Enzyme-Gelatin Electrochemical Biosensors: Scaling Down
Abstract
:1. Introduction

2. Experimental Section
2.1. Chemicals and Solutions
2.2. Electrode Preparation
2.3. Apparatus
3. Results and Discussion
3.1. Electrochemical Behaviour of HHC in Gelatin on Glassy Carbon Electrodes: Drop Dried (DD) versus Spin Coated (SC) Layers
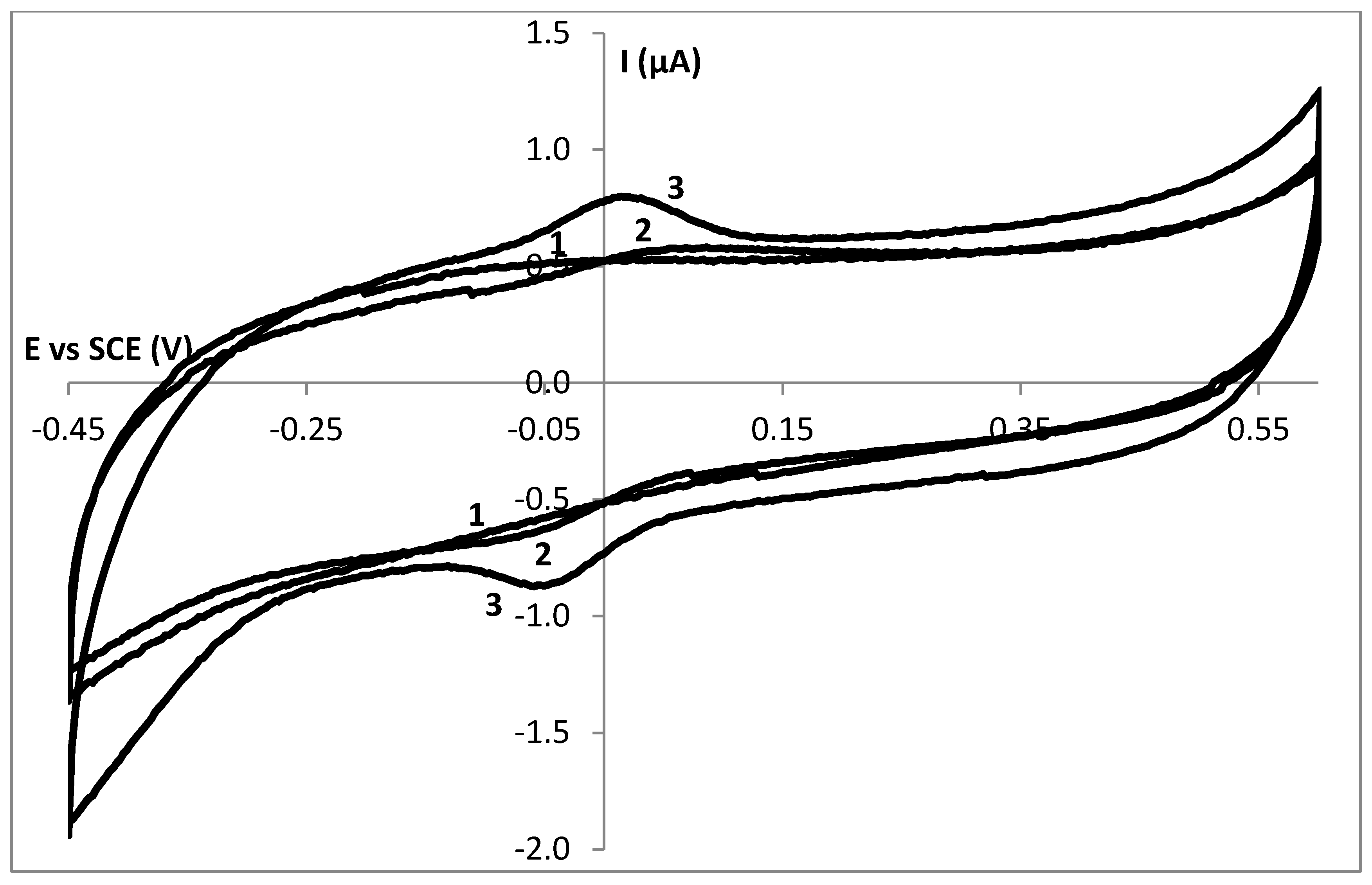
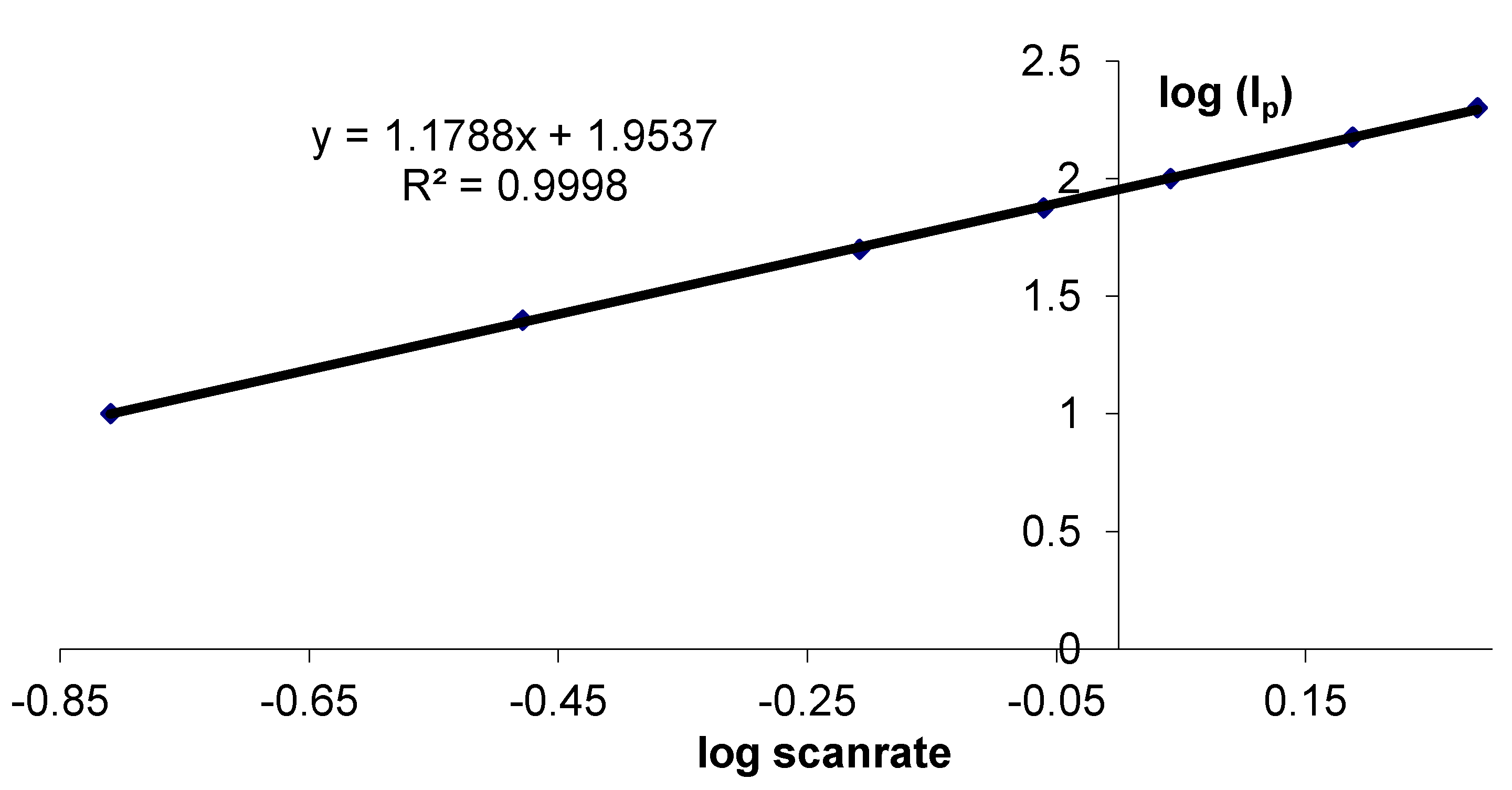
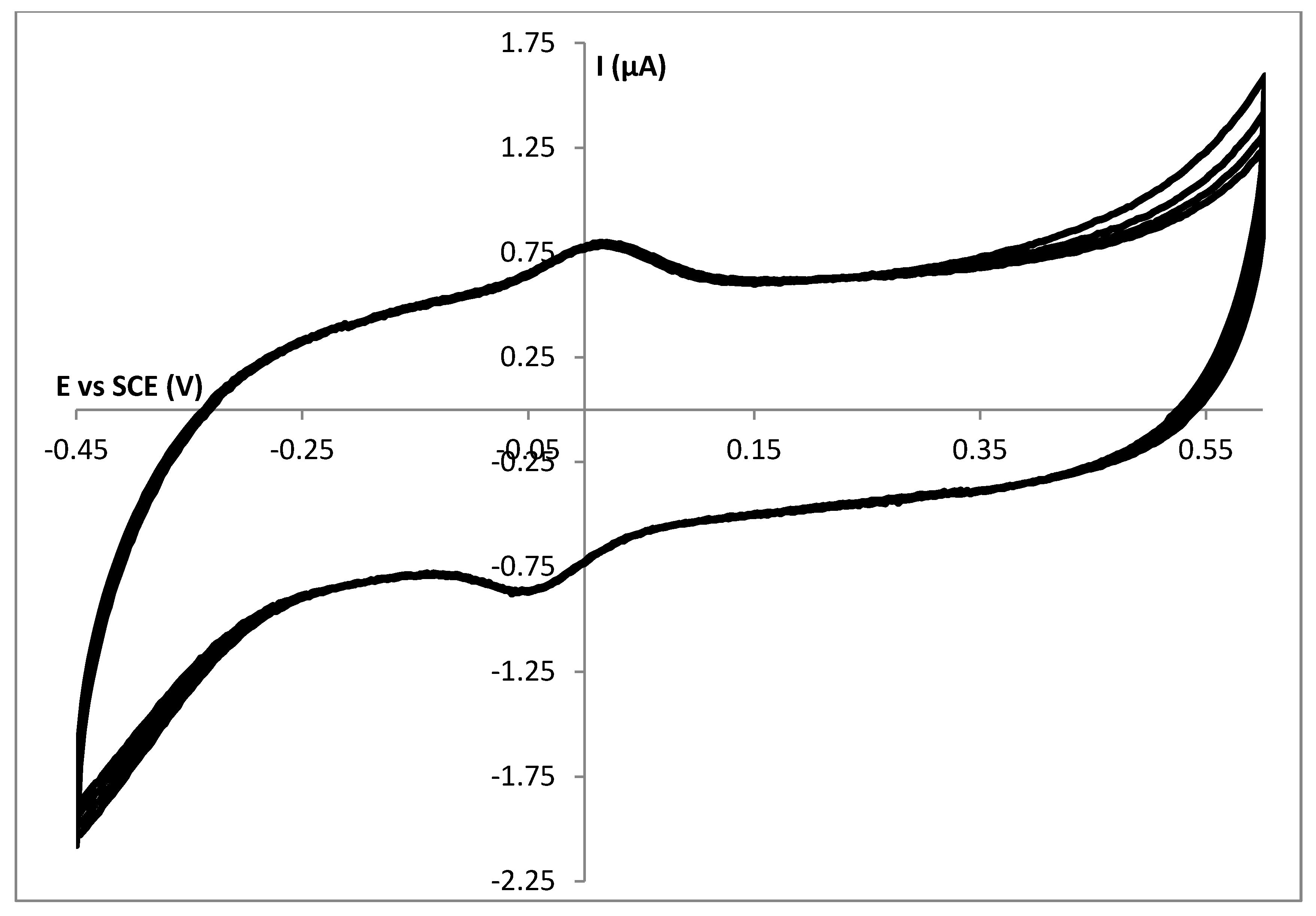
3.2. Stability Study of a SC Enzyme-Gelatin Layer
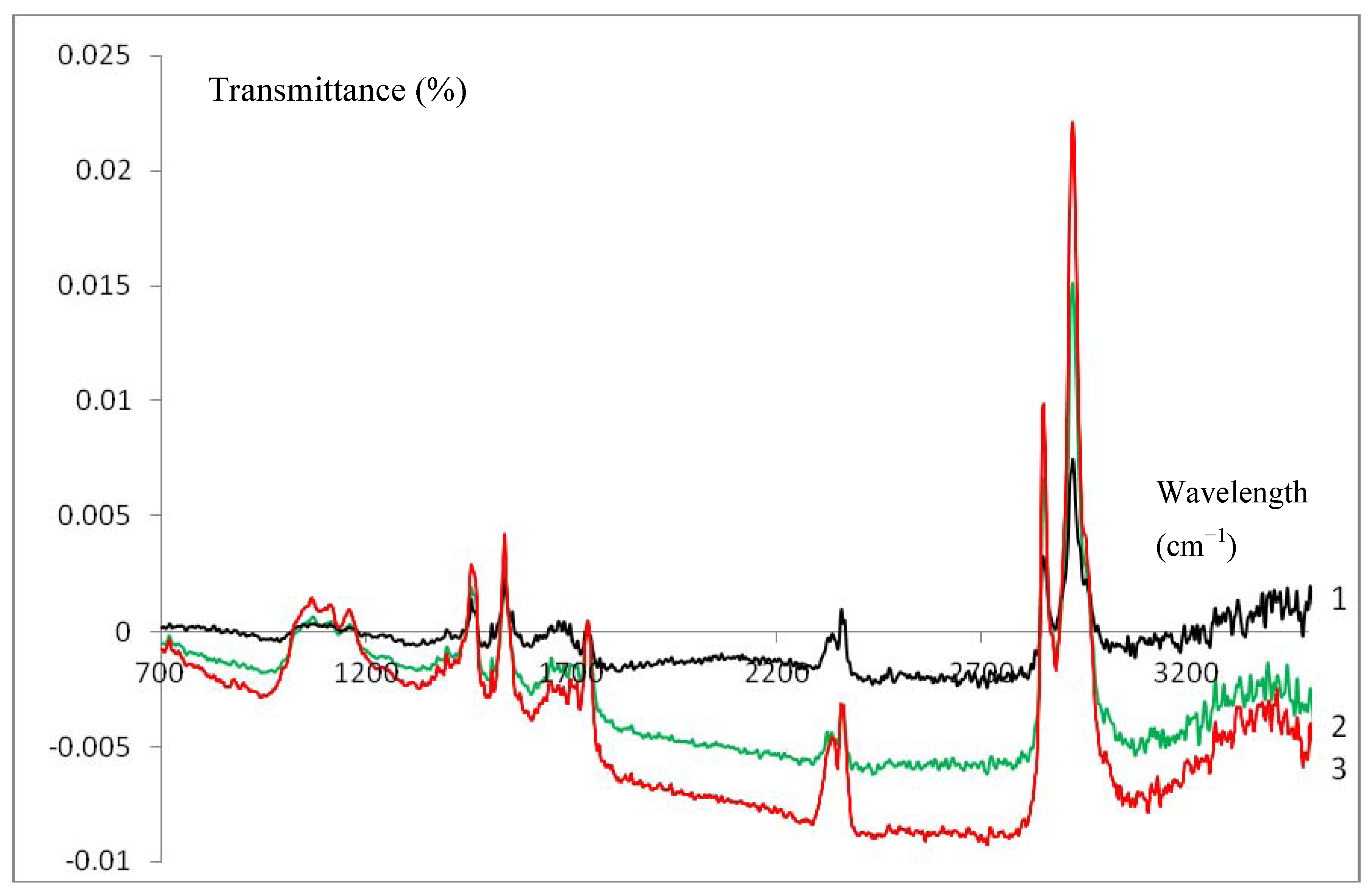
3.3. Infrared Characterization of a SC Enzyme-Gelatin Layer
3.4. Encapsulation of Cat in a SC Gelatin Film
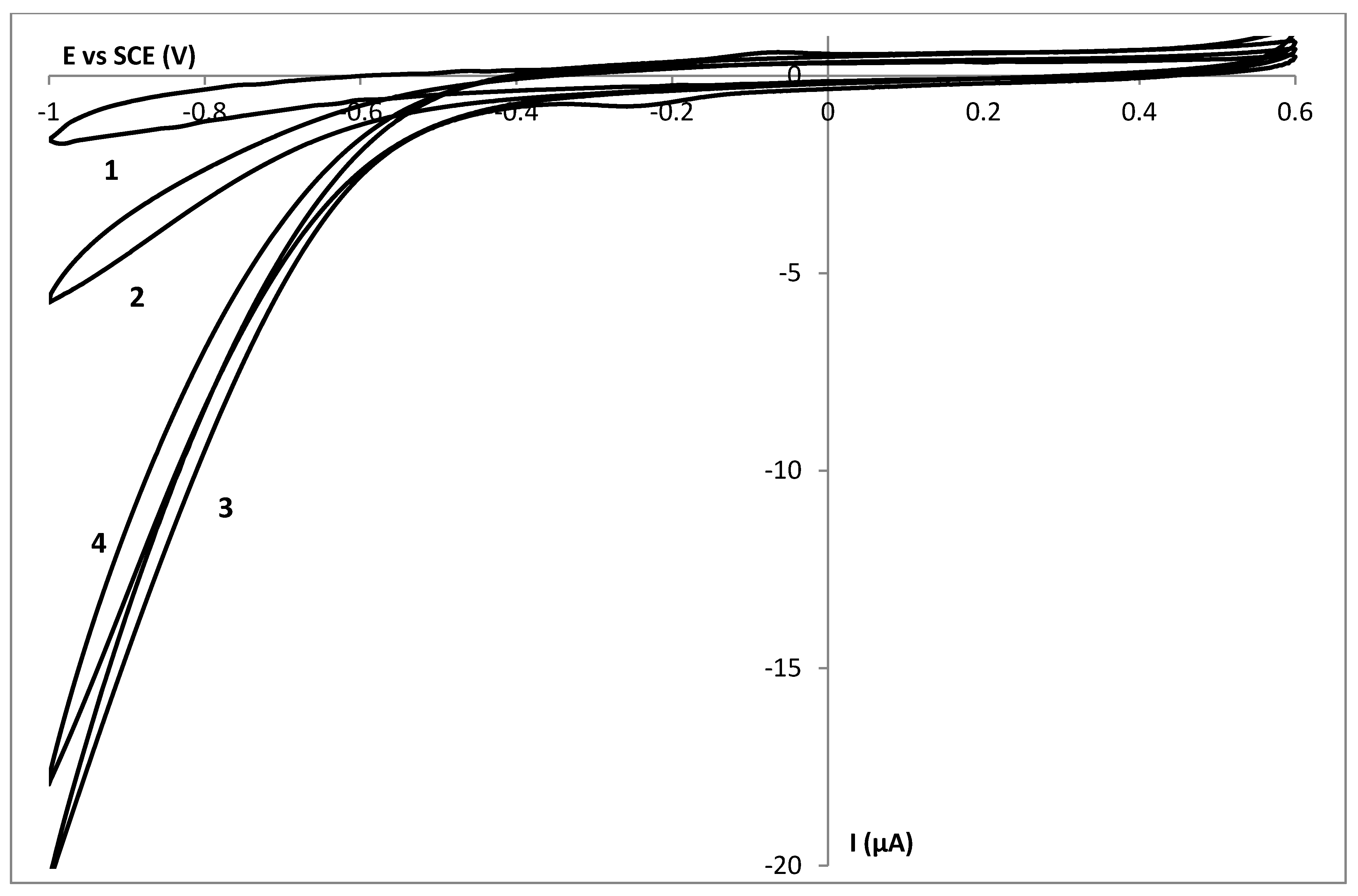
3.5. Thickness Determination

3.6. Characterization of Cat into Gel
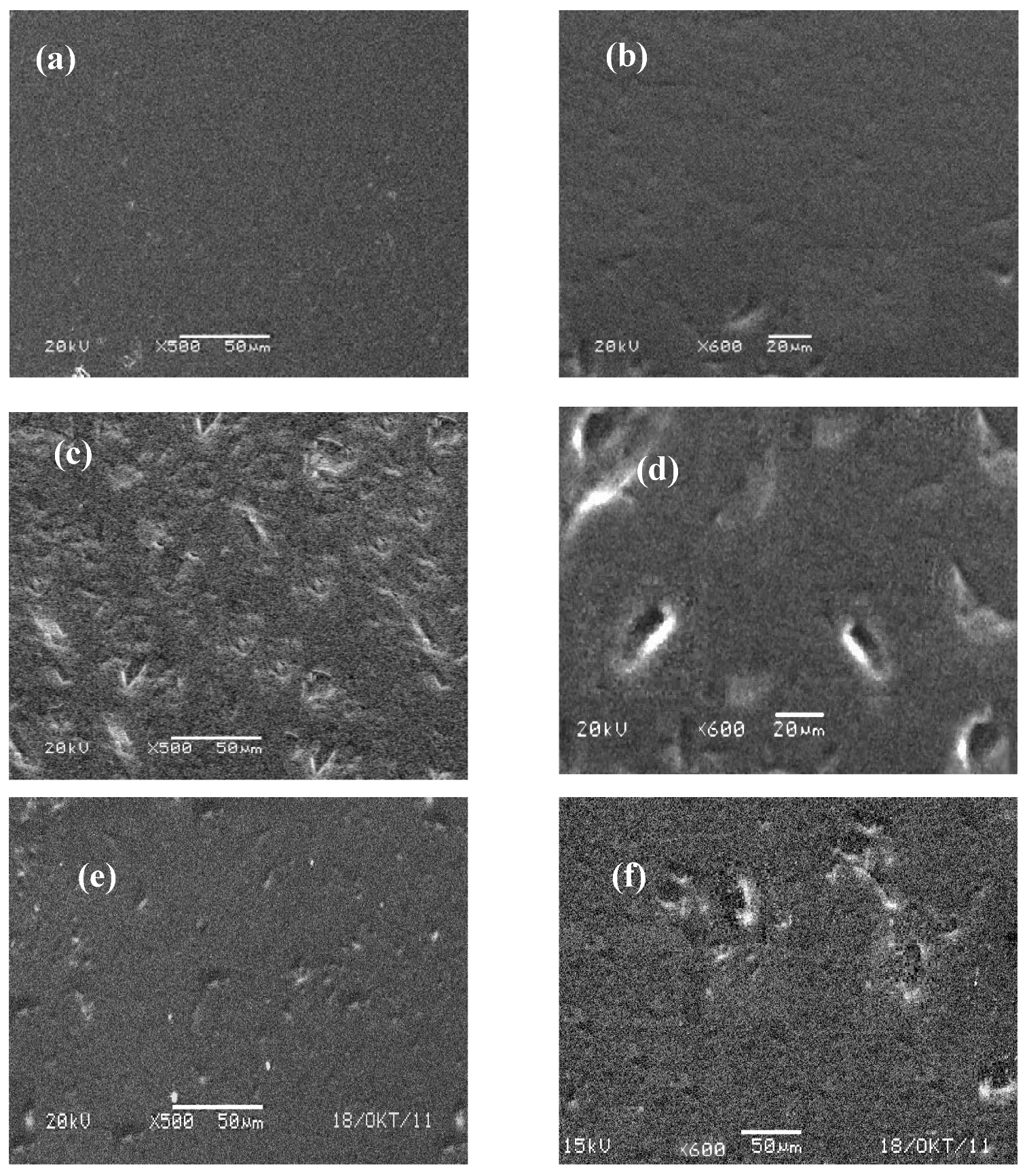
4. Conclusions
Acknowledgments
References
- Monosik, R.; Ukropcova, D.; Stredansky, M.; Sturdik, E. Multienzymatic amperometric biosensor based on gold and nanocomposite planar electrodes for glycerol determination in wine. Anal. Biochem. 2012, 421, 256–261. [Google Scholar]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as innovative tools for the detection of food borne pathogens. Biosens. Bioelectron. 