Metal–Phenolic Networks for Sensing Applications
Abstract
1. Introduction
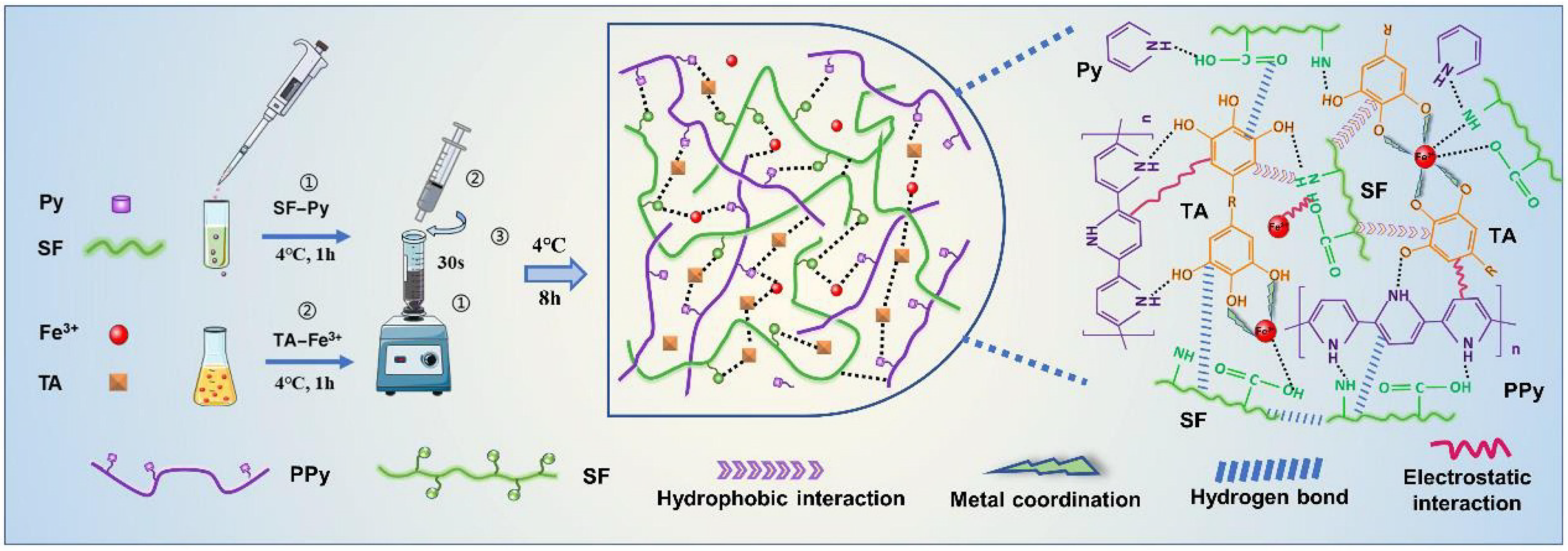
2. Synthesis and Advantages of MPNs
3. MPNs-Based Sensing Applications
3.1. Sensing of Small Molecules and Biological Species
3.1.1. Optical Sensing
3.1.2. Electrochemical Sensing
3.2. Immunoassays
3.2.1. Optical Immunoassays
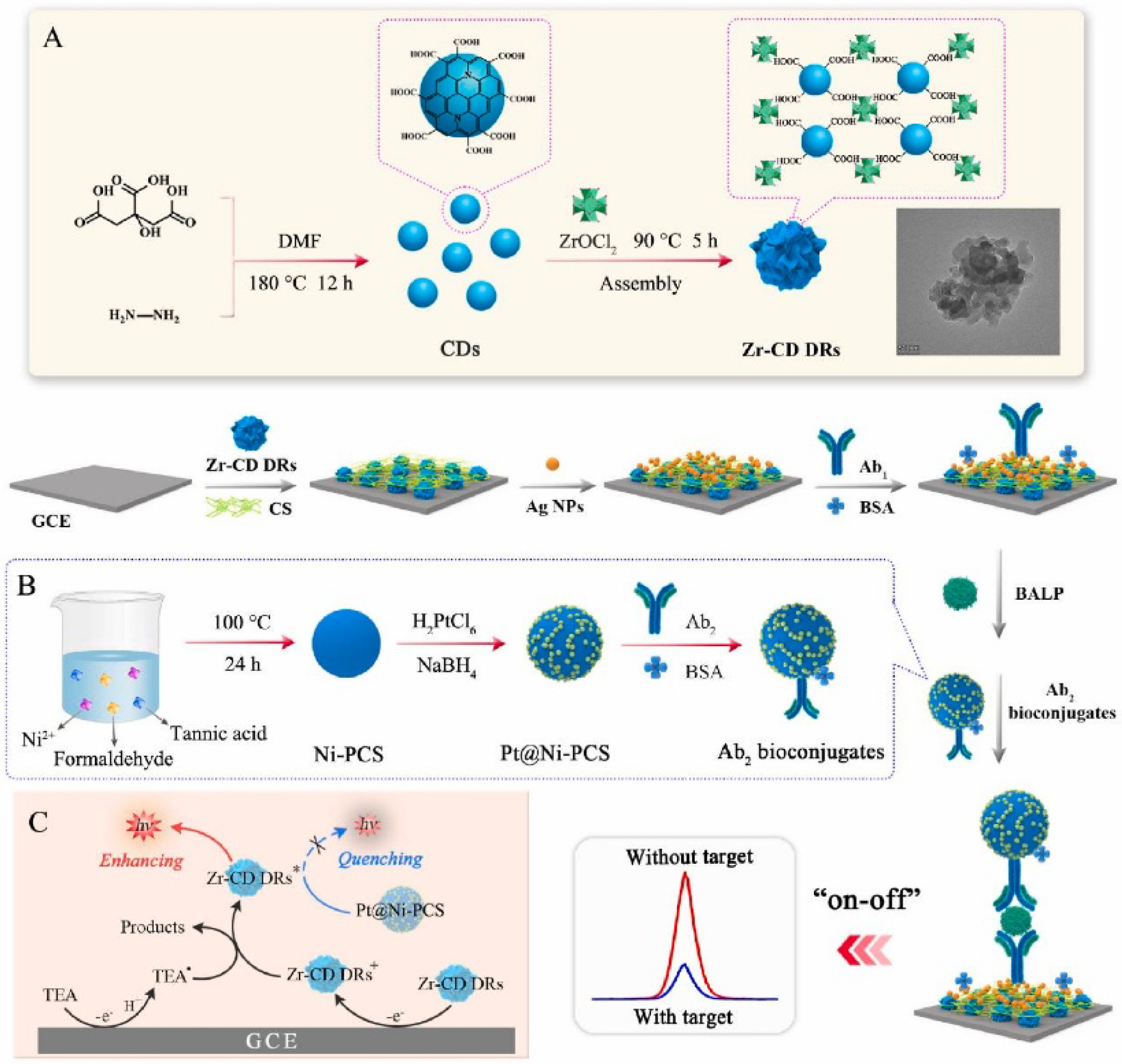
3.2.2. Electrochemical Immunosensors
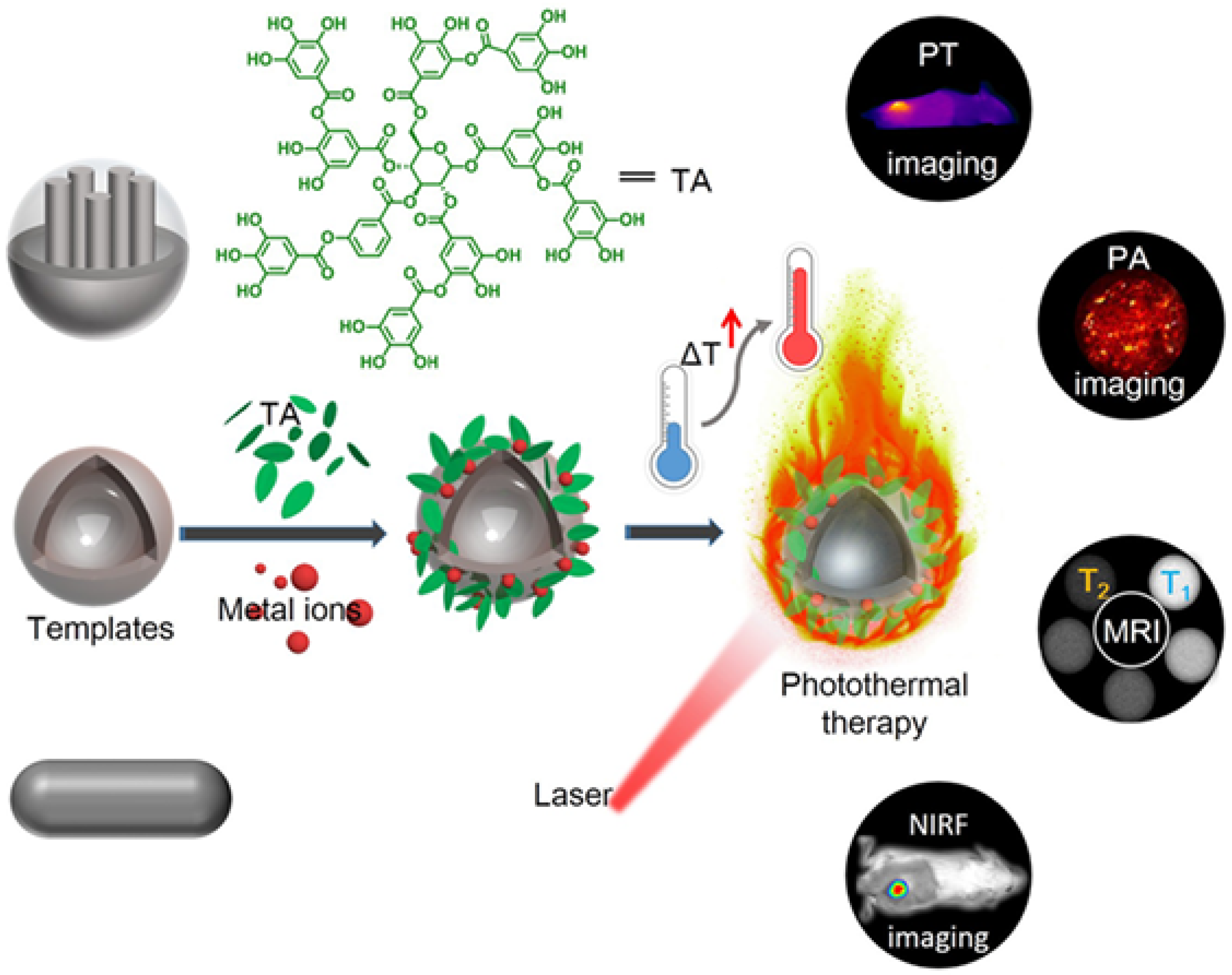
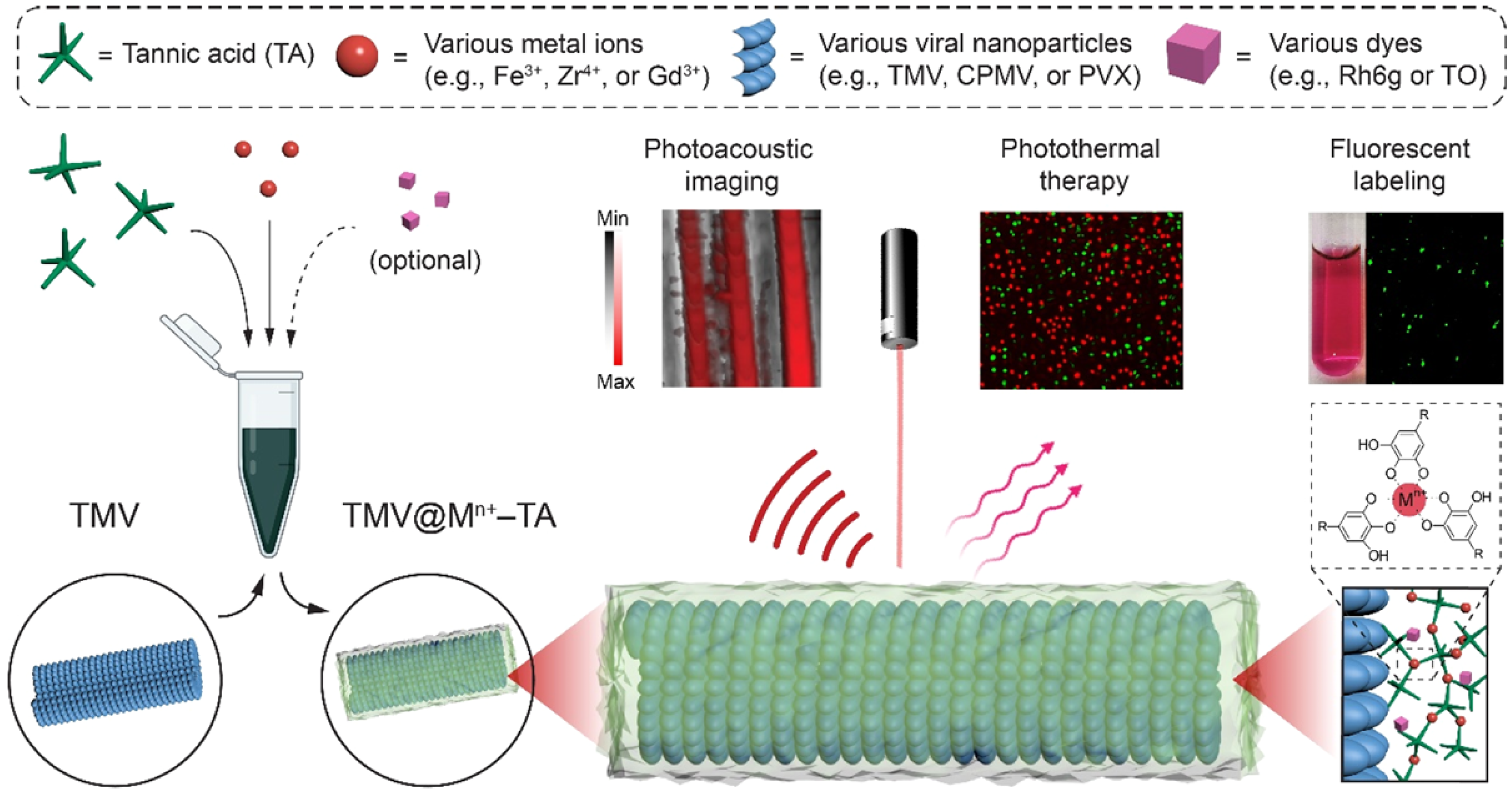
3.3. Bioimaging
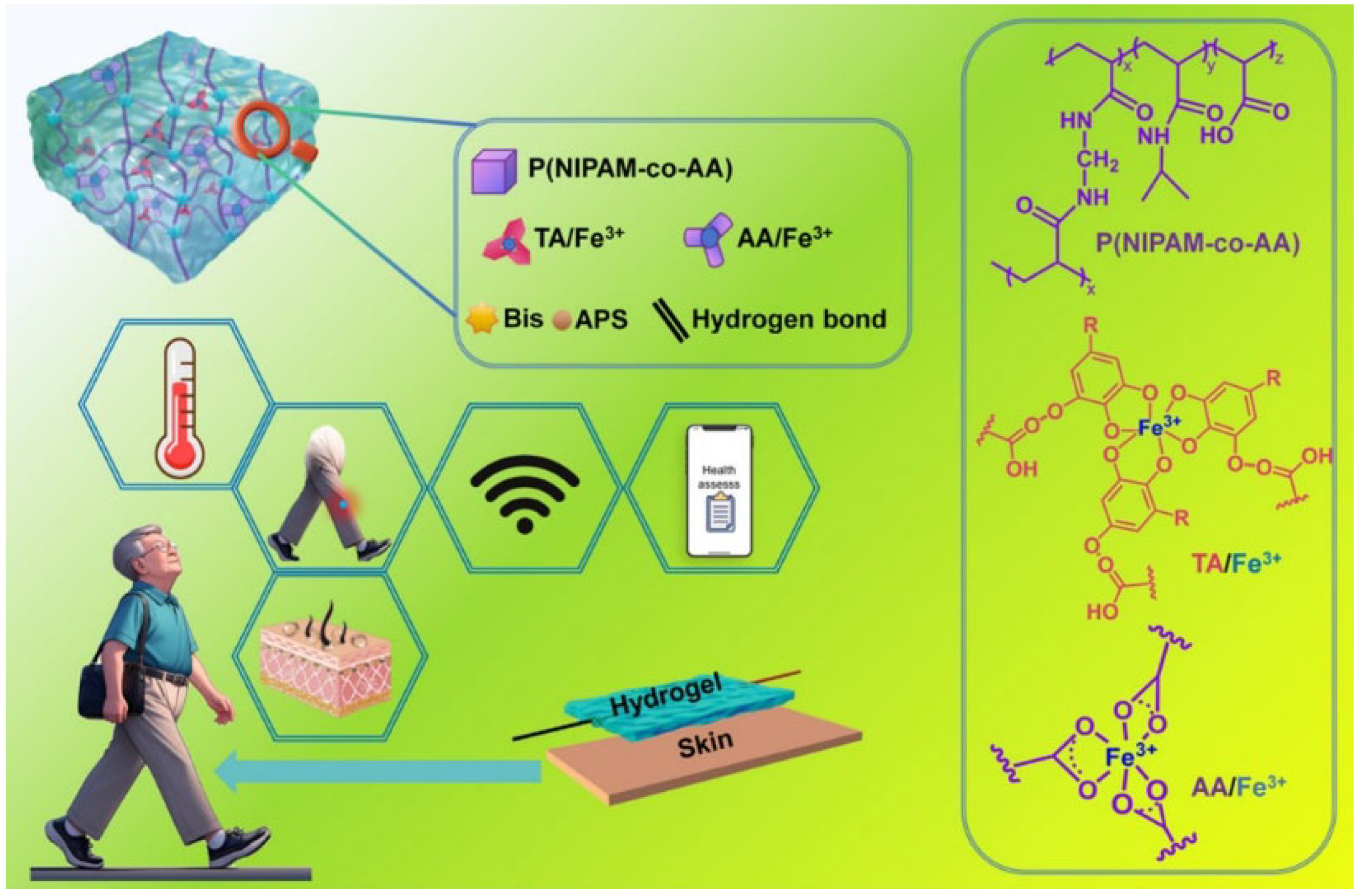
3.4. Wearable Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in biosensors and diagnostic technologies using nanostructures and nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Masson, J.F. Ultralow concentration sensing: What should we be aware of? ACS Sens. 2023, 8, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Chen, C.-D. Progress in nanomaterials-based electrochemical biosensors for beta-amyloid peptides as the biomarkers of Alzheimer’s disease. Int. J. Electrochem. Sci. 2022, 17, 220342. [Google Scholar] [CrossRef]
- Yu, C.-X.; Xiong, F.; Liu, L.-L. Electrochemical biosensors with silver nanoparticles as signal labels. Int. J. Electrochem. Sci. 2020, 15, 3869–3890. [Google Scholar] [CrossRef]
- Xia, N.; Gao, F.; Zhang, J.; Wang, J.; Huang, Y. Overview on the development of electrochemical immunosensors by the signal amplification of enzyme- or nanozyme-based catalysis plus redox cycling. Molecules 2024, 29, 2796. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Huang, Y.T. Inorganic-organic hybrid nanomaterials for therapeutic and diagnostic imaging applications. Int. J. Mol. Sci. 2011, 12, 3888–3927. [Google Scholar] [CrossRef]
- Hu, Q.; Ding, D.; Tang, Y. Inorganic–organic hybrid materials to detect urinary biomarkers: Recent progress and future prospects. Mater. Chem. Front. 2022, 6, 2011–2033. [Google Scholar] [CrossRef]
