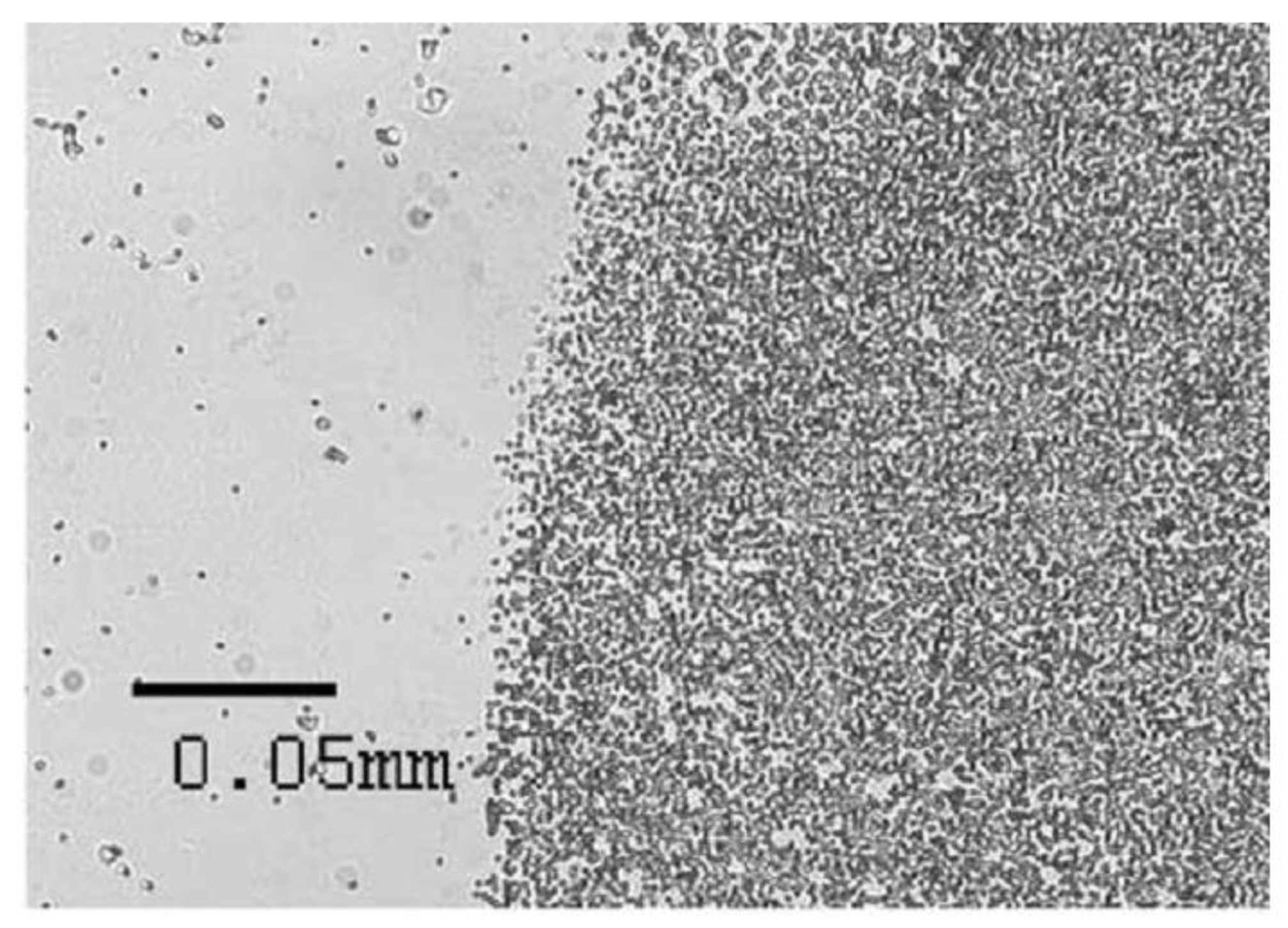
Lab-on-a-Chip Magneto-Immunoassays: How to Ensure Contact between Superparamagnetic Beads and the Sensor Surface
Abstract
:1. Introduction
2. Magnetic Approach

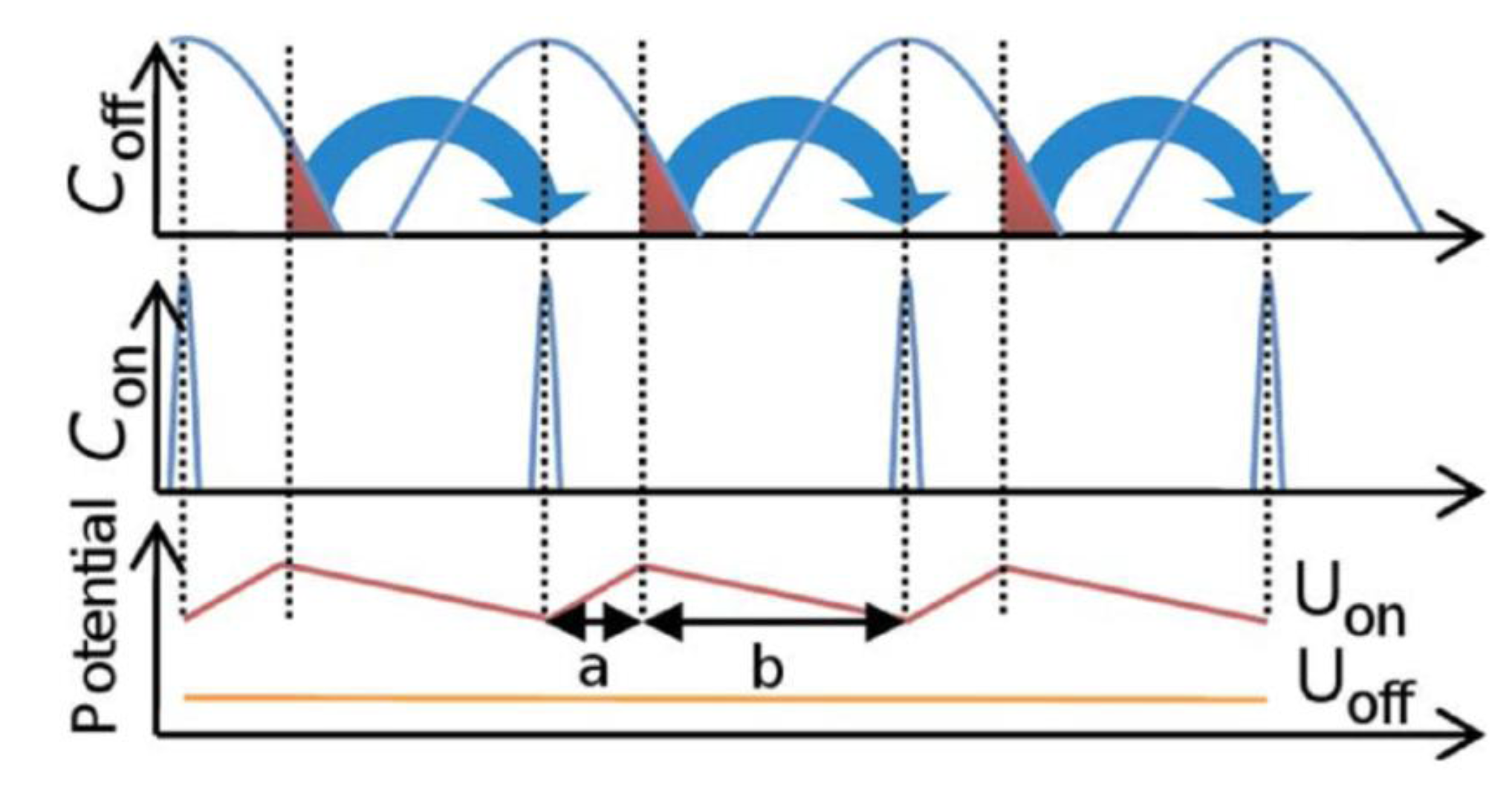
3. Hydrodynamic Approach
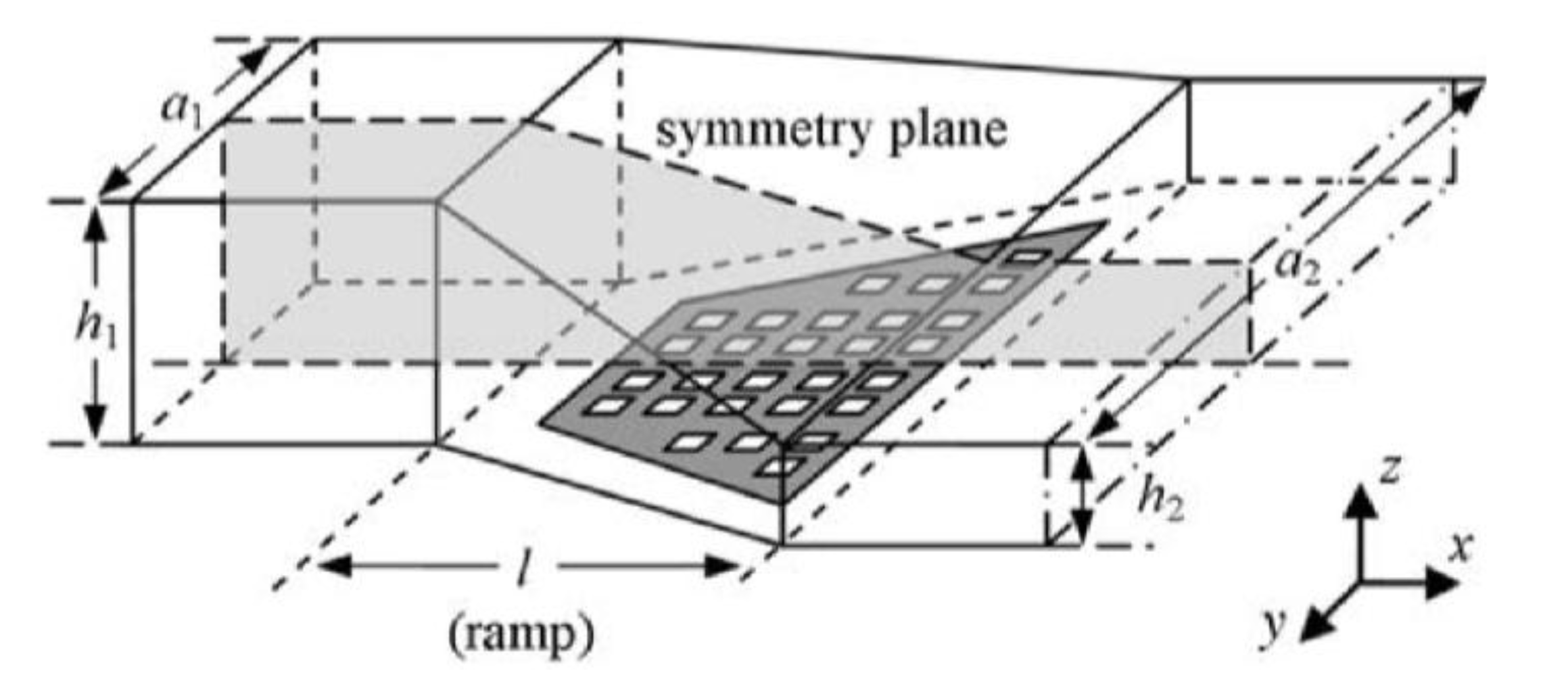
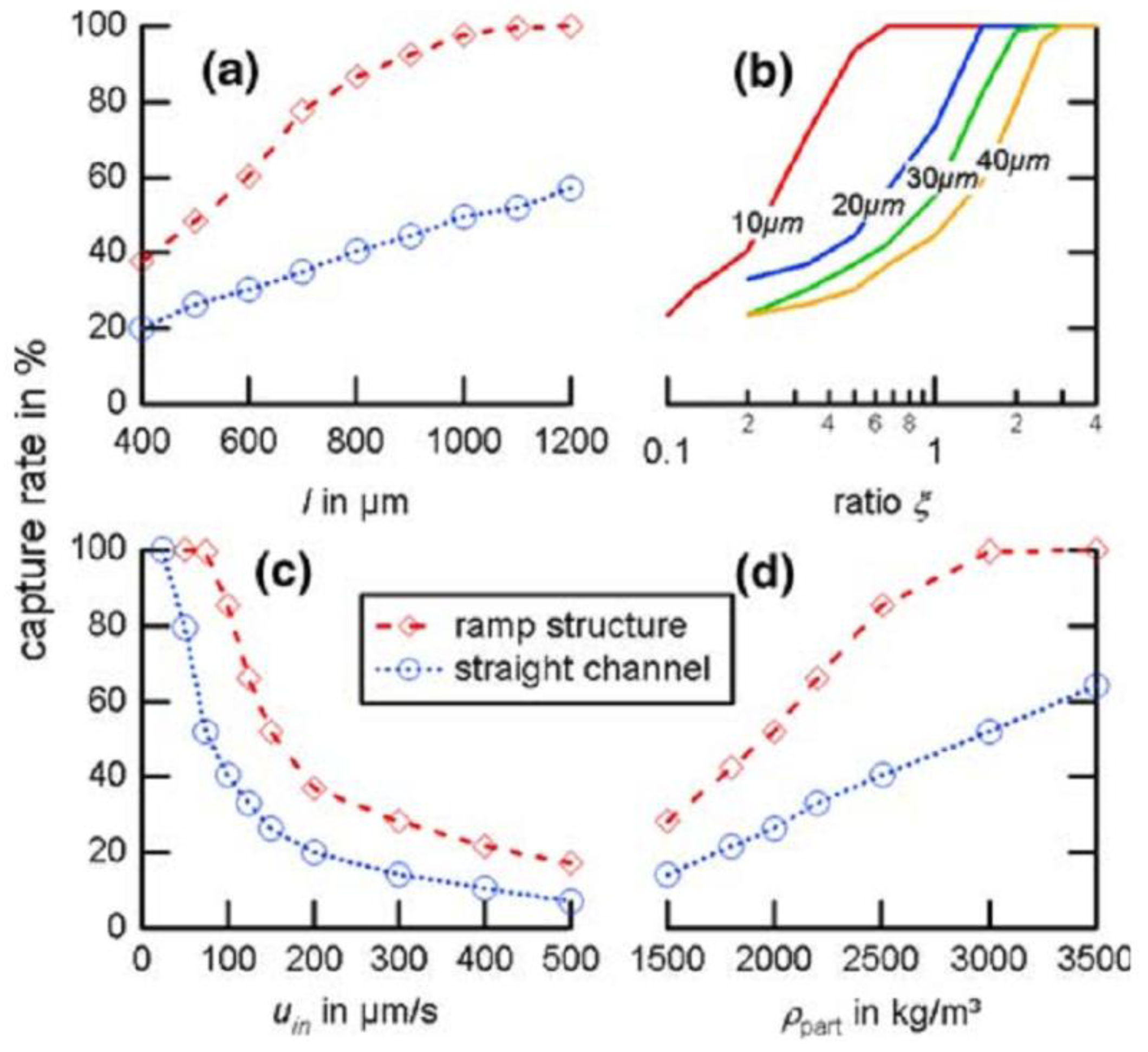
4. Acoustofluidic Approach
5. Nanogranular GMR
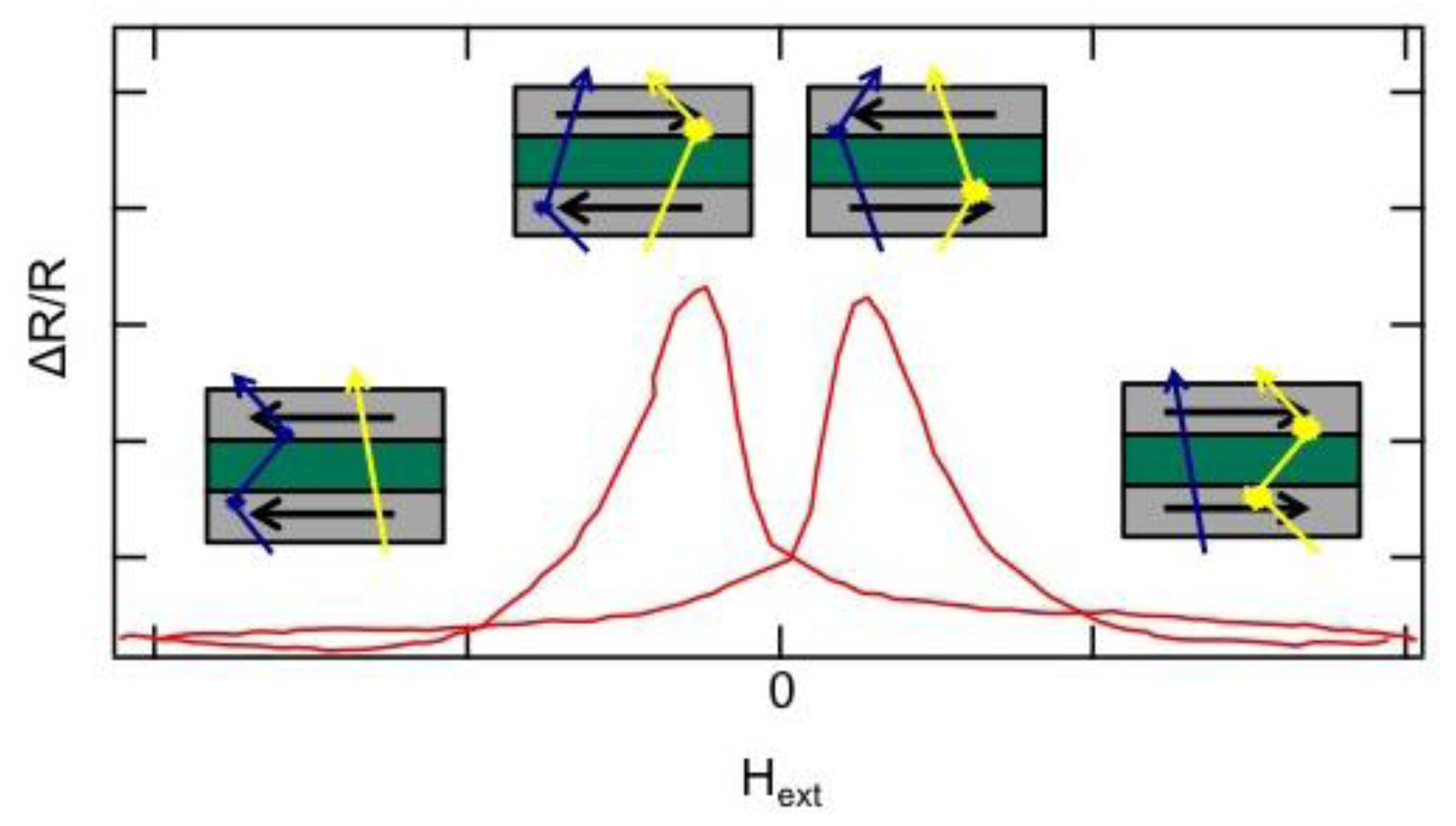
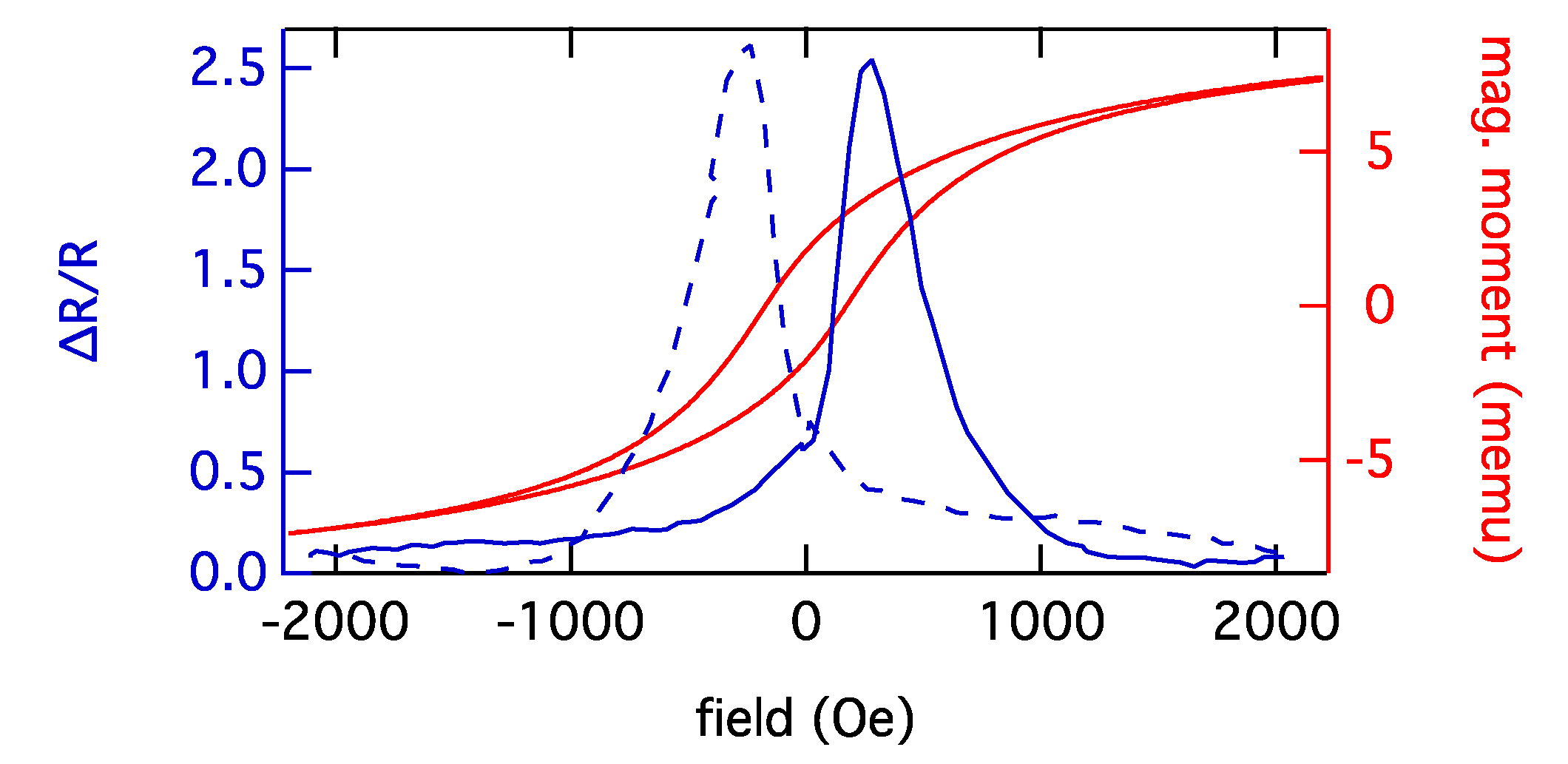

Acknowledgments
Conflicts of Interest
References
- Chan, C.P.Y.; Cheung, Y.C.; Renneberg, R.; Seydack, M. New trends in immunoassays. Adv. Biochem. Eng. Biotechnol. 2008, 109, 123–154. [Google Scholar]
- Mattiasson, B.; Teeparuksapun, K.; Hedstrom, M. Immunochemical binding assays for detection and quantification of trace impurities in biotechnological production. Trends Biotechnol. 2010, 28, 20–27. [Google Scholar] [CrossRef]
