Recent Advances in Flexible Materials for Wearable Optical Biosensors
Abstract
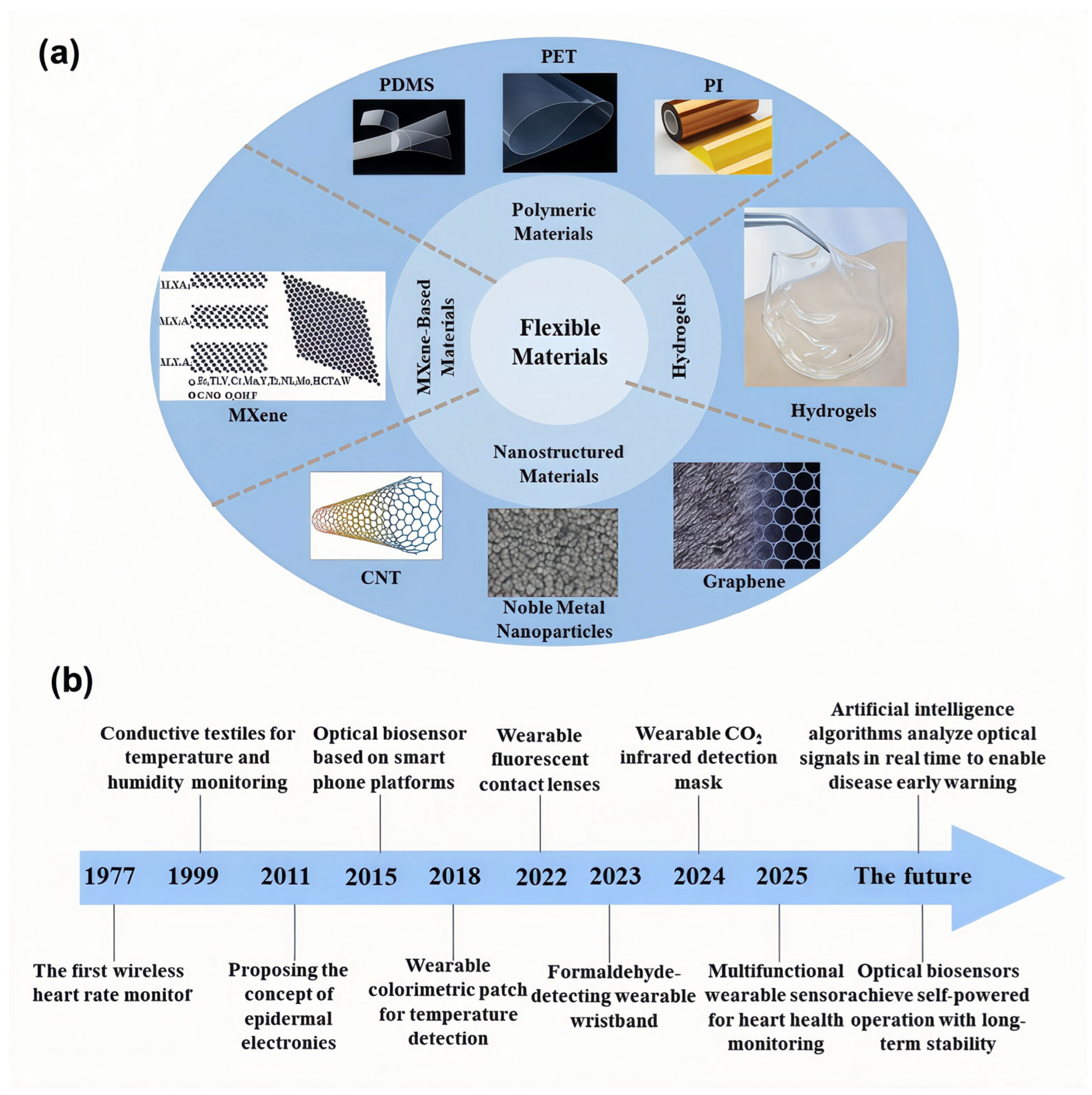
1. Introduction
2. Technological Innovations in Flexible Materials
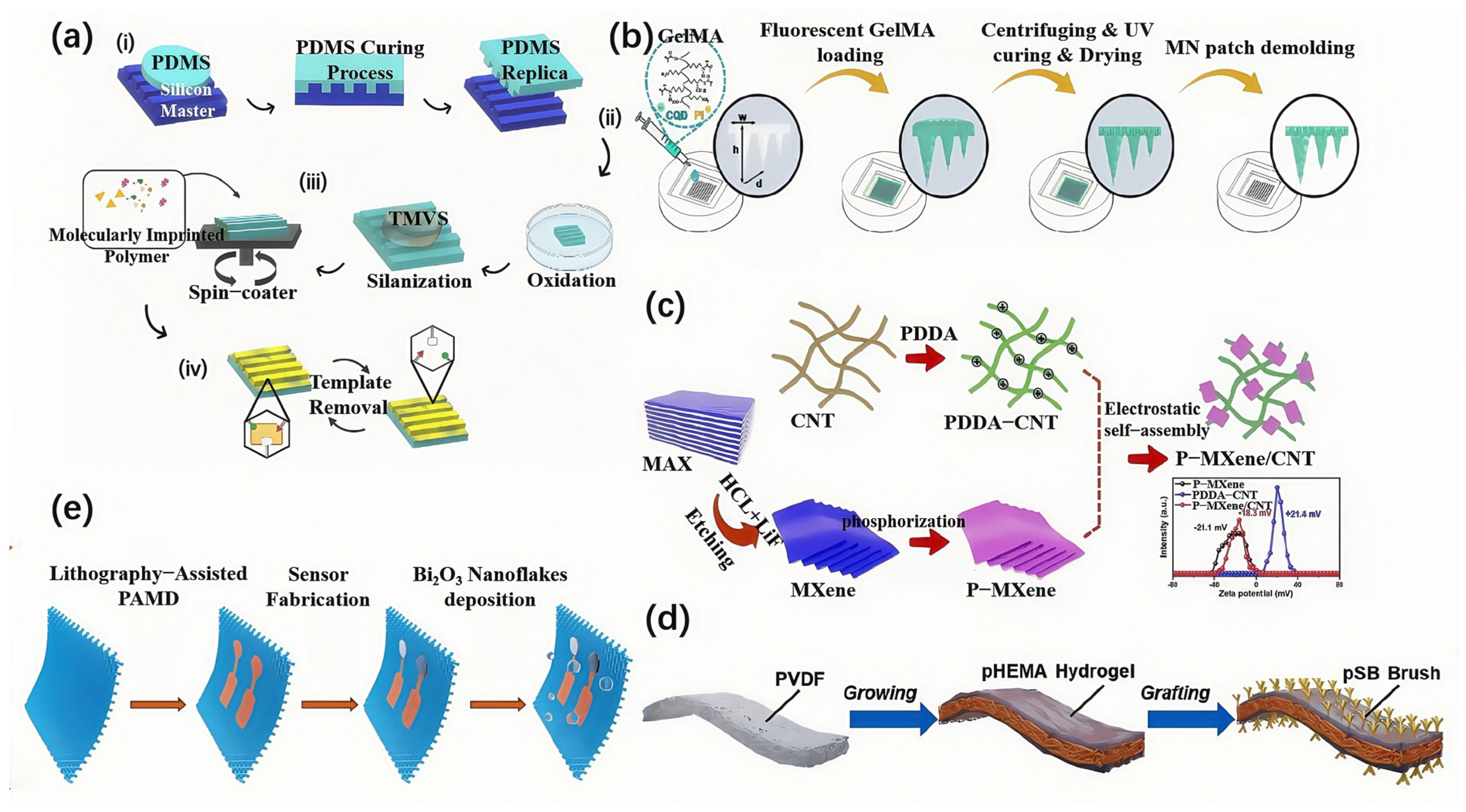
2.1. Polymer Substrates and Their Derivatives
2.2. Nanostructured Materials
2.3. MXene-Based Materials
2.4. Hydrogels and Conductive Composites
2.5. Textile-Based Platforms and Hybrid Composites
2.6. Thin-Film of Inorganic Non-Metallic Material
3. Optical Sensing Mechanisms for Wearable Biosensors
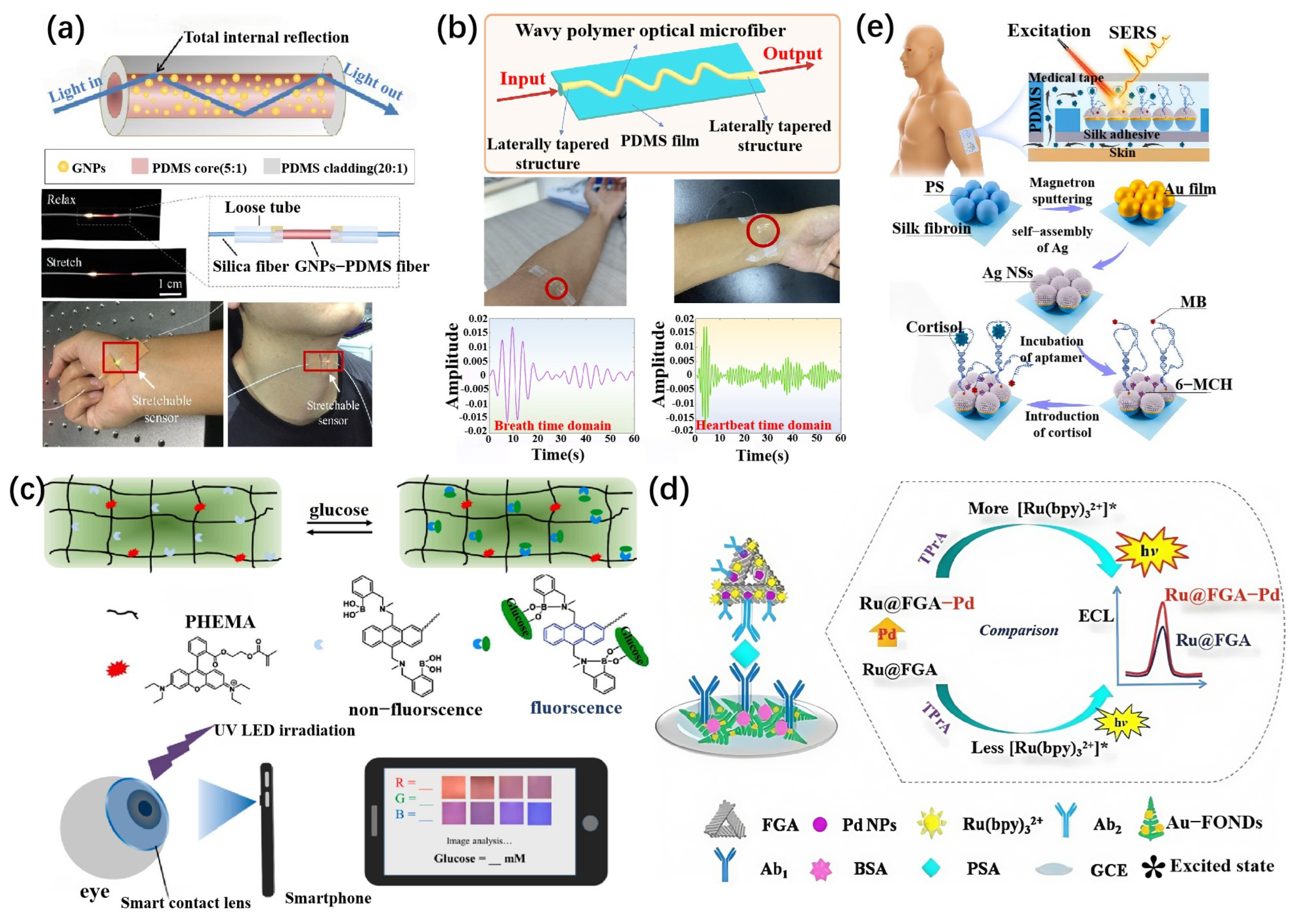
3.1. Principles of Flexible Optical Biosensors and Signal Conversion
3.2. Surface Plasmon Resonance (SPR) and Localized Surface Plasmon Resonance (LSPR)
3.3. Optical Fiber Sensing Mechanism
3.4. Fluorescence Sensing Mechanism
3.5. Chemiluminescence and Electrochemiluminescence
3.6. Surface-Enhanced Raman Spectroscopy (SERS)
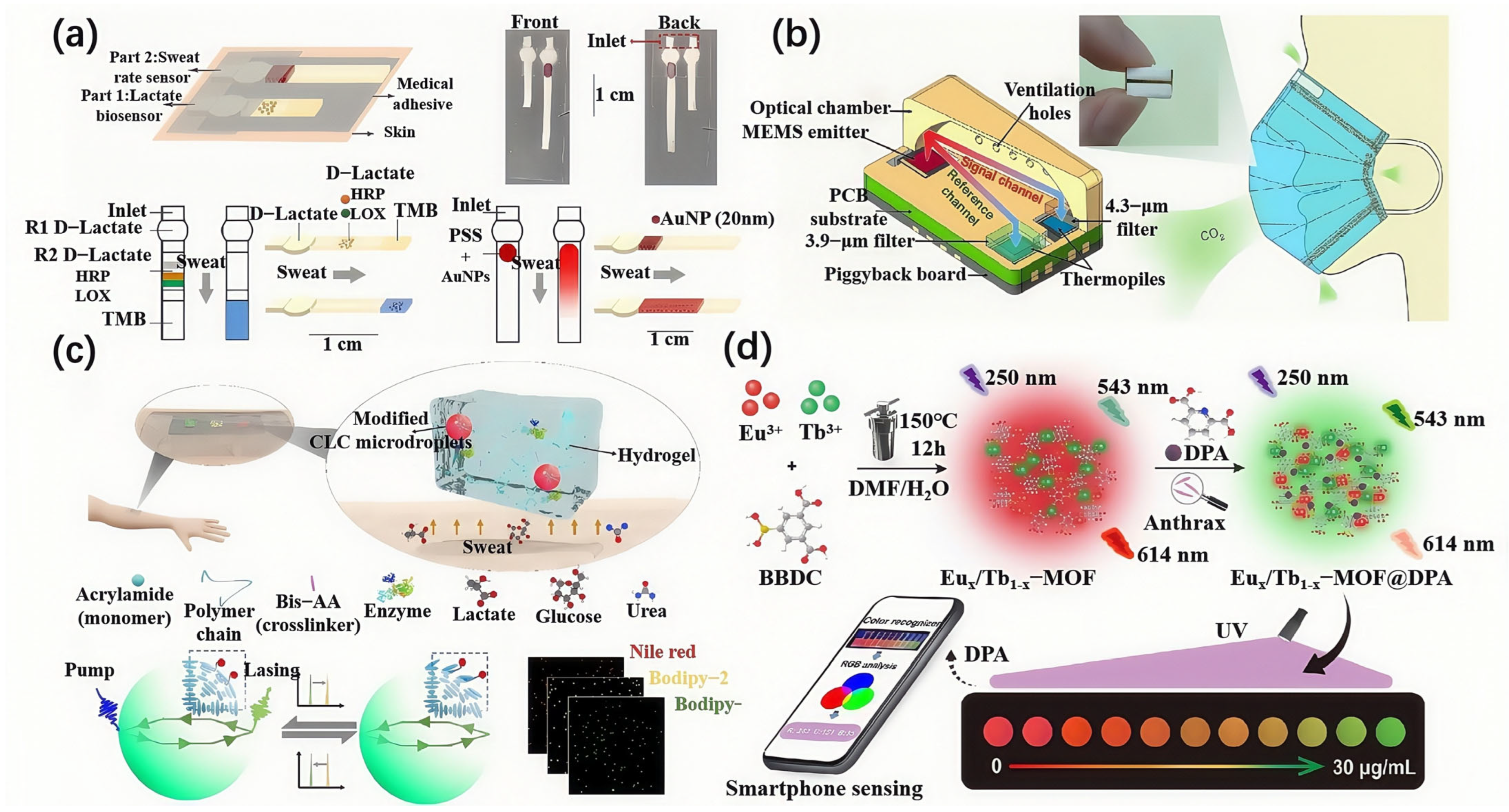
4. Integration of Flexible Materials and Optical Sensing Technologies
4.1. Miniaturization and Integration of Optical Sensors
4.2. Development of Multifunctional Sensors
4.3. Integration of Smart Features and Wireless Transmission Technologies
5. Challenges in the Development of Wearable Optical Biosensors
5.1. Material Stability and Performance Under Environmental Conditions
5.2. Biocompatibility and Long-Term Wearability
5.3. Signal Interference and Sensitivity Optimization
5.4. Cost and Manufacturing Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDMS | Polydimethylsiloxane |
| PI | Polyimide |
| PET | Polyethylene terephthalate |
| CRP | C-reactive protein |
| VSC | Volatile sulfur compounds |
| 0D/1D/2D | Zero/One/Two-dimensional |
| SPR | Surface plasmon resonance |
| LSPR | Localized Surface Plasmon Resonance |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| CPs | Polyimide aerogel-based paper |
| ECL | Electrochemiluminescence |
| CVD | Chemical vapor deposition |
| IFE | Internal filter effect |
| PEG | Polyethylene Glycol |
| PAM | Polyacrylamide, |
| PVA | Polyvinyl alcohol |
| pHEMA | Poly(hydroxyethyl methacrylate) |
| pSB | Poly(sulfobetaine) |
| PVDF | Polyvinylidene fluoride |
| PAHS | Polycyclic aromatic hydrocarbons |
| UV | Ultraviolet |
| GGFF | Graphene-coated glass fiber fabric |
| POF | Polymer optical fibers |
| GNPs | Gold nanoparticles |
| FDM | Fused deposition modeling |
| FBG | Fiber bragg gratings |
| PLA | Polylactic acid |
| HEMA | Hydroxyethyl methacrylate |
| CMC | Carboxymethyl cellulose |
| MOF | Metal-organic framework |
| ROS | Reactive oxygen species |
| PSA | Prostate specific antigen |
| SERS | Surface-enhanced raman spectroscopy |
| HR | Heart rate |
| CMOS | Complementary metal-oxide-semiconductor |
| TMDCs | Transition metal dichalcogenides |
References
- Kozma, P.; Kehl, F.; Ehrentreich-Forster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of integrated optical biosensors for point-of-care applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Fojtů, M.; Latiff, N.M.; Pumera, M. Two-dimensional materials in biomedical, biosensing and sensing applications. Chem. Soc. Rev. 2021, 50, 619–657. [Google Scholar] [CrossRef]
- Mostufa, S.; Rezaei, B.; Ciannella, S.; Yari, P.; Gómez-Pastora, J.; He, R.; Wu, K. Advancements and perspectives in optical biosensors. ACS Omega 2024, 9, 24181–24202. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Yakoh, A.; Jampasa, S.; Chaiyo, S.; Chailapakul, O. Electrochemical and optical biosensors for biological sensing applications. ScienceAsia 2020, 46, 245–253. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Ashraf, G.; Chen, W.; Asif, M.; Aziz, A.; Zhong, Z.T.; Iftikhar, T.; Zhao, Y.D. Topical advancements in electrochemical and optical signal amplification for biomolecules detection: A comparison. Mater. Today Chem. 2022, 26, 101119. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Zhou, Z.; Xiao, J.; Xu, T.; Zhang, X. Epidermal wearable optical sensors for sweat monitoring. Commun. Mater. 2024, 5, 77. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zheng, T.; Wu, H.; Wu, Q.; Wang, Y. Wearable optical fiber sensors in medical monitoring applications: A review. Sensors 2023, 23, 6671. [Google Scholar] [CrossRef]
- Rasheed, S.; Kanwal, T.; Ahmad, N.; Fatima, B.; Najam-ul-Haq, M.; Hussain, D. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends Anal. Chem. 2024, 173, 117640. [Google Scholar] [CrossRef]
- Nichols, J.H. Utilizing point-of-care testing to optimize patient care. eJIFCC 2021, 32, 140–144. [Google Scholar] [PubMed] [PubMed Central]
