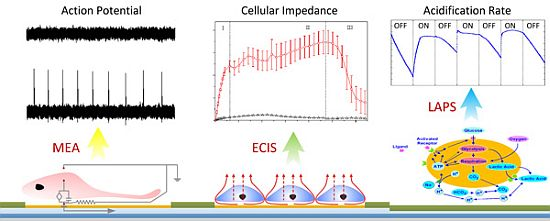
Microfabricated Electrochemical Cell-Based Biosensors for Analysis of Living Cells In Vitro
Abstract
:1. Introduction
2. Principles of Electrochemical Cell-Based Biosensors
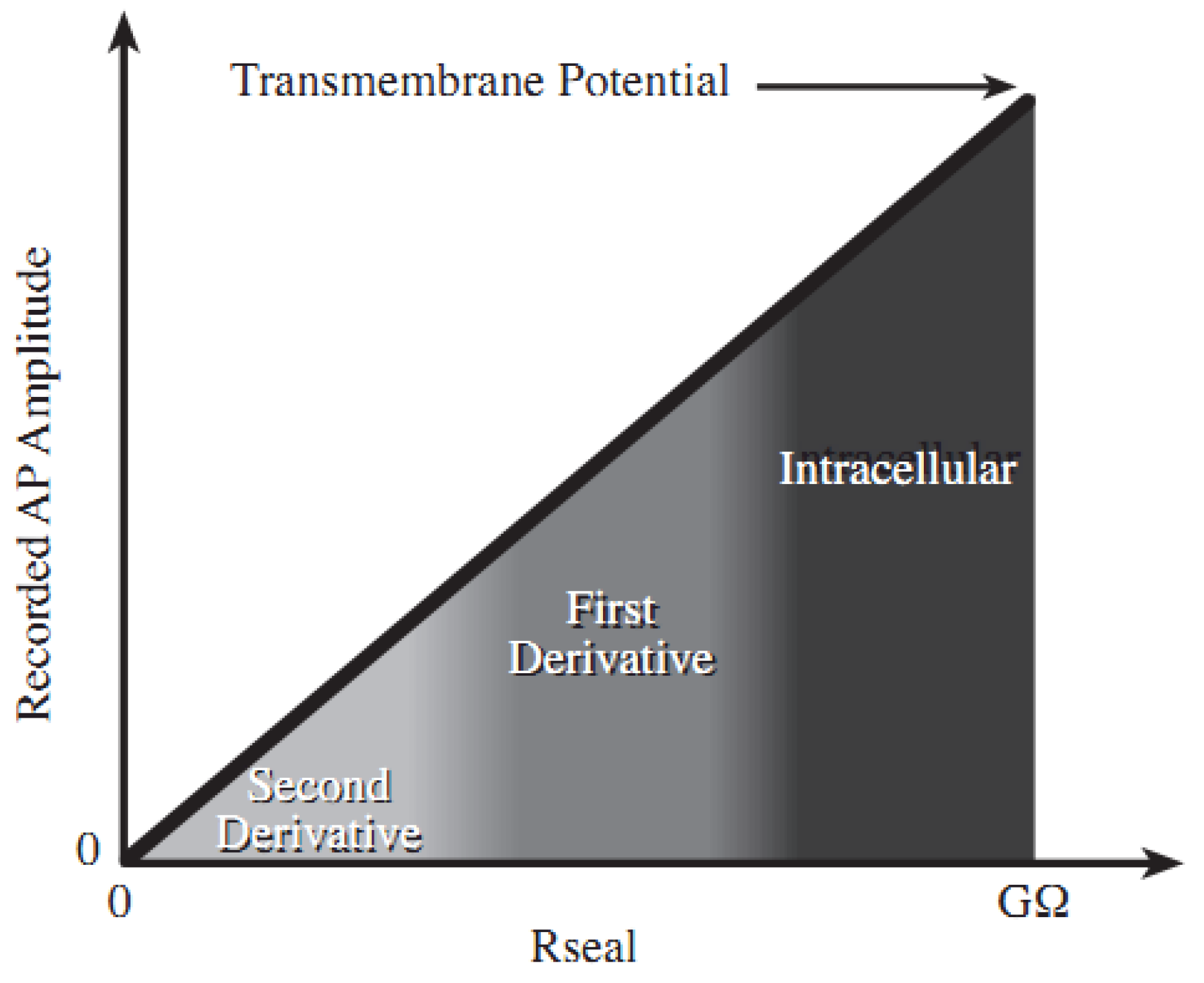
2.1. Theory and Structure of Microelectrode Array


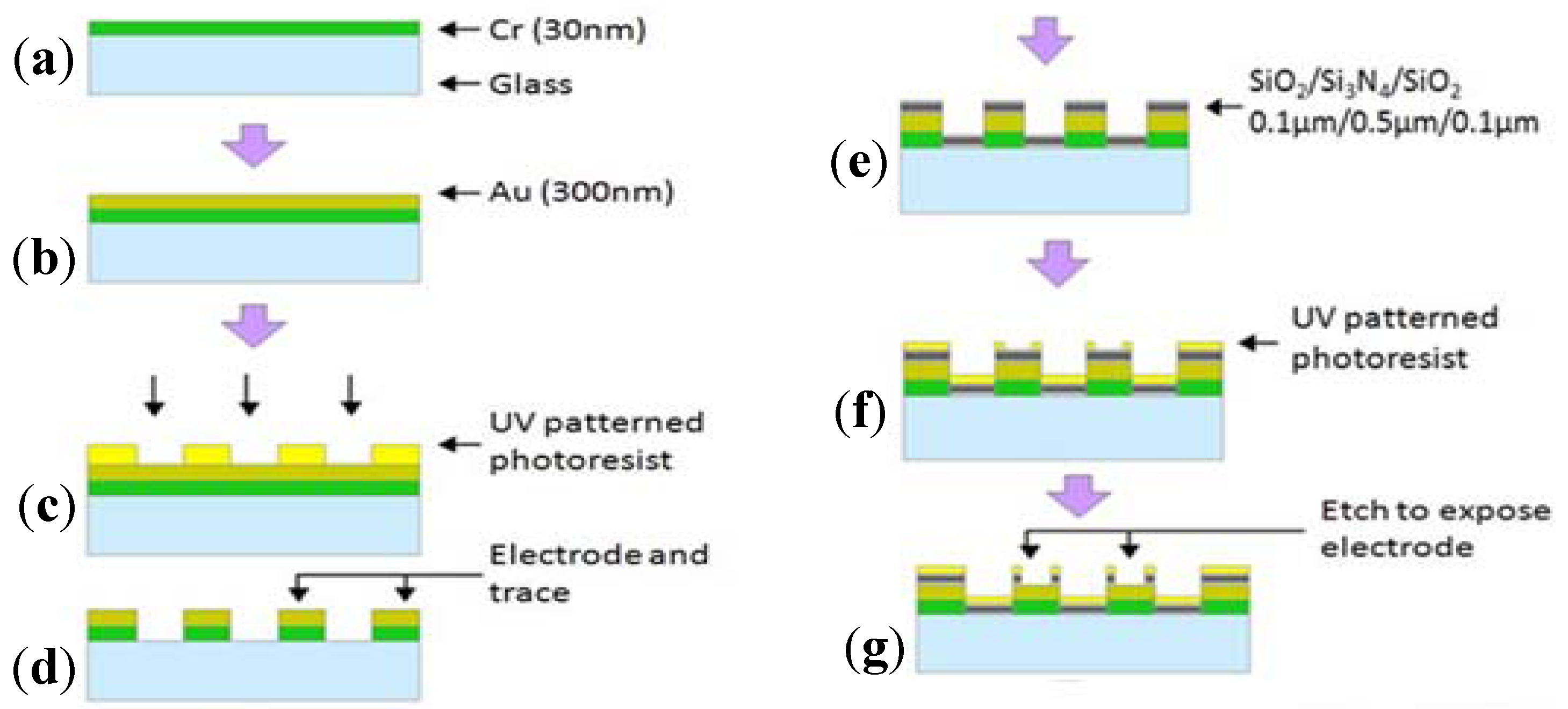
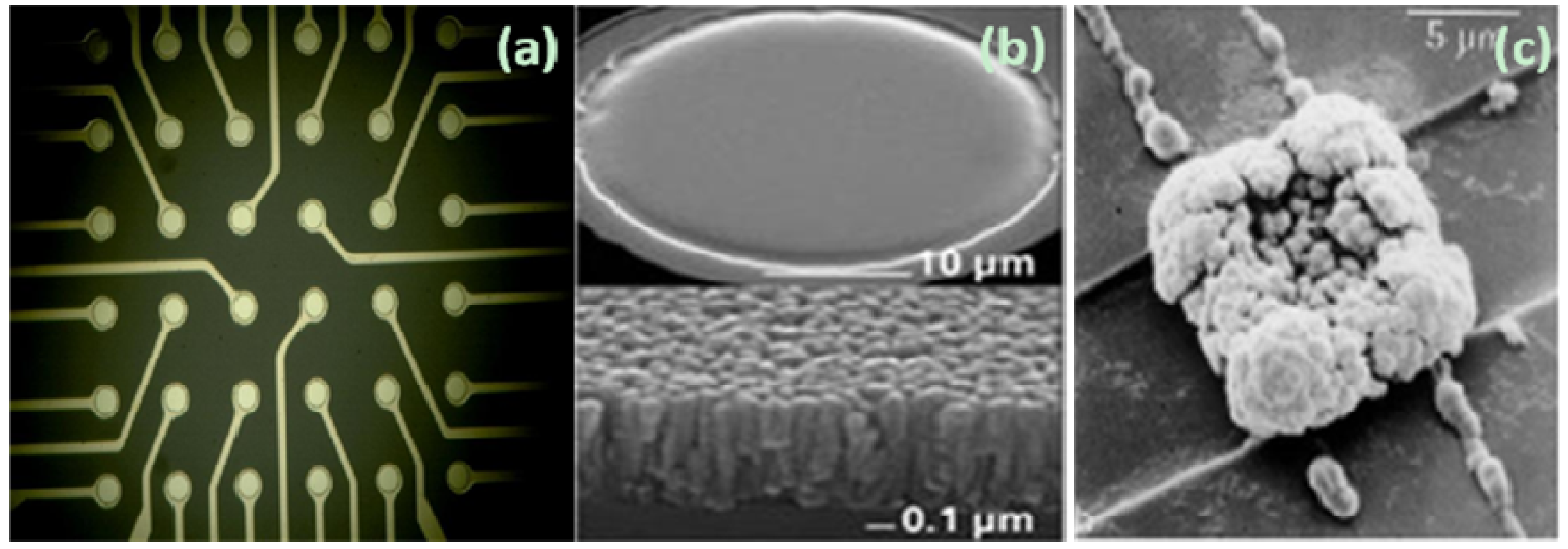
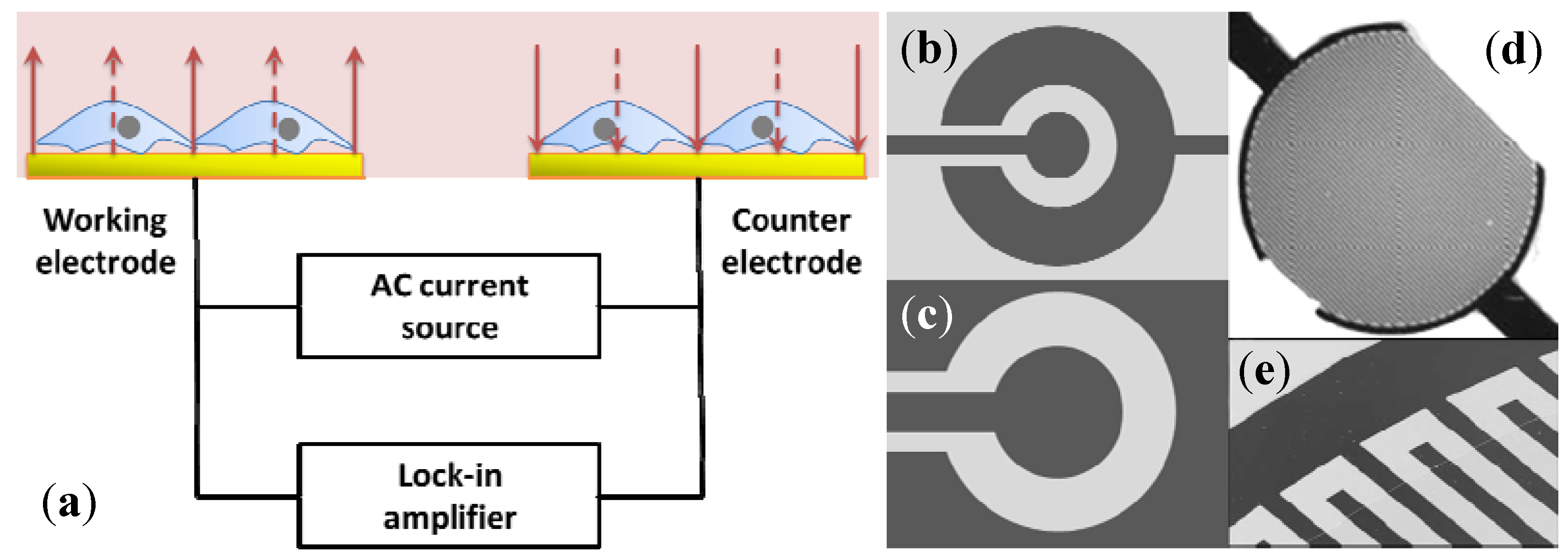
2.2. Electric Cell-Substrate Impedance Sensor
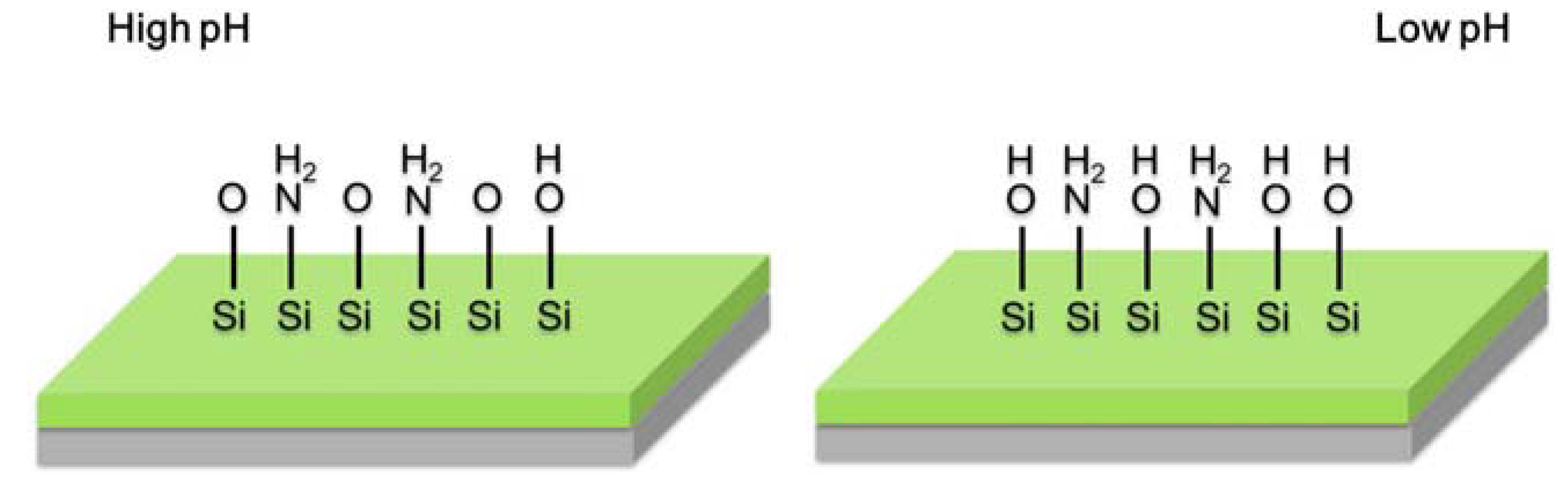
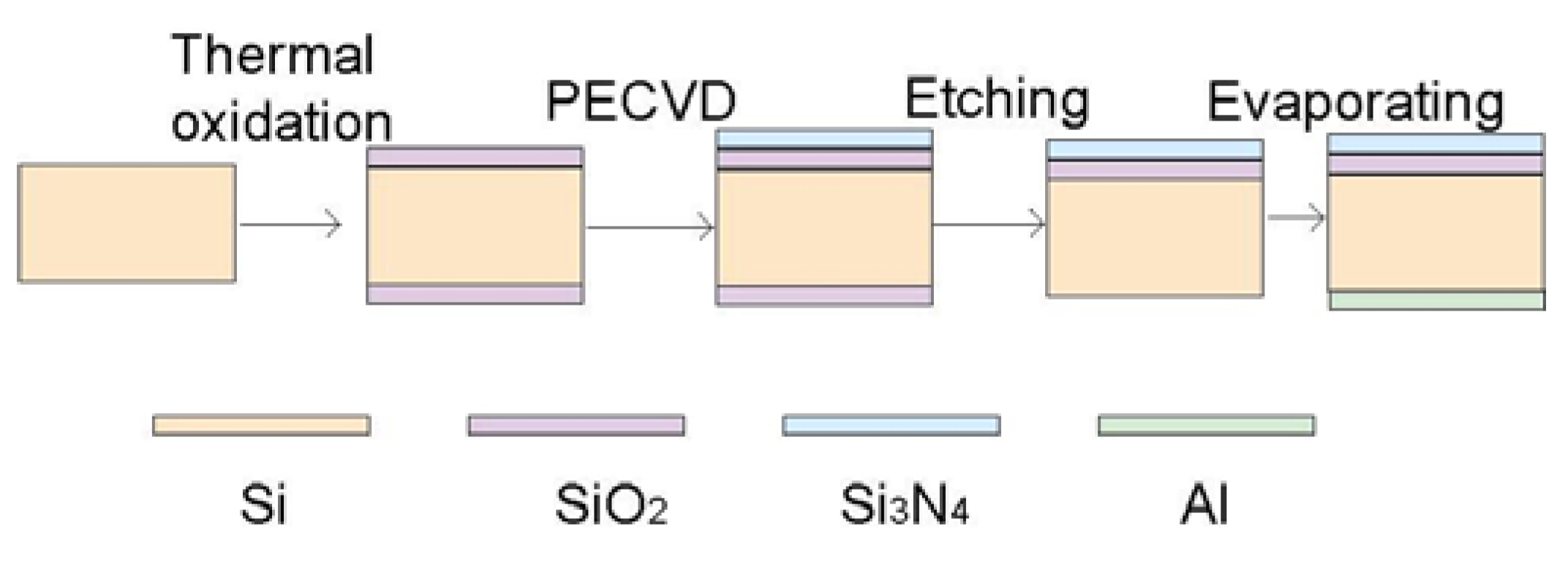
2.3. Light Addressable Potentiometric Sensor
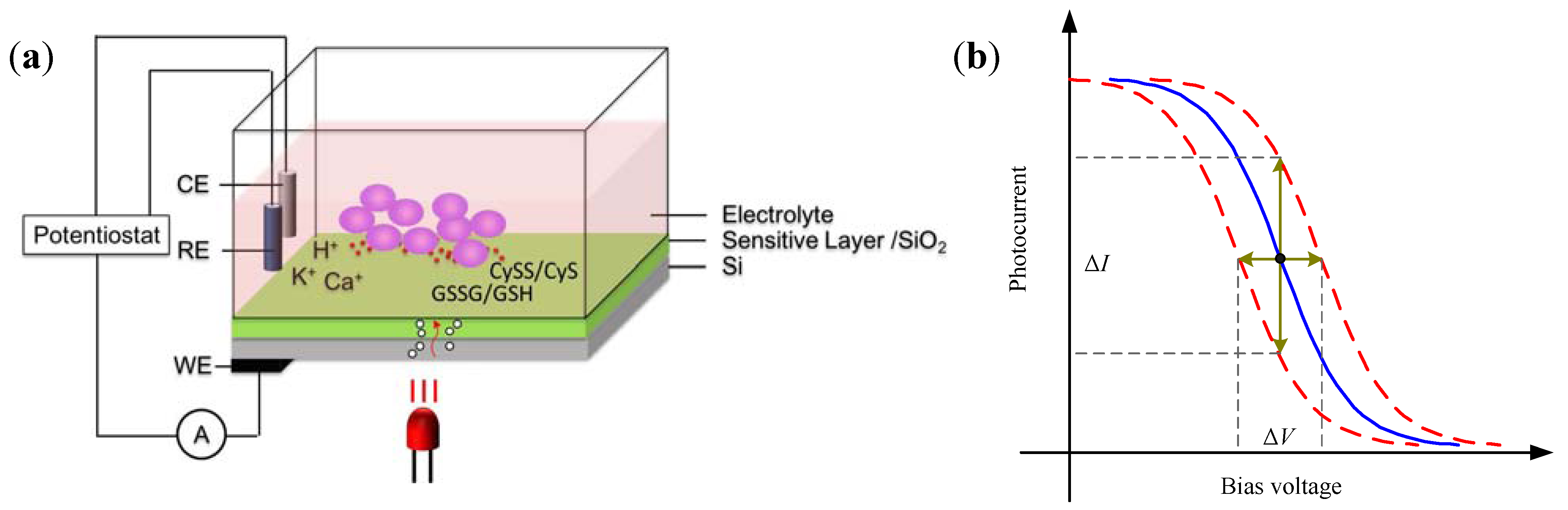

3. Applications in Biochemical Monitoring of Living Cells
3.1. Cellular Electrophysiology Detection
3.1.1. Cardiomyocyte Detection
3.1.2. Brain Slice Detection

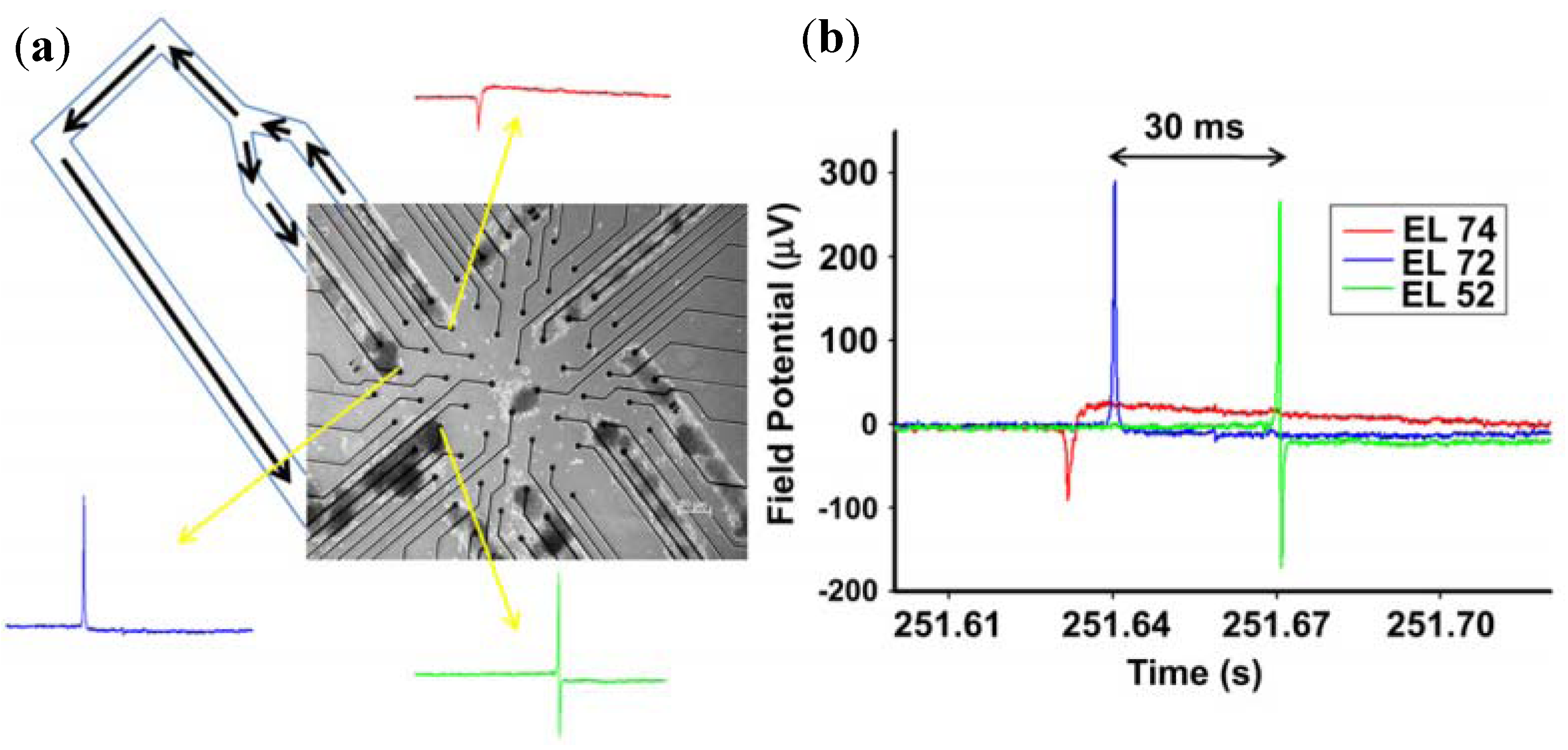
3.1.3. Dissociated Neural Network Detection

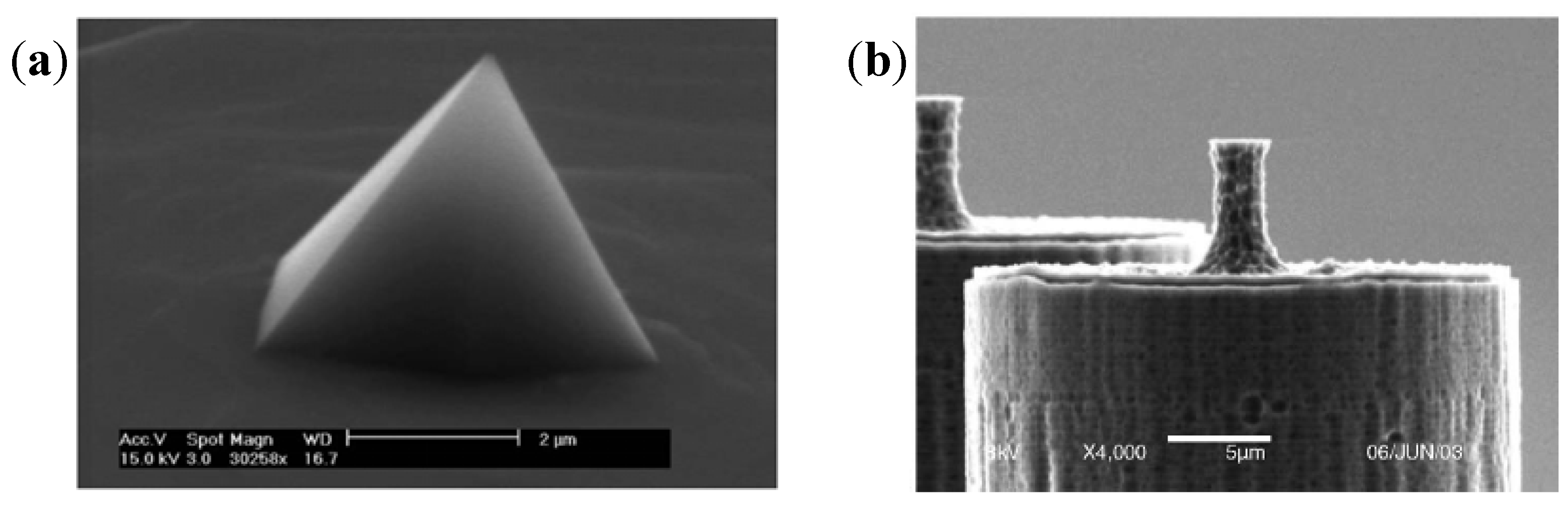
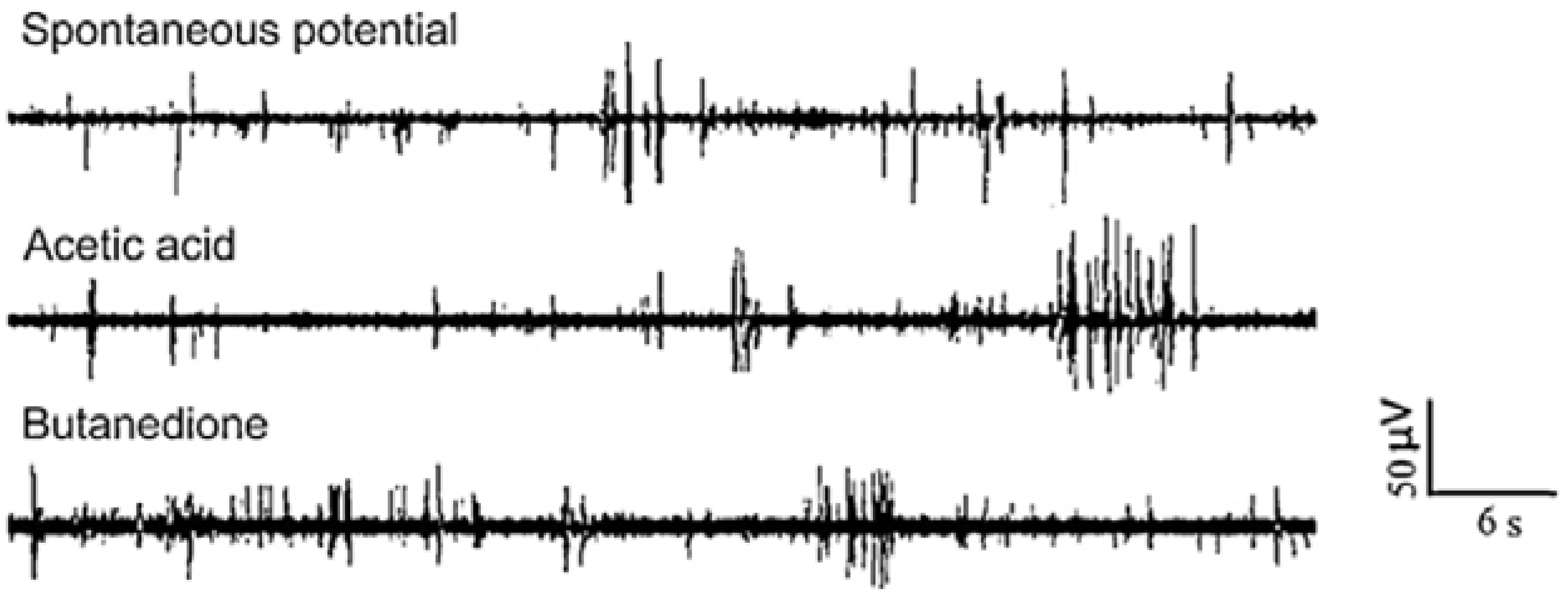
3.1.4. Other Electrogenic Tissues or Cells Detection
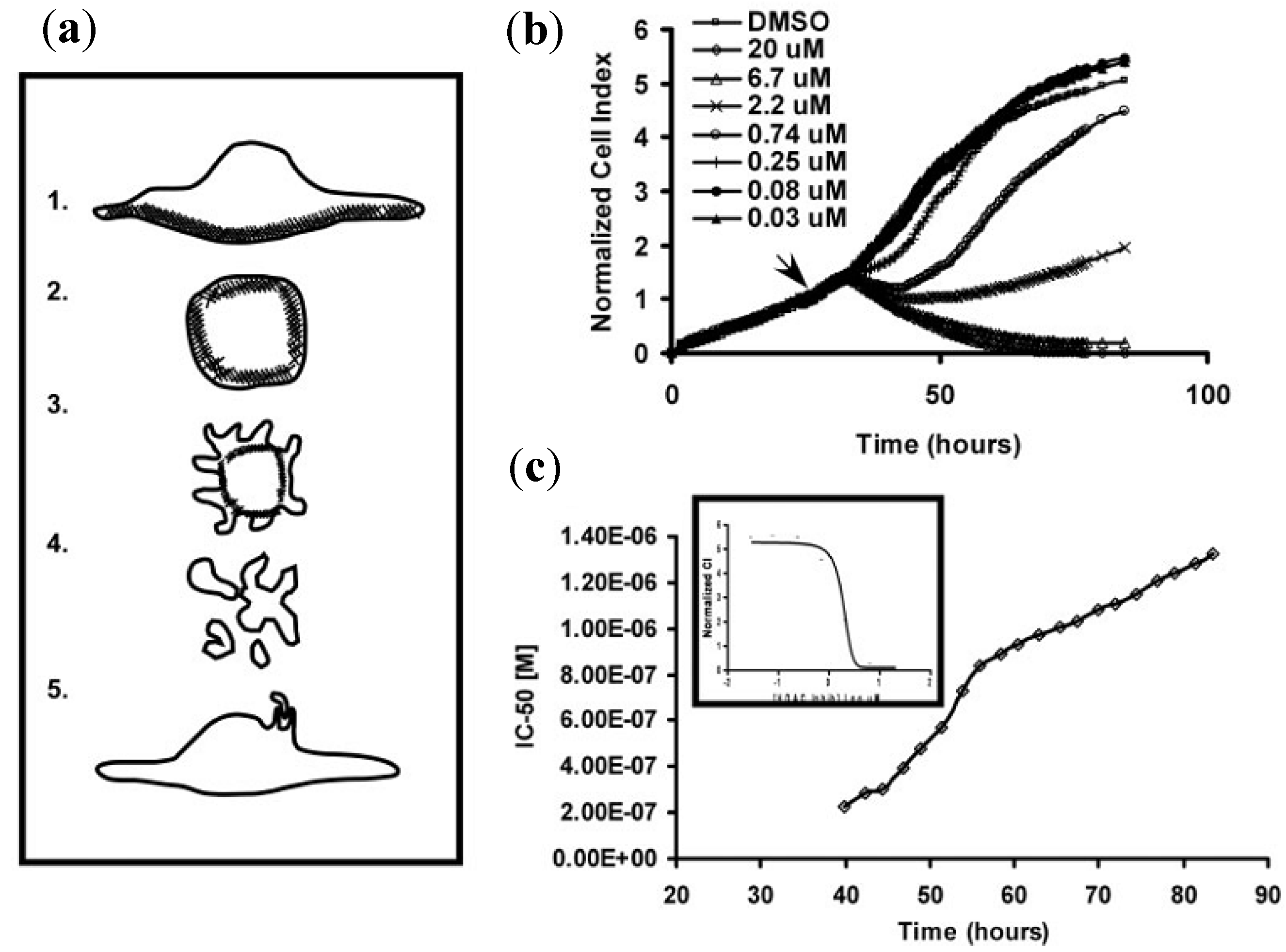
3.2. Cellular Morphology Detection
3.2.1. Cell Adhesion and Proliferation

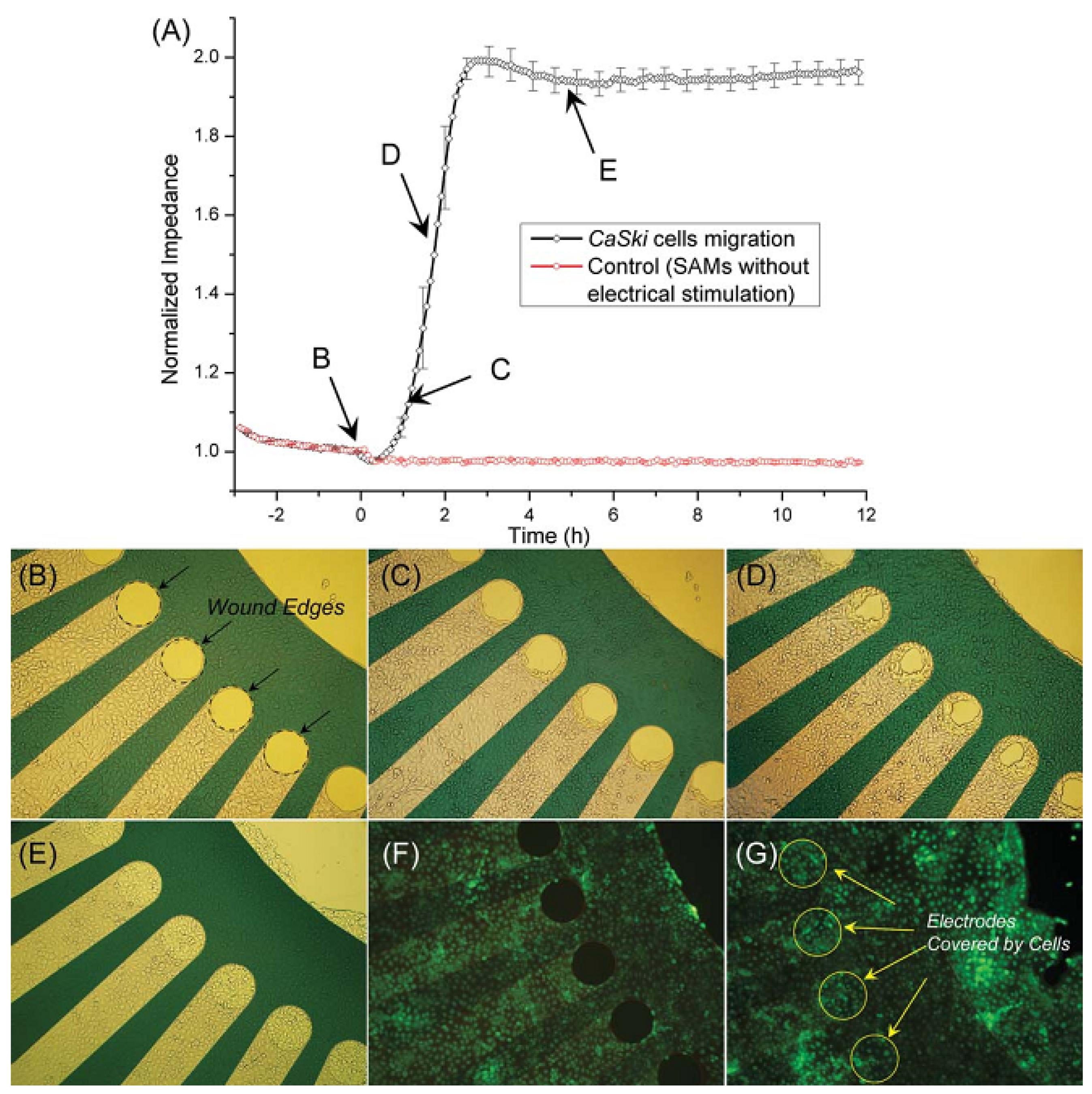
3.2.2. Cell Migration and Metastasis

3.3. Cellular Metabolism Detection
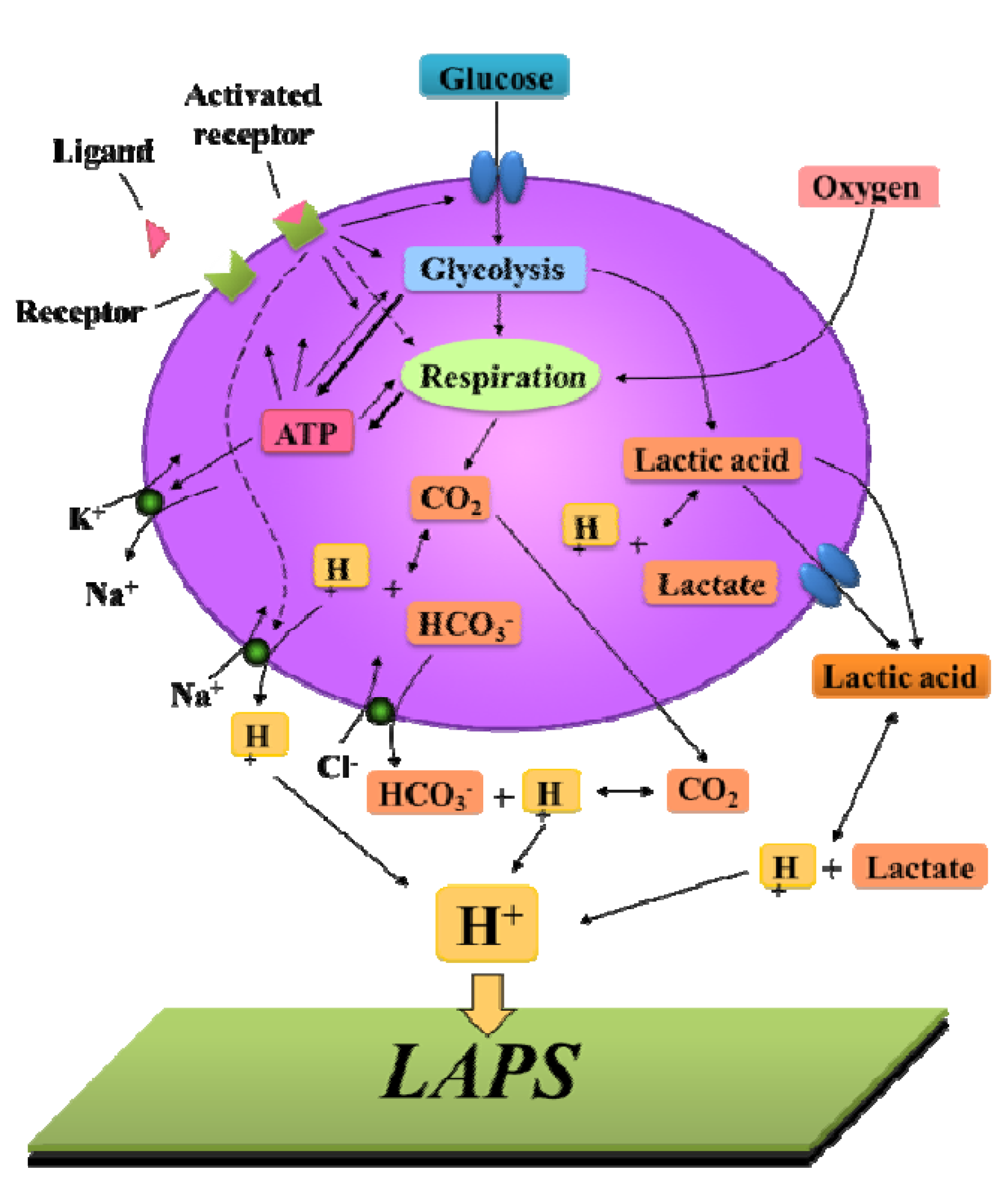
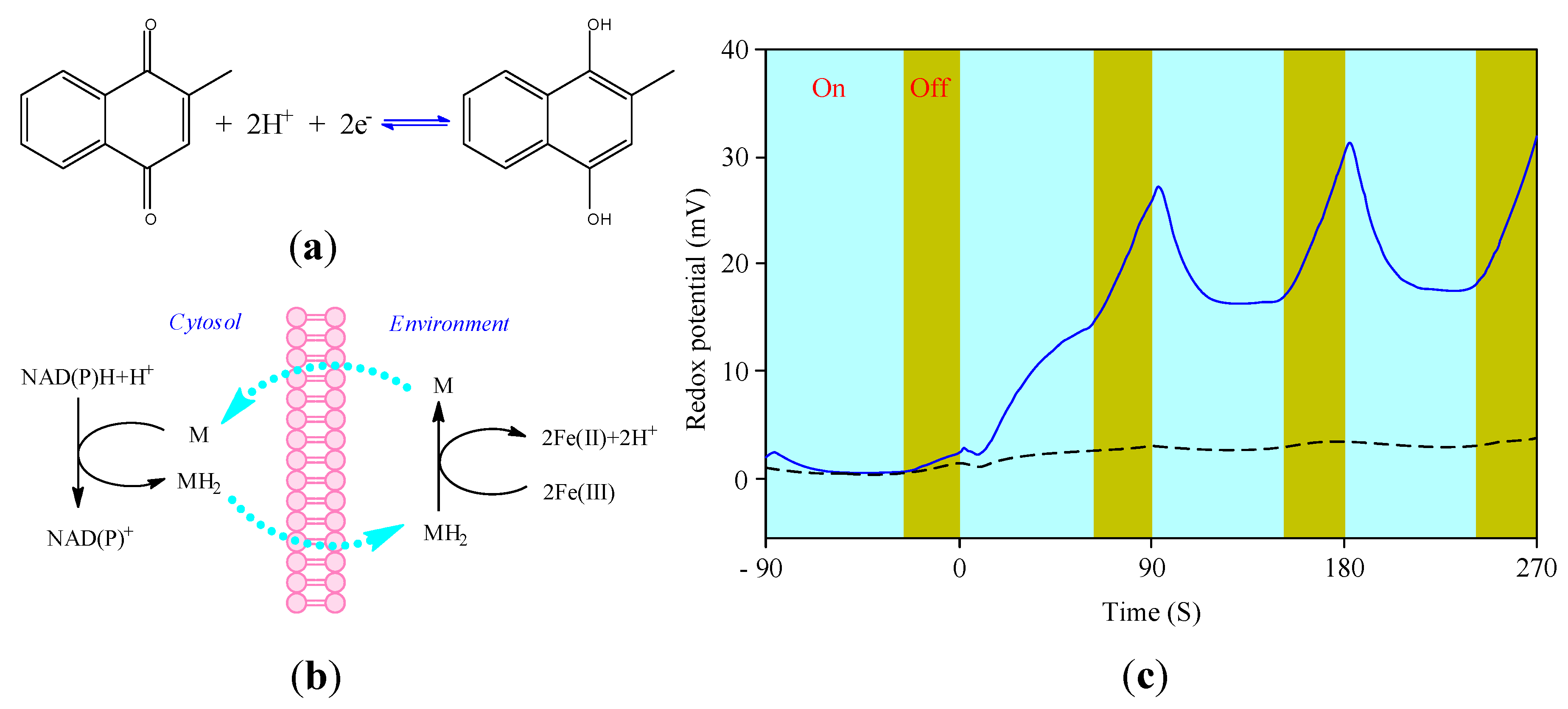
3.4. Cellular Redox Potential Detection
3.5. Cytotoxicity Assays and Drug Screening
3.5.1. Drug Analysis Using MEA
| Stimulus and concentration | Cellular component | Description | Reference |
|---|---|---|---|
| N-methyl-D-aspartate (NMDA) (10 μM) | Cortical neurons | NMDA drastically increased the bursting activity. The spike rates and frequencies were increased, but the pattern and shape regularity of bursting neural signal were decreased. | [141] |
| γ-aminobutyrate (GABA) (30 μM) | Mouse spinal nerve cells | The neural signal bursting was totally inhibited at 30 μM GABA. | [142] |
| Dopamine (15–100 μM) | Cortical neurons | Dopamine dispersed correlations between individual neuronal activities while preserving the global distribution of correlations at the network level. | [143] |
| APV (NMDA receptor antagonist) (40–100 μM) | Spinal cord networks | The rate of neural signal bursting was reduced by half. | [144] |
| Strychnine (0 nM–20 μM) | Spinal cord networks | Neural signal bursting was increased at 5–20 nM and coordinated above 5 μM. | [145] |
| GABA (10 μM), Bicuculline (10 μM) | Olfactory placode neurons | GABA almost immediately inhibited firing activities. Bicuculline had the facilitatory effect or lack of effect. | [151] |
| Eserine (10–150 μM) | Cortical cultures | The increase of bursting and spiking at 10 and 25 mM was concentration dependent, but it was inhibited at concentrations above 50 mM. | [147] |
| Ethanol (40 mM) | Embryonic murine neuronal networks | Spontaneously active frontal cortex cultures showed repeatable, concentration-dependent sensitivities to ethanol, with initial inhibition at 20 mM and a spike rate 50% effective concentration (EC50) of 48.8 ± 5.4 mM. | [148] |
| Tetrodotoxin (TTX, 100 μM) | Retina | The transient ON-response of ganglion cells was blocked by the sodium channel antagonist TTX. | [150] |
| Zn | Cortical neurons | The enhanced, coordinated bursting and spiking were increased, followed by irreversible activity decay. | [64] |
| Botulinum toxin (BoNT, 10 ng/mL) | Cortical networks | The duration and number of spikes were increased. | [149] |
3.5.2. Drug Analysis Using ECIS
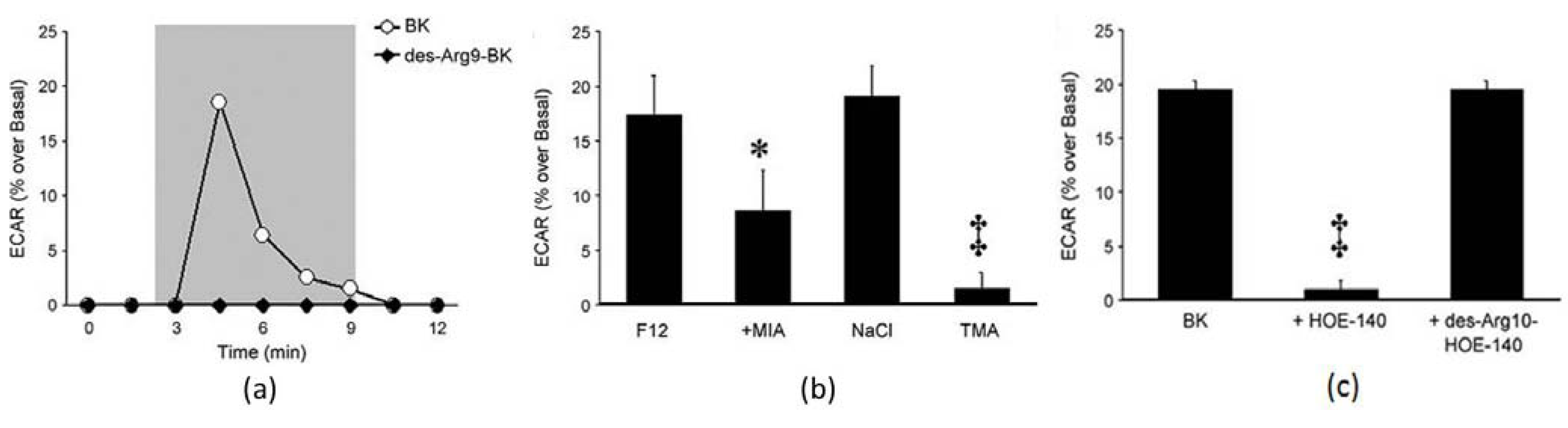
3.5.3. Drug Analysis Using LAPS
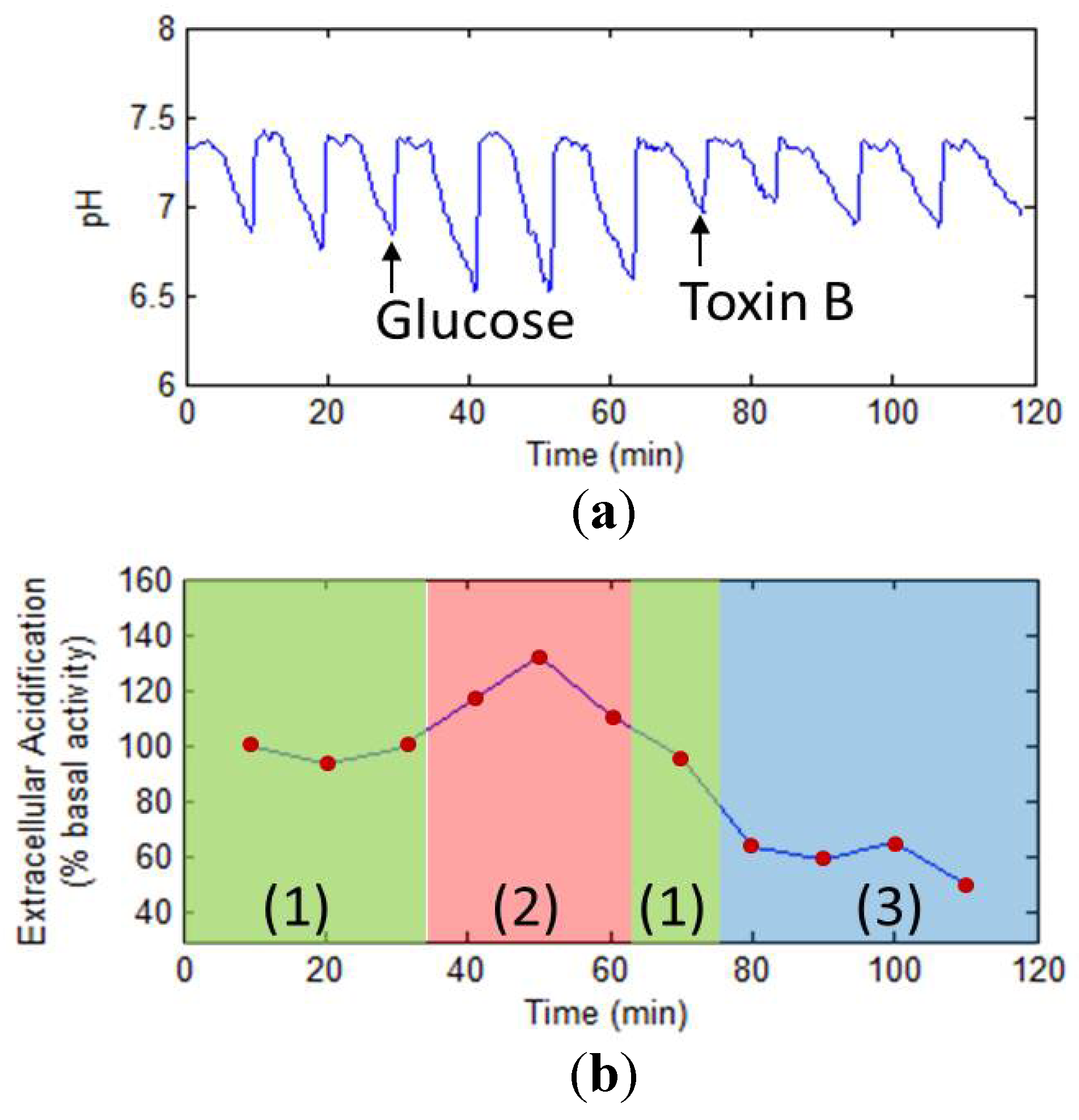
4. Development Trends of Electrochemical Cell-Based Biosensors
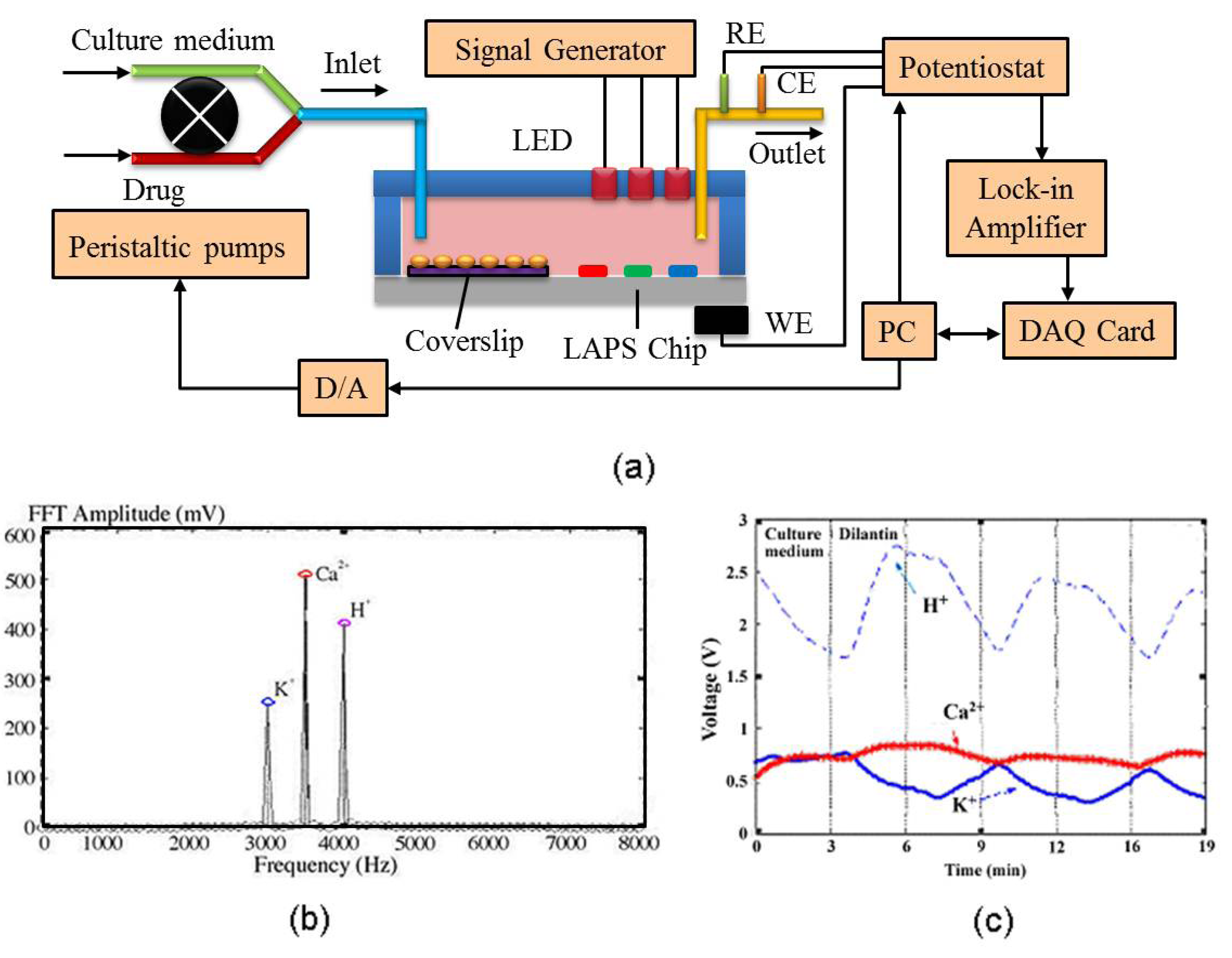
4.1. Integrated Sensors for Multi-Parameters Monitoring
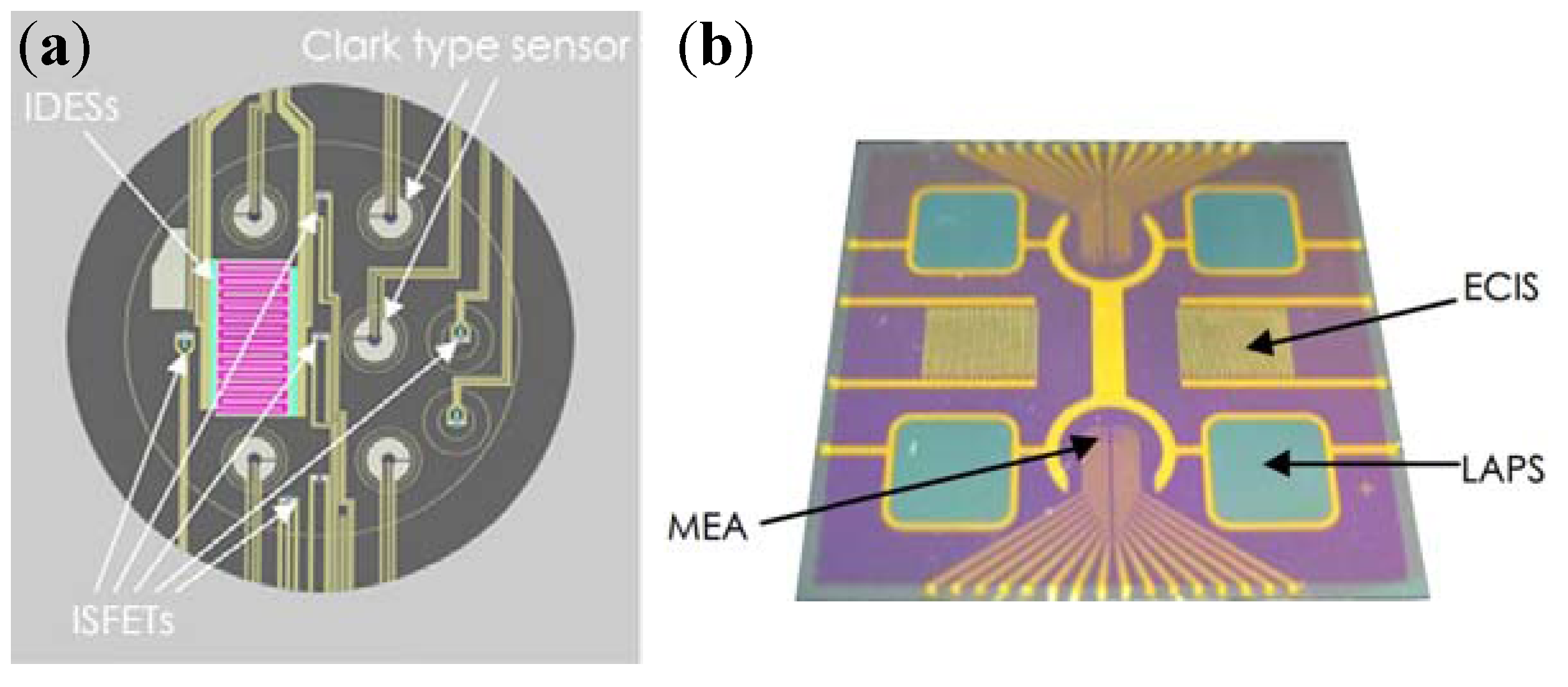
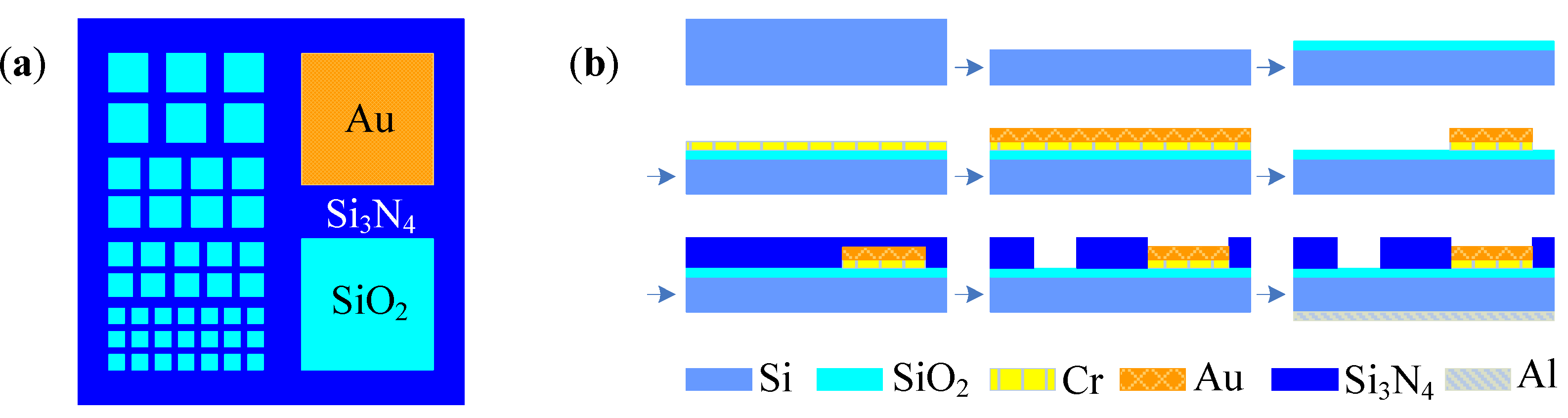
4.2. Cell-Based Electrochemical Biosensor with Microfluidic Technology

4.3. Cell-Based Electrochemical Biosensor with Nanotechnology
5. Conclusions
Acknowledgments
References
- Bery, M.N.; Grivell, M.B. An electrochemical description of metabolism. In Bioelectrochemistry of Cells and Tissues; Walz, D., Berg, H., Milazzo, G., Eds.; Birkhauser Verlag: Basel, Switzerland, 1995; pp. 134–158. [Google Scholar]
- Nonner, W.; Eisenberg, B. Electrodiffusion in ionic channels of biological membranes. J. Mol. Liq. 2000, 87, 149–162. [Google Scholar] [CrossRef]
- Du, D.; Cai, J.; Ju, H.X.; Yan, F.; Chen, J.; Jiang, X.Q.; Chen, H.Y. Construction of a biomimetic zwitterionic interface for monitoring cell proliferation and apoptosis. Langmuir 2005, 21, 8394–8399. [Google Scholar] [CrossRef]
- Du, D.; Liu, S.L.; Chen, J.; Ju, H.X.; Lian, H.Z.; Li, J.X. Colloidal gold nanoparticle modified carbon paste interface for studies of tumor cell adhesion and viability. Biomaterials 2005, 26, 6487–6495. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Ju, H.X. Immobilization and electrochemical behavior of gold nanoparticles modified leukemia K562 cells and application in drug sensitivity test. Electrochem. Commun. 2007, 9, 293–298. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, L.; Lei, J.P.; Ding, S.J.; Ju, H.X. Effective cell capture with tetrapeptide-functionalized carbon nanotubes and dual signal amplification for cytosensing and evaluation of cell surface carbohydrate. Anal. Chem. 2008, 80, 3867–3872. [Google Scholar]