- Hwang, E.; Jung, H.S. Metal-organic complex-based chemodynamic therapy agents for cancer therapy. Chem. Commun. 2020, 56, 8332–8341. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Jin, X.; Liu, H.; Sun, K.; Wang, X.; Li, M.; Wang, P.; Chang, Y.; Wang, T.; et al. An encounter between metal ions and natural products: Natural products-coordinated metal ions for the diagnosis and treatment of tumors. J. Nanobiotech. 2024, 22, 726. [Google Scholar] [CrossRef]
- Xia, N.; Gao, F.; Liu, G.; Chang, Y.; Liu, L. Pyrroloquinoline quinone exhibiting peroxidase-mimicking property for colorimetric assays. Anal. Chim. Acta 2025, 1335, 343468. [Google Scholar] [CrossRef]
- Guo, X.; Luo, W.; Wu, L.; Zhang, L.; Chen, Y.; Li, T.; Li, H.; Zhang, W.; Liu, Y.; Zheng, J.; et al. Natural products from herbal medicine self-assemble into advanced bioactive materials. Adv. Sci. 2024, 11, 2403388. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y.; Zhang, J.; Liu, J.; Wang, A.; Ding, L. Recent development of copper-based nanozymes for biomedical applications. Adv. Healthc. Mater. 2024, 13, e2302023. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Liu, B.; Meng, Q.; Yuan, M.; Ma, X.; Wang, J.; Wang, M.; Li, K.; Ma, P.; et al. Facile synthesis of Fe-based metal-quinone networks for mutually enhanced mild photothermal therapy and ferroptosis. Angew. Chem. Int. Ed. 2025, 64, e202414879. [Google Scholar]
- Cong, C.; Ma, H. Advances of electroactive metal-organic frameworks. Small 2023, 19, e2207547. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, R.; Cao, Z.; Du, X. Smart controlled-release nanopesticides based on metal-organic frameworks. Chem. Commun. 2024, 60, 6082. [Google Scholar] [CrossRef]