- Vashist, S. Point-of-care diagnostics: Recent advances and trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef]
- Liu, L.; Dou, Y.; Wang, J.; Zhao, Y.; Kong, W.; Ma, C.; He, D.; Wang, H.; Zhang, H.; Chang, A.; et al. Recent advances in flexible temperature sensors: Materials, mechanism, fabrication, and applications. Adv. Sci. 2024, 11, 202405003. [Google Scholar] [CrossRef]
- Zhu, Y.; Moyle, W.; Hong, M.; Aw, K. From sensors to care: How robotic skin is transforming modern healthcare—A mini review. Sensors 2025, 25, 2895. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ju, H. Label-free optical biosensors based on a planar optical waveguide. Biochip J. 2013, 7, 295–318. [Google Scholar] [CrossRef]
- Khani, S.; Hayati, M. Optical biosensors using plasmonic and photonic crystal band-gap structures for the detection of basal cell cancer. Sci. Rep. 2022, 12, 5246. [Google Scholar] [CrossRef]
- Khani, S.; Danaie, M.; Rezaei, P. Hybrid all-optical infrared metal-insulator-metal plasmonic switch incorporating photonic crystal bandgap structures. Photonics Nanostructures Fundam. Appl. 2020, 40, 100802. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.C. Comprehensive review on two dimensional nanomaterials for optical biosensors: Present progress and outlook. Sustain. Mater. Technol. 2024, 40, e00900. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.C. Development and integration of a hierarchical Pd/WO3 acetone-sensing device for real-time exhaled breath monitoring with disposable face mask. J. Hazard. Mater. 2024, 463, 132872. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Zang, J.; An, Q.; Li, B.; Zhang, Z.; Gao, L.; Xue, C. A novel wearable device integrating ECG and PCG for cardiac health monitoring. Microsyst. Nanoeng. 2025, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Meng, Z.; Zhuang, J.; Xu, X.; Hao, W.; Dou, S.X.; Du, Y. Electronic band engineering in elemental 2D materials. Adv. Mater. Interfaces 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling experimental evidence for graphenelike two-dimensional silicon. Phys. Rev. Lett. 2012, 108, 155501. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Pires, L.; Zamora, A.; Merkoçi, A. Graphene-based biosensors: Going simple. Adv. Mater. 2016, 29, 1604905. [Google Scholar] [CrossRef]
- Agrawal, N.; Saha, C.; Kumar, C.; Singh, R.; Zhang, B.; Jha, R.; Kumar, S. Detection of l-cysteine using silver nanoparticles and graphene oxide immobilized tapered SMS optical fiber structure. IEEE Sens. J. 2020, 20, 11372–11379. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Resende, S.; Fernandes, J.; Sousa, P.C.; Calaza, C.; Frasco, M.F.; Freitas, P.P.; Goreti, F.; Sales, M. Fabrication and sensing properties of a molecularly imprinted polymer on a photonic PDMS substrate for the optical detection of C-reactive protein. Chem. Eng. J. 2024, 485, 149924. [Google Scholar] [CrossRef]
- Li, X.; Luo, C.; Fu, Q.; Zhou, C.; Ruelas, M.; Wang, Y.; He, J.; Wang, Y.; Zhang, Y.S.; Zhou, J. A Transparent, Wearable fluorescent mouthguard for high-sensitive visualization and accurate localization of hidden dental lesion sites. Adv. Mater. 2020, 32, e2000060. [Google Scholar] [CrossRef]
- Tabatabaee, R.S.; Naghdi, T.; Peyravian, M.; Kiani, M.A.; Golmohammadi, H. An invisible dermal nanotattoo-based smart wearable sensor for eDiagnostics of jaundice. ACS Nano 2024, 18, 28012–28025. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, S.; Hao, C.; Ji, T.; Liu, Y.; Wang, Y. Carbon nanotube cross-linked phosphorus-doped MXene for capacitive pressure microsensors. J. Mater. Chem. A 2024, 12, 19891–19898. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Shi, Y.; Yu, X.; Zhang, X.; Ma, X.; Su, J.; Ding, R.; Lin, Y. Epidermal secretion-purified biosensing patch with hydrogel sebum filtering membrane and unidirectional flow microfluidic channels. Biomaterials 2025, 313, 122810. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, K.; Ma, X.; Huang, L.; Hu, X.; Wang, P.; Zhang, Y.; Chen, F.; Huang, M.; Wu, J.; et al. Washable textile biosensors enabled by nanostructured oxides with fast ion diffusion. Device 2024, 2, 100503. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Ramanathan, S.; Gopinath, S.C.B. Revolutionizing biosensing through cutting-edge nanomaterials: An in-depth exploration of recent technological advances. Nano-Struct. Nano-Objects 2024, 38, 101128. