Fabrication of Biocompatible, Vibrational Magnetoelastic Materials for Controlling Cellular Adhesion
Abstract
:1. Introduction
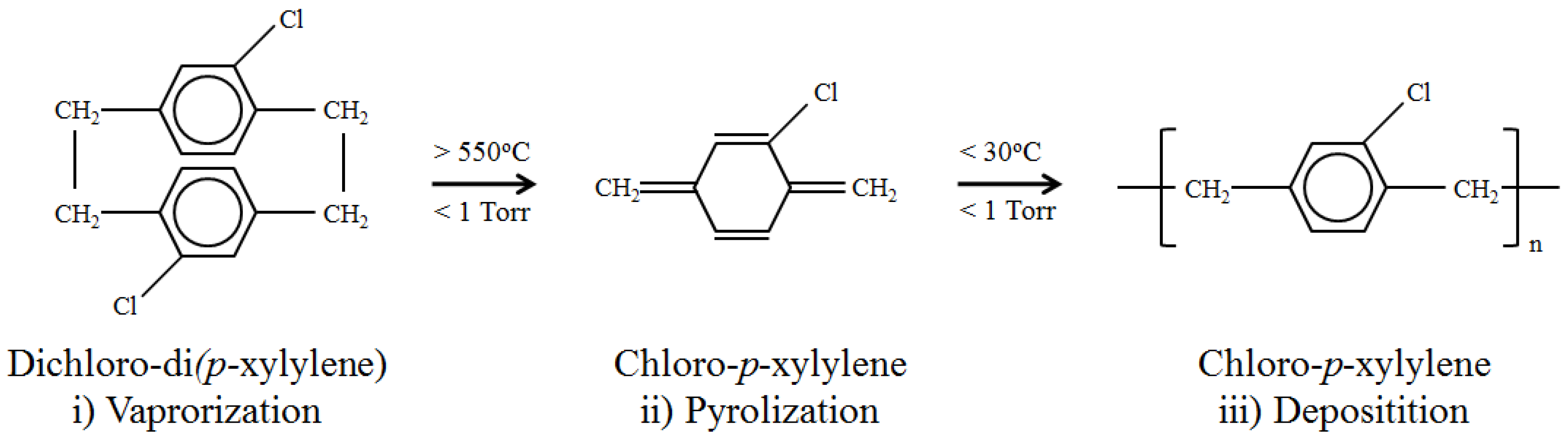
2. Materials and Methods
2.1. ME Material Preparation and Parylene-C Coating
2.2. Oxygen Plasma Etching
2.3. Surface Characterization
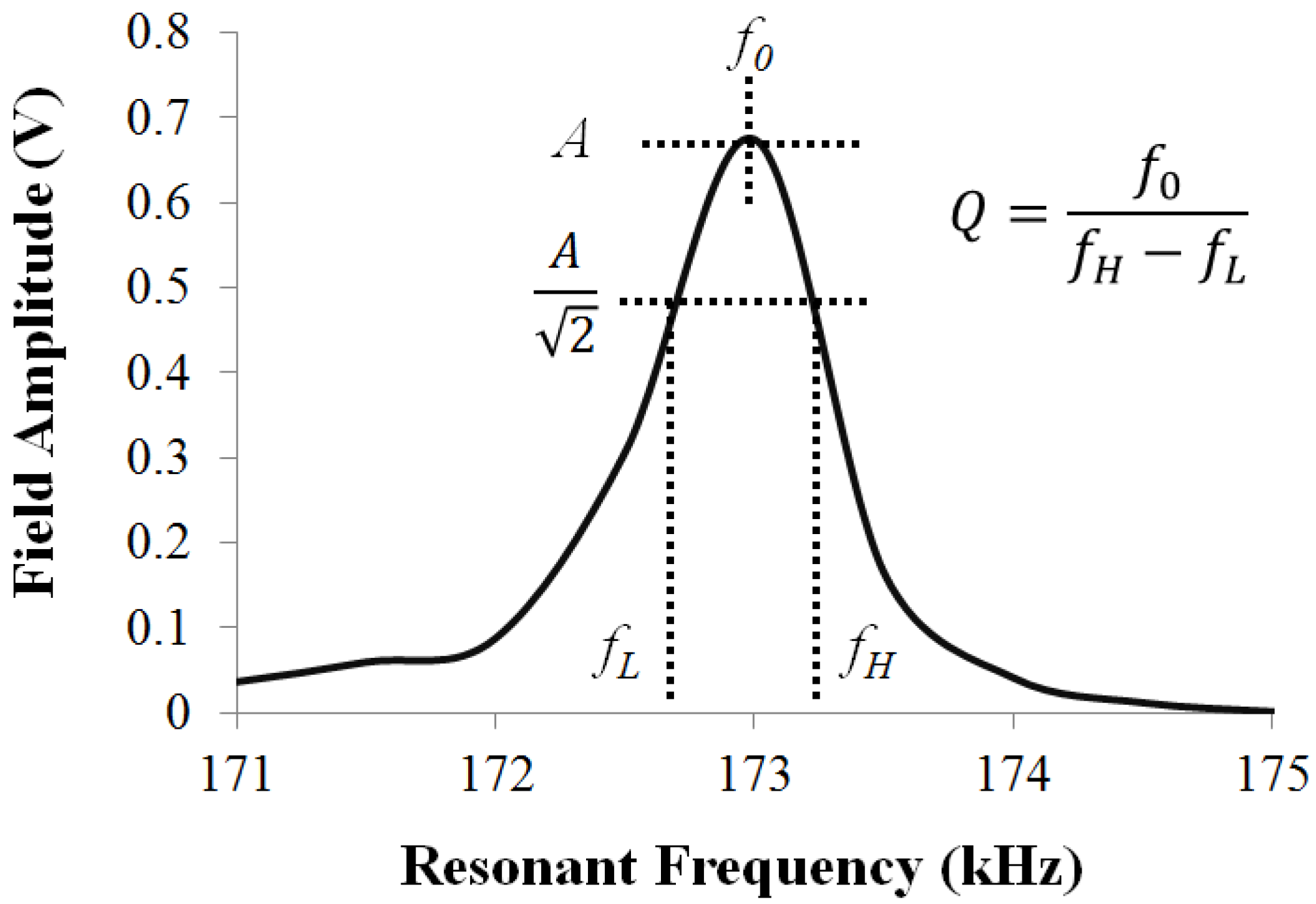
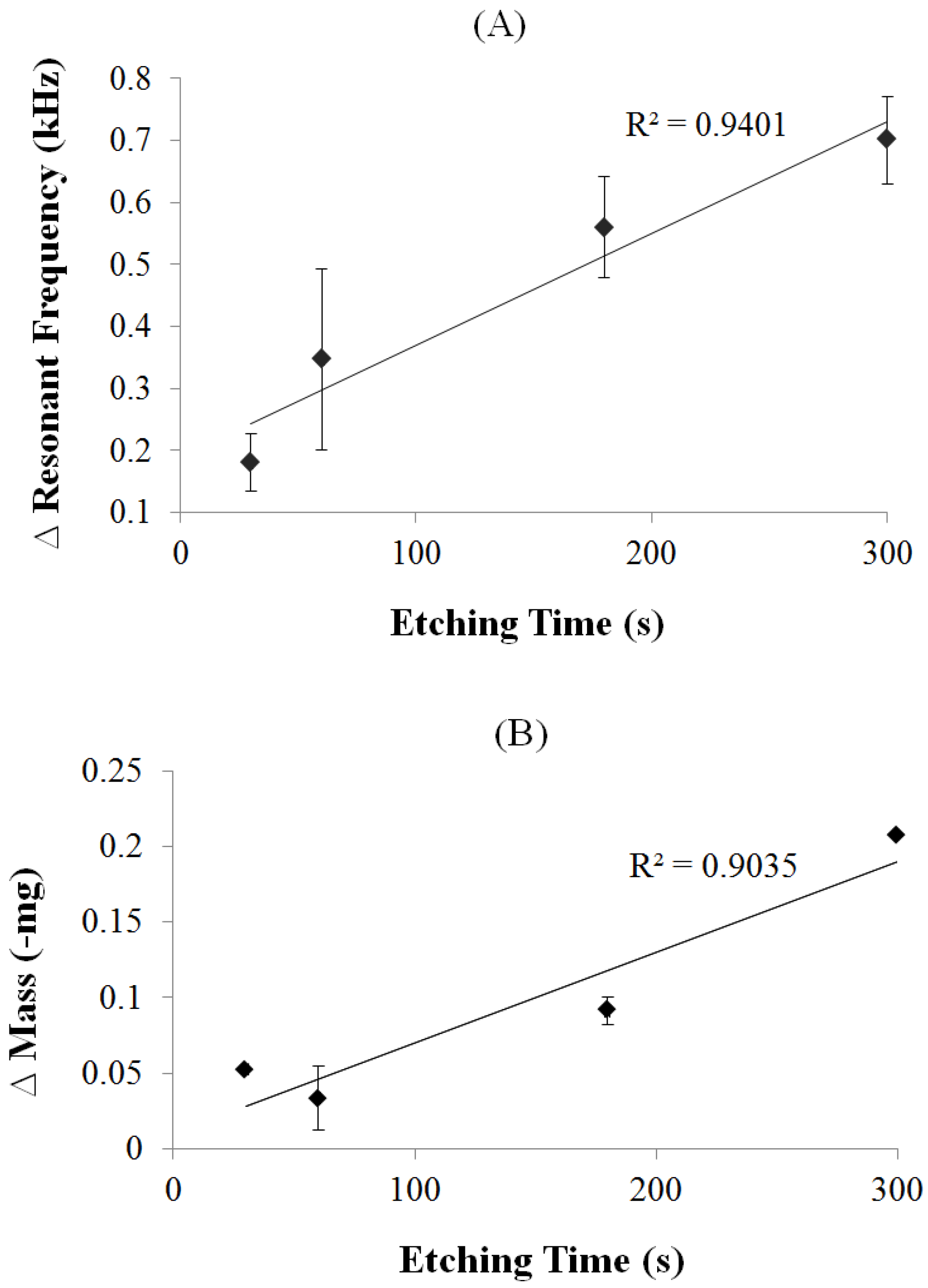
2.4. Effect of Coating on the ME Vibration
2.5. Protein Adsorption
2.6. Cell Culture and Adhesion
2.7. In Vivo Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Secondary Field Characterization
| Parylene-C Thickness (µm) | Mass Loading (mg) |
|---|---|
| 0 | 14.40 |
| 5 | 15.46 |
| 10 | 17.14 |
| 20 | 18.82 |
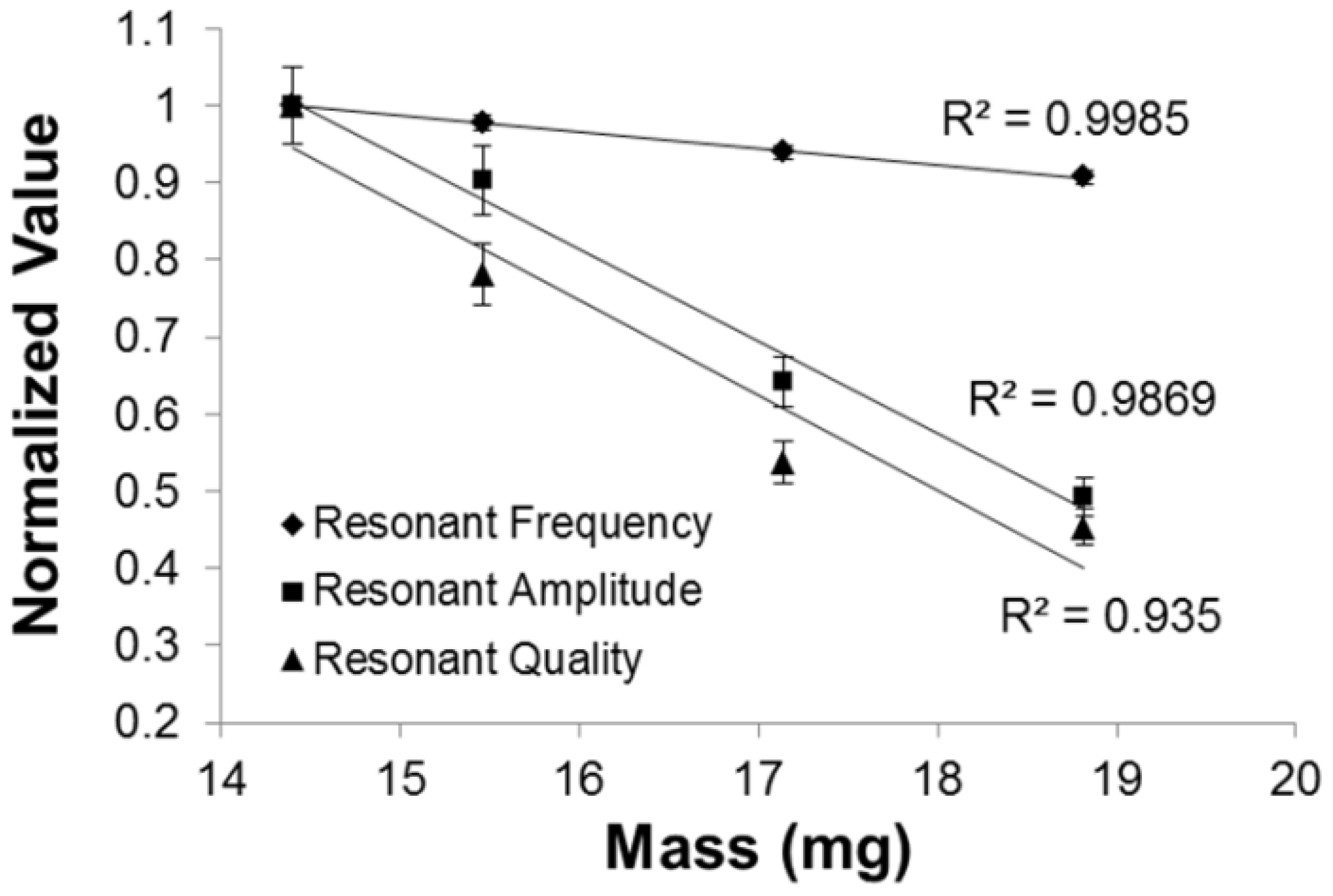
3.2. Parylene-C Functionalization via Oxygen Plasma Etching