2011, 28, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Yin, L.F.; Dai, Y.R.; Jiang, F.; Niu, J.F. Application of electrochemical enzyme biosensor in environmental pollution monitoring. Prog. Chem. 2012, 24, 131–143. [Google Scholar]
- Chandra, P.; Zaidi, S.A.; Noh, H.B.; Shim, Y.B. Separation and simultaneous detection of anticancer drugs in a microfluidic device with an amperometric biosensor. Biosens. Bioelectron. 2011, 28, 326–332. [Google Scholar] [CrossRef]
- Gil, E.D.; de Melo, G.R. Electrochemical biosensors in pharmaceutical analysis. Braz. J. Pharm. Sci. 2010, 46, 375–391. [Google Scholar] [CrossRef]
- 6. Yalcır, F.; Çevik, E.; Senel, M.; Baykal, A. Development of an amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto nickel ferrite nanoparticle-chitosan composite. Nano-Micro Lett. 2011, 3, 91–98. [Google Scholar]
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications; John Wiley and Sons: New York, NY, USA, 2008. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar]
- Rodrigues, R.C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilization. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, G.; Huang, Y.; Bai, H.; Lu, X. A new hydrogen peroxide biosensor based on gold nanoelectrode ensembles/multiwalled carbon nanotubes/chitosan film-modified electrode. Colloids Surfaces A 2009, 340, 50–55. [Google Scholar] [CrossRef]
- Lai, M.E.; Bergel, A. Direct electrochemistry of catalase on glassy carbon electrodes. Bioelectrochemistry 2002, 55, 157–160. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Yang, W.; Li, Y.; Sun, C. Gold nanoparticles-mesoporous silica composite used as an enzyme immobilization matrix for amperometric glucose biosensor construction. Sens. Actuat. B 2007, 124, 179–186. [Google Scholar] [CrossRef]
- Wittlich, P.; Capan, E.; Schlieker, M.; Vorlop, K.-D.; Jahnz, U. Entrapment in Lentikats, encapsulation of various biocatalysts—Bacteria, fungi, yeast or enzymes intopolyvinyl alcohol based hydrogel particles. In Fundamentals of Cell Immobilization Biotechnology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 53–63. [Google Scholar]