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Sargazi, S.; Shojaei, S.; Farasati Far, B.; Mirinejad, S.; Cordani, M.; Khosravi, A.; Zarrabi, A.; Ghavami, S. Biotin-functionalized nanoparticles: An overview of recent trends in cancer detection. Nanoscale 2024, 16, 12750–12792. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent Advances in Two-dimensional materials-based kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2022, 22, 1069–1080. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, A.; Saji, J.; Umapathi, A.; Kumar, S.; Daima, H.K. Smart nanomaterials for cancer diagnosis and treatment. Nano Converg. 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Zeininger, L.; Weyandt, E.; Savagatrup, S.; Harvey, K.S.; Zhang, Q.; Zhao, Y.; Swager, T.M. Waveguide-based chemo- and biosensors: Complex emulsions for the detection of caffeine and proteins. Lab Chip 2019, 19, 1327–1331. [Google Scholar] [CrossRef]
- Walter, J.-G.; Eilers, A.; Alwis, L.S.; Roth, B.W.; Bremer, K. SPR biosensor based on polymer multi-mode optical waveguide and nanoparticle signal enhancement. Sensors 2020, 20, 2889. [Google Scholar] [CrossRef]
- Yadav, P.; Sahay, K.; Srivastava, M.; Verma, A.; Yadav, B.C. Emerging trends in self-healable nanomaterials for triboelectric nanogenerators: A comprehensive review and roadmap. Front. Energy 2023, 17, 727–750. [Google Scholar] [CrossRef]
- Jia, T.; Fan, Z.; Zheng, S.; Zhou, H.; Chen, H.; Ma, N.; Liu, C. MWCNTs/polyimide multilayered aerogel-based paper enabling high-temperature-resistant and flexible sensor. Chem. Eng. J. 2024, 492, 152230. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Xing, F. Graphene optical biosensors. Int. J. Mol. Sci. 2019, 20, 2461. [Google Scholar] [CrossRef]
- Ban, D.K.; Bandaru, P.R. Graphene and two-dimensional materials for biomolecule sensing. Annu. Rev. Biophys. 2023, 52, 487–507. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Lambert, A.S.; Morales-Luna, G.; Pisano, E.; Aguilar-Hernandez, I.; Mahlknecht, J.; Cheng, Q.; Ornelas-Soto, N. Development of a surface plasmon resonance based immunosensor for diclofenac quantification in water. Chemosphere 2023, 336, 139156. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xue, B.; Qi, P.; Chen, G.Y.; Zhou, X. A rapid and sensitive fluorescence biosensor for Hg2+ detection in environmental samples. Sens. Actuators Rep. 2022, 4, 100101. [Google Scholar] [CrossRef]
- Gonçalves, M.; Melikyan, A.; Minassian, H.; Makaryan, T.; Petrosyan, P.; Sargsian, T. Interband, surface plasmon and fano resonances in titanium carbide (MXene) nanoparticles in the visible to infrared range. Photonics 2021, 8, 36. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, S.; Wang, J.; Ding, Y.; Ding, C. Ultrasensitive ratiometric electrochemiluminescence sensor with an efficient antifouling and antibacterial interface of PSBMA@SiO2-MXene for oxytetracycline trace detection in the marine environment. Anal. Chem. 2023, 95, 16327–16334. [Google Scholar] [CrossRef]
- Huang, W.; Hu, G.-B.; Yao, L.-Y.; Yang, Y.; Liang, W.-B.; Yuan, R.; Xiao, D.-R. Matrix coordination-induced electrochemiluminescence enhancement of tetraphenylethylene-based hafnium metal-organic framework: An electrochemiluminescence chromophore for ultrasensitive electrochemiluminescence sensor construction. Anal. Chem. 2020, 92, 3380–3387. [Google Scholar] [CrossRef]
- Shi, Y.E.; Han, F.; Xie, L.; Zhang, C.; Li, T.; Wang, H.; Lai, W.F.; Luo, S.; Wei, W.; Wang, Z.; et al. A MXene of type Ti3C2Tx functionalized with copper nanoclusters for the fluorometric determination of glutathione. Microchim. Acta 2019, 187, 38. [Google Scholar] [CrossRef]
- Anasori, B.; Halim, J.; Lu, J.; Voigt, C.A.; Hultman, L.; Barsoum, M.W. Mo2TiAlC2: A new ordered layered ternary carbide. Scr. Mater. 2015, 101, 5–7. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and their applications in wearable sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. MXenes-based fiber-optic SPR sensor for colorectal cancer diagnosis. IEEE Sens. J. 2022, 22, 6661–6668. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, A.; Baloria, V.; Singh, P.; Gupta, G. Ultrahigh sensitive NO sensor based on WO3 film with ppb-level sensitivity. Ceram. Int. 2023, 49, 7853–7860. [Google Scholar] [CrossRef]