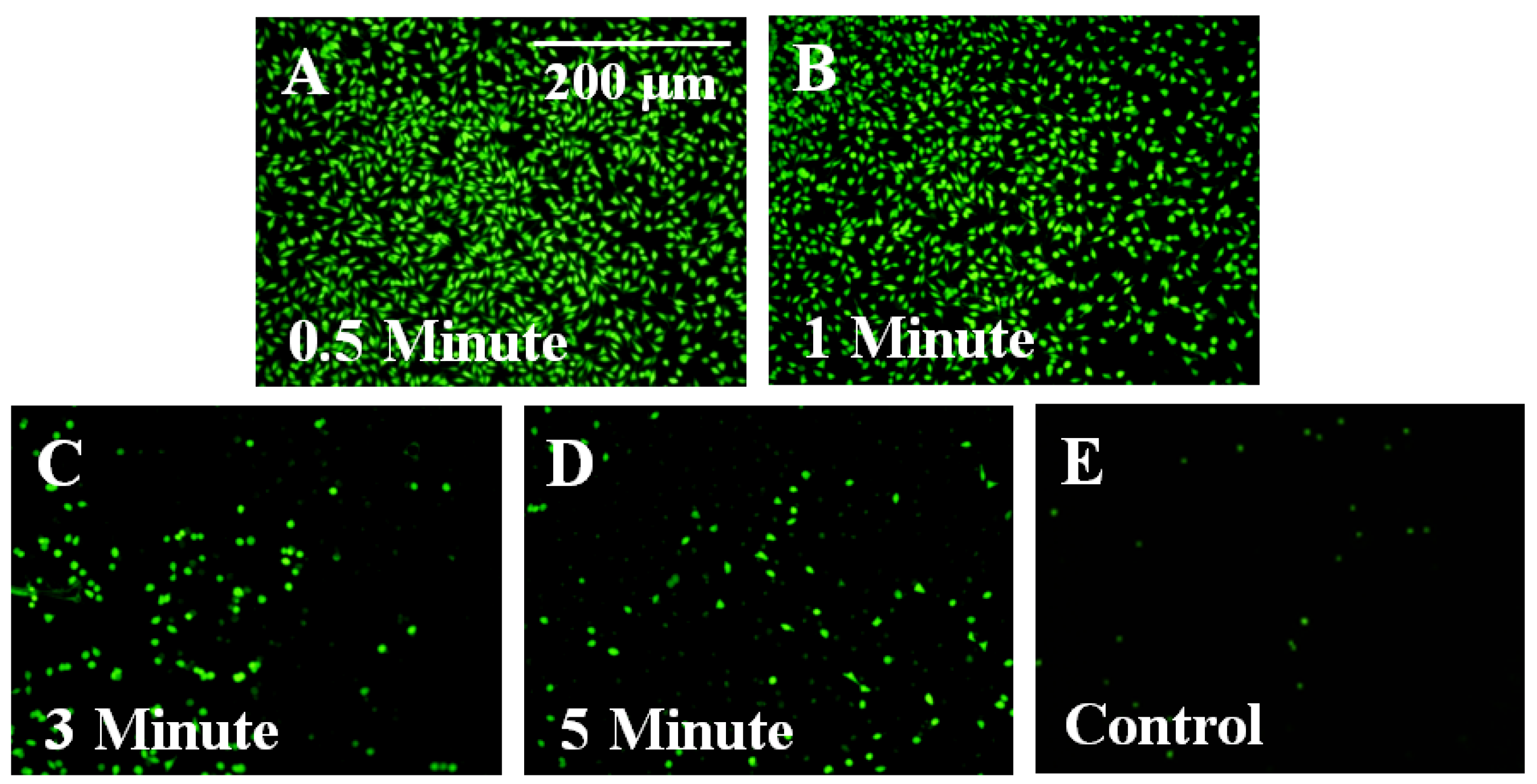
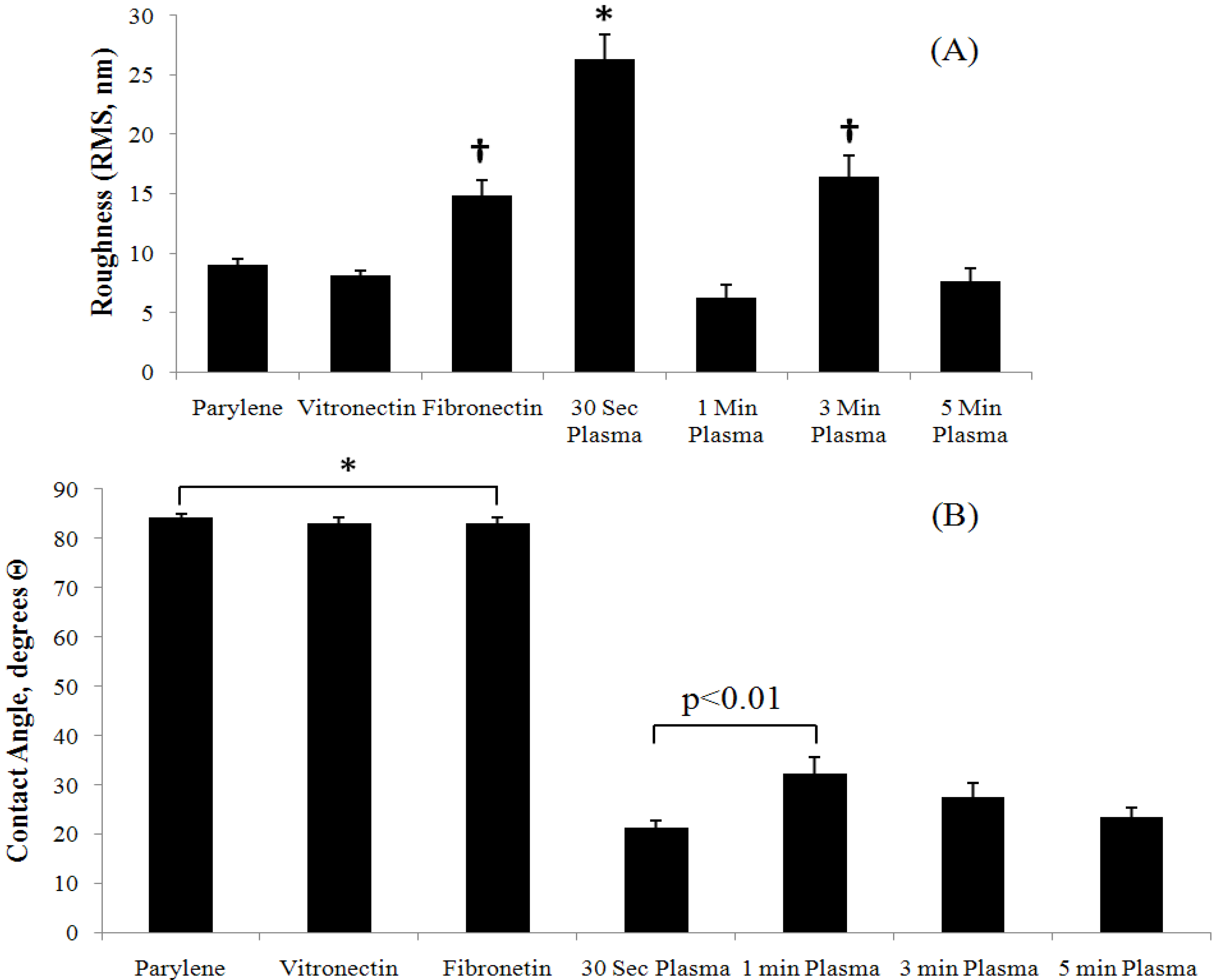
3.3. Control of Cellular Adhesion on Functionalized Parylene-C Surfaces
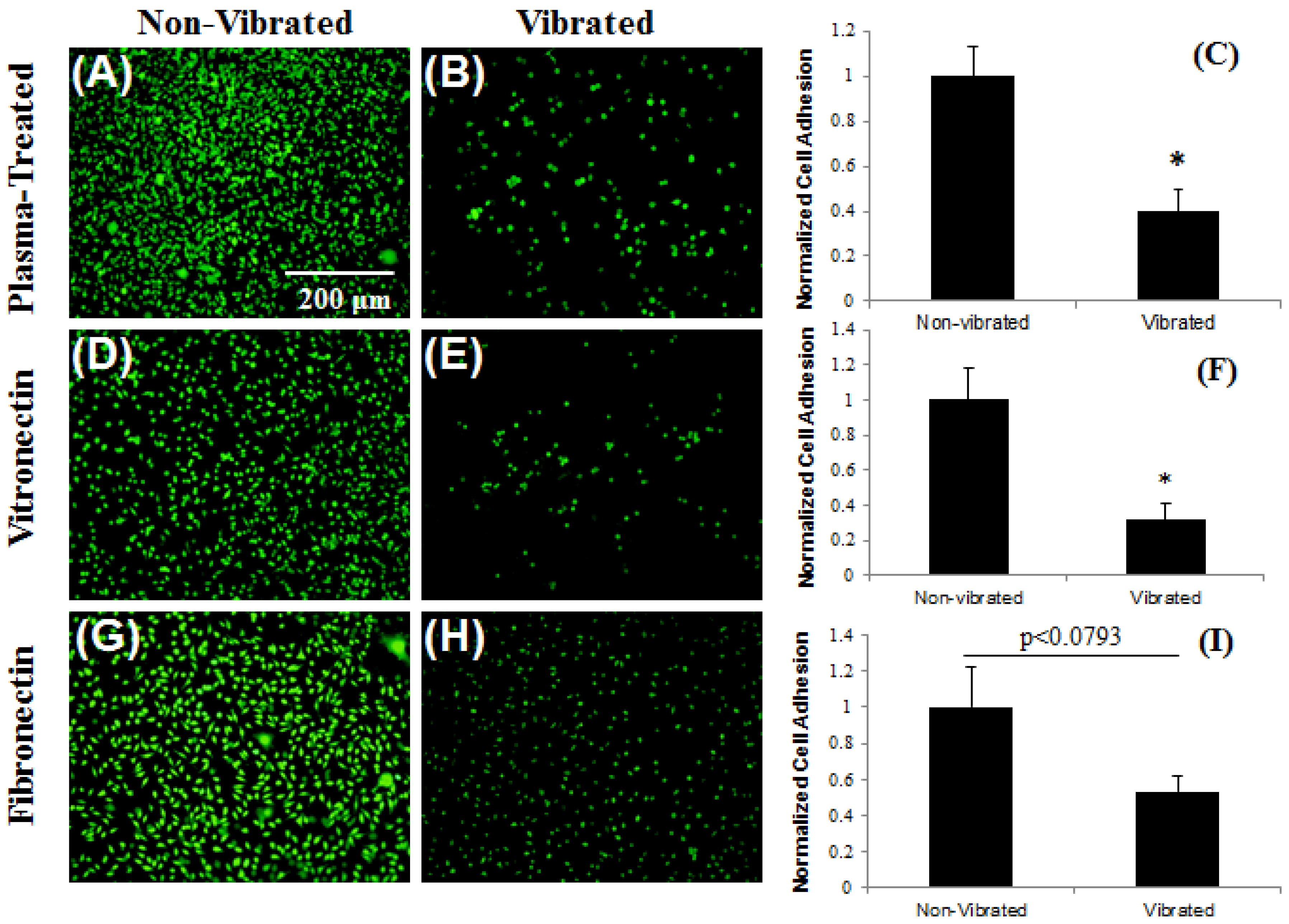
3.4. In Vivo Stability

4. Conclusions
Acknowledgments
References
- Chang, D.; Rodriguez, A.; Anderson, J. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 2007, 117, 524–529. [Google Scholar]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar]
- Winter, G. Transcutaneous implants: Reaction of the skin implant interface. J. Biomed. Mater. Res. Symp. 1974, 5, 99–113. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Janka, L.P.; Ong, K.G.; Rajachar, R.M. Magnetoelastic materials as novel bioactive coatings for control of cell adhesion. IEEE Trans. Biomed. Eng. 2010, 58, 698–704. [Google Scholar]