- Hao, C.; Ding, L.; Zhang, X.J.; Ju, H.X. Biocompatible conductive architecture of carbon nanofiber-doped chitosan prepared with controllable electrodeposition for cytosensing. Anal. Chem. 2007, 79, 4442–4447. [Google Scholar]
- Hu, H.L.; Jiang, H.; Wang, X.M.; Chen, B.A. Detection and distinguishability of leukemia cancer cells based on Au nanoparticles modified electrodes. Electrochem. Commun. 2008, 10, 1121–1124. [Google Scholar] [CrossRef]
- Shen, Q.; You, S.K.; Park, S.G.; Jiang, H.; Guo, D.D.; Chen, B.A.; Wang, X.M. Electrochemical biosensing for cancer cells based on TiO2/CNT nanocomposites modified electrodes. Electroanalysis 2008, 20, 2526–2530. [Google Scholar] [CrossRef]
- Jia, X.E.; Tan, L.; Zhou, Y.P.; Jiang, X.F.; Xie, Q.J.; Tang, H.; Yao, S.Z. Magnetic immobilization and electrochemical detection of leukemia K562 cells. Electrochem. Commun. 2009, 11, 141–144. [Google Scholar] [CrossRef]
- Zhong, X.; Bai, H.J.; Xu, J.J.; Chen, H.Y.; Zhu, Y.H. A reusable interface constructed by 3-aminophenylboronic acid functionalized multiwalled carbon nanotubes for cell capture, release, and cytosensing. Adv. Funct. Mater. 2010, 20, 992–999. [Google Scholar]
- Weng, J.; Zhang, Z.W.; Sun, L.P.; Wang, J.A. High sensitive detection of cancer cell with a folic acid-based boron-doped diamond electrode using an AC impedimetric approach. Biosens. Bioelectron. 2011, 26, 1847–1852. [Google Scholar]
- Zhang, J.J.; Cheng, F.F.; Zheng, T.T.; Zhu, J.J. Design and implementation of electrochemical cytosensor for evaluation of cell surface carbohydrate and glycoprotein. Anal. Chem. 2010, 82, 3547–3555. [Google Scholar] [CrossRef]
- Ding, L.; Du, D.; Wu, H.; Ju, H.X. A disposable impedance sensor for electrochemical study and monitoring of adhesion and proliferation of K562 leukaemia cells. Electrochem. Commun. 2007, 9, 953–958. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Q.J. Cell-Based Biosensors: Principles and Applications; Artech House Publishers: Norwood, MA, USA, 2010. [Google Scholar]
- Thomas, C.A., Jr.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, X.; Tsao, C.Y.; Wu, H.C.; Betz, J.; Payne, G.F.; Bentley, W.E.; Rubloff, G.W. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation. Lab Chip 2011, 11, 2316–2318. [Google Scholar]
- Hsiung, L.C.; Yang, C.H.; Chiu, C.L.; Chen, C.L.; Wang, Y.; Lee, H.; Cheng, J.Y.; Ho, M.C.; Wo, A.M. A planar interdigitated ring electrode array via dielectrophoresis for uniform patterning of cells. Biosens. Bioelectron. 2008, 24, 869–875. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue-culture with an applied electric-field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A Morphological biosensor for mammalian-cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Ehret, R.; Baumann, W.; Brischwein, M.; Schwinde, A.; Stegbauer, K.; Wolf, B. Monitoring of cellular behaviour by impedance measurements on interdigitated electrode structures. Biosens. Bioelectron. 1997, 12, 29–41. [Google Scholar] [CrossRef]
- Wegener, J.; Sieber, M.; Galla, H.J. Impedance analysis of epithelial and endothelial cell monolayers cultured on gold surfaces. J. Biochem. Biophys. Methods 1996, 32, 151–170. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Huang, X.Q.; Greve, D.W.; Domach, M.M. Fibroblast growth and H-7 protein kinase inhibitor response monitored in microimpedance sensor arrays. Biotechnol. Bioeng. 2004, 87, 138–144. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, Bm17, 70–71. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F.; Yu, J.; Chan, H.L.W.; Yang, M. The application of organic electrochemical transistors in cell-based biosensors. Adv. Mater. 2010, 22, 3655–3660. [Google Scholar]
- Cheng, Y.; Chen, K.S.; Meyer, N.L.; Yuan, J.; Hirst, L.S.; Chase, P.B.; Xiong, P. Functionalized SnO2 nanobelt field-effect transistor sensors for label-free detection of cardiac troponin. Biosens. Bioelectron. 2011, 26, 4538–4544. [Google Scholar] [CrossRef]
- Hafeman, D.G.; Parce, J.W.; Mcconnell, H.M. Light-addressable potentiometric sensor for biochemical systems. Science 1988, 240, 1182–1185. [Google Scholar]
- McConnell, H.M.; Owicki, J.C.; Parce, J.W.; Miller, D.L.; Baxter, G.T.; Wada, H.G.; Pitchford, S. The cytosensor microphysiometer: Biological applications of silicon technology. Science 1992, 257, 1906–1912. [Google Scholar]
- Wu, Y.C.; Wang, P.; Ye, X.S.; Zhang, Q.T.; Li, R.; Yan, W.M.; Zheng, X.X. A novel microphysiometer based on MLAPS for drugs screening. Biosens. Bioelectron. 2001, 16, 277–286. [Google Scholar] [CrossRef]
- Siu, W.M.; Cobbold, R.S.C. Basic properties of the electrolyte-SiO2-Si system: Physical and theoretical aspects. IEEE Trans.Electron Devices 1979, 26, 1805–1815. [Google Scholar] [CrossRef]
- Bove, M.; Grattarola, M.; Martinoia, S.; Verreschi, G. Interfacing cultured neurons to planar substrate microelectrodes: Characterization of the neuron-to-microelectrode junction. Bioelectrochem. Bioenerg. 1995, 38, 255–265. [Google Scholar] [CrossRef]
- Breckenridge, L.; Wilson, R.; Connolly, P.; Curtis, A.; Dow, J.; Blackshaw, S.; Wilkinson, C. Advantages of using microfabricated extracellular electrodes for in vitro neuronal recording. J. Neurosci. Res. 1995, 42, 266–276. [Google Scholar] [CrossRef]
- Regehr, W.G.; Pine, J.; Cohan, C.S.; Mischke, M.D.; Tank, D.W. Sealing cultured invertebrate neurons to embedded dish electrodes facilitates long-term stimulation and recording. J. Neurosci. Methods 1989, 30, 91–106. [Google Scholar] [CrossRef]
