An Electrochemical Immunosensor for Detection of Staphylococcus aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Bacteria Cultivation
2.3. Instrumentation and Techniques
2.4. Elaboration of the Immunosensor
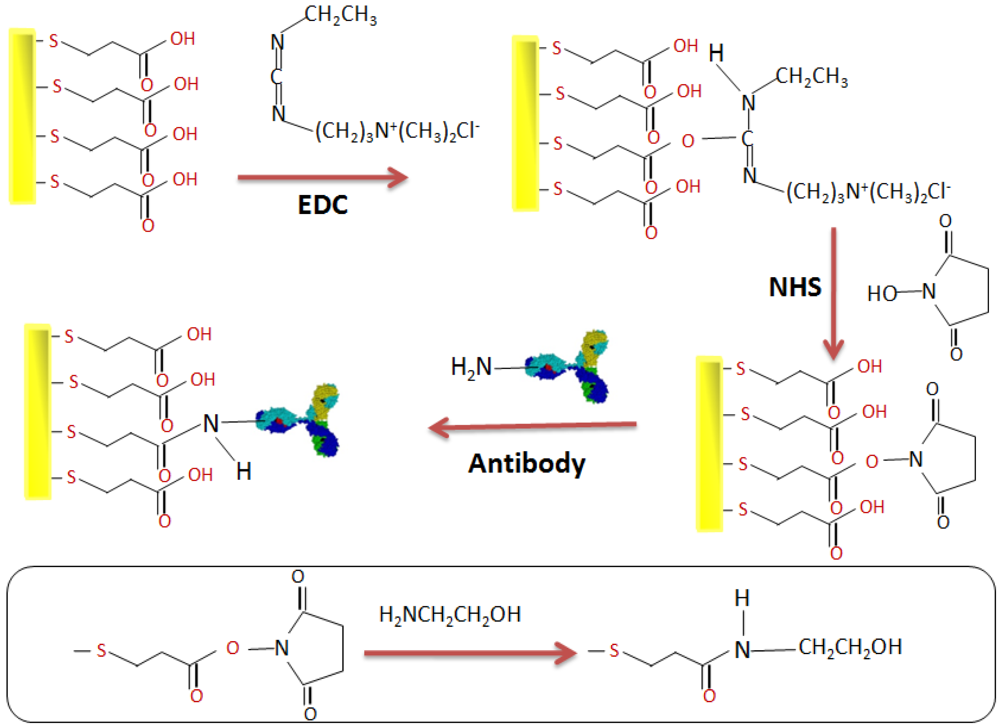
3. Results and Discussion
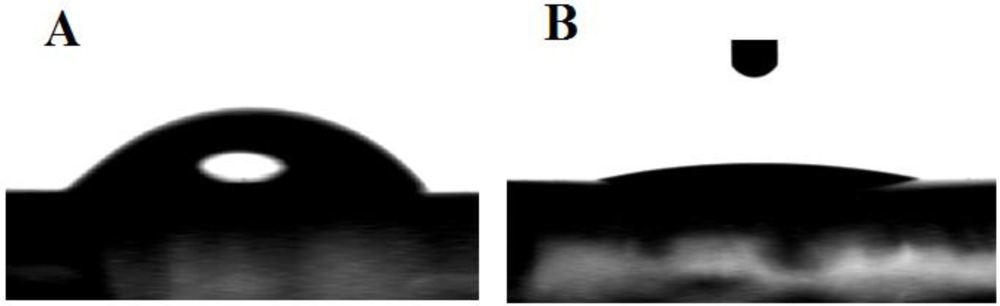
3.1. Characterization of the Functionalized Electrode

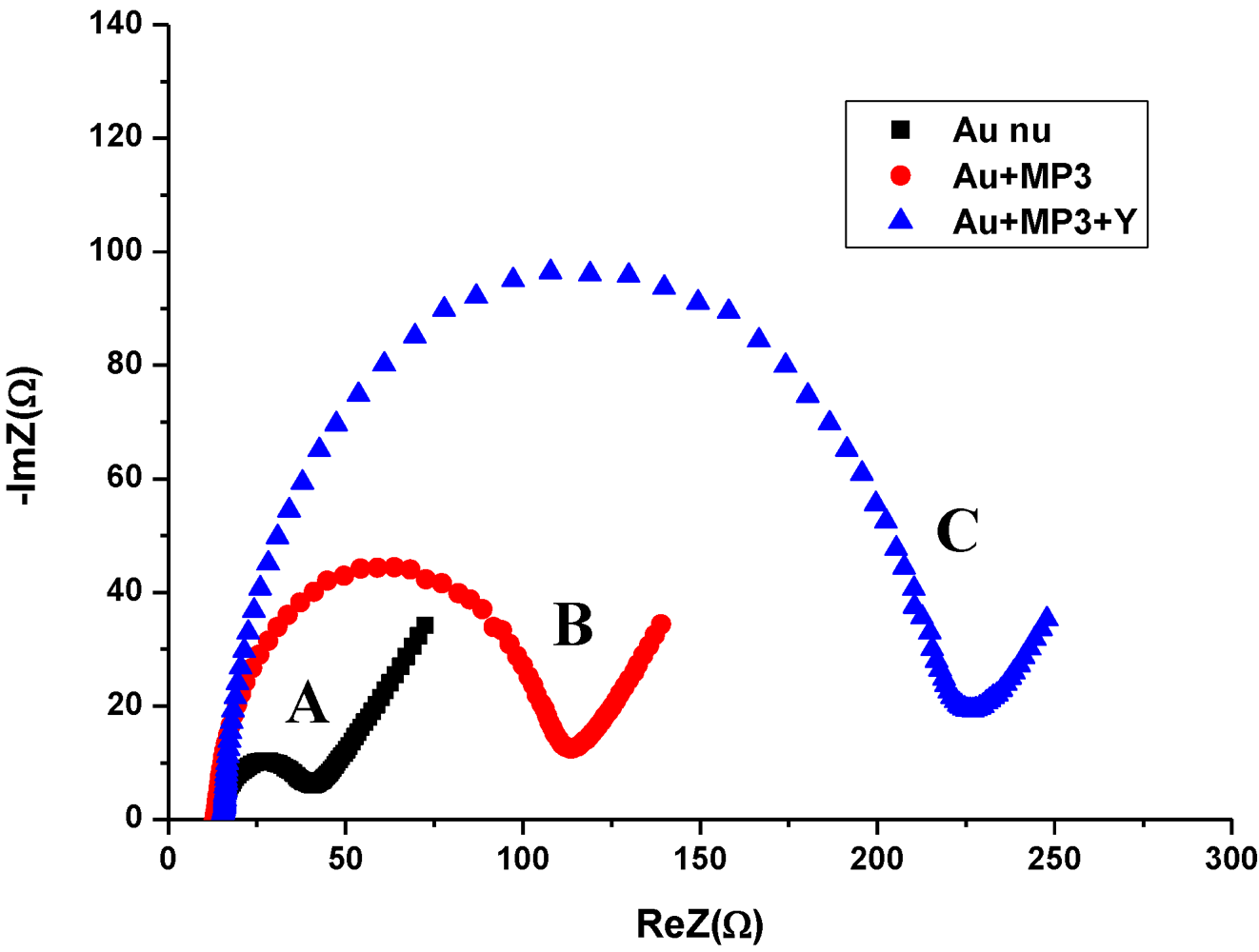
3.2. Impedance Measurements for the Detection of S. aureus Bacteria

- -
- A resistance related to the charge transfer rate of the redox reaction at the biofunctionalized electrode; RCT.
- -
- A constant phase element (CPE) that is related to the capacitance of the biofunctionalized Au electrode/electrolyte interface. CPE reflects the non-ideality of the double-layer at the biofunctionalized gold electrode/electrolyte interface due to the roughness and porosity of the biofilm.
- -
- A specific electrochemical element of diffusion, the Warburg element (Zw).