- Jain, M.K.; Schmidt, S.; Ong, K.G.; Mungle, C.; Grimes, C.A. Magnetoacoustic remote query temperature and humidity sensors. Smart Mater. Struct. 2000, 9, 502–510. [Google Scholar] [CrossRef]
- Jain, M.K.; Cai, Q.Y.; Grimes, C.A. A wireless micro-sensor for simultaneous measurement of pH, temperature, and pressure. Smart Mater. Struct. 2001, 10, 347–353. [Google Scholar]
- Kouzoudis, D.; Grimes, C.A. The frequency response of magnetoelastic sensors to stress and atmospheric pressure. Smart. Mater. Struct. 2000, 8, 885–889. [Google Scholar] [CrossRef]
- Grimes, C.A.; Stoyanov, P.G.; Kouzoudis, D.; Ong, K.G. Remote query pressure measurement using magnetoelastic sensors. Rev. Sci. Instrum. 1999, 70, 4711–4714. [Google Scholar]
- Grimes, C.A.; Kouzoudis, D. Remote query measurement of pressure, fluid-flow velocity, and humidity using magnetoelastic thick-film sensors. Sens. Actuat. 2000, 84, 205–212. [Google Scholar] [CrossRef]
- Grimes, C.A.; Kouzoudis, D.; Mungle, C. Simultaneous measurement of liquid density and viscosity using remote query magnetoelastic sensors. Rev. Sci. Instrum. 2000, 71, 3822–3824. [Google Scholar]
- Stoyanov, P.G.; Grimes, C.A. A remote query magnetostrictive viscosity sensor. Sens. Actuat. 2000, 80, 8–14. [Google Scholar]
- Loiselle, K.T.; Grimes, C.A. Viscosity measurements of viscous liquids using magnetoelastic thick-film sensors. Rev. Sci. Instrum 2000, 71, 1441–1446. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Cammers-Goodwin, A.; Grimes, C.A. A wireless remote query magnetoelastic CO2 sensor. J. Environ. Monit. 2000, 2, 556–560. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Grimes, C.A. A remote query magnetoelastic pH sensor. Sens. Actuat. B 2000, 71, 112–117. [Google Scholar]