- Grattarola, M.; Martinoia, S.; Massobrio, G.; Cambiaso, A.; Rosichini, R.; Tetti, M. Computer simulations of the responses of passive and active integrated microbiosensors to cell activity. Sens. Actuat. B: Chem. 1991, 4, 261–265. [Google Scholar] [CrossRef]
- Heuschkel, M.O.; Fejtl, M.; Raggenbass, M.; Bertrand, D.; Renaud, P. A three-dimensional multi-electrode array for multi-site stimulation and recording in acute brain slices. J. Neurosci. Methods 2002, 114, 135–148. [Google Scholar] [CrossRef]
- Koo, K.; Chung, H.; Yu, Y.; Seo, J.; Park, J. Fabrication of pyramid shaped three-dimensional 8 × 8 electrodes for artificial retina. Sens. Actuat. A: Phys. 2006, 130, 609–615. [Google Scholar] [CrossRef]
- Gross, G.W.; Wen, W.Y.; Lin, J.W. Transparent indium-tin oxide electrode patterns for extracellular, multisite recording in neuronal cultures. J. Neurosci. Methods 1985, 15, 243–252. [Google Scholar] [CrossRef]
- Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 1980, 2, 19–31. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans. Biomed. Eng. 1986, 33, 242–247. [Google Scholar] [CrossRef]
- Xiao, C.; Lachance, B.; Sunahara, G.; Luong, J.H.T. An in-depth analysis of electric cell-substrate impedance sensing to study the attachment and spreading of mammalian cells. Anal. Chem. 2002, 74, 1333–1339. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Deng, C.; Xing, W.; Cheng, J. An automatic and quantitative on-chip cell migration assay using self-assembled monolayers combined with real-time cellular impedance sensing. Lab Chip 2008, 8, 872–878. [Google Scholar] [CrossRef]
- Chang, B.W.; Chen, C.H.; Ding, S.J.; Chen, D.C.H.; Chang, H.C. Impedimetric monitoring of cell attachment on interdigitated microelectrodes. Sens. Actuat. B: Chem. 2005, 105, 159–163. [Google Scholar]
- Parce, J.W.; Owicki, J.C.; Kercso, K.M.; Sigal, G.B.; Wada, H.; Muir, V.C.; Bousse, L.J.; Ross, K.L.; Sikic, B.I.; McConnell, H.M. Detection of cell-affecting agents with a silicon biosensor. Science 1989, 246, 243–247. [Google Scholar]
- Piras, L.; Adami, M.; Fenu, S.; Dovis, M.; Nicolini, C. Immunoenzymatic application of a redox potential biosensor. Anal. Chim. Acta 1996, 335, 127–135. [Google Scholar] [CrossRef]
- Bousse, L. The chemical Sensitivity of Electrolyte/Interface/Silicon Structures. Ph.D. Dissertation, Twente University of Technology, Enschede, The Netherlands. 1982. [Google Scholar]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar]
- Cheng, Y.; Xiong, P.; Yun, C.S.; Strouse, G.; Zheng, J.; Yang, R.; Wang, Z. Mechanism and optimization of pH sensing using SnO2 nanobelt field effect transistors. Nano Lett. 2008, 8, 4179–4184. [Google Scholar]
- Hafner, F. Cytosensor® Microphysiometer: Technology and recent applications. Biosens. Bioelectron. 2000, 15, 149–158. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Rabinowitz, J.D.; Vacchino, J.F.; Beeson, C.; McConnell, H.M. Potentiometric measurement of intracellular redox activity. J. Am. Chem. Soc. 1998, 120, 2464–2473. [Google Scholar] [CrossRef]
- Ward, K.; Hawkridge, F.; Brahim, S.; Lewis, K.L.; Rhoten, M.C. Non-biofouling, universal redox electrode and measurement system. U.S. Patent Application 20090134043, May 2009. [Google Scholar]
- Natarajan, A.; Stancescu, M.; Dhir, V.; Armstrong, C.; Sommerhage, F.; Hickman, J.J.; Molnar, P. Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform. Biomaterials 2011, 32, 4267–4274. [Google Scholar]
- Chen, M.Q.; Whittington, R.H.; Day, P.W.; Kobilka, B.K.; Giovangrandi, L.; Kovacs, G.T.A. A device for separated and reversible co-culture of cardiomyocytes. Biotechnol. Prog. 2010, 26, 1164–1171. [Google Scholar]
- Gholmieh, G.; Soussou, W.; Han, M.; Ahuja, A.; Hsiao, M.C.; Song, D.; Tanguay, A.R., Jr.; Berger, T.W. Custom-designed high-density conformal planar multielectrode arrays for brain slice electrophysiology. J. Neurosci. Methods 2006, 152, 116–129. [Google Scholar] [CrossRef]
- Hofmann, F.; Bading, H. Long term recordings with microelectrode arrays: Studies of transcription-dependent neuronal plasticity and axonal regeneration. J. Physiol.-Paris 2006, 99, 125–132. [Google Scholar] [CrossRef]
- Kiryushko, D.; Kofoed, T.; Skladchikova, G.; Holm, A.; Berezin, V.; Bock, E. A synthetic peptide ligand of neural cell adhesion molecule (NCAM), C3d, promotes neuritogenesis and synaptogenesis and modulates presynaptic function in primary cultures of rat hippocampal neurons. J. Biol. Chem. 2003, 278, 12325–12334. [Google Scholar]
- David-Pur, M.; Shein, M.; Hanein, Y.; Balasubramanian, K. Carbon nanotube-based neurochips. Methods Mol. Biol. 2010, 625, 171–177. [Google Scholar] [CrossRef]
- Charvet, G.; Rousseau, L.; Billoint, O.; Gharbi, S.; Rostaing, J.P.; Joucla, S.; Trevisiol, M.; Bourgerette, A.; Chauvet, P.; Moulin, C. BioMEATM: A versatile high-density 3D microelectrode array system using integrated electronics. Biosens. Bioelectron. 2010, 25, 1889–1896. [Google Scholar] [CrossRef]
- Rajaraman, S.; Choi, S.O.; Shafer, R.H.; Ross, J.D.; Vukasinovic, J.; Choi, Y.; DeWeerth, S.P.; Glezer, A.; Allen, M.G. Microfabrication technologies for a coupled three-dimensional microelectrode, microfluidic array. J. Micromech. Microeng. 2007, 17, 163–171. [Google Scholar]
- Chu, H.Y.; Kuo, T.Y.; Chang, B.; Lu, S.W.; Chiao, C.C.; Fang, W. Design and fabrication of novel three-dimensional multi-electrode array using SOI wafer. Sens. Actuat. A: Phys. 2006, 130, 254–261. [Google Scholar] [CrossRef]