- Kricka, L.J. Selected strategies for improving sensitivity and reliability of immunoassays. Clin. Chem. 1994, 40, 347–357. [Google Scholar]
- Hartwell, S.K.; Grudpan, K. Flow based immuno/bioassay and trends in micro-immuno/biosensors. Microchim. Acta 2012, 169, 201–220. [Google Scholar]
- Paek, S.H.; Cho, J.H.; Cho, I.H.; Kim, Y.K.; Oh, B.K. Immunosensors for point-of-care testing. Biochip J. 2007, 1, 1–16. [Google Scholar]
- Kim, Y.K. Signal transducing methods for immuno-sensing devices. Biochip J. 2007, 1, 145–150. [Google Scholar]
- Lequin, R.M. Enzyme immunoassay (EIA)/Enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Truszczynski, M.; Pejsak, Z. Importance of ELISA in epidemiology and control of animal infectious diseases. Medycyna Wet. 2005, 61, 10–13. [Google Scholar]
- Zangar, R.C.; Daly, D.S.; White, A.M. ELISA microarray technology as a high-throughput system for cancer biomarker validation. Expert Rev. Proteomics 2006, 3, 37–44. [Google Scholar] [CrossRef]
- Yoshihara, N. ELISA for diagnosis of infections by viruses. Jpn. J. Clin. Med. 1995, 53, 2277–2282. [Google Scholar]
- Gelpi, E. Radioimmunoassay and the development of RIA-HPLC procedures: An updated literature survey. Trends Anal. Chem. 1985, 4, R13–R14. [Google Scholar]
- Yalow, R.S. Practices and pitfalls in immunologic methodology. Adv. Prostaglandin Thromboxane Leukot. Res. 1986, 16, 327–338. [Google Scholar]
- Goldberg, M.E.; Djavadiohaniance, L. Methods for measurement of antibody antigen affinity based on ELISA and RIA. Curr. Opin. Immunol. 1993, 5, 278–281. [Google Scholar] [CrossRef]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [CrossRef]
- McKie, A.; Vyse, A.; Maple, C. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect. Dis. 2002, 2, 18–24. [Google Scholar] [CrossRef]
- Gijs, M.A.M. Magnetic bead handling on-chip: New opportunities for analytical applications. Microfluid. Nanofluidics 2004, 1, 22–40. [Google Scholar]