- Cai, Q.Y.; Jain, M.K.; Grimes, C.A. A wireless, remote query ammonia sensor. Sens. Actuat. B 2000, 77, 614–619. [Google Scholar]
- Grimes, C.A.; Mungle, C.S.; Zeng, K.; Jain, M.K.; Dreschel, W.R.; Paulose, M.; Ong, K.G. Wireless magnetoelastic resonance sensors: A critical review. Sensors 2002, 2, 294–313. [Google Scholar]
- Meng, E.; Li, P.Y.; Tai, Y.C. Plasma removal of Parylene-C. J. Micromech. Microeng. 2008, 180, 045004. [Google Scholar]
- Chang, T.Y.; Yadav, V.G.; Leo, S.D.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarash, S.; Dokmeci, M.R. Cell an protein compatibility of Parylene-C surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar]
- Sharma, A.; Rieth, L.; Tathireddy, P.; Harrison, R.; Oppermann, H.; Klein, M.; Töpper, M.; Jung, E.; Normann, R.; Clark, G.; Solzbacher, F. Long term in vitro function stability and recording longevity of fully integrated wireless neural interfaces based on the Utah Slant Electrode Array. J. Neural Eng. 2011. [Google Scholar] [CrossRef]
- Lahann, J. Vapor-based polymer coatings for potential biomedical applications. Polym. Int. 2006, 55, 1361–1370. [Google Scholar]
- Fortin, J.B.; Lu, T.-M. A model for the chemical vapor deposition of poly(para-xylylene) (Parylene) thin films. Chem. Mater. 2002, 14, 1945–1949. [Google Scholar] [CrossRef]
- Prendegrass, C.J.; Middleton, C.A.; Blunn, G.W. Fibronectin functionalized hydroxyapatite coatings: Improving dermal fibroblast adhesion in vitro and in vivo. Adv. Eng. Mater. 2010, 12, B365–B373. [Google Scholar]