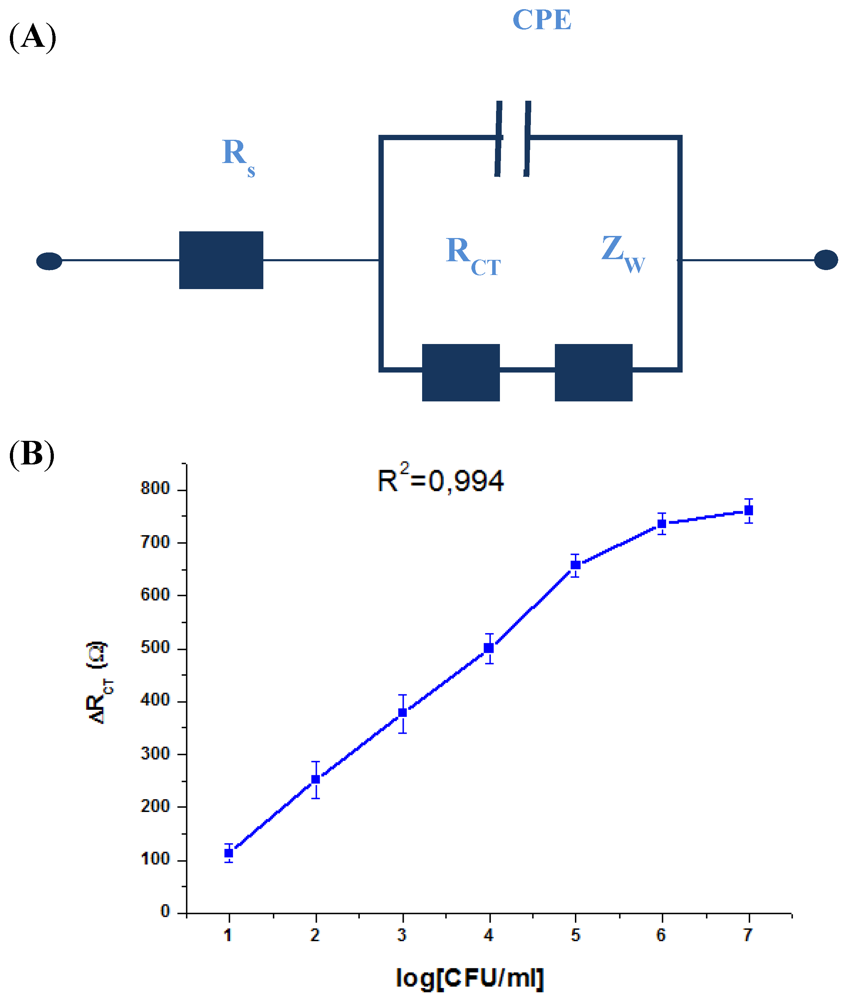
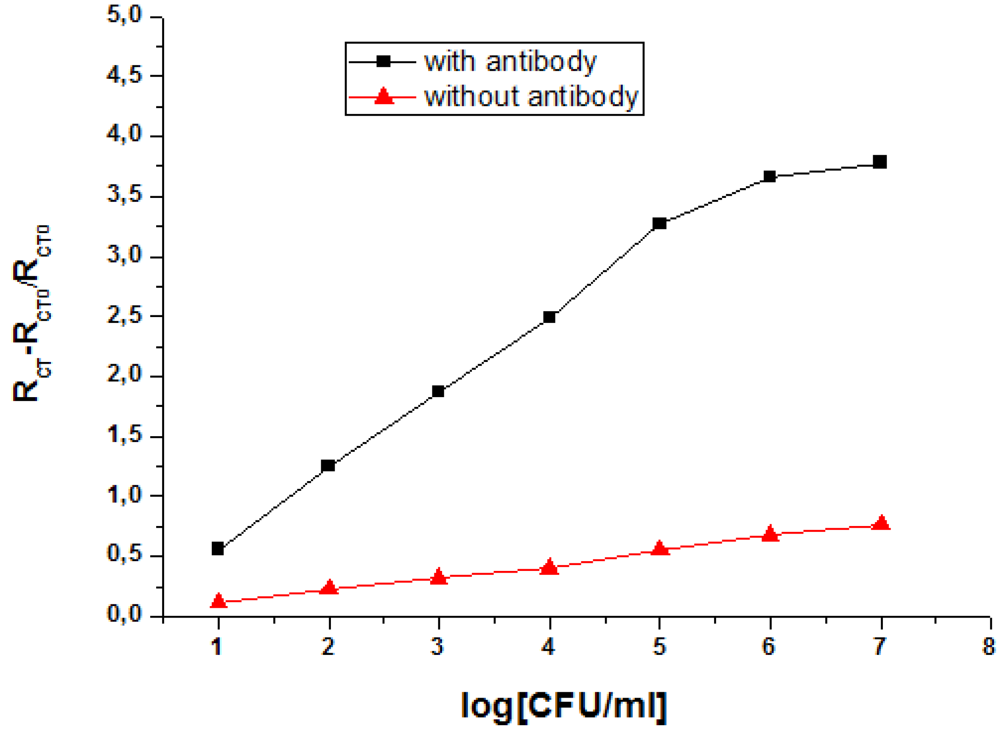
| Type of transducer | Detection Limit | Dynamic range | Reference |
|---|---|---|---|
| Impedance | 10 CFU/mL | 10–106 CFU/mL | This work |
| Impedance | 102 CFU/mL | 102–107 CFU/mL | [11] |
| Amperometry | 3.7 × 102 CFU/mL | 1.3 × 103–7.6 × 104 CFU/mL | [10] |
| SPR | 105 CFU/mL | - | [9] |
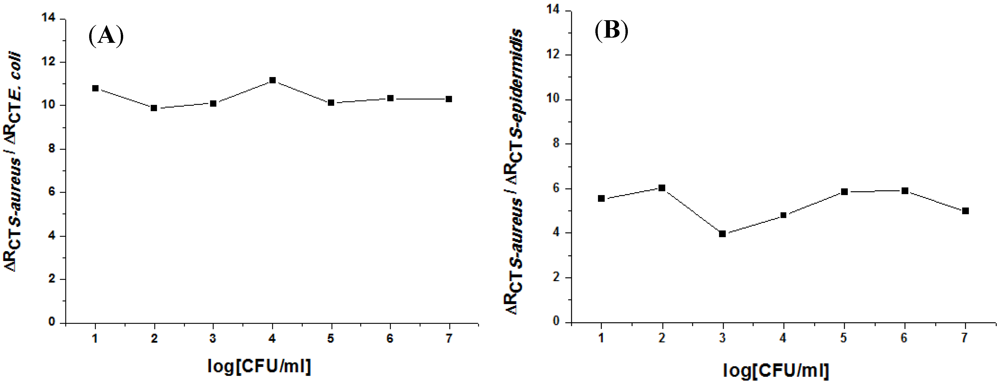
4. Conclusion
Acknowledgments
References
- Chaubeau-Duffour, C. Toxi-infections alimentaires d’origine staphylococcique. Le Point Vétérinaire 1992, 24, 33–40. [Google Scholar]
- De Buyser, M.-L.; Lapeyre, C. Mammites à staphylocoques et sécurité alimentaire. Le Point Vétérinaire 1994, 26, 79–82. [Google Scholar]
- Brisabois, A.; Lafarge, V.; Brouillaud, A.; De Buyser, M.L.; Collette, C.; Garin-Bastuji, B.; Thorel, M.F. Les germes pathogènes dans le lait et les produits laitiers: Situation en France et en Europe. Rev. Sci. Tech. 1997, 16, 452–471. [Google Scholar]
- Boujday, S.; Briandet, R.; Salmain, M.; Herry, J.-M.; Marnet, P.-G.; Gautier, M.; Pradier, C.-M. Detection of pathogenic Staphylococcus aureus bacteria by gold based immunosensors. Microchim. Acta 2008, 163, 203–209. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Zhou, L.; Ye, Z.; Wang, J.; Ying, Y.; Ruan, C.; Wang, R.; Li, Y. Label-free capacitive immunosensor based on quartz crystal Au electrode for rapid and sensitive detection of Escherichia coli O157:H7. Anal. Chim. Acta. 2011, 687, 89–96. [Google Scholar] [CrossRef]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. A mixed self-assembled monolayer-based surface plasmonimmunosensor for detection of E. coli O157:H7. Biosens. Bioelectron 2006, 7, 998–1006. [Google Scholar]