- De Wael, K.; De Belder, S.; Van Vlierberghe, S.; Van Steenberge, G.; Dubruel, P.; Adriaens, A. Electrochemical study of gelatin as a matrix for the immobilization of horse heart cytochrome c. Talanta 2010, 82, 1980–1985. [Google Scholar]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Disc. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- De Wael, K.; Bashir, Q.; Van Vlierberghe, S.; Dubruel, P.; Heering, H.A.; Adriaens, A. Eletrochemical determination of hydrogen peroxide with cytochrome c peroxidase and horse heart cytochrome c entrapped in a gelatin hydrogel. Bioelectrochemistry 2012, 83, 15–18. [Google Scholar] [CrossRef]
- 19. Li, N.; Xue, M-H.; Yao, H.; Zhu, J.-J. Reagentless biosensor for phenolic compounds based on tyrosinase entrapped within gelatine film. Anal. Bioanal. Chem. 2005, 383, 1127–1132. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterial 2001, 22, 763–768. [Google Scholar]
- Yalcıner, F.; Çevik, E.; Senel, M.; Baykal, A. Development of an amperometric hydrogen peroxide biosensor based on the immobilization of horseradish peroxidase onto nickel ferrite nanoparticle-chitosan composite. Nano-Micro Lett. 2011, 3, 91–98. [Google Scholar]