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 2010, 110, 1518–1563. [Google Scholar] [CrossRef]
- Liu, C.; Stakenborg, T.; Peeters, S.; Lagae, L. Cell manipulation with magnetic particles toward microfluidic cytometry. J. Appl. Phys. 2009, 105, 102014:1–102014:11. [Google Scholar]
- Weddemann, A.; Albon, C.; Auge, A.; Wittbracht, F.; Hedwig, P.; Akemeier, D.; Rott, K.; Meissner, D.; Jutzi, P.; Hütten, A. How to design magneto-based total analysis systems for biomedical applications. Biosens. Bioelectron. 2010, 26, 1152–1163. [Google Scholar] [CrossRef]
- Albon, C.; Weddemann, A.; Auge, A.; Rott, K.; Hütten, A. Tunneling magnetoresistance sensors for high resolutive particle detection. Appl. Phys. Lett. 2009, 95, 023101:1–023101:3. [Google Scholar]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22, 455–462. [Google Scholar] [CrossRef]
- Reiss, G.; Brückl, H.; Hütten, A.; Schotter, J.; Brzeska, M.; Panhorst, M.; Sudfeld, D. Magnetoresistive sensors and magnetic nanoparticles for biotechnology. J. Mater. Res. 2005, 20, 3294–3302. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Makarov, D.; Chenglin, Y.; Streubel, R.; Schmidt, O.G. Printable giant magnetoresistive devices. Adv. Mater. 2012, 24, 4518–4522. [Google Scholar] [CrossRef]
- Li, G.X.; Joshi, V.; White, R.L.; Wang, S.X.; Kem, J.T.; Webb, C.; Davis, R.W.; Sun, S.H. Detection of single micron-sized magnetic bead and magnetic nanoparticles using spin valve sensors for biological applications. J. Appl. Phys. 2003, 93, 7557–7559. [Google Scholar]
- Graham, D.L.; Ferreira, H.; Bernardo, J.; Freitas, P.P.; Cabral, J.M.S. Single magnetic microsphere placement and detection on-chip using current line designs with integrated spin valve sensors: Biotechnological applications. J. Appl. Phys. 2002, 91, 7786–7788. [Google Scholar] [CrossRef]