- Morin, F.O.; Takamura, Y.; Tamiya, E. Investigating neuronal activity with planar microelectrode arrays: Achievements and new perspectives. J. Biosci. Bioeng. 2005, 100, 131–143. [Google Scholar] [CrossRef]
- Streit, J.; Tscherter, A.; Heuschkel, M.O.; Renaud, P. The generation of rhythmic activity in dissociated cultures of rat spinal cord. Eur. J. Neurosci. 2001, 14, 191–202. [Google Scholar] [CrossRef]
- Robinson, H.; Kawahara, M.; Jimbo, Y.; Torimitsu, K.; Kuroda, Y.; Kawana, A. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. J. Neurophysiol. 1993, 70, 1606–1616. [Google Scholar]
- Pan, L.; Xiang, G.; Huang, L.; Yu, Z.; Cheng, J.; Xing, W.; Zhou, Y. Automatic positioning and sensing microelectrode array (APSMEA) for multi-site electrophysiological recordings. J. Neurosci. Methods 2008, 170, 123–129. [Google Scholar] [CrossRef]
- Jing, G.; Yao, Y.; Gnerlich, M.; Perry, S.; Tatic-Lucic, S. Towards a multi-electrode array (MEA) system for patterned neural networks. Procedia Chem. 2009, 1, 329–332. [Google Scholar]
- Berdondini, L.; Chiappalone, M.; Van Der Wal, P.; Imfeld, K.; De Rooij, N.F.; Koudelka-Hep, M.; Tedesco, M.; Martinoia, S.; Van Pelt, J.; Le Masson, G. A microelectrode array (MEA) integrated with clustering structures for investigating in vitro neurodynamics in confined interconnected sub-populations of neurons. Sens. Actuat. B: Chem. 2006, 114, 530–541. [Google Scholar] [CrossRef]
- Pearce, T.M.; Wilson, J.A.; Oakes, S.G.; Chiu, S.Y.; Williams, J.C. Integrated microelectrode array and microfluidics for temperature clamp of sensory neurons in culture. Lab Chip 2004, 5, 97–101. [Google Scholar]
- Dworak, B.J.; Wheeler, B.C. Novel MEA platform with PDMS microtunnels enables the detection of action potential propagation from isolated axons in culture. Lab Chip 2008, 9, 404–410. [Google Scholar]
- Parviz, M.; Gross, G.W. Quantification of zinc toxicity using neuronal networks on microelectrode arrays. NeuroToxicology 2007, 28, 520–531. [Google Scholar] [CrossRef]
- Kamioka, H.; Maeda, E.; Jimbo, Y.; Robinson, H.P.C.; Kawana, A. Spontaneous periodic synchronized bursting during formation of mature patterns of connections in cortical cultures. Neurosci. Lett. 1996, 206, 109–112. [Google Scholar] [CrossRef]
- Hescheler, J.; Halbach, M.; Egert, U.; Lu, Z.J.; Bohlen, H.; Fleischmann, B.K.; Reppel, M. Determination of electrical properties of ES cell-derived cardiomyocytes using MEAs. J. Electrocardiol. 2004, 37, 110–116. [Google Scholar] [CrossRef]
- Illes, S.; Fleischer, W.; Siebler, M.; Hartung, H.P.; Dihné, M. Development and pharmacological modulation of embryonic stem cell-derived neuronal network activity. Exp. Neurol. 2007, 207, 171–176. [Google Scholar] [CrossRef]
- Taketani, M.; Baudry, M. Advances in Network Electrophysiology: Using Multi-Electrode Arrays; Springer: Berlin/Heidelberg, Germany, 2006; pp. 321–331. [Google Scholar]
- Liu, Q.; Ye, W.; Xiao, L.; Du, L.; Hu, N.; Wang, P. Extracellular potentials recording in intact olfactory epithelium by microelectrode array for a bioelectronic nose. Biosens. Bioelectron. 2010, 25, 2212–2217. [Google Scholar]
- LeBaron, R.G.; Athanasiou, K.A. Extracellular matrix cell adhesion peptides: Functional applications in orthopedic materials. Tissue Eng. 2000, 6, 85–103. [Google Scholar] [CrossRef]
- Cuvelier, D.; Théry, M.; Chu, Y.S.; Dufour, S.; Thiéry, J.P.; Bornens, M.; Nassoy, P.; Mahadevan, L. The universal dynamics of cell spreading. Curr. Biol. 2007, 17, 694–699. [Google Scholar]
- Luong, J.H.T.; Xiao, C.; Lachance, B.; Leabu, S.M.; Li, X.; Uniyal, S.; Chan, B. Extended applications of electric cell-substrate impedance sensing for assessment of the structure–function of α2β1 integrin. Anal. Chim. Acta 2004, 501, 61–69. [Google Scholar] [CrossRef]
- Bouafsoun, A.; Helali, S.; Othmane, A.; Kerkeni, A.; Prigent, A.F.; Jaffrézic-Renault, N.; Bessueille, F.; Léonard, D.; Ponsonnet, L. Evaluation of endothelial cell adhesion onto different protein/gold electrodes by EIS. Macromol. Biosci. 2007, 7, 599–610. [Google Scholar] [CrossRef]
- Kataoka, N.; Iwaki, K.; Hashimoto, K.; Mochizuki, S.; Ogasawara, Y.; Sato, M.; Tsujioka, K.; Kajiya, F. Measurements of endothelial cell-to-cell and cell-to-substrate gaps and micromechanical properties of endothelial cells during monocyte adhesion. Proc. Natil. Acad. Sci. USA 2002, 99, 15638–15643. [Google Scholar] [CrossRef]
- Atienza, J.M.; Yu, N.; Wang, X.; Xu, X.; Abassi, Y. Label-free and real-time cell-based kinase assay for screening selective and potent receptor tyrosine kinase inhibitors using microelectronic sensor array. J. Biomol. Screen. 2006, 11, 634–643. [Google Scholar] [CrossRef]
- Yu, N.; Atienza, J.M.; Bernard, J.; Blanc, S.; Zhu, J.; Wang, X.; Xu, X.; Abassi, Y.A. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: An approach to study G protein-coupled receptors. Anal. Chem. 2006, 78, 35–43. [Google Scholar]
- Cho, R.J.; Huang, M.; Campbell, M.J.; Dong, H.; Steinmetz, L.; Sapinoso, L.; Hampton, G.; Elledge, S.J.; Davis, R.W.; Lockhart, D.J. Transcriptional regulation and function during the human cell cycle. Nat. Genet. 2001, 27, 48–54. [Google Scholar]
- Tapon, N.; Moberg, K.H.; Hariharan, I.K. The coupling of cell growth to the cell cycle. Curr. Opin. Cell Biol. 2001, 13, 731–737. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Xing, W.; Yu, Z.; Guo, M.; Cheng, J. Real-time, label-free monitoring of the cell cycle with a cellular impedance sensing chip. Biosens. Bioelectron. 2010, 25, 990–995. [Google Scholar] [CrossRef]
