3D Printing Assisted Wearable and Implantable Biosensors
Abstract
1. Introduction to Biosensors
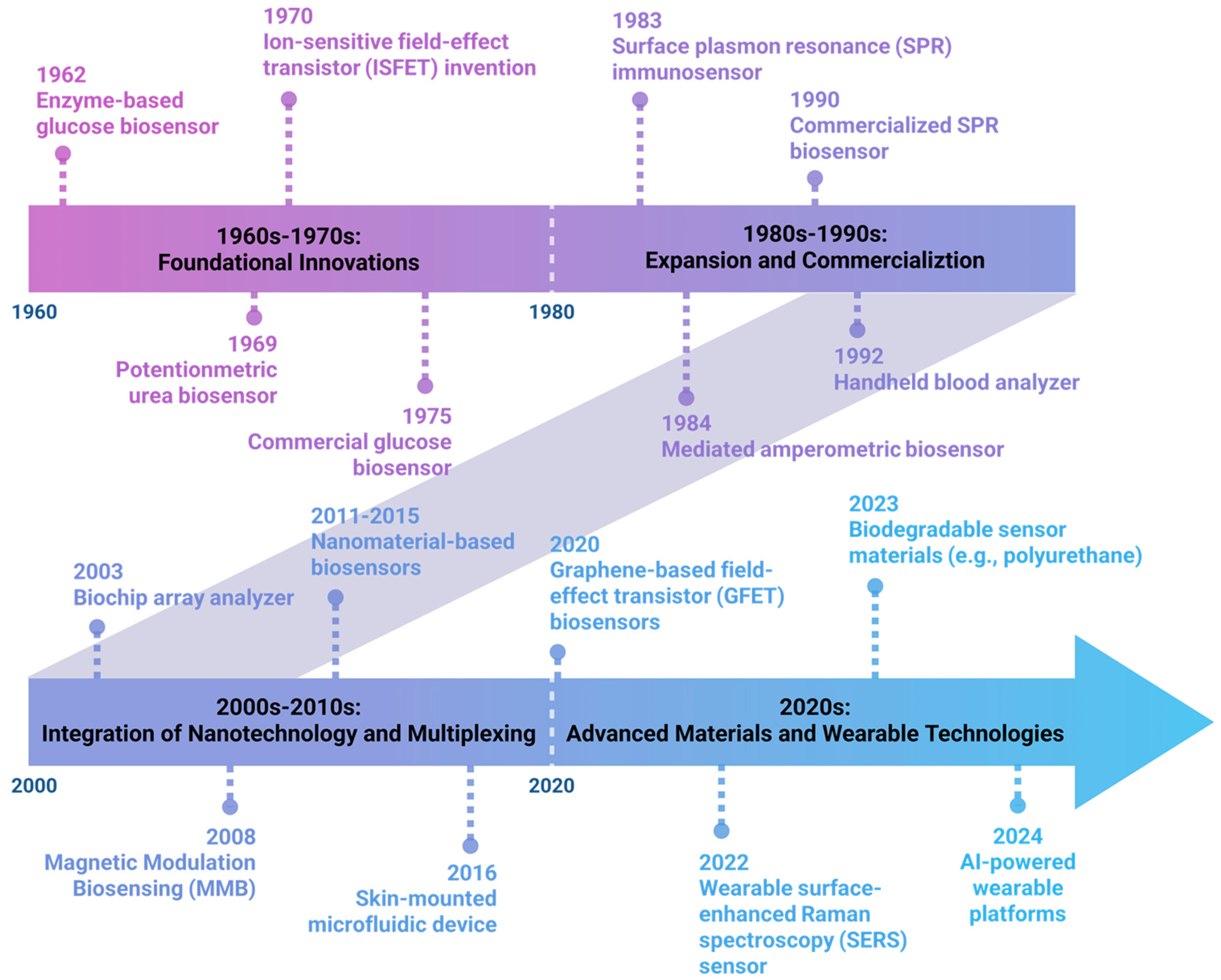
2. Wearable and Implantable Biosensors
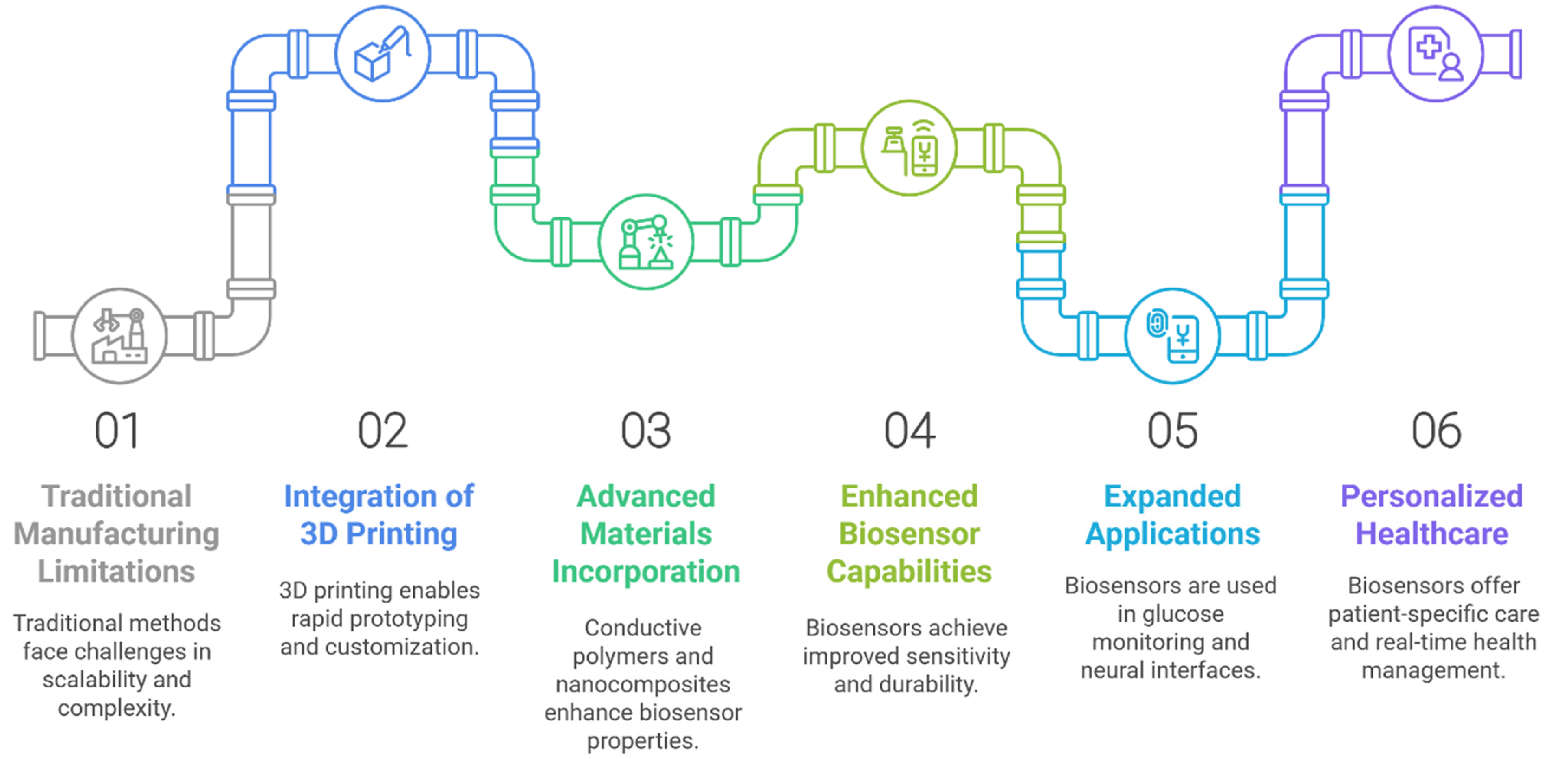
3. The Requirement of 3D Printing Technology
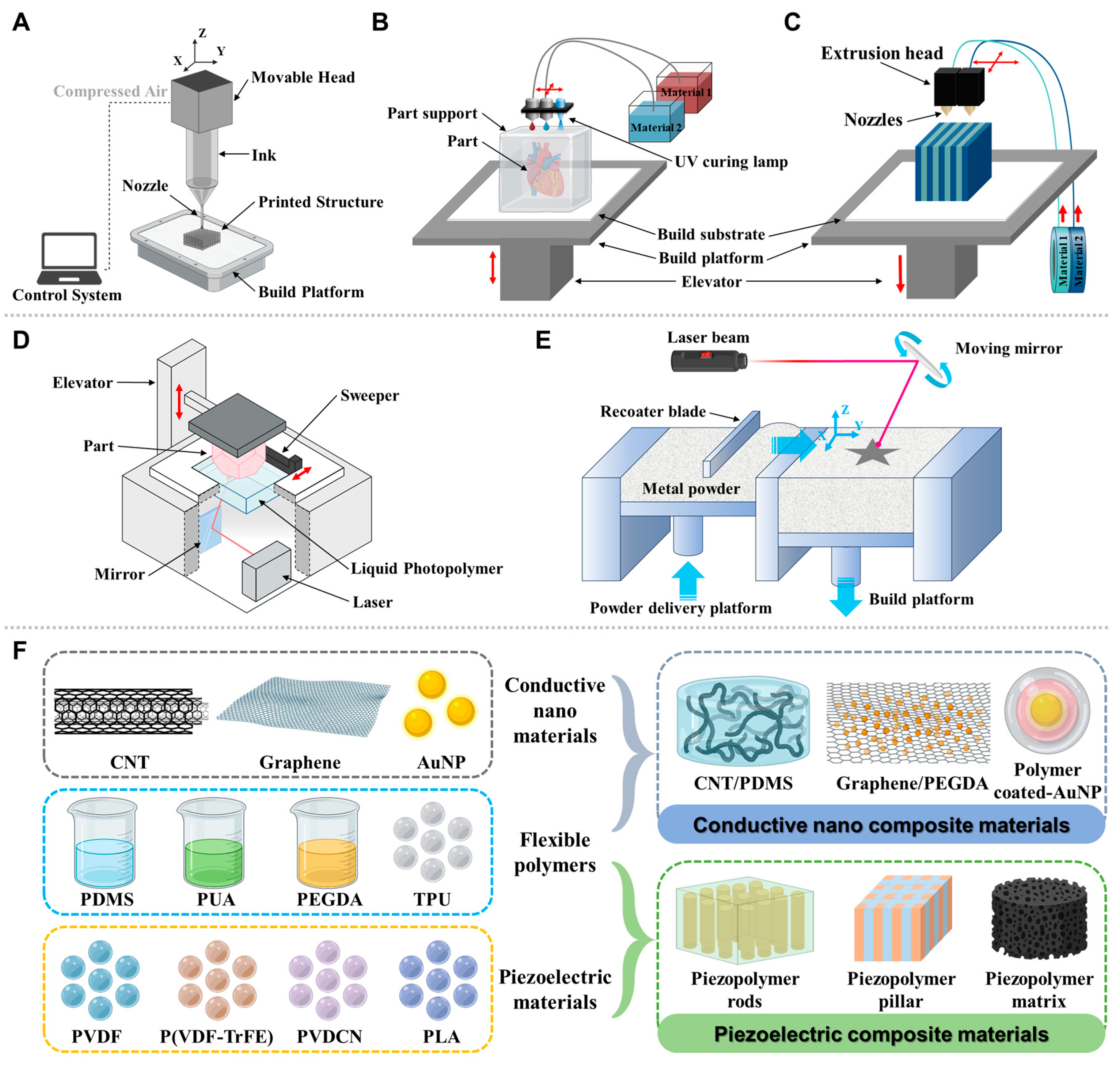
4. 3D Printing—Materials and Methods
| Fabrication Method | Typical Feature Size/Resolution | Sensor Transduction Type | Sensitivity/Limit of Detection | Fabrication Speed (per Device or Batch) | Approx. Cost per Device (Prototype vs. Scaled) | Notable Advantages/Limitations | References |
|---|---|---|---|---|---|---|---|
| SLA/DLP 3DPrinting | Sub-50 μm features achievable in some resins; practical ~50–200 μm walls and microchannels | Electrochemical, optical, or impinging microfluidic integration | Limit of quantity in pM–nM range for some electrochemical sensors; depends on electrode surface area and functionalization | Minutes to hours per device for single parts; rapid prototyping; multi-part assemblies possible | Prototype cost is low to moderate; scalable with batch printing | High-resolution smooth surfaces; post-processing (washing, curing, and sealing) can influence performance | [133,134] |
| FDM (thermoplastic) | Typical feature ~100–300 μm; printers ~50–100 μm with high-end nozzles; | Electrochemical, colorimetric, or integrated microfluidics | Limit of quantity often higher than SLA/DLP, but acceptable for glucose, urea, with surface modifications | Slow per device due to layer-by-layer deposition; batch printing feasible for simple housings | Low material cost; high-volume tooling not required; unit cost higher at small runs | Best for rugged housings and disposable cartridges; limited microchannel resolution | [118] |
| Powder-BasedSintering/SLS | ~100–200 μm features; complex geometries possible | Electrochemical, adhered membranes, microfluidic networks | Variable; often in μM–nM for optimized electrode surfaces; not all SLS surfaces are chemically active | Moderate; build time scales with part volume; post-processing (debinding, sintering) adds time | Moderate tooling; no molds, but material costs are higher; post-processing adds steps | Good for robust, solvent-resistant parts; surface chemistry can be challenging | [135] |
| Inkjet 3DPrinting (droplet-based) | High resolution for membranes and films; ~tens of micrometers in thickness | Optical, colorimetric, enzyme films | Often high sensitivity with surface coatings; limit of detection in μM–nM depending on biofunctionalization | Moderate; drop-on-demand patterns; faster for small arrays | Moderate for consumables; no tooling, scalable for arrays | Flexible sensor patterning and rapid multiplexing | [136] |
| Photolithography/Microfabrication | Sub-micron to micron-scale features (e.g., microfluidic channels) | Electrochemical, optical, and enzymatic | Limit of detection depending on electrode design; e.g., pM–nM range in optimized electrodes | High-volume throughput; batch processing possible | High upfront tooling (photomasks, molds) but very low per-unit cost at scale | Excellent control, repeatability, and scalability; long-established ecosystems | [137] |
| ScreenPrinting | 50–200 μm typical channel and electrode features | Electrochemical | Competitive Limit of detection for well-established assays (e.g., glucose) with functionalized inks | High-throughput; rapid batch production | Very low per-unit cost at scale; expensive for molds/tools upfront | Simple, cost-effective for disposable sensors; limited complex 3D geometry | [138] |
| InjectionMolding | Microfluidic channels down to ~100 μm in optimized molds | Electrochemical, optical | High signal-to-noise with well-defined net surfaces | Very high when production volumes are large | High tooling cost; low per-unit cost at scale | Best for mass production of disposable biosensors; long lead time to set up | [139] |
5. Applications for Wearable Biosensors by 3D Printing Technology
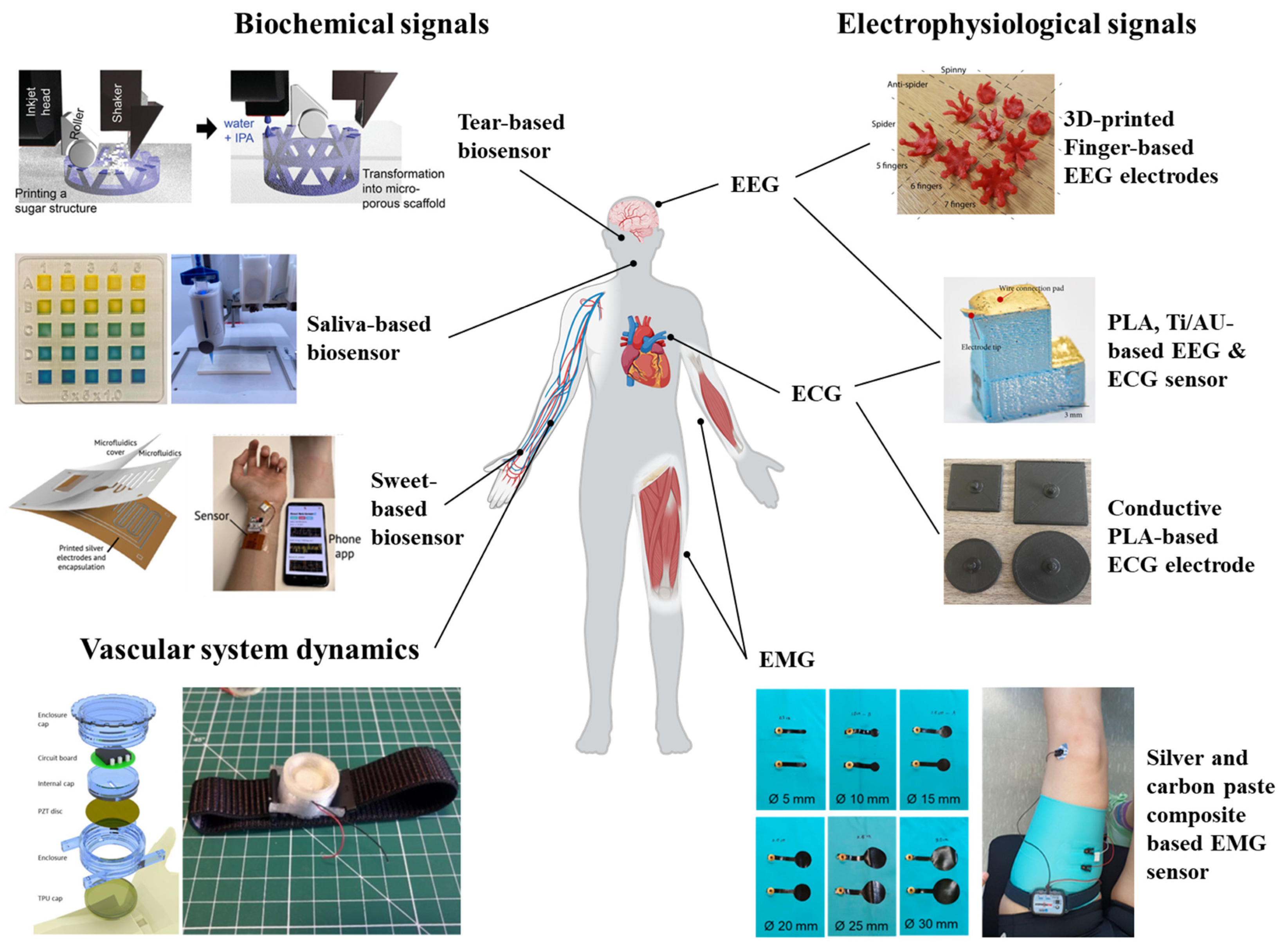
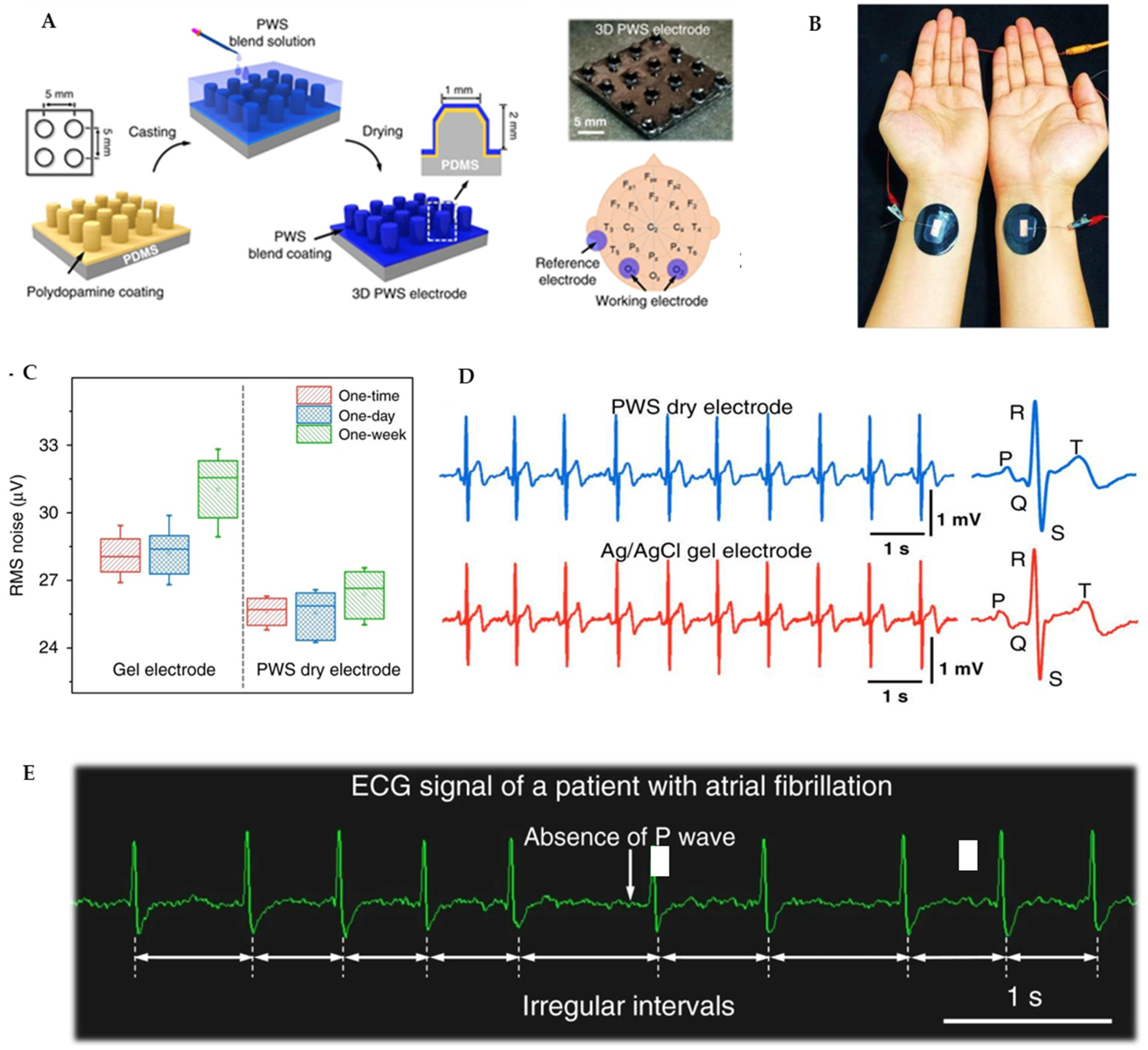
5.1. Electrophysiological Signals
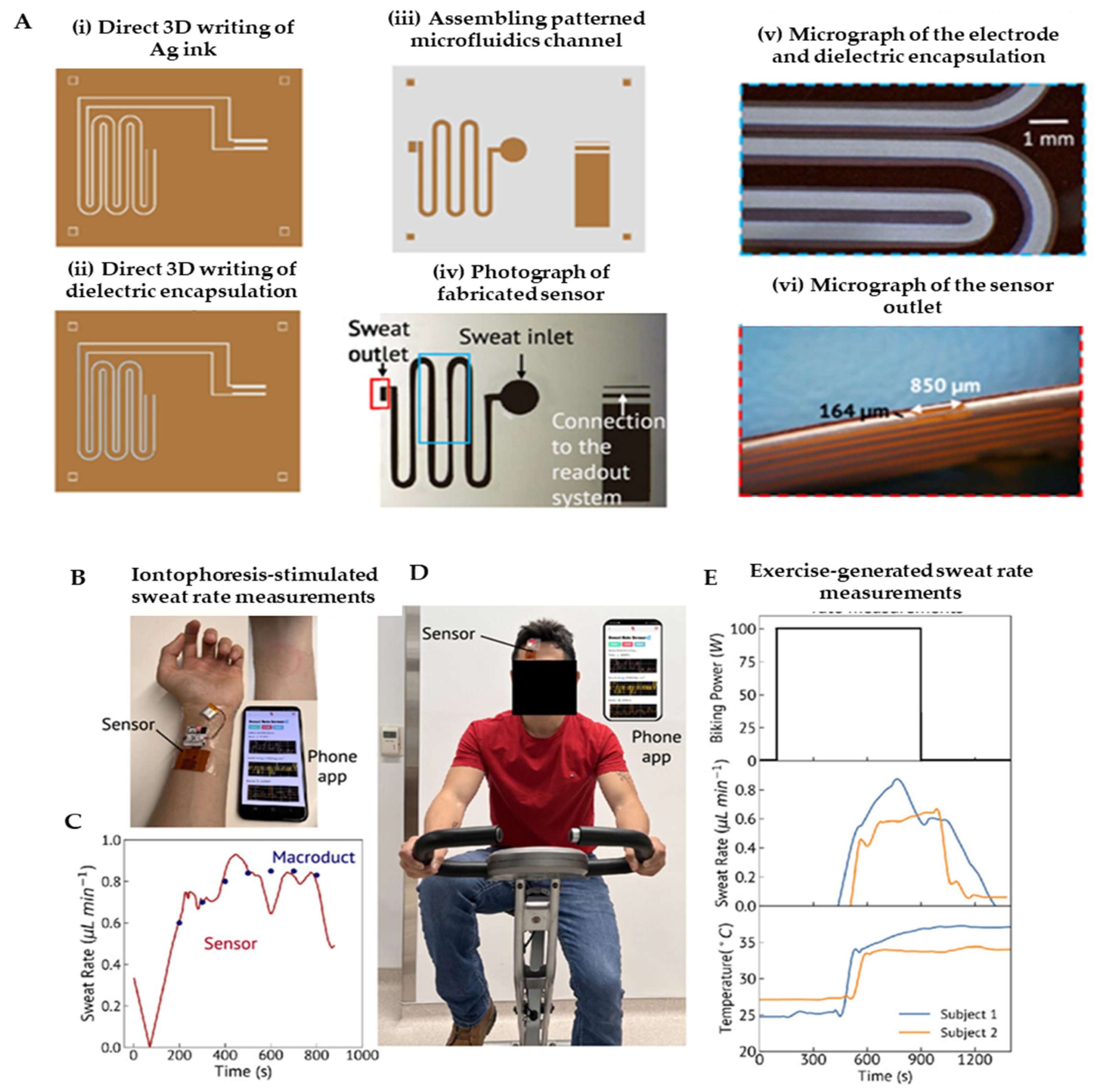
5.2. Biochemical Signals
5.3. Vascular System Dynamics
6. Implantable Devices by 3D Printing Technology
6.1. Implantable Biosensors for Neurological Applications
6.2. Bone Regeneration and Orthopedic Sensors
6.3. Tumor and Cancer Biomarker Sensors
6.4. Biocompatibility, Stability, and Regulatory Considerations
7. Challenges and Future Perspectives
7.1. Material Challenges in 3D Printing of Biosensors
7.2. Technical Challenges in 3D Printing of Biosensors
7.3. Operational Challenges
7.4. Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thevenot, D.R.; Tóth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Pure. Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Mehrotra, P. Monitoring and Control Blood and Tissue Oxygen. J. Oral. Biol. Craniofacial. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.; Shi, H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Fu, D.; Gao, C.; Liu, Y. 2D Material-Based Electrochemical Sensors for Early Diabetes Detection: A Review of Progress and Prospects. Int. J. Electrochem. Sci. 2025, 20, 101123. [Google Scholar] [CrossRef]
- Sharma, S.K.; Leblanc, R.M. Biosensors Based on β-Galactosidase Enzyme: Recent Advances and Perspectives. Anal. Biochem. 2017, 535, 1–11. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Botewad, S.N.; Gaikwad, D.K.; Girhe, N.B.; Thorat, H.N.; Pawar, P.P. Urea Biosensors: A Comprehensive Review. Biotech. App Biochem. 2023, 70, 485–501. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J.S. Cholesterol Biosensors: A Review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Wen, J.; Ma, Y.; Dai, G.; Mo, F.; Wang, J. A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors 2025, 25, 1045. [Google Scholar] [CrossRef]
- Sivagnanam, S.; Mahato, P.; Das, P. An Overview on the Development of Different Optical Sensing Platforms for Adenosine Triphosphate (ATP) Recognition. Org. Biomol. Chem. 2023, 21, 3942–3983. [Google Scholar] [CrossRef]