- Chen, S.; Yuan, R.; Chai, Y.; Zhang, L.; Wang, N.; Li, X. Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosens. Bioelectron. 2007, 22, 1268–1274. [Google Scholar] [CrossRef]
- Philip, A.; Clapp, F.; Dennis, E. Spectrophotometric determination of hydrogen peroxide with leuco Patent Blue Violet. Anal. Chim. Acta 1991, 243, 217–220. [Google Scholar] [CrossRef]
- Abbas, M.E.; Luo, W.; Zhu, L.; Zou, J.; Tang, H. Fluorometric determination of hydrogen peroxide in milk by using a Fenton reaction syste. Food Chem. 2010, 120, 327–331. [Google Scholar] [CrossRef]
- Uchida, S.; Satoh, Y.; Yamashiro, N.; Satoh, T. Determination of hydrogen peroxide in water by chemiluminescence detection, (II) theoretical analysis of luminol chemiluminescence processes. J. Nuclear Sci. Technol. 2004, 41, 898–906. [Google Scholar] [CrossRef]
- Prakash, P.A.; Yogeswaran, U.; Chen, S.M. A review on direct electrochemistry of catalase for electrochemical sensors. Sensors 2009, 9, 1821–1844. [Google Scholar] [CrossRef]
- Lai, M.E.; Bergel, A. Direct electrochemistry of catalase on glassy carbon electrodes. Bioelectrochemistry 2002, 55, 157–160. [Google Scholar] [CrossRef]