- Laczka, O.; Baldrich, E.; Muñoz, F.X.; del Campo, F. Detection of Escherichia coli and Salmonella typhimurium using interdigitated microelectrode capacitive immunosensors: The importance of transducer geometry. J. Anal. Chem. 2008, 80, 7239–7247. [Google Scholar] [CrossRef]
- Salam, F.; Tothill, I.E. Detection of Salmonella typhimurium using an electrochemical immunosensor. Biosens. Bioelectron. 2009, 24, 2630–2636. [Google Scholar] [CrossRef]
- Subramanian, A.; Irudayaraj, J.; Ryan, T. Mono and dithiol surfaces on surface plasmon resonance biosensors for detection of Staphylococcus aureus. Sens. Actuator. B 2006, 114, 192–198. [Google Scholar] [CrossRef]
- Escamilla-Gomez, V.; Campuzano, S.; Pedrero, M.; Pingarron, J.M. Electrochemical immunosensor designs for the determination of Staphylococcus aureus using 3,3-dithiodipropionic acid di(N-succinimidyl ester)-modified gold electrodes. Talanta 2008, 77, 876–881. [Google Scholar] [CrossRef]
- Tan, F.; Leung, P.H.M.; Liu, Z.B.; Zhang, Y.; Xiao, L.D.; Ye, W.W.; Zhang, X.; Yi, L.; Yang, M. A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens. Actuator. B 2011, 159, 328–335. [Google Scholar] [CrossRef]
- Grysakowski, B.; Jasielec, J.; Wierzba, B.; Sokalski, T.; Lewenstam, A.; Danielewski, M. Electrochemical Impedance Spectroscopy (EIS) of ion sensors: Direct modeling and inverse problem solving using the Nernst-Planck-Poisson (NPP) model and the HGS(FP) optimization strategy. J. Electroanal. Chem. 2011, 662, 143–149. [Google Scholar] [CrossRef]
- Prodromidis, M.I. Impedimetricimmunosensors—A review. Electrochim. Acta 2010, 55, 4227–4223. [Google Scholar] [CrossRef]
- Tamchang, S.W.; Biebuyck, H.A.; Whitesides, G.M.; Jeon, N.; Nuzzo, R.G. Self-assembled monolayers on gold generated from Alkanethiols with the structure RNHCOCH2SH. Langmuir 1995, 11, 4371–4382. [Google Scholar] [CrossRef]