- Bieberich, E.; Guiseppi-Elie, A. Neuronal differentiation and synapse formation of PC12 and embryonic stem cells on interdigitated microelectrode arrays: Contact structures for neuron-to-electrode signal transmission (NEST). Biosens. Bioelectron. 2004, 19, 923–931. [Google Scholar] [CrossRef]
- Maercker, C.; Rogge, T.; Mathis, H.; Ridinger, H.; Bieback, K. Development of live cell chips to monitor cell differentiation processes. Eng. Life Sci. 2008, 8, 33–39. [Google Scholar] [CrossRef]
- Diederichs, S.; Riechers, D.; Sempf, F.; Kall, S.; Kasper, C.; Griensven, M.; Scheper, T. Investigation of the effect of mechanical strain on the osteogenic differentiation of mesenchymal stem cells. Cells Cult. 2010, 579–589. [Google Scholar]
- Hediger, S.; Fontannaz, J.; Sayah, A.; Hunziker, W.; Gijs, M. Biosystem for the culture and characterisation of epithelial cell tissues. Sens. Actuat. B: Chem. 2000, 63, 63–73. [Google Scholar]
- Asami, K. Dielectric spectra of biological cells and tissues simulated by three-dimensional finite difference method. IFMBE Proc. 2007, 17, 98–101. [Google Scholar]
- Frampton, J.; Hynd, M.; Williams, J.; Shuler, M.; Shain, W. Three-dimensional hydrogel cultures for modeling changes in tissue impedance around microfabricated neural probes. J. Neural Eng. 2007, 4, 399–409. [Google Scholar] [CrossRef]
- Han, A.; Yang, L.; Frazier, A.B. Quantification of the heterogeneity in breast cancer cell lines using whole-cell impedance spectroscopy. Clin. Cancer Res. 2007, 13, 139–143. [Google Scholar]
- Linderholm, P.; Vannod, J.; Barrandon, Y.; Renaud, P. Bipolar resistivity profiling of 3D tissue culture. Biosens. Bioelectron. 2007, 22, 789–796. [Google Scholar] [CrossRef]
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J.; Shain, W.; Kipke, D.R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410–423. [Google Scholar] [CrossRef]
- Atienza, J.M.; Zhu, J.; Wang, X.; Xu, X.; Abassi, Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J. Biomol. Screen. 2005, 10, 795–805. [Google Scholar] [CrossRef]
- Webb, D.J.; Zhang, H.Y.; Horwitz, A.F. Cell migration. In Cell Migration: Developmental Methods and Protocols; Guan, J.-L., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2005; Volume 294, pp. 3–11. [Google Scholar]
- Noiri, E.; Hu, Y.; Bahou, W.F.; Keese, C.R.; Giaever, I.; Goligorsky, M.S. Permissive role of nitric oxide in endothelin-induced migration of endothelial cells. J. Biol. Chem. 1997, 272, 1747–1752. [Google Scholar]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar]
- Shaw, L.M. Tumor cell invasion assays. Methods Mol. Biol. 2005, 294, 97–105. [Google Scholar]
- Keese, C.R.; Bhawe, K.; Wegener, J.; Giaever, I. Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques 2002, 33, 842–851. [Google Scholar]
- Wang, G.; Zhang, W. The signaling network of tumor invasion. Histol. Histopathol. 2005, 20, 593–602. [Google Scholar]
- Ren, J.; Xiao, Y.; Singh, L.S.; Zhao, X.; Zhao, Z.; Feng, L.; Rose, T.M.; Prestwich, G.D.; Xu, Y. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cell. Cancer Res. 2006, 66, 3006–3014. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar]
- Chen, J.; Ye, L.; Zhang, L.; Jiang, W.G. Placenta growth factor, PLGF, influences the motility of lung cancer cells, the role of Rho associated kinase, Rock1. J. Cell. Biochem. 2008, 105, 313–320. [Google Scholar] [CrossRef]
- Earley, S.; Plopper, G.E. Phosphorylation of focal adhesion kinase promotes extravasation of breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 366, 476–482. [Google Scholar] [CrossRef]
- Saxena, N.K.; Taliaferro-Smith, L.T.; Knight, B.B.; Merlin, D.; Anania, F.A.; O’Regan, R.M.; Sharma, D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008, 68, 9712–9722. [Google Scholar] [CrossRef]
- Sgambato, A.; De Paola, B.; Migaldi, M.; Di Salvatore, M.; Rettino, A.; Rossi, G.; Faraglia, B.; Boninsegna, A.; Maiorana, A.; Cittadini, A. Dystroglycan expression is reduced during prostate tumorigenesis and is regulated by androgens in prostate cancer cells. J. Cell. Physiol. 2007, 213, 528–539. [Google Scholar]
- Taliaferro-Smith, L.; Nagalingam, A.; Zhong, D.; Zhou, W.; Saxena, N.; Sharma, D. LKB1 is required for adiponectin-mediated modulation of AMPK–S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 2009, 28, 2621–2633. [Google Scholar]
- Patani, N.; Douglas-Jones, A.; Mansel, R.; Jiang, W.; Mokbel, K. Tumour suppressor function of MDA-7/IL-24 in human breast cancer. Cancer Cell Int. 2010, 10, 29. [Google Scholar]
- Mandel, L.J. Energy metabolism of cellular activation, growth, and transformation. Curr. Top. Membr. Transp. 1986, 27, 261–291. [Google Scholar] [CrossRef]
- Hu, N.; Ha, D.; Wu, C.X.; Cheng, G.; Yu, H.; Wang, T.X.; Wu, J.Y.; Cai, H.; Liu, Q.J.; Wang, P. Design of microphysiometer based on multiparameter cell-based biosensors for quick drug analysis. J. Innov. Opt. Health Sci. 2012, 5, 1150005–1. [Google Scholar]
- Deuticke, B. Monocarboxylate transport in red blood cells: Kinetics and chemical modification. Methods Enzymol. 1989, 173, 300–329. [Google Scholar]
- Wada, H.; Owicki, J.; Bruner, L.; Miller, K.; Raley-Susman, K.; Panfili, P.; Humphries, G.; Parce, J. Measurement of cellular responses to toxic agents using a silicon microphysiometer. Altern. Anim. Test. Exp. (AATEX) 1992, 1, 154–164. [Google Scholar]
- Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Habata, Y.; Hinuma, S.; Onda, H.; Nishimura, O.; Fujino, M. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3*. J. Biol. Chem. 2000, 275, 21068–21074. [Google Scholar]
- Wille, K.; Paige, L.A.; Higgins, A.J. Application of the Cytosensor microphysiometer to drug discovery. Recept. Channels 2003, 9, 125–131. [Google Scholar] [CrossRef]
- Kramarenko, I.I.; Bunni, M.A.; Morinelli, T.A.; Raymond, J.R.; Garnovskaya, M.N. Identification of functional bradykinin B2 receptors endogenously expressed in HEK293 cells. Biochem. Pharmacol. 2009, 77, 269–276. [Google Scholar]
- Needham, J.; Needham, D.M. The oxidation-reduction potential of protoplasm: A review. Protoplasma 1926, 1, 255–294. [Google Scholar] [CrossRef]
- Allyn, W.P.; Baldwin, I. Oxidation-reduction potentials in relation to the growth of an aerobic form of bacteria. J. Bacteriol. 1932, 23, 369–398. [Google Scholar]
- Ward, W.E. The apparent oxidation-reduction potentials of bright platinum electrodes in synthetic media cultures of bacteria. J. Bacteriol. 1938, 36, 337–355. [Google Scholar]
- Kwong, S.C.; Randers, L.; Rao, G. On-line assessment of metabolic activities based on culture redox potential and dissolved oxygen profiles during aerobic fermentation. Biotechnol. Prog. 1992, 8, 576–579. [Google Scholar] [CrossRef]
- Cater, D.; Phillips, A.; Silver, I. The measurement of oxidation-reduction potentials, pH, and oxygen tension in tumours. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957, 146, 382–399. [Google Scholar] [CrossRef]
- Eyer, K.; Heinzle, E. On-line estimation of viable cells in a hybridoma culture at various DO levels using ATP balancing and redox potential measurement. Biotechnol. Bioeng. 1996, 49, 277–283. [Google Scholar] [CrossRef]
- Higareda, A.E.; Possani, L.D.; Ramirez, O.T. The use of culture redox potential and oxygen uptake rate for assessing glucose and glutamine depletion in hybridoma cultures. Biotechnol. Bioeng. 1997, 56, 555–563. [Google Scholar] [CrossRef]
- Relchart, O.; Szakmar, K.; Jozwiak, A.; Felfoldi, J.; Baranyai, L. Redox potential measurement as a rapid method for microbiological testing and its validation for coliform determination. Int. J. Food Microbiol. 2007, 114, 143–148. [Google Scholar] [CrossRef]
- Zheng, J.D.; Xu, G.; Chu, J.; Wang, Y.; Zhuang, Y.; Zhang, S. Using oxidation-reduction potential to optimize lactic acid production by Lactobacillus paracasei. Chin. J. Bioprocess Eng. 2008, 6, 73–77. [Google Scholar]
- Koo, B.S.; Gong, Y.J.; Kim, S.Y.; Kim, C.W.; Lee, H.C. Improvement of coenzyme Q(10) production by increasing the NADH/NAD(+) ratio in Agrobacterium tumefaciens. Biosci. Biotechnol. Biochem. 2010, 74, 895–898. [Google Scholar] [CrossRef]
- Hwang, C.; Sinskey, A. The role of oxidation-reduction potential in monitoring growth of cultured mammalian cells. In Production of Biologicals from Animal Cells in Culture; Spier Raymond G.J.B. Meignier, B., Ed.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 548–569. [Google Scholar]
- Carelli, S.; Ceriotti, A.; Cabibbo, A.; Fassina, G.; Ruvo, M.; Sitia, R. Cysteine and glutathione secretion in response to protein disulfide bond formation in the ER. Science 1997, 277, 1681–1684. [Google Scholar]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized Redox State of Glutathione in the Endoplasmic-Reticulum. Science 1992, 257, 1496–1502. [Google Scholar]
- Bellomo, G.; Palladini, G.; Vairetti, M. Intranuclear distribution, function and fate of glutathione and glutathione-S-conjugate in living rat hepatocytes studied by fluorescence microscopy. Microsc. Res. Tech. 1997, 36, 243–252. [Google Scholar] [CrossRef]
- Natarajan, A.; Molnar, P.; Sieverdes, K.; Jamshidi, A.; Hickman, J. Microelectrode array recordings of cardiac action potentials as a high throughput method to evaluate pesticide toxicity. Toxicol. in vitro 2006, 20, 375–381. [Google Scholar] [CrossRef]
- Borkholder, D.A. Cell Based Biosensors Using Microelectrodes. Ph.D. Thesis, Stanford University, Stanford, CA, USA. 1998. [Google Scholar]
- Halbach, M.D.; Egert, U.; Hescheler, J.; Banach, K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell. Physiol. Biochem. 2003, 13, 271–284. [Google Scholar] [CrossRef]
- Reppel, M.; Igelmund, P.; Egert, U.; Juchelka, F.; Hescheler, J.; Drobinskaya, I. Effect of cardioactive drugs on action potential generation and propagation in embryonic stem cell-derived cardiomyocytes. Cell. Physiol. Biochem. 2007, 19, 213–224. [Google Scholar]
- Gilchrist, K.H. Characterization and validation of cell-based biosensors. Ph.D. Thesis, Stanford University, Stanford, CA, USA. 2003. [Google Scholar]
- Yeung, C.; Sommerhage, F.; Wrobel, G.; Offenhäusser, A.; Chan, M.; Ingebrandt, S. Drug profiling using planar microelectrode arrays. Anal. Bioanal. Chem. 2007, 387, 2673–2680. [Google Scholar] [CrossRef]
- Tsai, C.T.; Tseng, C.D.; Hwang, J.J.; Wu, C.K.; Yu, C.C.; Wang, Y.C.; Chen, W.P.; Lai, L.P.; Chiang, F.T.; Lin, J.L. Tachycardia of atrial myocytes induces collagen expression in atrial fibroblasts through transforming growth factor β1. Cardiovasc. Res. 2011, 89, 805–815. [Google Scholar] [CrossRef]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological application of microelectrode arrays in drug discovery and basic research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef]
- Meyer, T.; Leisgen, C.; Gonser, B.; Günther, E. QT-screen: High-throughput cardiac safety pharmacology by extracellular electrophysiology on primary cardiac myocytes. Assay Drug Dev. Technol. 2004, 2, 507–514. [Google Scholar] [CrossRef]