- Qing, X.; Liu, Z.; Vananroye, A.; Franceschini, F.; Bouropoulos, N.; Katsaounis, A.; Taurino, I.; Fardim, P. Self-healing and transparent ionic conductive PVA/pullulan/borax hydrogels with multi-sensing capabilities for wearable sensors. Int. J. Biol. Macromol. 2025, 284, 137841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Wang, P.; Yu, Y.; Zhou, M.; Xu, B.; Cui, L.; Wang, Q. Stretchable, transparent, self-adhesive, anti-freezing and ionic conductive nanocomposite hydrogels for flexible strain sensors. Eur. Polym. J. 2023, 186, 111824. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Liang, J.; Zhao, X.; Li, B.; Zang, J.; Gao, L.; Zhang, Z.; Xue, C. Ti3C2Tx-MXene/PET textile-based flexible pressure sensor for wearable pulse monitoring. J. Mater. Chem. C 2023, 11, 15638–15648. [Google Scholar] [CrossRef]
- Zhang, M.; Su, H.; Zhang, C.; Sun, Z.; Jiang, Z. Smart optical fiber fabric based on side-emitting and side-coupling for pulse and blood oxygen measurement. Text. Res. J. 2023, 93, 3382–3392. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Zhao, T.; Chen, K.; Zhang, T. Highly sensitive skin-like wearable optical sensor for human physiological signals monitoring. Opt. Fiber Technol. 2024, 82, 103652. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Qiao, S.; Zou, Y.; Danto, S.; Richardson, K.; Musgraves, J.D.; Lu, N.; Hu, J. Integrated flexible chalcogenide glass photonic devices. Nat. Photonics 2014, 8, 643–649. [Google Scholar] [CrossRef]
- Notaros, M.; Dyer, T.; Garcia Coleto, A.; Hattori, A.; Fealey, K.; Kruger, S.; Notaros, J. Mechanically-flexible wafer-scale integrated-photonics fabrication platform. Sci. Rep. 2024, 14, 10623. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y.; Dai, X.; Li, S.; Li, B.; Zhang, X. Flexible pressure sensor based on Pt/PI network with high sensitivity and high thermal resistance. Chem. Eng. J. 2024, 494, 152996. [Google Scholar] [CrossRef]
- Jha, R.; Mishra, P.; Kumar, S. Advancements in optical fiber-based wearable sensors for smart health monitoring. Biosens. Bioelectron. 2024, 254, 116232. [Google Scholar] [CrossRef]
- Sagar Shrikrishna, N.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Zong, R.; Pan, L.; Li, X.; Yu, X.; Yang, C.; Kong, L.; Dai, Q. Stretchable and highly sensitive optical strain sensors for human-activity monitoring and healthcare. ACS Appl. Mater. Interfaces 2019, 11, 33589–33598. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Chatterjee, S. 2—The origin of photoplethysmography. In Photoplethysmography; Allen, J., Kyriacou, P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 17–43. [Google Scholar]
- Liu, C.; Hu, F.; Yang, W.; Xu, J.; Chen, Y. A critical review of advances in surface plasmon resonance imaging sensitivity. TrAC Trends Anal. Chem. 2017, 97, 354–362. [Google Scholar] [CrossRef]
- Capelli, D.; Scognamiglio, V.; Montanari, R. Surface plasmon resonance technology: Recent advances, applications and experimental cases. TrAC Trends Anal. Chem. 2023, 163, 117079. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Ma, L.; Wang, Q.; Wang, H.; Leal-Junior, A.; Li, X.; Marques, C.; Min, R. Optical microfiber intelligent sensor: Wearable cardiorespiratory and behavior monitoring with a flexible wave-shaped polymer optical microfiber. ACS Appl. Mater. Interfaces 2024, 16, 8333–8345. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Zhang, Y.; Fan, D.; Pang, X.; Wei, Q.; Du, B. 3D nanostructured palladium-functionalized graphene-aerogel-supported Fe3O4 for enhanced Ru(bpy)32+-based electrochemiluminescent immunosensing of prostate specific antigen. ACS Appl. Mater. Interfaces 2017, 9, 35260–35267. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, K.; Wei, J.; Xu, Z.; Yang, K.; Wu, L.; Zong, S.; Wang, Z. Wearable microfluidic SERS patch based on silk fibroin for the non-invasive monitoring of sweat cortisol and pH. Sens. Actuators B Chem. 2025, 427, 137152. [Google Scholar] [CrossRef]
- El-Helou, A.J.; Liu, Y.; Chen, C.; Wang, F.; Altug, H.; Reece, P.J.; Zhu, Y. Optical metasurfaces for the next-generation biosensing and bioimaging. Laser Photonics Rev. 2025, 19, 2401715. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Xu, J.; Dai, L.H. Flexible microwave plasmonic sensor for noninvasive measurement of blood glucose. In Proceedings of the 2021 13th Global Symposium on Millimeter-Waves & Terahertz (GSMM), Nanjing, China, 23–26 May 2021; pp. 1–3. [Google Scholar] [CrossRef]
- Jiao, L.; Zhong, N.; Zhao, X.; Ma, S.; Fu, X.; Dong, D. Recent advances in fiber-optic evanescent wave sensors for monitoring organic and inorganic pollutants in water. TrAC Trends Anal. Chem. 2020, 127, 115892. [Google Scholar] [CrossRef]