- Xia, N.; Chang, Y.; Zhou, Q.; Ding, S.; Gao, F. An overview of the design of metal-organic frameworks-based fluorescent chemosensors and biosensors. Biosensors 2022, 12, 928. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and application of electrochemical sensors with metal-organic frameworks as the electrode materials or signal tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, L.; Ju, Y.; Dai, Y. Recent advances in metal-phenolic networks for cancer theranostics. Small 2021, 17, 2100314. [Google Scholar] [CrossRef]
- Li, L.; Pan, J.; Huang, M.; Sun, J.; Wang, C.; Xu, H. Metal-phenolic networks: A promising frontier in cancer theranostics. Int. J. Nanomed. 2024, 19, 11379–11395. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Caruso, F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Su, X.; Wang, P.; Chen, H.; Kuang, X.; Liu, J. Tannic acid-based metal-phenolic networks as a versatile platform to mediate cell therapy. Sci. China Mater. 2024, 67, 3833–3848. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Feng, Y.; Li, P.; Wei, J. Engineering functional mesoporous materials from plant polyphenol based coordination polymers. Coord. Chem. Rev. 2022, 468, 214649. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, H.; Richardson, J.J.; Xu, W.; Chen, J.; Zhou, J.; Caruso, F. Metal-phenolic network composites: From fundamentals to applications. Chem. Soc. Rev. 2024, 53, 10800–10826. [Google Scholar] [CrossRef]
- Wu, S.; Gan, W.; Zhou, X.; Bin, Z.; Chen, J.; Li, W.; Lai, H.; Wang, Z. Enhancement of hydrogen-sensing properties of Pd-modified WO3 nanocubes via tannic acid-assisted surface functionalization. ACS Sens. 2025, 10, 3600–3609. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired metal-polyphenol materials: Self-healing and beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef]
- Park, J.; Brust, T.F.; Lee, H.J.; Lee, S.C.; Watts, V.J.; Yeo, Y. Polydopamine-based simple and versatile surface modification of polymeric nano drug carriers. ACS Nano 2014, 8, 3347–3356. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef]