- Wankhade, U.A.; Thakare, Y.N.; Hardas, B.M.; Pande, R.S. Cortisol Detection Methods for Stress Monitoring: Current Insight and Future Prospect: A Review. IEEE Sens. J. 2024, 24, 23389–23400. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Electrochemical Biosensors for Hormone Analyses. Biosens. Bioelectron. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Psoma, S.D.; Kanthou, C. Wearable Insulin Biosensors for Diabetes Management: Advances and Challenges. Biosensors 2023, 13, 719. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M.; Barros Azeredo, N.F.; Santos, B.G.; De Oliveira, T.G.; De Oliveira Lins, R.S.; Dos Santos Lima, I.; Angnes, L. Electrochemical Nanomaterial-Based Sensors/Biosensors for Drug Monitoring. CTMC 2023, 23, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Garzón, V.; Pinacho, D.; Bustos, R.-H.; Garzón, G.; Bustamante, S. Optical Biosensors for Therapeutic Drug Monitoring. Biosensors 2019, 9, 132. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Nisha Pauline, J.M.; Ramaraju, P.; Mohanasundaram, S.; Nguyen Vo, D.-V. Biosensor for Heavy Metals Detection in Wastewater: A Review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef]
- Sassolas, A.; Prieto-Simón, B.; Marty, J.-L. Biosensors for Pesticide Detection: New Trends. AJAC 2012, 03, 210–232. [Google Scholar] [CrossRef]
- Ali, A.A.; Altemimi, A.B.; Alhelfi, N.; Ibrahim, S.A. Application of Biosensors for Detection of Pathogenic Food Bacteria: A Review. Biosensors 2020, 10, 58. [Google Scholar] [CrossRef]
- Guliy, O.I.; Zaitsev, B.D.; Larionova, O.S.; Borodina, I.A. Virus Detection Methods and Biosensor Technologies. Biophysics 2019, 64, 890–897. [Google Scholar] [CrossRef]
- V, K. DNA Biosensors-A Review. J. Bioeng. Biomed. Sci. 2017, 07. [Google Scholar] [CrossRef]
- Du, Y.; Dong, S. Nucleic Acid Biosensors: Recent Advances and Perspectives. Anal. Chem. 2017, 89, 189–215. [Google Scholar] [CrossRef]
- Khanmiri, H.H.; Yazdanfar, F.; Mobed, A.; Rezamohammadi, F.; Rahmani, M.; Haghgouei, T. Biosensors; Noninvasive Method in Detection of C-Reactive Protein (CRP). Biomed. Microdevices 2023, 25, 27. [Google Scholar] [CrossRef]
- Gerdan, Z.; Saylan, Y.; Denizli, A. Biosensing Platforms for Cardiac Biomarker Detection. ACS Omega. 2024, 9, 9946–9960. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Shakouri, S.K.; Dolati, S. Biosensors: A Novel Approach to and Recent Discovery in Detection of Cytokines. Cytokine 2020, 136, 155272. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr. Monitor and Control of Blood and Tissue Oxygen Tensions. Trans.- Am. Soc. Artif. Intern. Organs. 1956, 2, 41–48. [Google Scholar]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. New York Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Cass, A.E.G.; Davis, G.; Francis, G.D.; Hill, H.A.O.; Aston, W.J.; Higgins, I.J.; Plotkin, E.V.; Scott, L.D.L.; Turner, A.P.F. Ferrocene-Mediated Enzyme Electrode for Amperometric Determination of Glucose. Anal. Chem. 1984, 56, 667–671. [Google Scholar] [CrossRef]
- Heller, A. Electrical Wiring of Redox Enzymes. Acc. Chem. Res. 1990, 23, 128–134. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Montalvo, J.G. Urea-Specific Enzyme Electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165. [Google Scholar] [CrossRef]
- Barcelona, M.J.; Liljestrand, H.M.; Morgan, J.J. Determination of Low Molecular Weight Volatile Fatty Acids in Aqueous Samples. Anal. Chem. 1980, 52, 321–325. [Google Scholar] [CrossRef]
- Wollenberger, U. Chapter 2 Third Generation Biosensors—Integrating Recognition and Transduction in Electrochemical Sensors. In Comprehensive Analytical Chemistry; Gorton, L., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 65–130. ISBN 978-0-444-50715-0. [Google Scholar]
- Sekretaryova, A.N.; Vokhmyanina, D.V.; Chulanova, T.O.; Karyakina, E.E.; Karyakin, A.A. Reagentless Biosensor Based on Glucose Oxidase Wired by the Mediator Freely Diffusing in Enzyme Containing Membrane. Anal. Chem. 2012, 84, 1220–1223. [Google Scholar] [CrossRef]
- Kranz, C.; Wohlschläger, H.; Schmidt, H.; Schuhmann, W. Controlled Electrochemical Preparation of Amperometric Biosensors Based on Conducting Polymer Multilayers. Electroanalysis 1998, 10, 546–552. [Google Scholar] [CrossRef]
- Gerard, M. Application of Conducting Polymers to Biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical Applications of Aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized Total Chemical Analysis Systems: A Novel Concept for Chemical Sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Nanomaterial-Based Electrochemical Biosensors. Analyst 2005, 130, 421. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene Based Sensors and Biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef]
- Hai, X.; Feng, J.; Chen, X.; Wang, J. Tuning the Optical Properties of Graphene Quantum Dots for Biosensing and Bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic Diagnostic Technologies for Global Public Health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Yadav, R.; Vaishnav, Y.; Verma, S.; Kaur, A.; Manjunath, K.; Pandey, A. Point of Care Diagnostics—Using Nanomaterials as Detection Probes. RJPT 2023, 3483–3488. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Bhui, A.K.; Singh, P.; Baig, Y.R.; Shukla, S.; Sen, S.; Dey, A.; Patel, R. Materials Advancement, Biomaterials, and Biosensors. In Mechanical Engineering in Biomedical Applications; Srivastava, J.P., Kozak, D., Ranjan, V., Kumar, P., Kumar, R., Tayal, S., Eds.; Wiley: Weinheim, Germany, 2024; pp. 291–325. ISBN 978-1-394-17452-2. [Google Scholar]
- Pandya, A.; Mahato, K. (Eds.) Advances in Materials for Wearable Biosensors. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2025; Volume 215, pp. 155–179. ISBN 978-0-443-34447-3. [Google Scholar]
- Abdelhamid, M.A.A.; Ki, M.-R.; Pack, S.P. Biominerals and Bioinspired Materials in Biosensing: Recent Advancements and Applications. IJMS 2024, 25, 4678. [Google Scholar] [CrossRef]
- Swan, H.J.C.; Ganz, W.; Forrester, J.; Marcus, H.; Diamond, G.; Chonette, D. Catheterization of the Heart in Man with Use of a Flow-Directed Balloon-Tipped Catheter. N. Engl. J. Med. 1970, 283, 447–451. [Google Scholar] [CrossRef]
- Sanders, G.D.; Hlatky, M.A.; Owens, D.K. Cost-Effectiveness of Implantable Cardioverter–Defibrillators. N. Engl. J. Med. 2005, 353, 1471–1480. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Moulart, F.; Bourgois, R.; Puers, R.; Van Schuylenbergh, K.; Barbosa, M.; Paiva, O.; Rodes, F.; Bégueret, J.B.; et al. Concept, Design and Fabrication of Smart Orthopedic Implants. Med. Eng. Phys. 2000, 22, 469–479. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, 70–71. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface Plasmon Resonance for Gas Detection and Biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Jönsson, U.; Fägerstam, L.; Ivarsson, B.; Johnsson, B.; Karlsson, R.; Lundh, K.; Löfås, S.; Persson, B.; Roos, H.; Rönnberg, I. Real-Time Biospecific Interaction Analysis Using Surface Plasmon Resonance and a Sensor Chip Technology. Biotechniques 1991, 11, 620–627. [Google Scholar] [PubMed]