- Thomas, N.; Singh, V.; Kuss, S. Optical fibers in analytical electrochemistry: Recent developments in probe design and applications. TrAC Trends Anal. Chem. 2021, 136, 116196. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, J.; Sharma, I. Fiber optic evanescent wave absorption-based sensors: A detailed review of advancements in the last decade (2007–18). Optik 2019, 183, 1008–1025. [Google Scholar] [CrossRef]
- Cheng-Yu, H.; Ahmed Abro, Z.; Yi-Fan, Z.; Ahmed Lakho, R. An FBG-based smart wearable ring fabricated using FDM for monitoring body joint motion. J. Ind. Text. 2019, 50, 1660–1673. [Google Scholar] [CrossRef]
- Tamura, T.; Hamachi, I. Recent progress in design of protein-based fluorescent biosensors and their cellular applications. ACS Chem. Biol. 2014, 9, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, A.; Campbell, R.E. Designs and applications of fluorescent protein-based biosensors. Curr. Opin. Chem. Biol. 2010, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Gong, M.; Zhan, Y.; Yu, Z.; Shen, C.; Zhang, Y.; Yu, L.; Chen, Z. Integrated photo-inspired antibacterial polyvinyl alcohol/carboxymethyl cellulose hydrogel dressings for pH real-time monitoring and accelerated wound healing. Int. J. Biol. Macromol. 2023, 238, 124123. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Liang, H.; Zhou, H.; Wang, X.; Heng, X.; Zhang, Z.; Gan, J.; Yang, Z. Stretchable and strain-decoupled fluorescent optical fiber sensor for body temperature and movement monitoring. ACS Photonics 2022, 9, 1415–1424. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Electro-chemiluminescent biosensing. Anal. Bioanal. Chem. 2007, 390, 155–168. [Google Scholar] [CrossRef]
- Yeh, H.-W.; Ai, H.-W. Development and applications of bioluminescent and chemiluminescent reporters and biosensors. Annu. Rev. Anal. Chem. 2019, 12, 129–150. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; Zhao, J.; Li, H.; Xu, J.; Gao, Z.; Ding, C.; Song, Y.Y. A “test-to-treat” pad for real-time visual monitoring of bacterial infection and on-site performing smart therapy strategies. ACS Nano 2023, 17, 13296–13309. [Google Scholar] [CrossRef]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced raman spectroscopy–based biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable plasmonic paper–based microfluidics for continuous sweat analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef] [PubMed]
- Lv, E.; Wang, T.; Yue, X.; Wang, H.; Zeng, J.; Shu, X.; Wang, J. Wearable SERS sensor based on bionic sea urchin-cavity structure for dual in-situ detection of metabolites and VOCs gas. Chem. Eng. J. 2024, 499, 156020. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Fang, M.; Li, H.; Xie, X.; Wang, H.; Jiang, Y.; Li, T.; Zhang, B.; Jiang, X.; Cao, Y.; Zhang, R.; et al. Imaging intracellular metabolite and protein changes in live mammalian cells with bright fluorescent RNA-based genetically encoded sensors. Biosens. Bioelectron. 2023, 235, 115411. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jin, L.; Shi, G.; Xu, Z.; Guo, Y.; Yang, B.; Yang, Y.; Wu, J.; Sun, D.; Ma, J. Flexible wearable optical microfiber sensor for identifying bending direction and body temperature. Opt. Laser Technol. 2024, 179, 111281. [Google Scholar] [CrossRef]
- Fereja, T.; Hymete, A.; Gunasekaran, T. A recent review on chemiluminescence reaction, principle and application on pharmaceutical analysis. ISRN Spectrosc. 2013, 2013, 230858. [Google Scholar] [CrossRef]
- Wu, R.; Yao, Z.; Chen, Z.; Ge, X.; Su, L.; Wang, S.; Wu, Y.; Song, J. Ultrasound-activated NIR chemiluminescence for deep tissue and tumor foci imaging. Anal. Chem. 2023, 95, 11219–11226. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L. Trends and advances in wearable plasmonic sensors utilizing surface-enhanced raman spectroscopy (SERS): A comprehensive review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Zhao, Z.; Lin, Y. Printable photonic materials and devices for smart healthcare. Adv. Mater. 2025. early view. [Google Scholar] [CrossRef]
- Vaquer, A.; Baron, E.; de la Rica, R. Wearable analytical platform with enzyme-modulated dynamic range for the simultaneous colorimetric detection of sweat volume and sweat biomarkers. ACS Sens. 2021, 6, 130–136. [Google Scholar] [CrossRef]
- Inamori, G.; Kamoto, U.; Nakamura, F.; Isoda, Y.; Uozumi, A.; Matsuda, R.; Shimamura, M.; Okubo, Y.; Ito, S.; Ota, H. Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals. Sci. Adv. 2021, 7, eabe3793. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Wang, Y.; Zhou, H.; Lu, Z.; Li, T. Ultra-compact dual-channel integrated CO2 infrared gas sensor. Microsyst. Nanoeng. 2024, 10, 151. [Google Scholar] [CrossRef]