- Geng, H.; Zhong, Q.Z.; Li, J.; Lin, Z.; Cui, J.; Caruso, F.; Hao, J. Metal ion-directed functional metal-phenolic materials. Chem. Rev. 2022, 122, 11432–11473. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Yang, H.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Recent advances of Fe(III)/Fe(II)-MPNs in biomedical applications. Pharmaceutics 2023, 15, 1323. [Google Scholar] [CrossRef]
- Chen, Z.; Swislocka, R.; Choinska, R.; Marszalek, K.; Dabrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the correlation between the molecular structure and biological activities of metal-phenolic compound complexes: Research and description of the role of metal ions in improving the antioxidant activities of phenolic compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, F.; Liu, Y.; Yang, M.; Zhang, B.; Bai, Z.; Zhao, B.; Li, X. Recent advances of copper-based metal phenolic networks in biomedical applications. Colloid. Surf. B Biointerf. 2024, 244, 114163. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal-phenolic networks as versatile coating materials for biomedical applications. ACS Appl. Bio. Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent advances in the development and antimicrobial applications of metal-phenolic networks. Adv. Sci. 2022, 9, e2202684. [Google Scholar] [CrossRef]
- Qin, J.; Guo, N.; Yang, J.; Chen, Y. Recent advances of metal-polyphenol coordination polymers for biomedical applications. Biosensors 2023, 13, 776. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Yu, J.; Zhang, Y.; Zhou, Y. Applications of metal-phenolic networks in nanomedicine: A review. Biomater. Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Cao, H.; Yang, L.; Tian, R.; Wu, H.; Gu, Z.; Li, Y. Versatile polyphenolic platforms in regulating cell biology. Chem. Soc. Rev. 2022, 51, 4175–4198. [Google Scholar] [CrossRef]
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927. [Google Scholar] [CrossRef]