- Erickson, K.A.; Wilding, P. Evaluation of a Novel Point-of-Care System, the i-STAT Portable Clinical Analyzer. Clin. Chem. 1993, 39, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Molloy, R.M.; Mc Connell, R.I.; Lamont, J.V.; FitzGerald, S.P. Automation of Biochip Array Technology for Quality Results. Clin. Chem. Lab. Med. (CCLM) 2005, 43. [Google Scholar] [CrossRef]
- Danielli, A.; Porat, N.; Ehrlich, M.; Arie, A. Magnetic Modulation Biosensing for Rapid and Homogeneous Detection of Biological Targets at Low Concentrations. CPB 2010, 11, 128–137. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Ning, Y. Silicon Nanowire Biosensor and Its Applications in Disease Diagnostics: A Review. Anal. Chim. Acta 2012, 749, 1–15. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A Soft, Wearable Microfluidic Device for the Capture, Storage, and Colorimetric Sensing of Sweat. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano. 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Liu, L.; Martinez Pancorbo, P.; Xiao, T.; Noguchi, S.; Marumi, M.; Segawa, H.; Karhadkar, S.; Gala De Pablo, J.; Hiramatsu, K.; Kitahama, Y.; et al. Highly Scalable, Wearable Surface-Enhanced Raman Spectroscopy. Adv. Opt. Mater. 2022, 10, 2200054. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Zhang, X.; Zhou, B.; Wen, S.; Zhou, Y.; Chen, S.; Jiang, L.; Jerrams, S.; Zhou, F. Biodegradable Polyurethane Fiber-Based Strain Sensor with a Broad Sensing Range and High Sensitivity for Human Motion Monitoring. ACS Sustain. Chem. Eng. 2022, 10, 8788–8798. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Jiang, F.; Yu, S.; Ji, F.; Sun, T.; Zhang, H.; Zhu, Y.; Chang, H.; Li, T.; et al. Fully Integrated Wearable Control System for Micro/Nanorobot Navigation. Int. J. Extrem. Manuf. 2025, 7, 035505. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; De Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Punjiya, M.; Rezaei, H.; Zeeshan, M.A.; Sonkusale, S. A Flexible pH Sensing Smart Bandage with Wireless CMOS Readout for Chronic Wound Monitoring. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 1700–1702. [Google Scholar]
- Liao, Y.-T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-µW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid-State Circuits 2012, 47, 335–344. [Google Scholar] [CrossRef]
- Dehghani, M.; Dangelico, R.M. Smart Wearable Technologies: Current Status and Market Orientation through a Patent Analysis. In Proceedings of the 2017 IEEE International Conference on Industrial Technology (ICIT), Toronto, ON, Canada, 2–25 March 2017; pp. 1570–1575. [Google Scholar]
- Sun, B.; Zhang, Z. Photoplethysmography-Based Heart Rate Monitoring Using Asymmetric Least Squares Spectrum Subtraction and Bayesian Decision Theory. IEEE Sens. J. 2015, 15, 7161–7168. [Google Scholar] [CrossRef]
- Holz, C.; Wang, E.J. Glabella: Continuously Sensing Blood Pressure Behavior Using an Unobtrusive Wearable Device. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–23. [Google Scholar] [CrossRef]
- Kedambaimoole, V.; Kumar, N.; Shirhatti, V.; Nuthalapati, S.; Sen, P.; Nayak, M.M.; Rajanna, K.; Kumar, S. Laser-Induced Direct Patterning of Free-Standing Ti3C2 MXene Films for Skin Conformal Tattoo Sensors. ACS Sens. 2020, 5, 2086–2095. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable Sensors: Modalities, Challenges, and Prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Wazni, O.M.; Callahan, T.; Kanj, M.; Hakim, A.H.; Wolski, K.; Wilkoff, B.L.; Saliba, W.; Lindsay, B.D. Using a Novel Wireless System for Monitoring Patients after the Atrial Fibrillation Ablation Procedure: The iTransmit Study. Heart Rhythm. 2015, 12, 554–559. [Google Scholar] [CrossRef]
- Po-Jui, C.; Rodger, D.C.; Saati, S.; Humayun, M.S.; Yu-Chong, T. Microfabricated Implantable Parylene-Based Wireless Passive Intraocular Pressure Sensors. J. Microelectromech. Syst. 2008, 17, 1342–1351. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable Sweat Sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Prieto-Simon, B.; Voelcker, N.H. Skin in the Diagnostics Game: Wearable Biosensor Nano- and Microsystems for Medical Diagnostics. Nano Today 2020, 30, 100828. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D Printing of Strain Sensors within Highly Stretchable Elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, Q.; Fu, J.; Chen, Q.; Zhu, J.; Sun, Y.; He, Y. Multimaterial 3D Printing of Highly Stretchable Silicone Elastomers. ACS Appl. Mater. Interfaces 2019, 11, 23573–23583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guo, S.; Hirdler, T.; Eide, C.; Fan, X.; Tolar, J.; McAlpine, M.C. 3D Printed Functional and Biological Materials on Moving Freeform Surfaces. Adv. Mater. 2018, 30, 1707495. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Raisa, S.A.; Kumar, P.; Kalkal, A.; Kumar, N.; Packirisamy, G.; Manhas, S. Optimization, Fabrication, and Characterization of Four Electrode-Based Sensors for Blood Impedance Measurement. Biomed. Microdevices 2021, 23, 9. [Google Scholar] [CrossRef]
- Gonzalez-Macia, L.; Morrin, A.; Smyth, M.R.; Killard, A.J. Advanced Printing and Deposition Methodologies for the Fabrication of Biosensors and Biodevices. Analyst 2010, 135, 845. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.-B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of Nanomaterials: A Crucial Step in Biosensor Fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef]
- Pradhan, R.; Kalkal, A.; Jindal, S.; Packirisamy, G.; Manhas, S. Four Electrode-Based Impedimetric Biosensors for Evaluating Cytotoxicity of Tamoxifen on Cervical Cancer Cells. RSC Adv. 2021, 11, 798–806. [Google Scholar] [CrossRef]
- Abdollahi, S.; Markvicka, E.J.; Majidi, C.; Feinberg, A.W. 3D Printing Silicone Elastomer for Patient—Specific Wearable Pulse Oximeter. Adv Healthc. Mater. 2020, 9, 1901735. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Lu, B.; Li, D.; Tian, X. Development Trends in Additive Manufacturing and 3D Printing. Engineering 2015, 1, 085–089. [Google Scholar] [CrossRef]
- 3D Printing Market Size and Share | Industry Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/3d-printing-industry-analysis (accessed on 20 February 2025).