- Nie, N.; Gong, X.; Gong, C.; Qiao, Z.; Wang, Z.; Fang, G.; Chen, Y.C. A wearable thin-film hydrogel laser for functional sensing on skin. Anal. Chem. 2024, 96, 9159–9166. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Wu, Y.; Wang, S.; Jiang, X.; Zhang, Z.; Li, S. Smartphone-integrated ratiometric fluorescence sensing platform based on bimetallic metal-organic framework nanowires for anthrax biomarker detection. Microchim. Acta 2023, 190, 484. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-ear SpO2: A tool for wearable, unobtrusive monitoring of core blood oxygen saturation. Sensors 2020, 20, 4879. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Huang, C.; Zhao, M.; Cheng, Y.; Abu Jawdeh, E.G.; Bada, H.S.; Chen, L.; Yu, G. Simultaneous measurements of tissue blood flow and oxygenation using a wearable fiber-free optical sensor. J. Biomed. Opt. 2021, 26, 012705. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Ma, J.; Xu, M. Multimodal soft sensor integrated with hydrogel-optoelectronic for wrist motion monitoring. Measurement 2025, 239, 115486. [Google Scholar] [CrossRef]
- Guntner, A.T.; Schenk, F.M. Environmental formaldehyde sensing at room temperature by smartphone-assisted and wearable plasmonic nanohybrids. Nanoscale 2023, 15, 3967–3977. [Google Scholar] [CrossRef]
- Dong, W.; Sheng, K.; Huang, B.; Xiong, K.; Liu, K.; Cheng, X. Stretchable self-powered TENG sensor array for human-robot interaction based on conductive ionic gels and LSTM neural network. IEEE Sens. J. 2024, 24, 37962–37969. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, Y.; Yang, R.; Zhang, G. 2D-materials-based wearable biosensor systems. Biosensors 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Chen, X.; Chen, X.; Peng, S.; Song, M. Advancements in MXene composite materials for wearable sensors: A review. Sensors 2024, 24, 4092. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, D.; Li, B.; Zhao, W.; Xie, D.; Mei, Y.; Liu, P. Ultra-Wide Range, High sensitivity piezoresistive sensor based on triple periodic minimum surface construction. Small 2023, 19, e2301378. [Google Scholar] [CrossRef]
- Bi, S.; Song, Y.; Hou, G.; Li, H.; Yang, N.; Liu, Z. Design and preparation of flexible graphene/nonwoven composites with simultaneous broadband absorption and stable properties. Nanomaterials 2023, 13, 634. [Google Scholar] [CrossRef]
- Focsan, M.; Craciun, A.M.; Potara, M.; Leordean, C.; Vulpoi, A.; Maniu, D.; Astilean, S. Flexible and tunable 3D gold nanocups platform as plasmonic biosensor for specific dual LSPR-SERS immuno-detection. Sci. Rep. 2017, 7, 14240. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Lu, D.; Shao, C.; Li, X.; Liu, Y. Highly electron-depleted ZnO/ZnFe2O4/Au hollow meshes as an advanced material for gas sensing application. Sens. Actuators B Chem. 2019, 297, 126769. [Google Scholar] [CrossRef]
- Xu, S.; Qian, W.; Zhang, D.; Zhao, X.; Zhang, X.; Li, C.; Bowen, C.R.; Yang, Y. A coupled photo-piezo-catalytic effect in a BST-PDMS porous foam for enhanced dye wastewater degradation. Nano Energy 2020, 77, 105305. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kang, J.; Song, S.; Lee, K.; Hwang, S.-W.; Ko, S.H.; Jeon, H.; Han, J.-H.; Lee, W. An ultrathin organic–inorganic integrated device for optical biomarker monitoring. Nat. Electron. 2024, 7, 914–923. [Google Scholar] [CrossRef]
- Asgari, S.; Sun, L.; Lin, J.; Weng, Z.; Wu, G.; Zhang, Y.; Lin, M. Nanofibrillar cellulose/Au@Ag nanoparticle nanocomposite as a SERS substrate for detection of paraquat and thiram in lettuce. Microchim. Acta 2020, 187, 390. [Google Scholar] [CrossRef] [PubMed]
- Turasan, H.; Cakmak, M.; Kokini, J. Fabrication of zein-based electrospun nanofiber decorated with gold nanoparticles as a SERS platform. J. Mater. Sci. 2019, 54, 8872–8891. [Google Scholar] [CrossRef]
- Ali, S.M.; Noghanian, S.; Khan, Z.U.; Alzahrani, S.; Alharbi, S.; Alhartomi, M.; Alsulami, R. Wearable and flexible sensor devices: Recent advances in designs, fabrication methods, and applications. Sensors 2025, 25, 1377. [Google Scholar] [CrossRef]