- Gross, G.W.; Harsch, A.; Rhoades, B.K.; Göpel, W. Odor, drug and toxin analysis with neuronal networks in vitro: Extracellular array recording of network responses. Biosens. Bioelectron. 1997, 12, 373–393. [Google Scholar] [CrossRef]
- Xiang, G.; Pan, L.; Huang, L.; Yu, Z.; Song, X.; Cheng, J.; Xing, W. Microelectrode array-based system for neuropharmacological applications with cortical neurons cultured in vitro. Biosens. Bioelectron. 2007, 22, 2478–2484. [Google Scholar]
- Gross, G.W.; Rhoades, B.; Jordan, R. Neuronal networks for biochemical sensing. Sens. Actuat. B: Chem. 1992, 6, 1–8. [Google Scholar]
- Eytan, D.; Minerbi, A.; Ziv, N.; Marom, S. Dopamine-induced dispersion of correlations between action potentials in networks of cortical neurons. J. Neurophysiol. 2004, 92, 1817–1824. [Google Scholar] [CrossRef]
- Keefer, E.W.; Gramowski, A.; Gross, G.W. NMDA receptor-dependent periodic oscillations in cultured spinal cord networks. J. Neurophysiol. 2001, 86, 3030–3042. [Google Scholar]
- Harsch, A.; Ziegler, C.; Gopel, W. Strychnine analysis with neuronal networks in vitro: Extracellular array recording of network responses. Biosens. Bioelectron. 1997, 12, 827–835. [Google Scholar] [CrossRef]
- Gross, G.W.; Rhoades, B.K.; Azzazy, H.M.E.; Wu, M.C. The use of neuronal networks on multielectrode arrays as biosensors. Biosens. Bioelectron. 1995, 10, 553–567. [Google Scholar]
- Keefer, E.W.; Norton, S.J.; Boyle, N.A.J.; Talesa, V.; Gross, G.W. Acute toxicity screening of novel AChE inhibitors using neuronal networks on microelectrode arrays. NeuroToxicology 2001, 22, 3–12. [Google Scholar] [CrossRef]
- Xia, Y.; Gross, G.W. Histiotypic electrophysiological responses of cultured neuronal networks to ethanol. Alcohol 2003, 30, 167–174. [Google Scholar] [CrossRef]
- Scarlatos, A.; Cadotte, A.; DeMarse, T.; Welt, B. Cortical networks grown on microelectrode arrays as a biosensor for botulinum toxin. J. Food Sci. 2008, 73, E129–E136. [Google Scholar] [CrossRef]
- Guenther, E.; Herrmann, T.; Stett, A. The retinasensor: An in vitro tool to study drug effects on retinal signaling. In Advances in Network Electrophysiology: Using Multi-Electrode Arrays; Taketani, M., Baudry, M., Eds.; Springer Science+Business Media Inc.: New York, NY, USA, 2006; pp. 321–331. [Google Scholar]
- Suyama, K.; Daikoku, S.; Funabashi, T.; Kimura, F. Effects of GABA and bicuculline on the electrical activity of rat olfactory placode neurons derived at E13. 5 and cultured for 1 week on multi-electrode dishes. Endocr. J. 2004, 51, 171–176. [Google Scholar] [CrossRef]
- Omori, T.; Saijo, K.; Kato, M.; Hayashi, M.; Itagaki, H.; Miyazaki, S.; Ohno, T.; Sugawara, H.; Teramoto, N.; Tanaka, N. Validation study on five cytotoxicity assays by JSAAE II. Statistical analysis. Altern. Anim. Test. Exp. 1998, 5, 39–58. [Google Scholar]
- Clemedson, C.; Barile, F.; Chesne, C.; Cottin, M.; Curren, R.; EKWALL, B.; Ferro, M.; Gomez-Lechon, M.; Imai, K.; Janus, J. MEIC evaluation of acute systemic toxicity. Part VII. Prediction of human toxicity by results from testing of the first 30 reference chemicals with 27 further in vitro assays. ATLA. Altern. Lab. Anim. 2000, 28, 161–200. [Google Scholar]
- Botham, P.A. Acute systemic toxicity. ILAR J. 2002, 43, 27–30. [Google Scholar]
- Botham, P. Acute systemic toxicity—Prospects for tiered testing strategies. Toxicol. in vitro 2004, 18, 227–230. [Google Scholar] [CrossRef]
- Xing, J.Z.; Zhu, L.; Jackson, J.A.; Gabos, S.; Sun, X.J.; Wang, X.; Xu, X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. [Google Scholar] [CrossRef]
- Keese, C.; Karra, N.; Dillon, B.; Goldberg, A.; Giaever, I. Cell-substratum interactions as a predictor of cytotoxicity. In Vitro Mol. Toxicol. 1998, 11, 183–192. [Google Scholar]
- Xiao, C.; Luong, J.H.T. On-line monitoring of cell growth and cytotoxicity using electric cell-substrate impedance sensing (ECIS). Biotechnol. Prog. 2003, 19, 1000–1005. [Google Scholar]
- Xing, J.Z.; Zhu, L.; Gabos, S.; Xie, L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol. in vitro 2006, 20, 995–1004. [Google Scholar] [CrossRef]
- Brischwein, M.; Motrescu, E.; Cabala, E.; Otto, A.; Grothe, H.; Wolf, B. Functional cellular assays with multiparametric silicon sensor chips. Lab Chip 2003, 3, 234–240. [Google Scholar] [CrossRef]
- Otto, A.M.; Brischwein, M.; Niendorf, A.; Henning, T.; Motrescu, E.; Wolf, B. Microphysiological testing for chemosensitivity of living tumor cells with multiparametric microsensor chips. Cancer Detect. Prev. 2003, 27, 291–296. [Google Scholar] [CrossRef]
- Geisler, T.; Ressler, J.; Harz, H.; Wolf, B.; Uhl, R. Automated multiparametric platform for high-content and high-throughput analytical screening on living cells. IEEE Trans. Autom. Sci. Eng. 2006, 3, 169–176. [Google Scholar] [CrossRef]
- Ceriotti, L.; Kob, A.; Drechsler, S.; Ponti, J.; Thedinga, E.; Colpo, P.; Ehret, R.; Rossi, F. Online monitoring of BALB/3T3 metabolism and adhesion with multiparametric chip-based system. Anal. Biochem. 2007, 371, 92–104. [Google Scholar]
- Becker, B.; Lob, V.; Janzen, N.; Grundl, D.; Ilchmann, F.; Wolf, B. Automated multi-parametric label free 24 channel real-time screening system. IFMBE Proc. 2008, 20, 186–189. [Google Scholar] [CrossRef]
- Xi, B.; Yu, N.; Wang, X.; Xu, X.; Abassi, Y. The application of cell-based label-free technology in drug discovery. Biotechnol. J. 2008, 3, 484–495. [Google Scholar]