- Lagae, L.; Wirix-Speetjens, R.; Das, J.; Graham, D.; Ferreira, H.; Freitas, P.P.P.; Borghs, G.; de Boeck, J. On-chip manipulation and magnetization assessment of magnetic bead ensembles by integrated spin-valve sensors. J. Appl. Phys. 2002, 91, 7445–7447. [Google Scholar] [CrossRef]
- Besse, P.A.; Boero, G.; Demierre, M.; Pott, V.; Popovic, R. Detection of a single magnetic microbead using a miniaturized Hall sensor. Appl. Phys. Lett. 2002, 80, 4199–4201. [Google Scholar] [CrossRef]
- Miller, M.M.; Prinz, G.A.; Cheng, S.F.; Bounnak, S. Detection of a micron-sized magnetic sphere using a ring-shaped anisotropic magnetoresistance-based sensor: A model for a magnetoresistance-based biosensor. Appl. Phys. Lett. 2002, 81, 2211–2213. [Google Scholar] [CrossRef]
- Li, F.; Gooneratne, C.; Kosel, J. Magnetic Biosensor System to Detect Biological Targets. In Proceedings of 2012 International Conference on Electromagnetics in Advanced Applications (ICEAA), Cape Town, South Africa, 2–7 September 2012; pp. 1238–1241.