- Costa, S.A.; Azevedo, H.S.; Reis, R.L. Enzyme immobilization in biodegradable polymers for biomedical applications. In Biodegradable Systems in Tissue Engineering and Regenerative Medicine; Reis, R.L., San Román, J., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 301–318. [Google Scholar]
- Ertas, N. Specific determination of hydrogen peroxide with a catalase biosensor based on mercury thin film electrodes. Turk. J. Chem. 2000, 24, 95–99. [Google Scholar]
- Schubert, D.W.; Dunkel, T. Spin coating from a molecular point of view: Its concentration regimes, influence of molar mass and distribution. Mat. Res. Innov. 2003, 7, 314–321. [Google Scholar] [CrossRef]
- Ges, I.A.; Baudenbacher, F. Enzyme electrodes to monitor glucose consumption of single cardiac myocytes in sub-nanoliter volumes. Biosens. Bioelectron. 2010, 25, 1019–1024. [Google Scholar] [CrossRef]
- Sirohi, R.S.; Genshaw, M.A. Electrochemical ellipsometric study of gold. J. Electrochem. Soc. 1969, 116, 910. [Google Scholar]
- Heering, H.A.; Wiertz, F.G.M.; Dekker, C.; De Vries, S. Direct immobilization of native yeast Iso-1 cytochrome c on bare gold: Fast electron relay to redox enzymes and zeptomole protein-film voltammetry. J. Am. Chem. Soc. 2004, 126, 11103–11112. [Google Scholar]
- Hawkridge, F.; Kuwana, T. Indirect coulometric titration of biological electron-transport components. Anal. Chem. 1973, 45, 1021–1027. [Google Scholar] [CrossRef]
- Guoz, Z.; Zhang, H.; Gai, P.; Duan, J. Direct electrochemistry of cytochrome c entrapped in agarose hydrogel by protein film voltammetry. Russ. J. Electrochem. 2011, 47, 175–180. [Google Scholar] [CrossRef]
- Schlereth, D.D. Characterization of protein monolayers by surface plasmon resonance combined with cyclic voltammetry ‘in situ’. J. Electroanal. Chem. 1999, 646, 198–207. [Google Scholar]
- Li, M.; He, P.; Zhang, Y.; Hu, N. An electrochemical investigation of hemoglobin and catalase incorporated in collagen films. Biochim. Biophys. Acta 1749, 43–51. [Google Scholar]
- Noel, M., Vasu. Cyclic Voltammetry and the Frontiers of Electrochemistry; Aspect Publications: London, UK, 1990. [Google Scholar]