- Lin, G.; Zhao, L.; Jin, H.; Wang, S.; Wang, N.; Dai, M.; Lin, X. Designing metal–phenolic networks in biomedicine. Appl. Mater. Today 2025, 45, 102822. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, H.C.; Yang, T.; Yue, X.Z.; Yi, S.S. Functions of metal-phenolic networks and polyphenol derivatives in photo (electro)catalysis. Chem. Commun. 2023, 59, 13690–13702. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cui, M.; Cai, C.; Zhang, N.; Chen, S.; Wang, Z.; Liu, Q.; Zhang, X.; Ren, S.; An, H. Application of metal polyphenol nanonetworks in phototherapy. Coord. Chem. Rev. 2025, 539, 216743. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Huang, H.; Qin, J.; Wang, G.; Guo, Z.; Yu, X.; Zhao, Y.; Wei, J. Synthesis of spiny metal–phenolic coordination crystals as a sensing platform for sequence-specific detection of nucleic acids. CrystEngComm 2018, 20, 7626–7630. [Google Scholar] [CrossRef]
- Chen, J.; Spoljaric, S.; Calatayud-Sanchez, A.; Alvarez-Brana, Y.; Caruso, F. Engineering metal-phenolic network nanoparticles via microfluidics. ACS Appl. Mater. Interfaces 2023, 15, 48050–48059. [Google Scholar] [CrossRef]
- Chen, J.; Pan, S.; Zhou, J.; Lin, Z.; Qu, Y.; Glab, A.; Han, Y.; Richardson, J.J.; Caruso, F. Assembly of bioactive nanoparticles via metal–phenolic complexation. Adv. Mater. 2022, 34, 2108624. [Google Scholar] [CrossRef]
- Xu, W.; Lin, Z.; Pan, S.; Chen, J.; Wang, T.; Cortez-Jugo, C.; Caruso, F. Direct assembly of metal-phenolic network nanoparticles for biomedical applications. Angew. Chem. Int. Ed. 2023, 62, e202312925. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Ping, Y.; Wang, C.; Gao, N.; Yin, X.; Gu, C.; Ding, D.; Brinker, C.J.; Li, G. Controlled fabrication of functional capsules based on the synergistic interaction between polyphenols and MOFs under weak basic condition. ACS Appl. Mater. Interfaces 2017, 9, 14258–14264. [Google Scholar] [CrossRef]
- Ping, Y.; Guo, J.; Ejima, H.; Chen, X.; Richardson, J.J.; Sun, H.; Caruso, F. PH-Responsive capsules engineered from metal-phenolic networks for anticancer drug delivery. Small 2015, 11, 2032–2036. [Google Scholar] [CrossRef]