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistive technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Edelstein, R.L.; Tamanaha, C.R.; Sheehan, P.E.; Miller, M.M.; Baselt, D.R.; Whitman, L.J.; Colton, R.J. The BARC biosensor applied to the detection of biological warfare agents. Biosens. Bioelectron. 2000, 14, 805–813. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Pühler, A.; Reiss, G.; Brückl, H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef]
- Koets, M.; van der Wijk, T.; van Eemeren, J.T.W.M.; van Amerongen, A.; Prins, M.W.J. Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens. Bioelectron. 2009, 24, 1893–1898. [Google Scholar] [CrossRef]
- Mujika, M.; Arana, S.; Castano, E.; Tijero, M.; Vilares, R.; Ruano-López, J.M.; Cruz, A.; Sainz, L.; Berganza, J. Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network. Biosens. Bioelectron. 2009, 24, 1253–1258. [Google Scholar] [CrossRef]
- Manteca, A.; Mujika, M.; Arana, S. GMR sensors: Magnetoresistive behavior optimization for biological detection by means of superparamagnetic nanoparticles. Biosens. Bioelectron. 2011, 26, 3705–3709. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef]
- Iverson, B.D.; Garimella, S.V. Recent advances in microscale pumping technologies: A review and evaluation. Microfluid. Nanofluidics 2008, 5, 145–174. [Google Scholar] [CrossRef]
- Luo, Y.; Qin, J.H.; Lin, B.C. Methods for pumping fluids on biomedical lab-on-a-chip. Front. Biosci. 2009, 14, 3913–3924. [Google Scholar]
- Sharp, J.M.; Clapp, A.R.; Dickinson, R.B. Measurement of long-range forces on a single yeast cell using a gradient optical trap and evanescent wave light scattering. Colloids Surf. B 2003, 27, 355–364. [Google Scholar] [CrossRef]
- Sinha, A.; Ganguly, R.; Puri, I.K. Magnetic separation from superparamagnetic particle suspensions. J. Magn. Magn. Mater. 2009, 321, 2251–2256. [Google Scholar] [CrossRef]
- Deng, T.; Whitesides, G.M.; Radhakrishnan, M.; Zabow, G.; Prentiss, M. Manipulation of magnetic microbeads in suspension using micromagnetic systems fabricated with soft lithography. Appl. Phys. Lett. 2001, 78, 1775–1777. [Google Scholar] [CrossRef]
- Weddemann, A.; Wittbracht, F.; Auge, A.; Hütten, A. A hydrodynamic switch: Microfluidic separation system for magnetic beads. Appl. Phys. Lett. 2009, 94, 173501:1–173501:3. [Google Scholar]
- Lee, C.S.; Lee, H.; Westervelt, R.M. Microelectromagnets for the control of magnetic nanoparticles. Appl. Phys. Lett. 2001, 79, 3308–3310. [Google Scholar] [CrossRef]
- Auge, A.; Weddemann, A.; Wittbracht, F.; Hütten, A. Magnetic ratchet for biotechnological applications. Appl. Phys. Lett. 2009, 94, 183507:1–183507:3. [Google Scholar]
- Helmich, L. Separation of Magnetically Functionalized Cells in a Microfluidic Rocking Ratchet. Master Thesis, Bielefeld University, Bielefeld, Germany, April 2013. [Google Scholar]
- Weddemann, A.; Wittbracht, F.; Auge, A.; Hütten, A. Positioning system for particles in microfluidic structures. Microfluid. Nanofluidics 2009, 7, 849–855. [Google Scholar] [CrossRef]
- Kappe, D.; Hütten, A. Positioning System for Particles in Microfluidic Structures. In Proceedings of Comsol Conference 2012, Milan, Italy, 10–12 October 2012.
- Chiu, Y.J.; Cho, S.H.; Mei, Z.; Lien, V.; Wu, T.F.; Lo, Y.H. Universally applicable three-dimensional hydrodynamic microfluidic flow focusing. Lab Chip 2013, 13, 1803–1809. [Google Scholar] [CrossRef]
- Wiklund, M.; Radel, S.; Hawkes, J.J. Acoustofluidics 21: Ultrasound-enhanced immunoassays and particle sensors. Lab Chip 2013, 13, 25–39. [Google Scholar] [CrossRef]
- Zourob, M.; Hawkes, J.J.; Coakley, W.T.; Treves Brown, B.J.; Fielden, P.R.; McDonnell, M.B.; Goddard, N.J. Optical leaky waveguide sensor for detection of bacteria with ultrasound attractor force. Anal. Chem. 2005, 77, 6163–6168. [Google Scholar] [CrossRef]
- Hawkes, J.J.; Long, M.J.; Coakley, W.T.; McDonnell, M.B. Ultrasonic deposition of cells on a surface. Biosens. Bioelectron. 2004, 19, 1021–1028. [Google Scholar] [CrossRef]