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D Printing. Nat. Mater 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Mengüç, Y.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive Soft Strain Sensors via Multicore–Shell Fiber Printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-Printing of Lightweight Cellular Composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef]
- Muth, J.T.; Dixon, P.G.; Woish, L.; Gibson, L.J.; Lewis, J.A. Architected Cellular Ceramics with Tailored Stiffness via Direct Foam Writing. Proc. Natl. Acad. Sci. USA 2017, 114, 1832–1837. [Google Scholar] [CrossRef]
- Mueller, J.; Raney, J.R.; Shea, K.; Lewis, J.A. Architected Lattices with High Stiffness and Toughness via Multicore-Shell 3D Printing. Adv. Mater. 2018, 30, 1705001. [Google Scholar] [CrossRef]
- Raney, J.R.; Compton, B.G.; Mueller, J.; Ober, T.J.; Shea, K.; Lewis, J.A. Rotational 3D Printing of Damage-Tolerant Composites with Programmable Mechanics. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 1198–1203. [Google Scholar] [CrossRef]
- Abshirini, M.; Charara, M.; Marashizadeh, P.; Saha, M.C.; Altan, M.C.; Liu, Y. Functional Nanocomposites for 3D Printing of Stretchable and Wearable Sensors. Appl. Nanosci. 2019, 9, 2071–2083. [Google Scholar] [CrossRef]
- Guo, S.; Yang, X.; Heuzey, M.-C.; Therriault, D. 3D Printing of a Multifunctional Nanocomposite Helical Liquid Sensor. Nanoscale 2015, 7, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Marro, A.; Bandukwala, T.; Mak, W. Three-Dimensional Printing and Medical Imaging: A Review of the Methods and Applications. Curr. Probl. Diagn. Radiol. 2016, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Yi, Q.; Hoang, E.; Esfandyarpour, R. A 3D Printed Wearable Bioelectronic Patch for Multi-Sensing and In Situ Sweat Electrolyte Monitoring. Adv. Mater. Technol. 2021, 6, 2001021. [Google Scholar] [CrossRef]
- Paterson, A.M.; Donnison, E.; Bibb, R.J.; Ian Campbell, R. Computer-Aided Design to Support Fabrication of Wrist Splints Using 3D Printing: A Feasibility Study. Hand Ther. 2014, 19, 102–113. [Google Scholar] [CrossRef]
- Choi, J.; Bandodkar, A.J.; Reeder, J.T.; Ray, T.R.; Turnquist, A.; Kim, S.B.; Nyberg, N.; Hourlier-Fargette, A.; Model, J.B.; Aranyosi, A.J.; et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 2019, 4, 379–388. [Google Scholar] [CrossRef]
- Wei, P.; Leng, H.; Chen, Q.; Advincula, R.C.; Pentzer, E.B. Reprocessable 3D-Printed Conductive Elastomeric Composite Foams for Strain and Gas Sensing. ACS Appl. Polym. Mater. 2019, 1, 885–892. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Qin, J.; Lowe, S.E.; Zhang, S.; Zhao, H.; Zhong, Y.L. Recent Progress of Direct Ink Writing of Electronic Components for Advanced Wearable Devices. ACS Appl. Electron. Mater. 2019, 1, 1718–1734. [Google Scholar] [CrossRef]
- Gao, W. Skin-Interfaced Wearable Sweat Biosensors. Meet. Abstr. 2021, MA2021-01, 1389. [Google Scholar] [CrossRef]
- Parupelli, S.K.; Desai, S. The 3D Printing of Nanocomposites for Wearable Biosensors: Recent Advances, Challenges, and Prospects. Bioengineering 2023, 11, 32. [Google Scholar] [CrossRef]
- Chen, C.; Fu, Y.; Sparks, S.S.; Lyu, Z.; Pradhan, A.; Ding, S.; Boddeti, N.; Liu, Y.; Lin, Y.; Du, D.; et al. 3D-Printed Flexible Microfluidic Health Monitor for In Situ Sweat Analysis and Biomarker Detection. ACS Sens. 2024, 9, 3212–3223. [Google Scholar] [CrossRef]
- Hussain, A.; Abbas, N.; Ali, A. Inkjet Printing: A Viable Technology for Biosensor Fabrication. Chemosensors 2022, 10, 103. [Google Scholar] [CrossRef]
- Ho, D.H.; Hong, P.; Han, J.T.; Kim, S.; Kwon, S.J.; Cho, J.H. 3D-Printed Sugar Scaffold for High-Precision and Highly Sensitive Active and Passive Wearable Sensors. Adv. Sci. 2020, 7, 1902521. [Google Scholar] [CrossRef] [PubMed]
- Mass, M.; Veiga, L.S.; Garate, O.; Longinotti, G.; Moya, A.; Ramón, E.; Villa, R.; Ybarra, G.; Gabriel, G. Fully Inkjet-Printed Biosensors Fabricated with a Highly Stable Ink Based on Carbon Nanotubes and Enzyme-Functionalized Nanoparticles. Nanomaterials 2021, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet Printed (Bio)Chemical Sensing Devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805. [Google Scholar] [CrossRef]
- Baldini, G.; Albini, A.; Maiolino, P.; Cannata, G. An Atlas for the Inkjet Printing of Large-Area Tactile Sensors. Sensors 2022, 22, 2332. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, Q.; Xiao, J.; Zheng, M.; Zhang, D.; Yang, J. An Inkjet-Printed Smartphone-Supported Electrochemical Biosensor System for Reagentless Point-of-Care Analyte Detection. Sens. Actuators B Chem. 2021, 346, 130447. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, N.; Sachdeva, A. Flexible Wearable Devices Using Extrusion-Based 3D Printing Approach: A Review. Mater. Today Proc. 2024, 113, 79–86. [Google Scholar] [CrossRef]
- Bakhtiari, H.; Aamir, M.; Tolouei-Rad, M. Effect of 3D Printing Parameters on the Fatigue Properties of Parts Manufactured by Fused Filament Fabrication: A Review. Appl. Sci. 2023, 13, 904. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalysis Overview: Additive Manufactured Biosensors Using Fused Filament Fabrication. Anal. Methods 2024, 16, 2625–2634. [Google Scholar] [CrossRef]
- Silva, L.R.G.; Lopes, C.E.C.; Tanaka, A.A.; Dantas, L.M.F.; Silva, I.S.; Stefano, J.S. Electrochemical Biosensors 3D Printed by Fused Deposition Modeling: Actualities, Trends, and Challenges. Biosensors 2025, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.S.; Guterres, E.; Silva, L.R.; Rocha, R.G.; Brazaca, L.C.; Richter, E.M.; Abarza Muñoz, R.A.; Janegitz, B.C. New Conductive Filament Ready-to-Use for 3D-Printing Electrochemical (Bio)Sensors: Towards the Detection of SARS-CoV-2. Anal. Chim. Acta 2022, 1191, 339372. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.; Razak, K.A.; Ab Wahab, M.N.; Hamzah, H.H. Recent Progress of Conductive 3D-Printed Electrodes Based upon Polymers/Carbon Nanomaterials Using a Fused Deposition Modelling (FDM) Method as Emerging Electrochemical Sensing Devices. RSC Adv. 2021, 11, 16557–16571. [Google Scholar] [CrossRef] [PubMed]
- Matham, M.V.; Pae, J.Y.; Medwal, R.; Rawat, R.S. Synthesis and the development of graphene-layered substrates for flexible wearable biosensors. In Proceedings of the 3rd International Conference on Theoretical and Applied Nanoscience and Nanotechnology, London, UK, 13–15 July 2025. [Google Scholar]
- Li, S.; Shan, Y.; Chen, J.; Chen, X.; Shi, Z.; Zhao, L.; He, R.; Li, Y. 3D Printing and Biomedical Applications of Piezoelectric Composites: A Critical Review. Adv Mater. Technol. 2025, 10, 2401160. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, S.; Ravichandran, D.; Ramanathan, A.; Sobczak, M.T.; Sacco, A.F.; Patil, D.; Thummalapalli, S.V.; Pulido, T.V.; Lancaster, J.N.; et al. 3D-Printed Polymeric Biomaterials for Health Applications. Adv Healthc. Mater. 2025, 14, 2402571. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Wei, K.; Sun, J.; Gao, Q.; Yang, X.; Ye, Y.; Ji, J.; Sun, X. 3D “Honeycomb” Cell/Carbon Nanofiber/Gelatin Methacryloyl (GelMA) Modified Screen-Printed Electrode for Electrochemical Assessment of the Combined Toxicity of Deoxynivalenol Family Mycotoxins. Bioelectrochemistry 2021, 139, 107743. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT:PSS-Based Conductive Textiles and Their Applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef]
- Seiti, M.; Giuri, A.; Corcione, C.E.; Ferraris, E. Advancements in Tailoring PEDOT: PSS Properties for Bioelectronic Applications: A Comprehensive Review. Biomater. Adv. 2023, 154, 213655. [Google Scholar] [CrossRef]
- Yu, J.; Wan, R.; Tian, F.; Cao, J.; Wang, W.; Liu, Q.; Yang, H.; Liu, J.; Liu, X.; Lin, T.; et al. 3D Printing of Robust High-Performance Conducting Polymer Hydrogel-Based Electrical Bioadhesive Interface for Soft Bioelectronics. Small 2024, 20, 2308778. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, D.; Ma, Y.; Zhang, S.; Zhou, Y.; Chen, W.; Liu, T.; Cai, Y.; Fang, L.; Zhang, J.; et al. One-Step Electrochemical Modification of PEDOT:PSS/PBNPs Hybrid Hydrogel on the Screen-Printed Electrode Surface for Highly Sensitive Detection of Creatinine. ACS Appl. Mater. Interfaces 2024, 16, 70352–70361. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Xu, D.; Qiu, Y.; Hu, J.; Wan, H.; Hu, N.; Wang, P. PEDOT: PSS-Modified Organic Flexible and Implantable Microelectrode for Internal Bi-Directional Electrophysiology of Three-Dimensional Cardiomyocyte Spheroid. ACS Sens. 2025, 10, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, M.; Lu, X.; Xu, K.; Yu, M.; Yang, H.; Yin, J. A 3D Printable Gelatin Methacryloyl/Chitosan Hydrogel Assembled with Conductive PEDOT for Neural Tissue Engineering. Compos. Part B Eng. 2024, 273, 111241. [Google Scholar] [CrossRef]
- Kim, T.; Uddin, M.A.; Yi, Q.; Esfandyarpour, R. Molecularly Imprinted Polymer Based Cortisol Sensor with Organic Electrochemical Transistor for Wearable Applications. In Proceedings of the 2024 IEEE Biosensors Conference (BioSensors), Cambridge, UK, 28 July 2024. [Google Scholar]
- Liang, M.; Liu, X.; Chong, Y.; Ye, Z.; Zhao, L.; Yu, Q.; Tang, K.; Geng, A.; Hu, B.; Ge, G.; et al. Engineering Biosensors and Biomedical Detection Devices from 3D-Printed Technology. ECS Sens. Plus 2023, 2, 030604. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, S.; Liu, H.; Chen, X.; Chen, X.; Li, B.; Tang, W.; Meng, Q.; Ding, P.; Tian, H.; et al. 3D Printed Piezoelectric BNNTs Nanocomposites with Tunable Interface and Microarchitectures for Self-Powered Conformal Sensors. Nano Energy 2020, 77, 105300. [Google Scholar] [CrossRef]
- Raghavendra Rao, R.; Sharath, B.; Pradeep, S.; Hareesha, M.; Navaneet, D. Critical Review on Additive Manufacturing Based Biomedical and Biosensors Application. Proc. Inst. Mech. Eng. H. 2025, 239, 721–735. [Google Scholar] [CrossRef]
- Paul, A.A.; Aladese, A.D.; Marks, R.S. Additive Manufacturing Applications in Biosensors Technologies. Biosensors 2024, 14, 60. [Google Scholar] [CrossRef]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Ageyeva, T.; Horváth, S.; Kovács, J.G. In-Mold Sensors for Injection Molding: On the Way to Industry 4.0. Sensors 2019, 19, 3551. [Google Scholar] [CrossRef]
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.A.; Jones, A.K.P.; Casson, A.J. Flexible 3D-Printed EEG Electrodes. Sensors 2019, 19, 1650. [Google Scholar] [CrossRef]
- Cho, S.-J.; Byun, D.; Nam, T.-S.; Choi, S.-Y.; Lee, B.-G.; Kim, M.-K.; Kim, S. A 3D-Printed Sensor for Monitoring Biosignals in Small Animals. J. Healthc. Eng. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aloqalaa, Z.M. 3D-Printed Electrocardiogram Dry Electrodes Using Four Commercially Available Polylactic Acid Conductive Filaments. J. Sens. 2023, 2023, 8468466. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Jeong, W. EMG Measurement with Textile-Based Electrodes in Different Electrode Sizes and Clothing Pressures for Smart Clothing Design Optimization. Polymers 2020, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
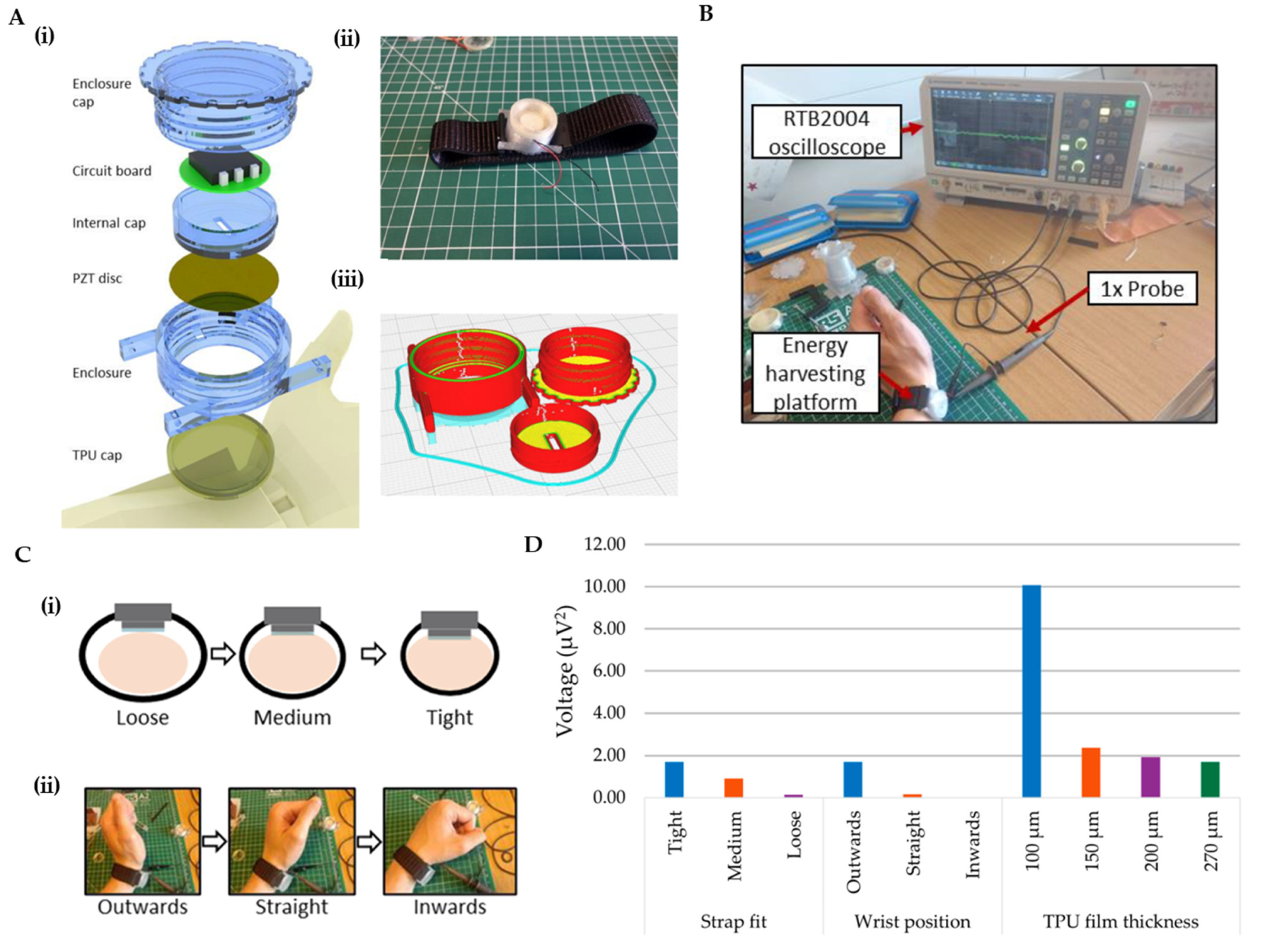
- Sobianin, I.; Psoma, S.D.; Tourlidakis, A. A 3D-Printed Piezoelectric Microdevice for Human Energy Harvesting for Wearable Biosensors. Micromachines 2024, 15, 118. [Google Scholar] [CrossRef]
- Islam, M.S.; Cha, S.; Hassan, M.F.; Cai, W.; Saniat, T.S.; Leach, C.R.; Khan, Y. Printed Wearable Sweat Rate Sensor for Continuous In Situ Perspiration Measurement. Adv. Intell. Syst. 2025, 7, 2400927. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Krakos, A.; Kubicki, W. 3D Printed Hydrogel Sensor for Rapid Colorimetric Detection of Salivary pH. Sensors 2024, 24, 3740. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully Organic Compliant Dry Electrodes Self-Adhesive to Skin for Long-Term Motion-Robust Epidermal Biopotential Monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef]
- Rauf, S.; Bilal, R.M.; Li, J.; Vaseem, M.; Ahmad, A.N.; Shamim, A. Fully Screen-Printed and Gentle-to-Skin Wet ECG Electrodes with Compact Wireless Readout for Cardiac Diagnosis and Remote Monitoring. ACS Nano. 2024, 18, 10074–10087. [Google Scholar] [CrossRef]
- Gharleghi, R.; Dessalles, C.A.; Lal, R.; McCraith, S.; Sarathy, K.; Jepson, N.; Otton, J.; Barakat, A.I.; Beier, S. 3D Printing for Cardiovascular Applications: From End-to-End Processes to Emerging Developments. Ann. Biomed. Eng. 2021, 49, 1598–1618. [Google Scholar] [CrossRef]
- Etana, B.B.; Malengier, B.; Timothy, K.; Wojciech, S.; Krishnamoorthy, J.; Van Langenhove, L. A Review on the Recent Developments in Design and Integration of Electromyography Textile Electrodes for Biosignal Monitoring. J. Ind. Text. 2023, 53, 1–34. [Google Scholar] [CrossRef]