- Kinfu, H.H.; Rahman, M.M. Separation performance of membranes containing ultrathin aurface xoating of metal-polyphenol network. Membranes 2023, 13, 481. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Zhang, H.; Deng, H.; Kuang, T.; Shen, Z.; Gu, Z. Biomimetic polyphenolic scaffolds with antioxidative abilities for improved bone regeneration. ACS Appl. Bio. Mater. 2023, 6, 4586–4591. [Google Scholar] [CrossRef]
- Vu, G.T.; Nguyen, L.M.; Nguyen Do, K.N.; Tran, D.L.; Vo, T.V.; Nguyen, D.H.; Vong, L.B. Preparation of metal-polyphenol modified zeolitic imidazolate framework-8 nanoparticles for cancer drug delivery. ACS Appl. Bio. Mater. 2025, 8, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Cui, X.; Gao, Z.; Li, M.; Zhang, X.; Ashokkumar, M.; Song, A.; Cui, J. Metal-phenolic network-coated nanoparticles as stabilizers for the engineering of pickering emulsions with bioactivity. ACS Appl. Mater. Interfaces 2024, 16, 27988–27997. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, X.; Yi, Q.; Xiong, J.; Han, Q.; Liang, Q. Metal-polyphenol network-mediated protein encapsulation strategy facilitating the separation of proteins and metabolites in biospecimens. Anal. Chem. 2023, 95, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Wakayama, C.; Inubushi, S.; Kunihisa, T.; Mizumoto, S.; Baba, M.; Tanino, H.; Ooya, T. Size dependency of selective cellular uptake of epigallocatechin gallate-modified gold nanoparticles for effective radiosensitization. ACS Appl. Bio. Mater. 2022, 5, 355–365. [Google Scholar] [CrossRef]
- Andrikopoulos, N.; Li, Y.; Nandakumar, A.; Quinn, J.F.; Davis, T.P.; Ding, F.; Saikia, N.; Ke, P.C. Zinc-epigallocatechin-3-gallate network-coated nanocomposites against the pathogenesis of amyloid-beta. ACS Appl. Mater. Interfaces 2023, 15, 7777–7792. [Google Scholar] [CrossRef]