- Bu, J.; Song, J. Breathable and durable tactile e-skin based on s-shaped nanofiber networks by patterned additive manufacturing. ACS Appl. Nano Mater. 2024, 7, 7154–7161. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, T.; Hao, M.; Hu, X.; Chen, Z.; Yang, B. Fabrication of breathable superhydrophobic composite fabric with moisture permeability and weak-electric-field bactericidal ability designed for protective clothing applications. Mater. Des. 2024, 237, 112612. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.T.; Zhang, X.; Pan, J.; Thean, A.V. Hybrid integration of wearable devices for physiological monitoring. Chem. Rev. 2024, 124, 10386–10434. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Z.; Jiang, C.; Zhang, L.; Tong, L. A multifunctional skin-like wearable optical sensor based on an optical micro-/nanofibre. Nanoscale 2020, 12, 17538–17544. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef]
- Liu, G.; Mu, Z.; Guo, J.; Shan, K.; Shang, X.; Yu, J.; Liang, X. Surface-enhanced raman scattering as a potential strategy for wearable flexible sensing and point-of-care testing non-invasive medical diagnosis. Front. Chem. 2022, 10, 1060322. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart gas sensors: Recent developments and future prospective. Nano-Micro Lett. 2024, 17, 54. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, G.; Xue, S.; Liu, S.; Ye, Q.; Zhou, F.; Liu, W. Laser manufactured-liquid metal nanodroplets intercalated Mxene as oil-based lubricant additives for reducing friction and wear. J. Mater. Sci. Technol. 2024, 187, 169–176. [Google Scholar] [CrossRef]
- Dutta, V.; Verma, R.; Gopalkrishnan, C.; Yuan, M.-H.; Batoo, K.M.; Jayavel, R.; Chauhan, A.; Lin, K.-Y.A.; Balasubramani, R.; Ghotekar, S. Bio-inspired synthesis of carbon-based nanomaterials and their potential environmental applications: A state-of-the-art review. Inorganics 2022, 10, 169. [Google Scholar] [CrossRef]
- Phung, T.H.; Gafurov, A.N.; Kim, I.; Kim, S.Y.; Kim, K.M.; Lee, T.M. IoT device fabrication using roll-to-roll printing process. Sci. Rep. 2021, 11, 19982. [Google Scholar] [CrossRef]
- Han, Y.; Cui, Y.; Liu, X.; Wang, Y. A review of manufacturing methods for flexible devices and energy storage devices. Biosensors 2023, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, H.; Chen, Z.; Hua, Q.; Shen, G.; Jiang, K. Revolutionizing wearable technology: Advanced fabrication techniques for body-conformable electronics. npj Flex. Electron. 2024, 8, 83. [Google Scholar] [CrossRef]
- Yang, S.; Ding, H.; Tan, J.; Zhang, Y.; Guo, Y.; Kang, R.; Zhu, X.; Wang, H.; Baughman, R.; Yin, Z.; et al. Multifaceted mechanical responsive metamaterials: Mechanisms, fabrications, and applications. Innovation 2025, 101070, In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Zhu, T.; Kang, Z.; Zeng, X.; Zhou, B.; Zhou, Y.; Mu, J.; Yin, Z. Strategies for achieving high-performance photomultiplication-type organic photodetectors and their promising applications. Nano Energy 2024, 132, 110399. [Google Scholar] [CrossRef]
| Material Category | Flexibility | Thermal Stability | Electrical Conductivity | Biocompatibility | Fabrication Process Specialty | Ref. | |
| Substrate Materials | PDMS | Excellent stretchable | Low (<150 °C) | Insulating (needs composites) | Excellent | Replica molding/soft lithography; microfluidics integration | [28,31,32] |
| Advantages: Low processing cost; Simple molding process; Optically transparent. Disadvantages: Low thermal stability; Hydrophobic surface. | |||||||
| PI | Moderate | High (>300 °C) | Insulating | Good, slightly brittle | Spin coating; photolithography; thin-film metallization | [29,64] | |
| Advantages: Good electrical insulation; Radiation resistance; High chemical stability. Disadvantages: Yellowish color, poor transparency; High brittleness; Prone to cracking under large deformation. | |||||||
| PET | Good, bendable, non-stretchable | Moderate (~150 °C) | Insulating | Moderate, needs surface treatment | Roll-to-roll printing; Inkjet/screen/gravure printing | [30,59] | |
| Advantages: High transparency; Low cost; Smooth; Mature roll-to-roll processing. Disadvantages: Poor biodegradability; Environmentally constrained; Polar surface, requires modification. | |||||||
| Hydrogels | Highly elastic, soft | Poor (temp sensitive) | Ionic conduction | Excellent, degradable | Photo/covalent/ionic crosslinking; 3D printing; dopant/biopolymer integration | [53,54,55] | |
| Advantages: Tissue-like softness; High water content; Conformal adhesion; Ionic conduction; Transparency. Disadvantages: Pure hydrogel has low ionic conductivity; Dehydration/freeze sensitivity. | |||||||
| Textiles | High, conformable | Variable (cotton < 200 °C; aramid > 400 °C) | Needs conductive yarns/coatings or ionic conduction | Good, breathable | Screen printing; embroidery/knitting of conductive yarns | [36,59,60] | |