- Asphahani, F.; Wang, K.; Thein, M.; Veiseh, O.; Yung, S.; Xu, J.; Zhang, M. Single-cell bioelectrical impedance platform for monitoring cellular response to drug treatment. Phys. Biol. 2011, 8, 015006. [Google Scholar] [CrossRef]
- Wang, W.; Foley, K.; Shan, X.; Wang, S.; Eaton, S.; Nagaraj, V.J.; Wiktor, P.; Patel, U.; Tao, N. Single cells and intracellular processes studied by a plasmonic-based electrochemical impedance microscopy. Nat. Chem. 2011, 3, 249–255. [Google Scholar]
- Poulain, B.; De Paiva, A.; Deloye, F.; Doussau, F.; Tauc, L.; Weller, U.; Dolly, J. Differences in the multiple step process of inhibition of neurotransmitter release induced by tetanus toxin and botulinum neurotoxins type A and B atAplysia synapses. Neuroscience 1996, 70, 567–576. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wang, P.; Ye, X.S.; Zhang, G.Y.; He, H.Q.; Yan, W.M.; Zheng, X.X.; Han, J.H.; Cui, D.F. Drug evaluations using a novel microphysiometer based on cell-based biosensors. Sens. Actuat. B: Chem. 2001, 80, 215–221. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Cai, H.; Du, L.; Liu, Q.; Wang, P. A photovoltage-based integrated sensor for nephrotoxicity evaluation under drug stimulation. In Proceeding of the 16th International Solid-State SensorsActuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 2122–2125.
- Adami, M.; Martini, M.; Piras, L. Characterization and enzymatic application of a redox potential biosensor based on a silicon transducer. Biosens. Bioelectron. 1995, 10, 633–638. [Google Scholar] [CrossRef]
- Primiceri, E.; Chiriacò, M.S.; Dioguardi, F.; Monteduro, A.G.; D’Amone, E.; Rinaldi, R.; Giannelli, G.; Maruccio, G. Automatic transwell assay by an EIS cell chip to monitor cell migration. Lab Chip 2011, 11, 4081–4086. [Google Scholar] [CrossRef]
- Wu, C.C.; Lin, W.C.; Fu, S.Y. The open container-used microfluidic chip using IrOx ultramicroelectrodes for the in situ measurement of extracellular acidification. Biosens. Bioelectron. 2011, 26, 4191–4197. [Google Scholar]
- Chao, T.C.; Ros, A. Microfluidic single-cell analysis of intracellular compounds. J. R. Soc. Interface 2008, 5, S139–S150. [Google Scholar] [CrossRef]
- Gao, Y.; Bhattacharya, S.; Chen, X.; Barizuddin, S.; Gangopadhyay, S.; Gillis, K.D. A microfluidic cell trap device for automated measurement of quantal catecholamine release from cells. Lab Chip 2009, 9, 3442–3446. [Google Scholar]
- Werdich, A.A.; Lima, E.A.; Ivanov, B.; Ges, I.; Anderson, M.E.; Wikswo, J.P.; Baudenbacher, F.J. A microfluidic device to confine a single cardiac myocyte in a sub-nanoliter volume on planar microelectrodes for extracellular potential recordings. Lab Chip 2004, 4, 357–362. [Google Scholar] [CrossRef]
- Gao, C.; Rilo, H.; Myneni, P.; Ahn, C. A new on-chip insulin biosensor for monitoring dynamic reponse of human islet cells. Spec. Publ.-R. Soc. Chem. 2004, 297, 581–583. [Google Scholar]
- Pereira Rodrigues, N.; Sakai, Y.; Fujii, T. Cell-based microfluidic biochip for the electrochemical real-time monitoring of glucose and oxygen. Sens. Actuat. B: Chem. 2008, 132, 608–613. [Google Scholar] [CrossRef]
- Fan, C.Y.; Tung, Y.C.; Takayama, S.; Meyhöfer, E.; Kurabayashi, K. Electrically programmable surfaces for configurable patterning of cells. Adv. Mater. 2008, 20, 1418–1423. [Google Scholar]
- Kaji, H.; Hashimoto, M.; Sekine, S.; Kawashima, T.; Nishizawa, M. Patterning adherent cells within microchannels by combination of electrochemical biolithography technique and repulsive dielectrophoretic force. Electrochemistry 2008, 76, 555–558. [Google Scholar] [CrossRef]
- Kaji, H.; Camci-Unal, G.; Langer, R.; Khademhosseini, A. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions. Engineering systems for the generation of patterned co-cultures for controlling cell-cell interactions 2011, 1810, 239–250. [Google Scholar]
- Li, Q.; Tang, D.; Tang, J.; Su, B.; Chen, G.; Wei, M. Magneto-controlled electrochemical immunosensor for direct detection of squamous cell carcinoma antigen by using serum as supporting electrolyte. Biosens. Bioelectron. 2011, 27, 153–159. [Google Scholar] [CrossRef]
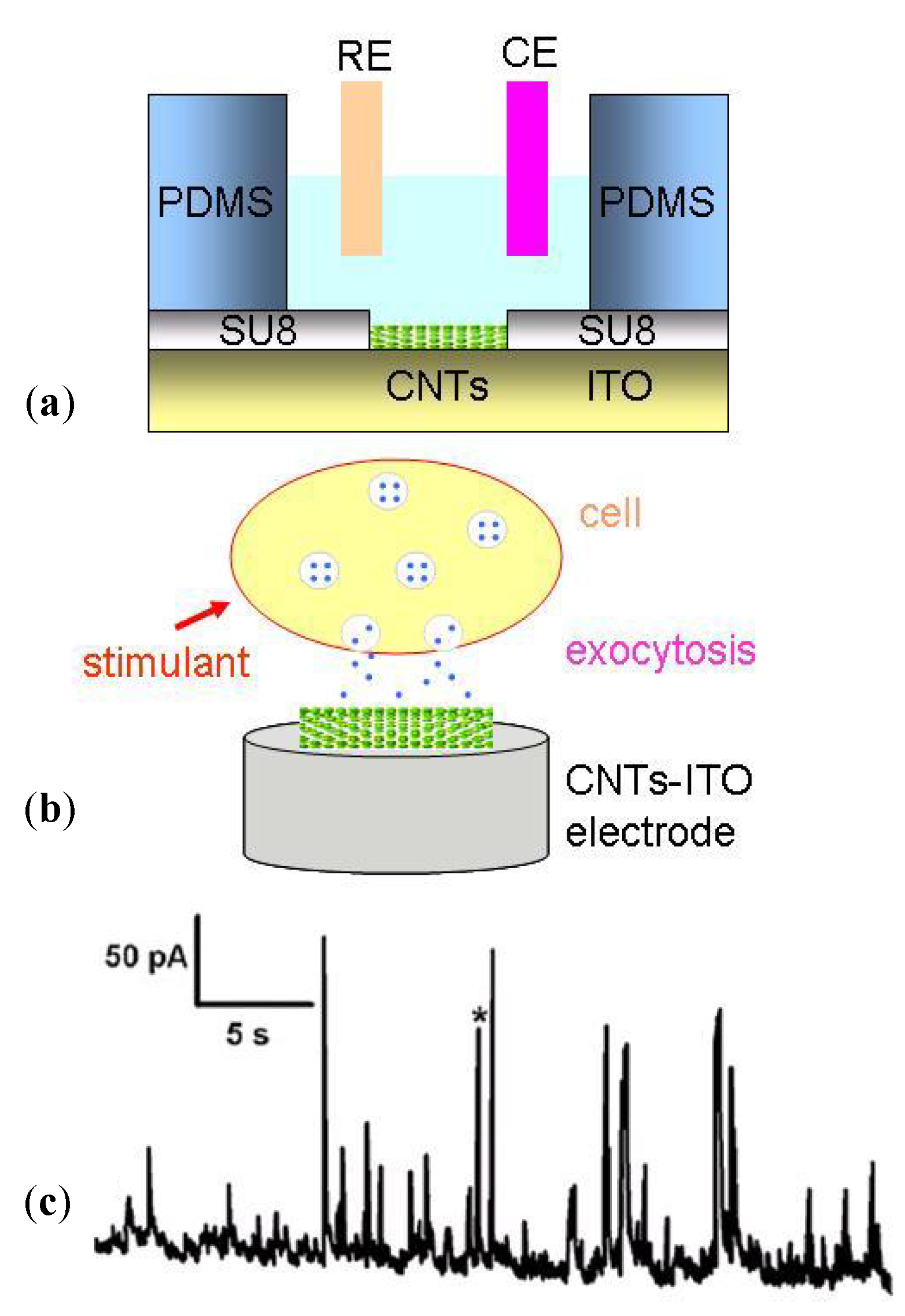
- Shi, B.X.; Wang, Y.; Zhang, K.; Lam, T.L.; Chan, H.L.W. Monitoring of dopamine release in single cell using ultrasensitive ITO microsensors modified with carbon nanotubes. Biosens. Bioelectron. 2011, 26, 2917–2921. [Google Scholar] [CrossRef]
- Merkoçi, A. Nanoparticles-based strategies for DNA, protein and cell sensors. Biosens. Bioelectron. 2010, 26, 1164–1177. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Wu, C.; Hu, N.; Zhou, J.; Du, L.; Wang, P. Microfabricated Electrochemical Cell-Based Biosensors for Analysis of Living Cells In Vitro. Biosensors 2012, 2, 127-170. https://doi.org/10.3390/bios2020127
Wang J, Wu C, Hu N, Zhou J, Du L, Wang P. Microfabricated Electrochemical Cell-Based Biosensors for Analysis of Living Cells In Vitro. Biosensors. 2012; 2(2):127-170. https://doi.org/10.3390/bios2020127
Chicago/Turabian StyleWang, Jun, Chengxiong Wu, Ning Hu, Jie Zhou, Liping Du, and Ping Wang. 2012. "Microfabricated Electrochemical Cell-Based Biosensors for Analysis of Living Cells In Vitro" Biosensors 2, no. 2: 127-170. https://doi.org/10.3390/bios2020127
APA StyleWang, J., Wu, C., Hu, N., Zhou, J., Du, L., & Wang, P. (2012). Microfabricated Electrochemical Cell-Based Biosensors for Analysis of Living Cells In Vitro. Biosensors, 2(2), 127-170. https://doi.org/10.3390/bios2020127