- Dai, Q.; Yu, Q.; Tian, Y.; Xie, X.; Song, A.; Caruso, F.; Hao, J.; Cui, J. Advancing metal-phenolic networks for visual information storage. ACS Appl. Mater. Interfaces 2019, 11, 29305–29311. [Google Scholar] [CrossRef]
- Wang, D.; Xing, J.; Zhang, Y.; Guo, Z.; Deng, S.; Guan, Z.; He, B.; Ma, R.; Leng, X.; Dong, K.; et al. Metal-phenolic networks for chronic wounds therapy. Int. J. Nanomed. 2023, 18, 6425–6448. [Google Scholar] [CrossRef]
- Fedenko, V.S.; Landi, M.; Shemet, S.A. Metallophenolomics: A novel integrated approach to study complexation of plant phenolics with metal/metalloid ions. Int. J. Mol. Sci. 2022, 23, 11370. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic acid-based metal phenolic networks for bio-applications: A review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Q.; Gu, J. Recent advances in colorimetric sensors based on nanozymes with peroxidase-like activity. Analyst 2023, 148, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Tang, Z. Recent progress in the design of analytical methods based on nanozymes. J. Mater. Chem. B 2021, 9, 8174–8184. [Google Scholar] [CrossRef] [PubMed]
- Chigozie, A.E.; Ravikumar, A.; Yang, X.; Tamilselvan, G.; Deng, Y.; Arunjegan, A.; Li, X.; Hu, Z.; Zhang, Z. A metal-phenolic coordination framework nanozyme exhibits dual enzyme mimicking activity and its application is effective for colorimetric detection of biomolecules. Anal. Methods 2024, 16, 3530–3538. [Google Scholar] [CrossRef]
- Davoodi-Rad, K.; Shokrollahi, A.; Shahdost-Fard, F.; Azadkish, K.; Madani-Nejad, E. A smartphone-based colorimetric assay using Cu-tannic acid nanosheets (Cu-TA NShs) as a laccase-mimicking nanozyme for visual detection of quercetin in vegetables. Microchim. Acta 2024, 191, 168. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Ouyang, Y.; Reheman, A.; Jiang, B. Enhanced peroxidase-like nanozyme: Copper-poly (tannic acid) for advanced colorimetric assay of total antioxidant capacity. RSC Adv. 2025, 15, 11401–11408. [Google Scholar] [CrossRef]