- Ting, S.W.; Periasamy, A.P.; Chen, S.M.; Saraswathi, R. Direct electrochemistry of catalase immobilized at electrochemically reduced graphene oxide modified electrode for amperometric H2O2 biosensor. Int. J. Electrochem. Sci. 2011, 14, 4438–4453. [Google Scholar]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Wael, K.; De Belder, S.; Pilehvar, S.; Van Steenberge, G.; Herrebout, W.; Heering, H.A. Enzyme-Gelatin Electrochemical Biosensors: Scaling Down. Biosensors 2012, 2, 101-113. https://doi.org/10.3390/bios2010101
De Wael K, De Belder S, Pilehvar S, Van Steenberge G, Herrebout W, Heering HA. Enzyme-Gelatin Electrochemical Biosensors: Scaling Down. Biosensors. 2012; 2(1):101-113. https://doi.org/10.3390/bios2010101
Chicago/Turabian StyleDe Wael, Karolien, Stijn De Belder, Sanaz Pilehvar, Geert Van Steenberge, Wouter Herrebout, and Hendrik A. Heering. 2012. "Enzyme-Gelatin Electrochemical Biosensors: Scaling Down" Biosensors 2, no. 1: 101-113. https://doi.org/10.3390/bios2010101
APA StyleDe Wael, K., De Belder, S., Pilehvar, S., Van Steenberge, G., Herrebout, W., & Heering, H. A. (2012). Enzyme-Gelatin Electrochemical Biosensors: Scaling Down. Biosensors, 2(1), 101-113. https://doi.org/10.3390/bios2010101