- Wan, R.; Liu, S.; Li, Z.; Li, G.; Li, H.; Li, J.; Xu, J.; Liu, X. 3D Printing of Highly Conductive and Strongly Adhesive PEDOT:PSS Hydrogel-Based Bioelectronic Interface for Accurate Electromyography Monitoring. J. Colloid Interface Sci. 2025, 677, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Lim, D.; Jeong, W. Development and Characterization of Embroidery-Based Textile Electrodes for Surface EMG Detection. Sensors 2022, 22, 4746. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Lee, J.C.-M.; Chuang, K.-C.; Chiu, C.-W. Photocured, Highly Flexible, and Stretchable 3D-Printed Graphene/Polymer Nanocomposites for Electrocardiography and Electromyography Smart Clothing. Prog. Org. Coat. 2023, 176, 107378. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Ruan, S.-J.; Lee, Y.-H. An Ultra-Low Power Surface EMG Sensor for Wearable Biometric and Medical Applications. Biosensors 2021, 11, 411. [Google Scholar] [CrossRef]
- Lee, M.S.; Paul, A.; Xu, Y.; Hairston, W.D.; Cauwenberghs, G. Characterization of Ag/AgCl Dry Electrodes for Wearable Electrophysiological Sensing. Front. Electron. 2022, 2, 700363. [Google Scholar] [CrossRef]
- Tong, A.; Perera, P.; Sarsenbayeva, Z.; McEwan, A.; De Silva, A.C.; Withana, A. Fully 3D-Printed Dry EEG Electrodes. Sensors 2023, 23, 5175. [Google Scholar] [CrossRef]
- De Fazio, R.; Cascella, I.; Visconti, P.; De Vittorio, M.; Al-Naami, B. EEG Signal Acquisition from the Forehead and Ears through Textile-Based 3D-Printed Electrodes to Be Integrated into a Sensorized Face-Mask for Astronauts’ Sleep Monitoring. In Proceedings of the 2024 Second Jordanian International Biomedical Engineering Conference (JIBEC), Amman, Jordan, 27 November 2024. [Google Scholar]
- Choi, Y.Y.; Ho, D.H.; Cho, J.H. Self-Healable Hydrogel–Liquid Metal Composite Platform Enabled by a 3D Printed Stamp for a Multimodular Sensor System. ACS Appl. Mater. Interfaces 2020, 12, 9824–9832. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D Printed Dry Electrode for ECG/EEG Recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Hwang, J.-Y.; Chung, J.; Jang, T.-M.; Seo, D.G.; Gao, Y.; Lee, J.; Park, H.; Lee, S.; et al. 3D Printed, Customizable, and Multifunctional Smart Electronic Eyeglasses for Wearable Healthcare Systems and Human—Machine Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 21424–21432. [Google Scholar] [CrossRef]
- Sung-Joon, C.; Tai-Seung, N.; Seok-Yong, C.; Myeong-Kyu, K.; Sohee, K. 3D Printed Multi-Channel EEG Sensors for Zebrafish. In Proceedings of the 2015 IEEE Sensors, Busan, Republic of Korea, 11 June 2015. [Google Scholar]
- Zhu, Z.; Park, H.S.; McAlpine, M.C. 3D Printed Deformable Sensors. Sci. Adv. 2020, 6, eaba5575. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Lu, J.; Cai, R.; Zhao, T.; Chen, Y.; Zhang, M.; Lu, X.; Chen, Y. Injectable, Tough and Adhesive Zwitterionic Hydrogels for 3D-Printed Wearable Strain Sensors. Chem. Eng. J. 2023, 475, 146340. [Google Scholar] [CrossRef]
- DemiRciOğlu, P.; Böğrekci, İ.; Uymaz, Ş.C. 3D Printed Holter Electrocardiogram (ECG). Int. J. 3D Print. Technol. Digit. Ind. 2022, 6, 429–437. [Google Scholar] [CrossRef]
- Abdou, A.; Mistry, N.; Krishnan, S. 3D Printed Dry Electrodes for Single-Lead Newborn ECG Monitoring. In Proceedings of the 2023 Computing in Cardiology (CinC), Atlanta, GA, USA, 1 October 2023. [Google Scholar]
- Alsharif, A.; Cucuri, N.; Dakhaikh, L.; Al-Modaf, F.; El-Atab, N. Structured 3D Printed Dry ECG Electrodes Using Copper Based Filament. ECS Trans. 2022, 109, 3–8. [Google Scholar] [CrossRef]
- Foster, M.; Erb, P.; Plank, B.; West, H.; Russenberger, J.; Gruen, M.; Daniele, M.; Roberts, D.L.; Bozkurt, A. 3D-Printed Electrocardiogram Electrodes for Heart Rate Detection in Canines. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2021. [Google Scholar]
- Ahmmed, P.; Reynolds, J.; Hamada, S.; Regmi, P.; Bozkurt, A. Novel 3D-Printed Electrodes for Implantable Biopotential Monitoring. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1 November 2021. [Google Scholar]
- Lopez-Larrea, N.; Criado-Gonzalez, M.; Dominguez-Alfaro, A.; Alegret, N.; Agua, I.D.; Marchiori, B.; Mecerreyes, D. Digital Light 3D Printing of PEDOT-Based Photopolymerizable Inks for Biosensing. ACS Appl. Polym. Mater. 2022, 4, 6749–6759. [Google Scholar] [CrossRef]
- Xing, L.; Casson, A.J. 3D-Printed, Directly Conductive and Flexible Electrodes for Personalized Electroencephalography. Sens. Actuators A Phys. 2023, 349, 114062. [Google Scholar] [CrossRef]
- Ramasamy, M.; Varadan, V.K. 3D Printing of Wearable Fractal-Based Sensor Systems for Neurocardiology and Healthcare. In Nanosensors, Biosensors, Info-Tech Sensors and 3D Systems; Varadan, V.K., Ed.; SPIE: Portland, OR, USA, 2017; Volume 10167, pp. 24–29. [Google Scholar]
- Schuhknecht, A.; Fadanelli, E.; Patel, M.; Hanson, A.J.; Maddipatla, D.; Atashbar, M.Z. Development of a Flexible and Conformable EEG Sensors Using 3D Printing Process. In Proceedings of the 2021 IEEE Sensors, Sydney, NJ, Australia, 31 October 2021. [Google Scholar]
- Krachunov, S.; Casson, A. 3D Printed Dry EEG Electrodes. Sensors 2016, 16, 1635. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Anwer, S.; Umer, W.; Antwi-Afari, M.F.; Xiao, E.B. Evaluation of Sweat-Based Biomarkers Using Wearable Biosensors for Monitoring Stress and Fatigue: A Systematic Review. Int. J. Occup. Saf. Ergon. 2024, 30, 677–703. [Google Scholar] [CrossRef]
- Yang, D.S.; Wu, Y.; Kanatzidis, E.E.; Avila, R.; Zhou, M.; Bai, Y.; Chen, S.; Sekine, Y.; Kim, J.; Deng, Y.; et al. 3D-Printed Epidermal Sweat Microfluidic Systems with Integrated Microcuvettes for Precise Spectroscopic and Fluorometric Biochemical Assays. Mater. Horiz. 2023, 10, 4992–5003. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Feng, Q.; Su, H.; Li, D.; Shang, Y.; Chen, H.; Li, B.; Dong, H. A Three-Dimensional-Printed Recyclable, Flexible, and Wearable Device for Visualized UV, Temperature, and Sweat pH Sensing. ACS Omega 2022, 7, 9834–9845. [Google Scholar] [CrossRef]
- Koukouviti, E.; Plessas, A.K.; Economou, A.; Thomaidis, N.; Papaefstathiou, G.S.; Kokkinos, C. 3D Printed Voltammetric Sensor Modified with an Fe(III)-Cluster for the Enzyme-Free Determination of Glucose in Sweat. Biosensors 2022, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Xia, Y.; Dong, H.; Shen, Y.; Feng, Z.; Hong, Y.; Zhu, H.; Yin, B.; Ji, Z.; Xu, Q.; et al. Microfluidic Wearable Electrochemical Sensor Based on MOF-Derived Hexagonal Rod-Shaped Porous Carbon for Sweat Metabolite and Electrolyte Analysis. Anal. Chem. 2024, 96, 16676–16685. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Cheng, Y.; Huang, W.; Xiang, L. Electrochemical Biosensors Based on Saliva Electrolytes for Rapid Detection and Diagnosis. J. Mater. Chem. B 2023, 11, 33–54. [Google Scholar] [CrossRef]
- Nguyen, M.-D.; Nguyen, K.-N.; Malo, S.; Banerjee, I.; Wu, D.; Du-Thumm, L.; Dauphin-Ducharme, P. Electrochemical Aptamer-Based Biosensors for Measurements in Undiluted Human Saliva. ACS Sens. 2023, 8, 4625–4635. [Google Scholar] [CrossRef]
- Wrobel Von Zuben, T.; Kalinke, C.; Campos Janegitz, B.; Gonçalves Salles, A.; Alves Bonacin, J. 3D-printed Amperometric Sensor for the Detection of Ethanol in Saliva. Electroanalysis 2023, 35, e202300044. [Google Scholar] [CrossRef]
- Sunil, N.; Unnathpadi, R.; Seenivasagam, R.K.; Abhijith, T.; Latha, R.; Sheen, S.; Pullithadathil, B. Development of an AI-Derived, Non-Invasive, Label-Free 3D-Printed Microfluidic SERS Biosensor Platform Utilizing Cu@Ag/Carbon Nanofibers for the Detection of Salivary Biomarkers in Mass Screening of Oral Cancer. J. Mater. Chem. B 2025, 13, 3405–3419. [Google Scholar] [CrossRef]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent Advances in 3D Printing Technologies for Wearable (Bio)Sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Rachim, V.P.; Park, S.-M. Review of 3D-Printing Technologies for Wearable and Implantable Bio-Integrated Sensors. Essays Biochem. 2021, 65, 491–502. [Google Scholar] [CrossRef]
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro Additive Manufacturing of Glucose Biosensors: A Feasibility Study. Anal. Chim. Acta 2018, 1043, 142–149. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Curto, V.F.; Seneci, C.A.; Wang, C.; Anastasova, S.; Vadgama, P.; Yang, G.-Z.; Boutelle, M.G. 3D Printed Microfluidic Device with Integrated Biosensors for Online Analysis of Subcutaneous Human Microdialysate. Anal. Chem. 2015, 87, 7763–7770. [Google Scholar] [CrossRef]
- Guan, H.; Zhong, T.; He, H.; Zhao, T.; Xing, L.; Zhang, Y.; Xue, X. A Self-Powered Wearable Sweat-Evaporation-Biosensing Analyzer for Building Sports Big Data. Nano Energy 2019, 59, 754–761. [Google Scholar] [CrossRef]
- Nolan, J.K.; Nguyen, T.N.H.; Le, K.V.H.; DeLong, L.E.; Lee, H. Simple Fabrication of Flexible Biosensor Arrays Using Direct Writing for Multianalyte Measurement from Human Astrocytes. SLAS Technol. 2020, 25, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.H.; Nolan, J.K.; Park, H.; Lam, S.; Fattah, M.; Page, J.C.; Joe, H.-E.; Jun, M.B.G.; Lee, H.; Kim, S.J.; et al. Facile Fabrication of Flexible Glutamate Biosensor Using Direct Writing of Platinum Nanoparticle-Based Nanocomposite Ink. Biosens. Bioelectron. 2019, 131, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Samper, I.C.; Gowers, S.A.N.; Rogers, M.L.; Murray, D.-S.R.K.; Jewell, S.L.; Pahl, C.; Strong, A.J.; Boutelle, M.G. 3D Printed Microfluidic Device for Online Detection of Neurochemical Changes with High Temporal Resolution in Human Brain Microdialysate. Lab Chip 2019, 19, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Calabria, D.; Lazzarini, E.; Pace, A.; Trozzi, I.; Zangheri, M.; Cinti, S.; Difonzo, M.; Valenti, G.; Guardigli, M.; Paolucci, F.; et al. Smartphone-Based 3D-Printed Electrochemiluminescence Enzyme Biosensor for Reagentless Glucose Quantification in Real Matrices. Biosens. Bioelectron. 2023, 227, 115146. [Google Scholar] [CrossRef]
- Wei, H.; Han, L.; Yin, R.; Yang, T.; Liu, Y.; Mou, C.; Pang, F.; Wang, T. Micro-3D Printed Concanavalin A Hydrogel Based Photonic Devices for High-Sensitivity Glucose Sensing. Sens. Actuators B Chem. 2023, 386, 133707. [Google Scholar] [CrossRef]
- Lee, J.; Maji, S.; Lee, H. Fabrication and Integration of a Low-cost 3D Printing-based Glucose Biosensor for Bioprinted Liver-on-a-chip. Biotechnol. J. 2023, 18, 2300154. [Google Scholar] [CrossRef]
- Adams, A.; Malkoc, A.; La Belle, J.T. The Development of a Glucose Dehydrogenase 3D-Printed Glucose Sensor: A Proof-of-Concept Study. J. Diabetes Sci. Technol. 2018, 12, 176–182. [Google Scholar] [CrossRef]
- Silva, M.N.T.; Rocha, R.G.; Richter, E.M.; Munoz, R.A.A.; Nossol, E. Nickel Oxy-Hydroxy/Multi-Wall Carbon Nanotubes Film Coupled with a 3D-Printed Device as a Nonenzymatic Glucose Sensor. Biosensors 2023, 13, 646. [Google Scholar] [CrossRef]
- Shar, A.; Glass, P.; Park, S.H.; Joung, D. 3D Printable One-Part Carbon Nanotube-Elastomer Ink for Health Monitoring Applications. Adv. Funct. Mater. 2023, 33, 2211079. [Google Scholar] [CrossRef]
- Tan, P.; Xi, Y.; Chao, S.; Jiang, D.; Liu, Z.; Fan, Y.; Li, Z. An Artificial Intelligence-Enhanced Blood Pressure Monitor Wristband Based on Piezoelectric Nanogenerator. Biosensors 2022, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.R.; Pozdin, V.A.; Young, A.T.; Erb, P.D.; Wisniewski, N.A.; Magness, S.T.; Daniele, M. Integrated Phosphorescence-Based Photonic Biosensor (iPOB) for Monitoring Oxygen Levels in 3D Cell Culture Systems. Biosens. Bioelectron. 2019, 123, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Contardi, U.A.; Morikawa, M.; Brunelli, B.; Thomaz, D.V. MAX30102 Photometric Biosensor Coupled to ESP32-Webserver Capabilities for Continuous Point of Care Oxygen Saturation and Heartrate Monitoring. In Proceedings of the 2nd International Electronic Conference on Biosensors, Rome, Italy, 14 October 2021. [Google Scholar]
- Nah, J.S.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A Wearable Microfluidics-Integrated Impedimetric Immunosensor Based on Ti3C2T MXene Incorporated Laser-Burned Graphene for Noninvasive Sweat Cortisol Detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar] [CrossRef]
- Parate, K.; Rangnekar, S.V.; Jing, D.; Mendivelso-Perez, D.L.; Ding, S.; Secor, E.B.; Smith, E.A.; Hostetter, J.M.; Hersam, M.C.; Claussen, J.C. Aerosol-Jet-Printed Graphene Immunosensor for Label-Free Cytokine Monitoring in Serum. ACS Appl. Mater. Interfaces 2020, 12, 8592–8603. [Google Scholar] [CrossRef]
- Padash, M.; Carrara, S. A 3D Printed Wearable Device for Sweat Analysis. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Bari, Italy, 7 July 2020. [Google Scholar]
- Wu, C.-H.; Ma, H.J.H.; Baessler, P.; Balanay, R.K.; Ray, T.R. Skin-Interfaced Microfluidic Systems with Spatially Engineered 3D Fluidics for Sweat Capture and Analysis. Sci. Adv. 2023, 9, eadg4272. [Google Scholar] [CrossRef]