| Advantages: Breathable; Conformable; Scalable; Hierarchical porosity aids sweat sampling. Disadvantages: Surface roughness/variability; Patterning challenges; Wash durability issues. | |||||||
| Functional Materials | Noble Metal Nanoparticles | Achieved via Compliant substrates/nano-meshes, particles themselves rigid | Good for Au; Ag less stable in sulfur/halide environments | Metallic (high) | Au generally good, Ag dose/size dependent-needs passivation | Nanoimprint/e-beam; transfer printing to elastomers/textiles | [39,40] |
| Advantages: High chemical stability; Surface plasmon effect. Disadvantages: High cost; Nanoparticles prone to agglomeration; High process requirements for bonding with flexible substrates. | |||||||
| Carbon-based Nanomaterials | Excellent | High in inert; oxidize > ~400–500 °C | High | Generally good after functionalization, dose/aggregation dependent | CVD growth; screen/inkjet/spray printing; laser reduction of GO | [41,42,43] | |
| Advantages: Large specific surface area; Lower cost. Disadvantages: Prone to stacking and agglomeration; Weak interfacial bonding with polymer substrates; High-cost. | |||||||
| MXene | Excellent as few-layer films/papers and coatings | Moderate; oxidation risk in high temperature | High | Promising but formulation/termination dependent | Selective etch of MAX; intercalation and surface functionalization | [48,49,50] | |
| Advantages: Rich surface functional groups; Excellent electrochemical performance; High specific capacitance. Disadvantages: Ambient oxidation; Requires encapsulation for long-term stability. | |||||||
| Thin-film of Inorganic Non-metallic Material | Good when t ≲ 100–500 nm and placed near neutral plane, small bending radius achievable | High for many oxides/chalcogenides (often >300 °C) | Semiconducting or insulating (tunable by doping/phase) | Generally good for oxides, composition-dependent | Lift-off/transfer to elastomers; CVD/ALD/sol-gel | [62,63] | |
| Advantages: Maintains superior optical/electronic properties; Flexible at nanoscale; Low optical. Disadvantages: Limited strain; Complex/expensive processing; Passivation often required. | |||||||
| Biosensor | Sample Requirements | Detection Range (M) | Sensitivity | Cycling Stability | Application | Ref. |
| SPR | Low sample volume; Label-free; Real-time detection | 10−12–10−6 | High Sensitivity, 103–105 RIU−1 | High (>50 cycles with regeneration) | Biomarker detection (proteins, DNA, small molecules); Clinical diagnostics | [67,75,90] |
| Optical Fiber Sensors | Low sample volume; Label-free; Real-time detection | 10−9–10−5 | Moderate to high Varies by Principle, ~10−8 M LOD | High (>50 cycles, depending on coating) | In vivo monitoring; Wearable biosensing; Environmental detection | [71,79,91] |
| Fluorescence Sensors | Low sample volume; Require fluorescent labeling; Real-time possible | 10−9–10−3 | High Sensitivity, ~10−7 M LOD | Moderate (signal drift after 5–10 cycles) | Enzyme assays; Immunoassays; Intracellular imaging | [23,82,83] |
| Chemiluminescence/ Electrochemiluminescence Sensors | Low sample volume; often label-free; Real-time detection not available | 10−12–10−9 | ~10−12 M LOD (ECL highly sensitive) | High (>100 cycles with stable electrode) | Clinical diagnostics (cardiac markers, cancer biomarkers, nucleic acids) | [86,92,93,94] |
| SERS | Low sample volume; Label-free; Real-time detection | 10−12–10−9 | Sensitivity single-molecule level (106–108 enhancement) | Moderate (substrate degradation after ~20 cycles) | Ultrasensitive detection of pathogens, DNA, proteins; Point-of-care diagnostics | [73,88,89,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Yang, K.; Wang, M.; Hou, W.; Ren, Q. Recent Advances in Flexible Materials for Wearable Optical Biosensors. Biosensors 2025, 15, 611. https://doi.org/10.3390/bios15090611
Xie L, Yang K, Wang M, Hou W, Ren Q. Recent Advances in Flexible Materials for Wearable Optical Biosensors. Biosensors. 2025; 15(9):611. https://doi.org/10.3390/bios15090611
Chicago/Turabian StyleXie, Linyan, Kai Yang, Mengfei Wang, Wenli Hou, and Qiongqiong Ren. 2025. "Recent Advances in Flexible Materials for Wearable Optical Biosensors" Biosensors 15, no. 9: 611. https://doi.org/10.3390/bios15090611
APA StyleXie, L., Yang, K., Wang, M., Hou, W., & Ren, Q. (2025). Recent Advances in Flexible Materials for Wearable Optical Biosensors. Biosensors, 15(9), 611. https://doi.org/10.3390/bios15090611





