepel, M.; Skladal, P. Investigation of highly sensitive piezoelectric immunosensors for 2,4-dichlorophenoxyacetic acid. Biosens. Bioelectron. 2001, 16, 253–260. [Google Scholar] [CrossRef]
- Pietrzyk, A.; Suriyanarayanan, S.; Kutner, W.; Chitta, R.; Zandler, M.E.; D’Souza, F. Molecularly imprinted polymer (MIP) based piezoelectric microgravimetry chemosensor for selective determination of adenine. Biosens. Bioelectron. 2010, 25, 2522–2529. [Google Scholar] [CrossRef]
- Horacek, J.; Skladal, P. Effect of organic solvents on immunoassays of environmental pollutants studied using a piezoelectric biosensor. Anal. Chim. Acta 2000, 412, 37–45. [Google Scholar] [CrossRef]
- Skladal, P. Piezoelectric quartz crystal sensors applied for bioanalytical assays and characterization of affinity interactions. J. Braz. Chem. Soc. 2003, 14, 491–502. [Google Scholar] [CrossRef]
- Dincel, A.; Basci, N.E. An experimental design approach to selecting the optimum LC conditions for the determination of local anaesthetics. Chromatographia 2007, 66, S81–S85. [Google Scholar] [CrossRef]
- Venkateshwaran, T.G.; Stewart, J.T. HPLC determination of morphine-hydromorphone-bupivacaine and morphine-hydromorphone-tetracaine mixtures in 0.9% sodium chloride injection. J. Liq. Chromatogr. 1995, 18, 565–578. [Google Scholar] [CrossRef]
- Fijalek, Z.; Baczynski, E.; Piwonska, A.; Warowna-Grzeskiewicz, M. Determination of local anaesthetics and their impurities in pharmaceutical preparations using HPLC method with amperometric detection. J. Pharm. Biomed. Anal. 2005, 37, 913–918. [Google Scholar] [CrossRef]
- Lindberg, R.L.P.; Pihlajamaki, K.K. High-performance liquid-chromatographic determination of bupivacaine in human-serum. J. Chromatogr. A 1984, 309, 369–374. [Google Scholar] [CrossRef]
- Shoukry, A.F.; Issa, Y.M.; Elshiekh, R.; Zareh, M. New ion-selective electrodes for determination of bupivacaine and oxybuprocaine. Anal. Lett. 1991, 24, 1581–1590. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Jiao, X. Lyotropic liquid crystal directed synthesis of nanostructured materials. Sci. Tech. Adv. Mater. 2009, 10. [Google Scholar] [CrossRef]
- Agha, H.; Fleury, J.B.; Galerne, Y. Micro-wires self-assembled and 3D-connected with the help of a nematic liquid crystal. Eur. Phys. J. E Soft Matter 2012, 35. [Google Scholar] [CrossRef]
- Haegel, F.H.; Schlupen, J.; Schultze, J.W.; Winkels, S.; Stromberg, C. Anodic polymerization of thiophene derivatives from microemulsions and liquid crystals. Electrochim. Acta 2001, 46, 3973–3984. [Google Scholar] [CrossRef]
- Song, G.; Han, J.; Bo, J.; Guo, R. Synthesis of polyaniline nanostructures in different lamellar liquid crystals and application to lubrication. J. Mater. Sci. 2009, 44, 715–720. [Google Scholar]
- Kawabata, K.; Yoneyama, H.; Goto, H. Uniaxially ordered conjugated polymer film prepared by electrochemical polymerization in a nematic liquid crystal with rubbing orientation method showing redox-driven tunable dichroism. Polym. Chem. 2010, 1, 1606–1608. [Google Scholar] [CrossRef]
- Yu, Z.X.; Westerlund, D. Direct injection of large volumes of plasma in a column-switching system for the analysis of local anaesthetics. II. Determination of bupivacaine in human plasma with an alkyl-diol silica precolumn. J. Chromatogr. A 1996, 725, 149–155. [Google Scholar] [CrossRef]
Appendix


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Suriyanarayanan, S.; Nawaz, H.; Ndizeye, N.; Nicholls, I.A. Hierarchical Thin Film Architectures for Enhanced Sensor Performance: Liquid Crystal-Mediated Electrochemical Synthesis of Nanostructured Imprinted Polymer Films for the Selective Recognition of Bupivacaine. Biosensors 2014, 4, 90-110. https://doi.org/10.3390/bios4020090
Suriyanarayanan S, Nawaz H, Ndizeye N, Nicholls IA. Hierarchical Thin Film Architectures for Enhanced Sensor Performance: Liquid Crystal-Mediated Electrochemical Synthesis of Nanostructured Imprinted Polymer Films for the Selective Recognition of Bupivacaine. Biosensors. 2014; 4(2):90-110. https://doi.org/10.3390/bios4020090
Chicago/Turabian StyleSuriyanarayanan, Subramanian, Hazrat Nawaz, Natacha Ndizeye, and Ian A. Nicholls. 2014. "Hierarchical Thin Film Architectures for Enhanced Sensor Performance: Liquid Crystal-Mediated Electrochemical Synthesis of Nanostructured Imprinted Polymer Films for the Selective Recognition of Bupivacaine" Biosensors 4, no. 2: 90-110. https://doi.org/10.3390/bios4020090
APA StyleSuriyanarayanan, S., Nawaz, H., Ndizeye, N., & Nicholls, I. A. (2014). Hierarchical Thin Film Architectures for Enhanced Sensor Performance: Liquid Crystal-Mediated Electrochemical Synthesis of Nanostructured Imprinted Polymer Films for the Selective Recognition of Bupivacaine. Biosensors, 4(2), 90-110. https://doi.org/10.3390/bios4020090