- Song, Y.; Tay, R.Y.; Li, J.; Xu, C.; Min, J.; Shirzaei Sani, E.; Kim, G.; Heng, W.; Kim, I.; Gao, W. 3D-Printed Epifluidic Electronic Skin for Machine Learning–Powered Multimodal Health Surveillance. Sci. Adv. 2023, 9, eadi6492. [Google Scholar] [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A Portable 3D Microfluidic Origami Biosensor for Cortisol Detection in Human Sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef]
- Laurila, M.-M.; Peltokangas, M.; Montero, K.L.; Verho, J.; Haapala, M.; Oksala, N.; Vehkaoja, A.; Mäntysalo, M. Self-Powered, High Sensitivity Printed e-Tattoo Sensor for Unobtrusive Arterial Pulse Wave Monitoring. Nano Energy 2022, 102, 107625. [Google Scholar] [CrossRef]
- Zhou, Z.-B.; Cui, T.-R.; Li, D.; Jian, J.-M.; Li, Z.; Ji, S.-R.; Li, X.; Xu, J.-D.; Liu, H.-F.; Yang, Y.; et al. Wearable Continuous Blood Pressure Monitoring Devices Based on Pulse Wave Transit Time and Pulse Arrival Time: A Review. Materials 2023, 16, 2133. [Google Scholar] [CrossRef]
- Charlton, P.H.; Paliakaitė, B.; Pilt, K.; Bachler, M.; Zanelli, S.; Kulin, D.; Allen, J.; Hallab, M.; Bianchini, E.; Mayer, C.C.; et al. Assessing Hemodynamics from the Photoplethysmogram to Gain Insights into Vascular Age: A Review from VascAgeNet. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H493–H522. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Luo, W.; Young, D.J. A 3D-Printed Wearable Ring Sensor for Long-Term Accurate Monitoring of Human Cardiovascular Condition. In Proceedings of the 2022 IEEE Sensors, Dallas, TX, USA, 30 October 2022. [Google Scholar]
- Chen, W.-L.; Lin, C.-H.; Yang, T.-L.; Lin, C.-W.; Kan, C.-D. Custom-Designed Sensors Embedded 3D-Printed Wearable Device for Improving the Hemodialysis-Related Vascular Dysfunction Detection. THC 2023, 31, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, R.; Wu, X. Recent Progress in the Development of Conductive Hydrogels and the Application in 3D Printed Wearable Sensors. RSC Appl. Polym. 2023, 1, 132–157. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Nie, S.; Meng, K.; Lin, Z. Recent Progress of Wearable Triboelectric Nanogenerator-Based Sensor for Pulse Wave Monitoring. Sensors 2023, 24, 36. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Yang, F.; Wei, H.; Zhang, Z.; Wang, Y.; Wang, J.; Li, C.; Carlos, C.; Dong, Y.; et al. Multifunctional Artificial Artery from Direct 3D Printing with Built-In Ferroelectricity and Tissue-Matching Modulus for Real-Time Sensing and Occlusion Monitoring. Adv. Funct. Mater. 2020, 30, 2002868. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-K.; Kim, D.-S.; Shanmugasundaram, A.; Park, S.A.; Kang, S.; Kim, S.-H.; Jeong, M.H.; Lee, D.-W. Wireless Pressure Sensor Integrated with a 3D Printed Polymer Stent for Smart Health Monitoring. Sens. Actuators B Chem. 2019, 280, 201–209. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, Z.; Wang, X.; Nie, J.; Huang, P.; Zhao, S. 3D-Printed Highly Stable Flexible Strain Sensor Based on Silver-Coated-Glass Fiber-Filled Conductive Silicon Rubber. Mater. Des. 2020, 193, 108788. [Google Scholar] [CrossRef]
- Le, T.; Song, B.; Liu, Q.; Bahr, R.A.; Moscato, S.; Wong, C.-P.; Tentzeris, M.M. A Novel Strain Sensor Based on 3D Printing Technology and 3D Antenna Design. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26 May 2015. [Google Scholar]
- Votzke, C.; Daalkhaijav, U.; Mengue, Y.; Johnston, M.L. Highly-Stretchable Biomechanical Strain Sensor Using Printed Liquid Metal Paste. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018. [Google Scholar]
- Agarwala, S.; Goh, G.L.; Yeong, W.Y. Aerosol Jet Printed Strain Sensor: Simulation Studies Analyzing the Effect of Dimension and Design on Performance. IEEE Access 2018, 6, 63080–63086. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Dinh Le, T.-S.; An, J.; Peh, Z.K.; Yeong, W.Y.; Kim, Y.-J. Wearable Bandage-Based Strain Sensor for Home Healthcare: Combining 3D Aerosol Jet Printing and Laser Sintering. ACS Sens. 2019, 4, 218–226. [Google Scholar] [CrossRef]
- Tetsu, Y.; Yamagishi, K.; Kato, A.; Matsumoto, Y.; Tsukune, M.; Kobayashi, Y.; Fujie, M.G.; Takeoka, S.; Fujie, T. Ultrathin Epidermal Strain Sensor Based on an Elastomer Nanosheet with an Inkjet-Printed Conductive Polymer. Appl. Phys. Express 2017, 10, 087201. [Google Scholar] [CrossRef]
- Siddique, S.; Park, J.G.; Andrei, P.; Liang, R. M3D Aerosol Jet Printed Buckypaper Multifunctional Sensors for Composite Structural Health Monitoring. Results Phys. 2019, 13, 102094. [Google Scholar] [CrossRef]
- Xiao, T.; Qian, C.; Yin, R.; Wang, K.; Gao, Y.; Xuan, F. 3D Printing of Flexible Strain Sensor Array Based on UV-Curable Multiwalled Carbon Nanotube/Elastomer Composite. Adv. Mater. Technol. 2021, 6, 2000745. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Haghniaz, R.; Rashidi, A.; Ahadian, S.; Sheikhi, A.; Chen, J.; Khademhosseini, A.; Milani, A.S.; Hoorfar, M.; et al. 3D-Printed Ultra-Robust Surface-Doped Porous Silicone Sensors for Wearable Biomonitoring. ACS Nano 2020, 14, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, E.; Fayazfar, H.; Liravi, F.; Jabari, E.; Toyserkani, E. Drop-on-Demand High-Speed 3D Printing of Flexible Milled Carbon Fiber/Silicone Composite Sensors for Wearable Biomonitoring Devices. Addit. Manuf. 2020, 32, 101016. [Google Scholar] [CrossRef]
- Huang, K.; Dong, S.; Yang, J.; Yan, J.; Xue, Y.; You, X.; Hu, J.; Gao, L.; Zhang, X.; Ding, Y. Three-Dimensional Printing of a Tunable Graphene-Based Elastomer for Strain Sensors with Ultrahigh Sensitivity. Carbon 2019, 143, 63–72. [Google Scholar] [CrossRef]
- Su, X.; Borayek, R.; Li, X.; Herng, T.S.; Tian, D.; Lim, G.J.H.; Wang, Y.; Wu, J.; Ding, J. Integrated Wearable Sensors with Bending/Stretching Selectivity and Extremely Enhanced Sensitivity Derived from Agarose-Based Ionic Conductor and Its 3D-Shaping. Chem. Eng. J. 2020, 389, 124503. [Google Scholar] [CrossRef]
- Yin, X.-Y.; Zhang, Y.; Cai, X.; Guo, Q.; Yang, J.; Wang, Z.L. 3D Printing of Ionic Conductors for High-Sensitivity Wearable Sensors. Mater. Horiz. 2019, 6, 767–780. [Google Scholar] [CrossRef]
- Vella, S.; Smithson, C.; Halfyard, K.; Shen, E.; Chrétien, M. Integrated Capacitive Sensor Devices Aerosol Jet Printed on 3D Objects. Flex. Print. Electron. 2019, 4, 045005. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D Printed Stretchable Tactile Sensors. Adv. Mater. 2017, 29, 1701218. [Google Scholar] [CrossRef]
- Lo, L.; Shi, H.; Wan, H.; Xu, Z.; Tan, X.; Wang, C. Inkjet-Printed Soft Resistive Pressure Sensor Patch for Wearable Electronics Applications. Adv Mater. Technol. 2020, 5, 1900717. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.; Cho, S.; Song, W.; Moon, S.; Park, C.; Park, S.; Myoung, J.M.; Jeong, U. Surface-Embedded Stretchable Electrodes by Direct Printing and Their Uses to Fabricate Ultrathin Vibration Sensors and Circuits for 3D Structures. Adv. Mater. 2017, 29, 1702625. [Google Scholar] [CrossRef]
- Jing, Q.; Choi, Y.S.; Smith, M.; Ou, C.; Busolo, T.; Kar-Narayan, S. Freestanding Functional Structures by Aerosol-Jet Printing for Stretchable Electronics and Sensing Applications. Adv. Mater. Technol. 2019, 4, 1900048. [Google Scholar] [CrossRef]
- Shi, G.; Lowe, S.E.; Teo, A.J.T.; Dinh, T.K.; Tan, S.H.; Qin, J.; Zhang, Y.; Zhong, Y.L.; Zhao, H. A Versatile PDMS Submicrobead/Graphene Oxide Nanocomposite Ink for the Direct Ink Writing of Wearable Micron-Scale Tactile Sensors. Appl. Mater. Today 2019, 16, 482–492. [Google Scholar] [CrossRef]
- Schouten, M.; Prakken, B.; Sanders, R.; Krijnen, G. Linearisation of a 3D Printed Flexible Tactile Sensor Based on Piezoresistive Sensing. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27 October 2019. [Google Scholar]
- Zou, Q.; Ma, Z.; Li, S.; Lei, Z.; Su, Q. Tunable Ionic Pressure Sensor Based on 3D Printed Ordered Hierarchical Mesh Structure. Sens. Actuators A Phys. 2020, 308, 112012. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, M.; Yu, G.; Tan, J.; Xuan, F. Extrusion Printing of Carbon Nanotube-Coated Elastomer Fiber with Microstructures for Flexible Pressure Sensors. Sens. Actuators A Phys. 2019, 299, 111625. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Shu, T.; Chen, Y.; Yang, W.; Liu, Y.; Long, J.; Xiong, W.; Xuan, F. 3D-Printed Coaxial Fibers for Integrated Wearable Sensor Skin. Adv. Mater. Technol. 2019, 4, 1900504. [Google Scholar] [CrossRef]
- Li, H.; Fang, X.; Li, R.; Liu, B.; Tang, H.; Ding, X.; Xie, Y.; Zhou, R.; Zhou, G.; Tang, Y. All-Printed Soft Triboelectric Nanogenerator for Energy Harvesting and Tactile Sensing. Nano Energy 2020, 78, 105288. [Google Scholar] [CrossRef]
- Nag, A.; Feng, S.; Mukhopadhyay, S.C.; Kosel, J.; Inglis, D. 3D Printed Mould-Based Graphite/PDMS Sensor for Low-Force Applications. Sens. Actuators A Phys. 2018, 280, 525–534. [Google Scholar] [CrossRef]
- Haque, R.I.; Chandran, O.; Lani, S.; Briand, D. Self-Powered Triboelectric Touch Sensor Made of 3D Printed Materials. Nano Energy 2018, 52, 54–62. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Q.; Clancy, T.; Fan, Q.; Wang, X.; Liu, X. 3D-Printed Strain-Gauge Micro Force Sensors. IEEE Sens. J. 2020, 20, 6971–6978. [Google Scholar] [CrossRef]
- Yi, Q.; Najafikhoshnoo, S.; Das, P.; Noh, S.; Hoang, E.; Kim, T.; Esfandyarpour, R. All-3D-Printed, Flexible, and Hybrid Wearable Bioelectronic Tactile Sensors Using Biocompatible Nanocomposites for Health Monitoring. Adv Mater. Technol. 2022, 7, 2101034. [Google Scholar] [CrossRef]
- Lo Presti, D.; Dimo, A.; Zoboli, L.; Bianchi, D.; Massaroni, C.; Altomare, V.; Grasso, A.; Oddo, C.M.; Gizzi, A.; Schena, E. A 3-D-Printed Tactile Probe Based on Fiber Bragg Grating Sensors for Noninvasive Breast Cancer Identification. IEEE Sens. J. 2023, 23, 24489–24499. [Google Scholar] [CrossRef]
- Bito, J.; Bahr, R.; Hester, J.; Kimionis, J.; Nauroze, A.; Su, W.; Tehrani, B.; Tentzeris, M.M. Inkjet-/3D-/4D-Printed Autonomous Wearable RF Modules for Biomonitoring, Positioning and Sensing Applications; George, T., Dutta, A.K., Islam, M.S., Eds.; SPIE: Anaheim, CA, USA, 2017; p. 101940Z. [Google Scholar]
- Su, W.; Wu, Z.; Fang, Y.; Bahr, R.; Raj, P.M.; Tummala, R.; Tentzeris, M.M. 3D Printed Wearable Flexible SIW and Microfluidics Sensors for Internet of Things and Smart Health Applications. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honololu, HI, USA, 9 June 2017. [Google Scholar]
- Kostecki, R.; Arman, A.; Zhang, B.; Yang, K.; Narayan, R.J.; Hutchinson, M.R.; Ebendorff-Heidepriem, H. Dynamic in Vivo Protein Carbonyl Biosensor for Measuring Oxidative Stress. Med. Devices Sens. 2020, 3, e10135. [Google Scholar] [CrossRef]
- Williams, N.X.; Noyce, S.; Cardenas, J.A.; Catenacci, M.; Wiley, B.J.; Franklin, A.D. Silver Nanowire Inks for Direct-Write Electronic Tattoo Applications. Nanoscale 2019, 11, 14294–14302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, J.; Song, J.; Li, Z. The Application of Impantable Sensors in the Musculoskeletal System: A Review. Front. Bioeng. Biotechnol. 2024, 12, 1270237. [Google Scholar] [CrossRef] [PubMed]
- Sadak, O.; Kuss, M.; Shi, W.; Duan, B.; Iverson, N.M. Development of a 3D Printed Liquid-Core Hydrogel Platform for Real-Time Carbon Nanotube Sensors: A Breakthrough in Minimally Invasive Health Monitoring. Meet. Abstr. 2024, MA2024-01, 855. [Google Scholar] [CrossRef]
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433. [Google Scholar] [CrossRef]
- Momin, M.; Feng, L.; Ahmed, S.; Ren, J.; Hossain, A.; Zhang, S.; Zhou, T. 3D-Printed Flexible Neural Probes for Recordings at Single-Neuron Level. Device 2024, 2, 100519. [Google Scholar] [CrossRef]
- Shao, Z.; Zhao, H.; Dunham, K.E.; Cao, Q.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Nanoneedle Electrodes for Dopamine Detection in Drosophila. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405634. [Google Scholar] [CrossRef]
- Shin, H.; Kim, K.; Lee, J.; Nam, J.; Baeg, E.; You, C.; Choi, H.; Kim, M.; Chung, C.K.; Kim, J.G.; et al. A Wireless Cortical Surface Implant for Diagnosing and Alleviating Parkinson’s Disease Symptoms in Freely Moving Animals. Adv. Healthc. Mater. 2025, 2405179. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ji, Y.; Deng, H.; Long, M.; Ge, S.; Su, Y.; Chan, S.Y.; Loh, X.J.; Zhuang, A.; et al. A Non-Invasive Smart Scaffold for Bone Repair and Monitoring. Bioact. Mater. 2023, 19, 499–510. [Google Scholar] [CrossRef]
- Lavdas, M.K.; Willing, R.; Lanting, B.A.; Teeter, M.G. Embedded Sensing Package for Temporary Bone Cement Spacers in Infected Total Knee Arthroplasty. J. Mech. Behav. Biomed. Mater. 2021, 115, 104301. [Google Scholar] [CrossRef] [PubMed]
- Kachare, A.; Goregaonkar, A.B.; Purohit, S.; Munde, K.; Renthlei, L. Surgical Planning and 3D-Printed Mesh Implant for Effective Bone Gap Management: A Case Report. J. Orthop. Case Rep. 2024, 14, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.J.; Kashlan, K.N.; Flanagan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J. 3D Printed Implantable Hydrogel Bioelectronics for Electrophysiological Monitoring and Electrical Modulation. Adv. Funct. Mater. 2024, 34, 2314471. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively Manufactured Medical Products—The FDA Perspective. 3D Print. Med. 2016, 2, 1. [Google Scholar] [CrossRef]