- Myrskog, A.; Anderson, H.; Aastrup, T.; Ingemarsson, B.; Liedberg, B. Esterification of self-assembled carboxylic-acid-terminated thiol monolayers in acid environment: A time-dependent study. Langmuir 2010, 26, 821–829. [Google Scholar] [CrossRef]
- Gawad, S.; Cheung, K.; Seger, U.; Bertsch, A.; Renaud, P. Dielectric spectroscopy in a micromachined flow cytometer: Theoretical and practical considerations. Lab Chip 2004, 4, 241–251. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Braiek, M.; Rokbani, K.B.; Chrouda, A.; Mrabet, B.; Bakhrouf, A.; Maaref, A.; Jaffrezic-Renault, N. An Electrochemical Immunosensor for Detection of Staphylococcus aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode. Biosensors 2012, 2, 417-426. https://doi.org/10.3390/bios2040417
Braiek M, Rokbani KB, Chrouda A, Mrabet B, Bakhrouf A, Maaref A, Jaffrezic-Renault N. An Electrochemical Immunosensor for Detection of Staphylococcus aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode. Biosensors. 2012; 2(4):417-426. https://doi.org/10.3390/bios2040417
Chicago/Turabian StyleBraiek, Mohamed, Karima Bekir Rokbani, Amani Chrouda, Béchir Mrabet, Amina Bakhrouf, Abderrazak Maaref, and Nicole Jaffrezic-Renault. 2012. "An Electrochemical Immunosensor for Detection of Staphylococcus aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode" Biosensors 2, no. 4: 417-426. https://doi.org/10.3390/bios2040417
APA StyleBraiek, M., Rokbani, K. B., Chrouda, A., Mrabet, B., Bakhrouf, A., Maaref, A., & Jaffrezic-Renault, N. (2012). An Electrochemical Immunosensor for Detection of Staphylococcus aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode. Biosensors, 2(4), 417-426. https://doi.org/10.3390/bios2040417