- Thevenot, P.T.; Nair, A.M.; Shen, J.; Lotfi, P.; Ko, C.Y.; Tang, L. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials 2010, 31, 3997–4008. [Google Scholar]
- Laschke, M.W.; Strohe, A.; Menger, M.D.; Alini, M.; Eglin, D. In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater. 2010, 6, 2020–2027. [Google Scholar]
- Kim, S.S.; Park, M.S.; Jeon, O.; Cho, C.Y.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Callahan, R.R.A.; Pruden, K.G.; Raupp, G.B.; Beaudoin, S.P. Downstream oxygen etching characteristics of polymers from the parylene family. J. Vac. Sci. Technol. B 2003, 21, 1496–1500. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Holmes, H.R.; Tan, E.L.; Ong, K.G.; Rajachar, R.M. Fabrication of Biocompatible, Vibrational Magnetoelastic Materials for Controlling Cellular Adhesion. Biosensors 2012, 2, 57-69. https://doi.org/10.3390/bios2010057
Holmes HR, Tan EL, Ong KG, Rajachar RM. Fabrication of Biocompatible, Vibrational Magnetoelastic Materials for Controlling Cellular Adhesion. Biosensors. 2012; 2(1):57-69. https://doi.org/10.3390/bios2010057
Chicago/Turabian StyleHolmes, Hal R., Ee Lim Tan, Keat Ghee Ong, and Rupak M. Rajachar. 2012. "Fabrication of Biocompatible, Vibrational Magnetoelastic Materials for Controlling Cellular Adhesion" Biosensors 2, no. 1: 57-69. https://doi.org/10.3390/bios2010057
APA StyleHolmes, H. R., Tan, E. L., Ong, K. G., & Rajachar, R. M. (2012). Fabrication of Biocompatible, Vibrational Magnetoelastic Materials for Controlling Cellular Adhesion. Biosensors, 2(1), 57-69. https://doi.org/10.3390/bios2010057