- Cândido, T.C.D.O.; Silva, D.N.D.; Borges, M.M.C.; Barbosa, T.G.; Trindade, S.O.D.D.; Pereira, A.C. 3D-Printed Electrochemical Sensors: A Comprehensive Review of Clinical Analysis Applications. Analytica 2024, 5, 552–575. [Google Scholar] [CrossRef]
- Podunavac, I.; Djocos, M.; Vejin, M.; Birgermajer, S.; Pavlovic, Z.; Kojic, S.; Petrovic, B.; Radonic, V. 3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes. Micromachines 2023, 14, 503. [Google Scholar] [CrossRef]
- Ahmadianyazdi, A.; Miller, I.J.; Folch, A. Tunable Resins with PDMS-like Elastic Modulus for Stereolithographic 3D-Printing of Multimaterial Microfluidic Actuators. Lab Chip 2023, 23, 4019–4032. [Google Scholar] [CrossRef]
- Remaggi, G.; Zaccarelli, A.; Elviri, L. 3D Printing Technologies in Biosensors Production: Recent Developments. Chemosensors 2022, 10, 65. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef]
- Koucheryavy, Y.; Yastrebova, A.; Martins, D.P.; Balasubramaniam, S. A Review on Bio-Cyber Interfaces for Intrabody Molecular Communications Systems. arXiv 2021, arXiv:10.48550/arXiv.2104.14944. [Google Scholar]
- Rajendran, J.; Esfandyarpour, R. Revolutionizing Personalized Health: The Frontier of Wearable Biomolecule Sensors Through 3D Printing Innovation. Biomed. Mater. Devices 2024, 3, 818–834. [Google Scholar] [CrossRef]

| Analyte | Bioreceptor | Detection Method (Transducer) | Applications | Reference |
|---|---|---|---|---|
| Glucose | Glucose oxidase, Glucose dehydrogenase | Electrochemical (Amperometric, Potentiometric), Optical | Diabetes monitoring, metabolic studies | [4] |
| Lactose | β-Galactosidase, Lactose oxidase | Electrochemical (Amperometric), Optical | Food quality control, lactose intolerance testing | [5] |
| Dopamine | Tyrosinase, Aptamers, MIPs | Electrochemical (Voltammetric, Amperometric), Optical | Neurological disorder diagnosis (Parkinson’s, schizophrenia) | [6] |
| Uric acid | Uricase, Aptamers | Electrochemical (Amperometric, Voltammetric) | Gout and kidney disorder diagnosis | [7] |
| Cholesterol | Cholesterol oxidase, Cholesterol esterase | Electrochemical (Amperometric, Potentiometric), Optical | Cardiovascular risk assessment | [8] |
| Lactic acid | Lactate oxidase | Electrochemical (Amperometric), Optical | Sports medicine, sepsis monitoring | [9] |
| ATP | Aptamers, Enzymes | Luminescent, Electrochemical | Cellular metabolism, cancer detection | [10] |
| Cortisol (Hormone) | Antibodies, Aptamers, MIPs | Electrochemical (Impedimetric), Optical (SPR, fluorescence) | Stress monitoring, endocrine disorder detection | [11] |
| Estrogen | Antibodies, Aptamers, MIPs | Electrochemical, Optical | Reproductive health, cancer diagnostics | [12] |
| Insulin | Antibodies, Aptamers | Electrochemical (Impedimetric), Optical | Diabetes management | [13] |
| Drugs (e.g., Antibiotics, Narcotics) | Aptamers, Antibodies, Enzymes | Electrochemical (Voltammetric), Optical (SPR, Fluorescence) | Drug abuse detection, therapeutic drug monitoring | [14,15] |
| Heavy metals (Pb2+, Hg2+, Cd2+) | DNAzymes, Aptamers | Electrochemical, Optical | Environmental monitoring, food/water safety | [16] |
| Pesticides (e.g., organophosphates) | Acetylcholinesterase (AChE) | Electrochemical (Amperometric), Optical | Agricultural and environmental safety | [17] |
| Pathogenic bacteria (E. coli, Salmonella) | Antibodies, Aptamers, Bacteriophages | Electrochemical (Impedimetric), Piezoelectric, Optical | Food safety, clinical diagnostics | [18] |
| Viruses (SARS-CoV-2, Influenza, HIV) | Antibodies, Aptamers, DNA probes | Electrochemical, Optical (SPR, plasmonic), Piezoelectric (QCM) | Infectious disease detection | [19] |
| DNA (genetic targets) | DNA probes, CRISPR-Cas systems | Electrochemical, Optical (FRET, SPR) | Genetic testing, personalized medicine | [20] |
| RNA (viral genomes, miRNA) | RNA aptamers, CRISPR-Cas | Electrochemical (Voltammetric), Optical (Fluorescence) | Viral diagnostics, cancer biomarker detection | [21] |
| CRP (C-reactive protein) | Antibodies, Aptamers | Electrochemical (Impedimetric), Optical (SPR) | Inflammation monitoring, cardiovascular risk | [22] |
| Troponin (Cardiac biomarker) | Antibodies, Aptamers | Electrochemical (Impedimetric), Optical | Heart attack (myocardial infarction) diagnosis | [23] |
| Cytokines (IL-6, TNF-α) | Antibodies, Aptamers | Electrochemical, Optical | Immune response monitoring, inflammatory disease | [24] |
| S. No. | Biosensing Application | 3D Printing Method | Statistical Data/Performance | Advantage | Social/Environmental Impact | Reference |
|---|---|---|---|---|---|---|
| ELECTROCARDIOGRAM (ECG) | ||||||
| 1 | Self-healable hydrogel–liquid metal ECG sensor | Custom 3D-printed molds | Effective ECG signal acquisition | Self-healing, flexible electrodes | Longer device lifespan, reduced waste | [158] |
| 2 | ECG and EEG dry electrode recording | 3D-printed electrode arrays | High resolution, repeatable | Affordable, scalable vs. gel electrodes | Accessible monitoring, low-cost healthcare | [159] |
| 3 | Multifunctional wearable biosensing (EEG, EOG, motion, and UV) | 3D-printed eyeglass frame | Demonstrated integrated biosensing | Customizable, multifunctional | Enhances personal healthcare and HMI | [160] |
| 4 | Underwater EEG sensing (zebrafish) | 3D-printed multichannel arrays | Feasible in aquatic conditions | Enables biosensing in non-human species | Advances marine neuroscience | [161] |
| 5 | On-body biosensing (aerogels) | Freeform closed-loop 3D printing | Functional printing on skin | Prints on moving, curved surfaces | Real-time, non-invasive monitoring | [80] |
| 6 | On-tissue electrical impedance sensing | Closed-loop 3D printing on deformable surfaces | Real-time EIT on porcine lung | Compensates for motion, deformation | Improves surgical and diagnostic tools | [162] |
| 7 | Smart clothing (ECG/EMG) | Photocuring-based 3D printing of graphene/polymer | Flexible, stretchable electrodes | Wearable, washable integration | Eco-friendly smart textiles | [153] |
| 8 | Wearable strain and heartbeat sensors | 3D printing of injectable DN hydrogels | Biocompatible, adhesive, tough | Better flexibility and adhesion vs. gels | Safer, reusable wearable healthcare | [163] |
| 9 | Remote ECG monitoring (Holter) | 3D-printed casing and electronics | Mobile app, SMS, GPRS enabled | Cost-effective, user-friendly | Remote healthcare, reduced hospital visits | [164] |
| 10 | Newborn ECG monitoring | 3D-printed dry electrodes | 92.1% accuracy (rapid HR) | Non-invasive, safe for neonates | Supports remote infant care | [165] |
| 11 | Customizable ECG electrodes | FFF with copper-based filament | Flat designs are optimal for conductivity | Adjustable structures for performance | Lower cost, reusable | [166] |
| 12 | Veterinary ECG (canines) | 3D-printed fur-friendly electrodes | In vivo trials: equivalent to sticky electrodes | Non-invasive, reusable | Enhances animal welfare, reduces waste | [167] |
| 13 | Subcutaneous ECG implants (animals) | Custom conductive 3D-printed electrodes | Comparable to commercial implants | Miniaturized, customizable | Biomedical and veterinary research boost | [168] |
| 14 | Flexible ECG/EMG biosensors | DLP printing of PEDOT inks | Conductivity: 10−1–10−2 S/cm | Superior to Ag/AgCl electrodes | Biocompatible, flexible wearables | [169] |
| ELECTROENCEPHALOGRAM (EEG) | ||||||
| 1 | EEG monitoring (SSVEPs) | Direct 3D printing of conductive flexible materials | Optimized electrical and mechanical performance | Low-cost, flexible, customizable electrodes | Affordable brain–computer interface applications | [170] |
| 2 | EEG and ECG monitoring in small animals | 3D-printed biosignal sensor fabrication | Time and cost-efficient fabrication | Alternative to microfabrication, non-invasive | Advances animal studies with lower cost | [141] |
| 3 | EMG, EDA, EEG, and strain sensing | High-resolution 3D printing with sugar scaffolds | High sensitivity, precision | Custom-fit, flexible, multimodal sensing | Promotes personalized wearable health tech | [110] |
| 4 | Neurocardiology wearable biosensing | 3D fabrication of flexible fractal-based sensors | Demonstrated functional wearable system | Low-cost, fractal design improves flexibility | Expands neurocardiology and remote healthcare | [171] |
| 5 | EEG monitoring | 3D printing of flexible, conformable sensors | Comparable signal quality to commercial electrodes | Enhanced comfort, long-term usability | Improves patient compliance in long studies | [172] |
| 6 | EEG monitoring | 3D printing with Ag/AgCl-coated electrodes | Reduced noise, improved impedance | Better performance than earlier 3D-printed sensors | Increases reliability for medical use | [140] |
| 7 | EEG monitoring (dry electrodes) | Low-cost 3D printing of dry electrodes | Comparable to wet electrodes | Reusable, cost-efficient, non-invasive | Accessible BCI applications, reduced waste | [173] |
| S. No. | Biosensing Application | 3D Printing Method | Statistical Data/Performance | Advantage | Social/Environmental Impact | Reference |
|---|---|---|---|---|---|---|
| GLUCOSE SENSOR | ||||||
| 1 | Electrochemical tattoo glucose sensor | Direct ink writing (DIW) | Sensitivity: 17.5 nA M−1; Range: 100–1000 µM | High sensitivity and specificity vs. screen printing | Non-invasive, wearable, and enhances continuous health monitoring | [185] |
| 2 | Glucose/lactose ratio in athletes | 3D-printed microfluidics (unspecified) | Real-time tissue metabolite tracking | Miniaturization, portability vs. conventional probes | Promotes athlete safety and performance monitoring | [186] |
| 3 | Self-powered sweat lactate sensor | Porous carbon film (3D-printed base) | Stable lactate detection with wireless data transfer | Energy autonomy, wearable vs. benchtop assays | Supports sports analytics and big-data-driven health | [187] |
| 4 | Multi-analyte biosensor (glucose, lactate, and neurotransmitters) | DIW | Flexible array; compatible with organ-on-chip | Multiplexing vs. single-analyte sensors | Advances neuroscience and clinical diagnostics | [188] |
| 5 | In vivo glutamate biosensor | DIW | High signal stability, PtNPs-based electrode | Direct integration, enhanced electrochemical activity | Enables real-time brain monitoring | [189] |
| 6 | Neurochemical monitoring (brain) | 3D-printed microfluidics | High temporal resolution microdialysis | Portable, integrated vs. bulky lab devices | Supports brain disorder studies and neurology research | [190] |
| 7 | Smartphone-enabled glucose biosensor | 3D-printed ECL device | Affordable, reagentless glucose detection | Point-of-care adaptability, reagent-free | Improves accessibility in low-resource settings | [191] |
| 8 | Photonic glucose sensor | DLP micro-3D printing | Sensitivity: 0.206 nm/mM; linear response | Optical detection vs. enzymatic electrochemistry | Environmentally friendly (UV-cured hydrogel); reusable | [192] |
| 9 | Liver-on-a-chip glucose biosensor | FDM with conductive PLA + MWCNT | Enhanced sensitivity via nanocomposites | Low-cost fabrication vs. lithography | Sustainable bioprinting; organ-on-chip integration | [193] |
| 10 | GDH-based glucose biosensor | 3D printing (unspecified) | Meets industrial performance standards | Robustness, manufacturability vs. manual assembly | Supports scalable diabetic treatment solutions | [194] |
| 11 | Disposable non-enzymatic glucose sensor | 3D-printed support + MWCNT/NiOOH | Stable electrochemical signals | Enzyme-free, cost-effective vs. enzymatic tests | Disposable design reduces costs and broadens testing access | [195] |
| OXYGEN SENSOR | ||||||
| 1 | Finger/toe wearable pulse oximeter | Freeform embedding (FRE) printing with PDMS | PDMS cuff customized to patient anatomy; accurate SpO2 and pulse monitoring | Patient-specific fit; better comfort and accuracy than rigid commercial probes | Reduces clinical device waste via custom fabrication; improves patient compliance | [85] |
| 2 | Flexible wireless smart bandage for wound oxygenation | 3D printing with TangoPlus (FLX930) | Bandage integrates a galvanic oximeter + printed elastomer; continuous wound oxygenation monitoring | Wearable, non-invasive wound care; replaces bulky equipment | Supports remote therapy for chronic wounds, reduces hospital visits | [196] |
| 3 | Blood pressure and oxygen monitoring wristband | Direct ink writing (DIW) | Substrate + electrodes printed via DIW; surface mount electronics assembled; integrated platform | Combines biosensing and electronics in one step; lightweight vs. traditional cuffs | Promotes home healthcare and reduces clinical dependency | [197] |