- Glynne-Jones, P.; Boltryk, R.J.; Hill, M.; Zhang, F.; Dong, L.; Wilkinson, J.S.; Melvin, T.; Harris, N.R.; Brown, T. Flexible acoustic particle manipulation device with integrated optical waveguide for enhanced microbead assays. Anal. Sci. 2009, 25, 285–291. [Google Scholar] [CrossRef]
- Oberti, S.; Neild, A.; Dual, J. Manipulation of micrometer sized particles within a micromachined fluidic device to form two-dimensional patterns using ultrasound. J. Acoust. Soc. Am. 2007, 121, 778–785. [Google Scholar] [CrossRef]
- González, I.; Fernández, L.J.; Gómez, T.E.; Berganzo, J.; Soto, J.L.; Carrato, A. A polymeric chip for micromanipulation and particle sorting by ultrasounds based on a multilayer configuration. Sens. Actuators B Chem. 2010, 144, 310–317. [Google Scholar] [CrossRef]
- Glynne-Jones, P.; Boltryk, R.J.; Hill, M.; Harris, N.R.; Baclet, P. Robust acoustic particle manipulator: A thin-reflector design for moving particles to a surface. J. Acoust. Soc. Am. 2009, 126, EL75–EL79. [Google Scholar] [CrossRef]
- Binasch, G.; Grünberg, P.; Saurenbach, F.; Zinn, W. Enhanced magnetoresistance in layered magnetic-structures with antiferromagnetic interlayer exchange. Phys. Rev. B 1989, 39, 4828–4830. [Google Scholar] [CrossRef]
- Baibich, M.N.; Broto, J.M.; Fert, A.; Vandau, F.N.; Petroff, F.; Eitenne, P.; Creuzet, G.; Friederich, A.; Chazelas, J. Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef]
- Mott, N.F. Electrons in transition metals. Adv. Phys. 1964, 13, 325–422. [Google Scholar] [CrossRef]
- Wedemann, A.; Ennen, I.; Regtmeier, A.; Albon, C.; Wolff, A.; Eckstädt, K.; Mill, N.; Peter, M.K.H.; Mattay, J.; Plattner, C.; et al. Review and outlook: From single nanoparticles to self-assembled monolayers and granular GMR sensors. Beilstein J. Nanotechnol. 2010, 1, 75–93. [Google Scholar]
- Berkowitz, A.E.; Mitchell, J.R.; Carey, M.J.; Young, A.P.; Zhang, S.; Spada, F.E.; Parker, F.T.; Hütten, A.; Thomas, G. Giant magnetoresistance in heterogeneous Cu-Co alloys. Phys. Rev. Lett. 1992, 68, 3745–3748. [Google Scholar] [CrossRef]
- Xiao, J.Q.; Jiang, J.S.; Chien, C.L. Giant magnetoresistance in nonmultilayer magnetic systems. Phys. Rev. Lett. 1992, 68, 3749–3752. [Google Scholar] [CrossRef]
- Meyer, J.; Rempel, T.; Schäfers, M.; Wittbracht, F.; Müller, C.; Patel, A.V.; Hütten, A. Giant magnetoresistance effects in gel-like matrices. Smart Mater. Struct. 2013, 22, 025032. [Google Scholar] [CrossRef]
- Allia, P.; Knobel, M.; Tiberto, P.; Vinai, F. Magnetic properties and giant magnetoresistance of melt-spun granular Cu100-x-Cox alloys. Phys. Rev. B 1995, 52, 15398–15411. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ding, J.; Si, L.; Cheung, W.Y.; Wong, S.P.; Wilson, I.H.; Suzuki, T. Magnetic domain structures and magnetotransport properties in Co-Ag granular thin films. Appl. Phys. A Mater. Sci. Proc. 2001, 73, 103–106. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eickenberg, B.; Meyer, J.; Helmich, L.; Kappe, D.; Auge, A.; Weddemann, A.; Wittbracht, F.; Hütten, A. Lab-on-a-Chip Magneto-Immunoassays: How to Ensure Contact between Superparamagnetic Beads and the Sensor Surface. Biosensors 2013, 3, 327-340. https://doi.org/10.3390/bios3030327
Eickenberg B, Meyer J, Helmich L, Kappe D, Auge A, Weddemann A, Wittbracht F, Hütten A. Lab-on-a-Chip Magneto-Immunoassays: How to Ensure Contact between Superparamagnetic Beads and the Sensor Surface. Biosensors. 2013; 3(3):327-340. https://doi.org/10.3390/bios3030327
Chicago/Turabian StyleEickenberg, Bernhard, Judith Meyer, Lars Helmich, Daniel Kappe, Alexander Auge, Alexander Weddemann, Frank Wittbracht, and Andreas Hütten. 2013. "Lab-on-a-Chip Magneto-Immunoassays: How to Ensure Contact between Superparamagnetic Beads and the Sensor Surface" Biosensors 3, no. 3: 327-340. https://doi.org/10.3390/bios3030327
APA StyleEickenberg, B., Meyer, J., Helmich, L., Kappe, D., Auge, A., Weddemann, A., Wittbracht, F., & Hütten, A. (2013). Lab-on-a-Chip Magneto-Immunoassays: How to Ensure Contact between Superparamagnetic Beads and the Sensor Surface. Biosensors, 3(3), 327-340. https://doi.org/10.3390/bios3030327