| 4 | Photonic biosensor for 3D cell culture (iPOB) | 3D-printed chamber with integrated biosensor (unspecified) | Phosphorescence-based oxygen monitoring; 3D-printed culture chamber allows gas exchange | High-resolution, non-invasive cell monitoring; better than manual sampling | Advances biomedical research while minimizing chemical waste | [198] |
| 5 | IoT-enabled photometric biosensor system (MAX30102) | 3D-printed case with MAX30102 sensor | Continuous SpO2 and HR monitoring; integrated with ESP32 + webserver for IoT | Portable, low-cost, real-time remote monitoring vs. hospital devices | Expands access to point-of-care diagnostics; low environmental burden | [199] |
| SWEAT SENSOR | ||||||
| 1 | Sweat electrolyte monitoring (multi-ion, real-time) | 3D printing of flexible bioelectronic patch (AIIW) | Real-time multi-ion tracking in human sweat | Low-cost, customizable, continuous biochemical monitoring | Noninvasive health tracking; democratizes personalized medicine | [100] |
| 2 | Cortisol detection for stress monitoring | 3D-printed microfluidic mold + laser-burned graphene with MXene | Continuous cortisol quantification in sweat | High sensitivity, non-invasive stress biosensing | Reduces reliance on blood tests; stress monitoring for mental health | [200] |
| 3 | Cytokine detection in serum | Aerosol Jet Printing (AJP) of graphene ink on polyamide | High sensitivity in real samples | Label-free, flexible immunosensing | Enables inflammation monitoring; minimal sample prep | [201] |
| 4 | Glucose detection in sweat | 3D-printed voltammetric sensor with Fe(III)-cluster | Enzyme-free, stable response under acidic sweat | Cost-effective, avoids enzyme instability | Portable, low-cost diabetes screening | [177] |
| 5 | Sweat analyte collection and analysis | Multi-Jet Modeling (MJM) with flexible polymers | Real-time sweat biofluid acquisition | Rapid, direct-on-skin collection | Enhances wearable diagnostics; reusability reduces waste | [202] |
| 6 | Sweat sample segmentation and spatial analysis | Digital Light Processing (DLP) for fluidic channels | Multi-compartment sweat capture (“sweatainer”) | Enables parallel analysis of different analytes | Advanced diagnostics, scalable to public health | [203] |
| 7 | Multimodal sensing (alcohol inhibition, behavior) | Extrusion-based 3D printing of elastic e-skin (e3-skin) | Continuous multimodal data; ML for behavioral prediction | Integrates biochemical + behavioral sensing | Supports substance abuse monitoring and safety | [204] |
| 8 | Smartphone-linked cortisol monitoring | Compact 3D-printed origami microfluidic sensor | Portable, low-cost, real-sweat analysis | Easy integration with smartphones | Expands access to stress diagnostics globally | [205] |
| S. No. | Biosensing Application | 3D Printing Method | Statistical Data/Performance | Advantage | Social/Environmental Impact | Reference |
|---|---|---|---|---|---|---|
| Blood Pressure Sensor | ||||||
| 1 | Ferroelectric artificial artery for BP and occlusion monitoring | Electric field-assisted 3D printing | In situ-poled artery with ferroelectric properties; real-time, battery-free BP sensing; thrombosis detection | Tissue-mimicking modulus; self-powered sensing, unlike battery-dependent cuffs | Reduces device replacement waste; improves patient safety through early clot detection | [213] |
| 2 | Wireless pressure sensor in a smart stent | 3D-printed biocompatible polymer stent + MEMS | Pressure sensor integrated into a stent, enabling wireless recording of biological signals | Combines structural implant + sensor; avoids invasive monitoring post-surgery | Enables continuous monitoring for cardiac patients; reduces the need for hospital readmission | [214] |
| 3 | Wearable ring sensor for BP waveform monitoring | 3D printing of ring housing + embedded MEMS | MEMS piezo-resistive sensor in 3D-printed ring; monitors BP waveforms and HRV | Comfortable, long-term use; Better fidelity than cuff-based devices | Promotes at-home monitoring; lowers healthcare system burden | [209] |
| S. No. | Biosensing Application | 3D Printing Method | Statistical Data/Performance | Advantage | Social/Environmental Impact | Reference |
|---|---|---|---|---|---|---|
| STRAIN SENSOR | ||||||
| 1 | Human joint motion monitoring | DIW with AGF/CF in PDMS | GF 8–10; FFT for load distinction | High stability, accurate joint tracking | Non-invasive rehab monitoring | [215] |
| 2 | Antenna-based strain sensing | FDM with Ninjaflex + ECA | Detects strain via antenna signal loss | Wireless, antenna-integrated sensing | Low-cost and scalable with consumer FDM | [216] |
| 3 | Motion and gesture detection | Embedded 3DP (e-3DP) | Reliable under 0–100% strain cycles | Liquid ink encapsulated, robust | Supports prosthetics and human–computer interaction | [78] |
| 4 | Human joint motion tracking | 3D printing of liquid metal in silicone | >375 cycles at 200% strain; near-zero hysteresis | Highly stretchable and durable | Safer for long-term wearable use | [217] |
| 5 | Wearable motion monitoring | Extrusion printing of MWCNT/PDMS | Strain up to 146%; GF = 12.15 | High linearity and stretchability | Promotes next-gen fitness/rehab devices | [97] |
| 6 | General wearable strain sensing | DIW with nanosilica-modified silicone | Tunable rheology; improved printability | Faster, accurate elastomer fabrication | Optimizes material efficiency | [79] |
| 7 | Structural and wearable monitoring | Aerosol Jet Printing (AgNP ink) | Optimized grid design; high precision | High-resolution, miniaturized sensors | Reduced waste via an additive approach | [218] |
| 8 | Wearable home healthcare | AJP + laser sintering on a bandage | Stable over 700 bending cycles | Low-cost, disposable, biocompatible | At-home continuous monitoring | [219] |
| 9 | Skin motion detection | Inkjet printing PEDOT:PSS + AuNP | GF 0.73 ± 0.1; 0–6% strain; ~1 μm thickness | Ultra-thin, epidermal precision | Minimally invasive, reduced material use | [220] |
| 10 | Structural health monitoring | AJP on Buckypaper (CNT) | High conductivity and flexibility | Direct integration in composites | Extends infrastructure lifetime | [221] |
| 11 | Motion detection (array) | DLP with UV-curable MWCNT/elastomer | Linear 0.01–45% strain; GF ≈ 8.94 | Multi-point, flexible, resilient | Supports robotics and wearable analytics | [222] |
| 12 | Robust wearable biomonitoring | FDM sacrificial molds + graphene coating | GF = 10 at 2–10% strain; >75% strain durability | Resistant to solvents and harsh cycles | Sustainable via mold reusability | [223] |
| 13 | Human joint motion detection | Material Jetting (silicone + CF) | High GF; flexible and foldable | Precise drop-on-demand fabrication | Energy efficient, scalable | [224] |
| 14 | Strain + VOC gas sensing | DIW TPU/CB foam | Linear up to ~80% strain; selective VOC response | Dual sensing capability (strain + gas) | Environmental VOC detection + wearable use | [103] |
| 15 | High-precision monitoring | DIW graphene/PDMS composite | Stable GF after 100 cycles | High sensitivity and repeatability | Enables precision diagnostics | [225] |
| 16 | Selective stretch/bend sensing | 3D elastomer molds + agarose ionic gel | GF = 17; up to 500% strain | Biocompatible, high stretch selectivity | Eco-friendly ionic materials | [226] |
| 17 | Strain and pressure sensing | DLP hydrogel (PAAm-PEGDA) | High sensitivity; static and dynamic detection | Capacitive, flexible, multi-sensing | Sustainable hydrogels for wearables | [227] |
| TACTILE SENSOR | ||||||
| 1 | Capacitive touch sensing on curved 3D surfaces | Aerosol jet printing (AJP) of AgNPs ink | Functional sensors on ABS, PC, PVC | Integrates on complex geometries | Expands IoT and robotics interfaces | [228] |
| 2 | Finger motion and pulse monitoring | Customized 3D printing on freeform surfaces | Skin-conforming detection of motion/pulse | Flexible, wearable integration | Enhances personalized health tracking | [229] |
| 3 | Soft pressure sensing (acoustic/pulse) | Inkjet printing of AgNPs on PDMS | Sensitivity: 0.48 kPa−1 | High reproducibility, wearable | Improves low-cost health electronics | [230] |
| 4 | Ultrathin vibration sensing | Direct ink writing (DIW) + chemical reduction | Detects subtle vibrations and weak pulses | Stretchable, ultra-thin electrodes | Wearable for biomedical and robotics | [231] |
| 5 | Strain and humidity sensing | Aerosol jet printing (Pt/AgNP inks, free-standing films) | Highly flexible free-standing structures | Enables multifunctional sensing | Supports sustainable wearable systems | [232] |
| 6 | Wearable tactile sensing (high strain tolerance) | DIW with PDMS/GO nanocomposite | Strain range ~40%, low resistivity | Improved mechanical robustness | Durable and reduces sensor replacement | [233] |
| 7 | Piezo-resistive tactile sensing | FDM with conductive filament | Achieved SINAD = 18 dB | Low-cost, 3D-printed, flexible | Scalable for robotics and prosthetics | [234] |
| 8 | Ionic pressure sensing (ultra-low pressure, pulse) | 3D-printed ordered hierarchical mesh | Sensitivity: 72.86 kPa; Durability: 7300 cycles | Tunable and durable | Real-time health + communication tools | [235] |
| 9 | Dual-mode resistive/capacitive pressure sensing | Extrusion printing of CNT-elastomer | Capacitive: 0.02 kPa, 25 ms; Resistive: 5 Pa, 20 ms | Rapid, multimodal detection | Useful for prosthetics and HMI devices | [236] |
| 10 | Integrated pressure and strain sensing | Coaxial extrusion AM of fibers | Detects shear, twist, bend, and press | Multifunctional e-skin | Human–machine interaction, robotics | [237] |
| 11 | Health monitoring, tissue engineering | Single-component CNT–silicone ink | High conductivity and flexibility | Simplifies fabrication | Reusable, supports medical bioplatforms | [196] |
| 12 | Tactile sensing + energy harvesting | Inkjet + DIW of triboelectric nanogenerator | All-printed TENG, tactile + power gen | Energy self-sufficient | Reduces battery waste in wearables | [238] |
| 13 | Multi-parameter sensing (force, temp, gas) | Mold-based 3D printing with PDMS/graphite | Low-force sensing patches | Low-cost, multi-signal monitoring | Affordable environmental diagnostics | [239] |
| 14 | Self-powered tactile sensing | 3D printing of soft triboelectric materials | Distinct responses to force/frequency | Operates without batteries | Promotes sustainable e-skin devices | [240] |
| 15 | Microforce sensing (µN resolution) | FDM + SLA | Detects micro-Newton forces | High sensitivity, customizable | Useful for biomedical microsurgery | [241] |
| 16 | Real-time wearable monitoring (respiration, pulse) | All-3D-printed hybrid nanocomposite sensors | Monitors multiple signals | Low-cost, biocompatible | Expands access to wearable healthcare | [242] |
| 17 | Breast cancer identification | 3D-printed tactile probe + FBG sensors | Improved force sensitivity, non-invasive | Overcomes the limits of manual palpation | Early cancer screening reduces biopsies | [243] |
| MISCELLANEOUS | ||||||
| 1 | RF electronics and sensors for biomonitoring | Inkjet/3D/4D printing on paper and polymer substrates | Demonstrated scalable RF modules | Low-cost, flexible, system-level integration | Enables affordable, wide-access wearable biomonitoring | [244] |
| 2 | Wearable smart health and food quality sensors | SLA 3D printing + metallization | Good sensitivity, IoT-enabled | Combines SIW and microfluidics, flexible design | Supports IoT in healthcare and food safety | [245] |
| 3 | Oxidative stress monitoring (protein carbonylation) | 3D printing of optical fiber biosensors | Dynamic in vivo protein carbonyl detection | Real-time, non-invasive stress monitoring | Applications in chronic disease, sports, and livestock health | [246] |
| 4 | Wearable biomedical devices and electronic tattoos | Aerosol jet printing (AJP) of silver nanowires | High conductivity, strong adhesion | Ultra-thin, flexible, fast drying | Eco-friendly, reusable e-tattoos for health monitoring | [247] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maji, S.; Kwak, M.; Kumar, R.; Lee, H. 3D Printing Assisted Wearable and Implantable Biosensors. Biosensors 2025, 15, 619. https://doi.org/10.3390/bios15090619
Maji S, Kwak M, Kumar R, Lee H. 3D Printing Assisted Wearable and Implantable Biosensors. Biosensors. 2025; 15(9):619. https://doi.org/10.3390/bios15090619
Chicago/Turabian StyleMaji, Somnath, Myounggyu Kwak, Reetesh Kumar, and Hyungseok Lee. 2025. "3D Printing Assisted Wearable and Implantable Biosensors" Biosensors 15, no. 9: 619. https://doi.org/10.3390/bios15090619
APA StyleMaji, S., Kwak, M., Kumar, R., & Lee, H. (2025). 3D Printing Assisted Wearable and Implantable Biosensors. Biosensors, 15(9), 619. https://doi.org/10.3390/bios15090619