- Ravikumar, A.; Natraj, V.; Sivalingam, Y.; Surya, V.J.; Han, W.; Zheng, F.; Liu, N. Flexible Cu-TA/PVDF composite film for efficient piezo-catalysis and biomolecule detection. Surf. Interf. 2023, 39, 102994. [Google Scholar] [CrossRef]
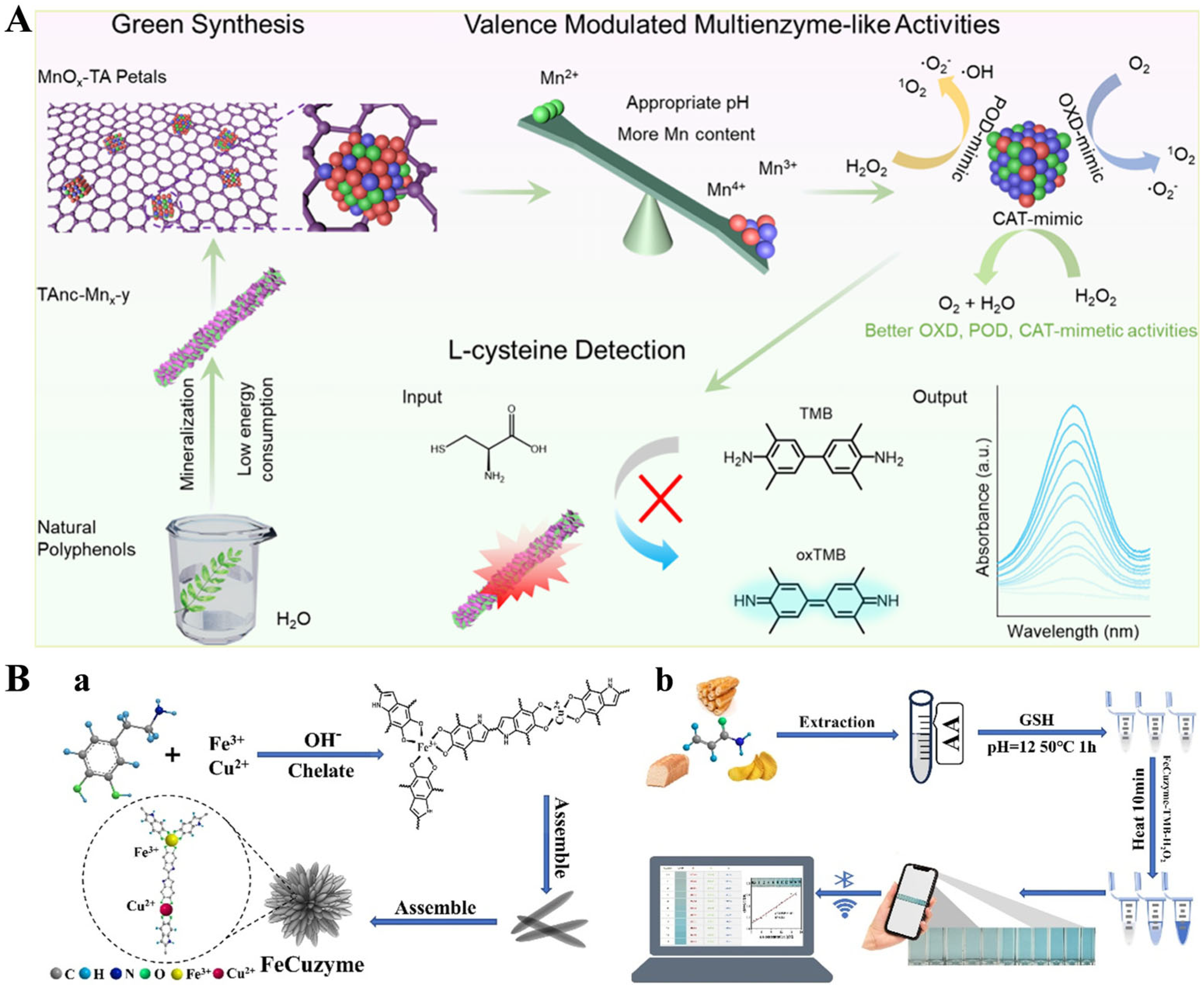
- Wei, Z.; Yang, L.; Ou, M.; Xie, Y.; Zhao, C. Tailoring the enzyme-mimetic activity and surface nanostructure of metal-phenolic platform for colorimetric detection of l-cysteine. ACS Sustainable Chem. Eng. 2024, 12, 3608–3620. [Google Scholar] [CrossRef]
- Chen, S.; Liu, F.; Cai, T.; Wang, R.; Ning, F.; Peng, H. Dimensionality engineering of flower-like bimetallic nanozyme with high peroxidase-activity for naked-eye and on-site detection of acrylamide in thermally processed foods. Nano Mater. Sci. 2025, 7, 123–133. [Google Scholar] [CrossRef]
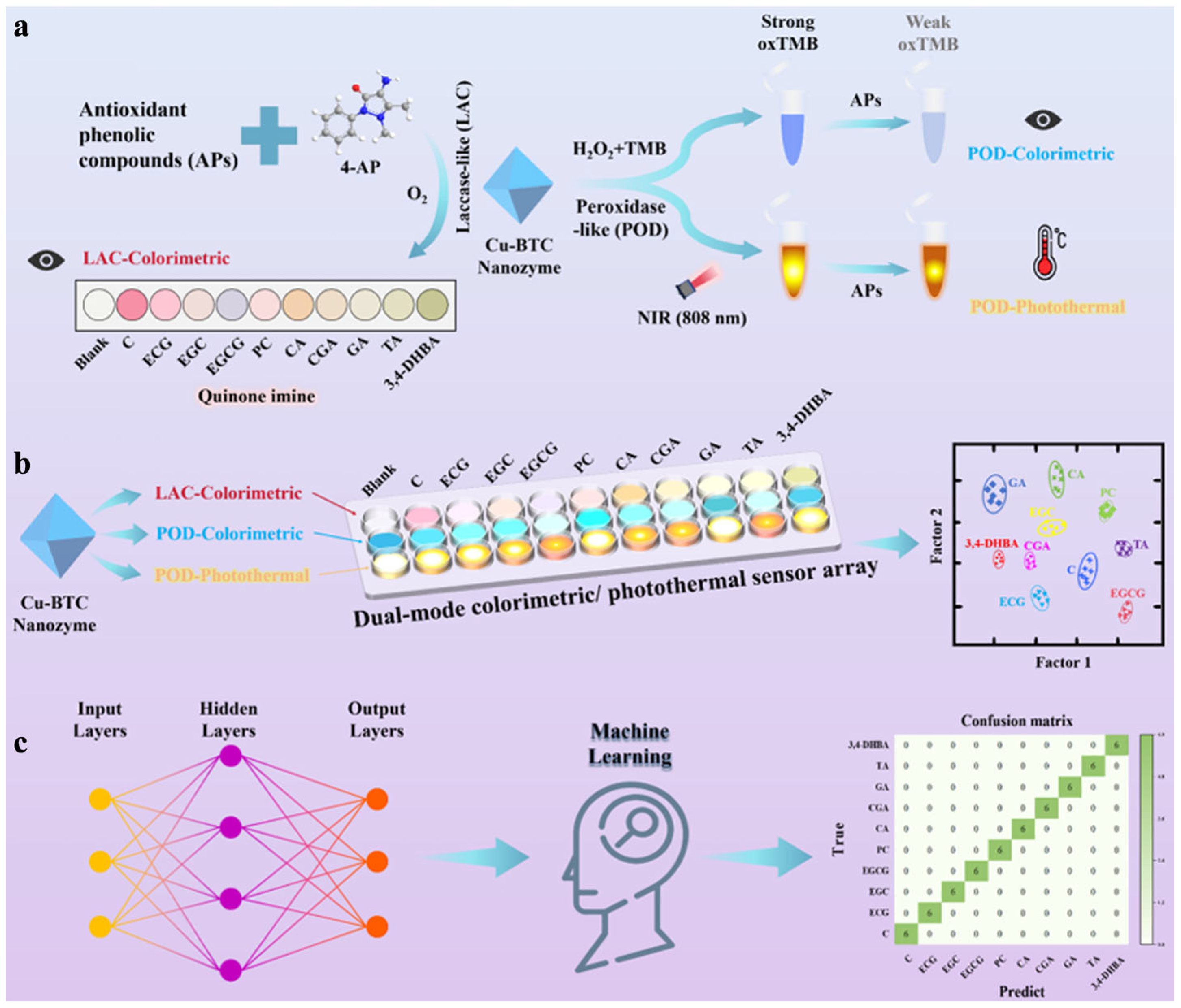
- Xu, J.; Wang, Y.; Li, Z.; Liu, F.; Jing, W. Machine learning assisted multi-signal nanozyme sensor array for the antioxidant phenolic compounds intelligent recognition. Food Chem. 2025, 471, 142826. [Google Scholar] [CrossRef]
- Jing, W.; Yang, Y.; Shi, Q.; Wang, Y.; Liu, F. Machine learning-based nanozyme sensor array as an electronic tongue for the discrimination of endogenous phenolic compounds in food. Anal. Chem. 2024, 96, 16027–16035. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, Y.; Gu, Y.; Xiang, T.; Shen, H.; Wang, Y.; Hu, Z.; Zheng, Z.; Yu, Z.; Wu, Q.; et al. Metal-polyphenol multistage competitive coordination system for colorimetric monitoring meat freshness. Adv. Mater. 2025, 37, 2503246. [Google Scholar] [CrossRef]
- Lai, H.; Xu, F.; Wang, L. A review of the preparation and application of magnetic nanoparticles for surface-enhanced Raman scattering. J. Mater. Sci. 2018, 53, 8677–8698. [Google Scholar] [CrossRef]
- Karthick Kannan, P.; Shankar, P.; Blackman, C.; Chung, C.H. Recent Advances in 2D Inorganic Nanomaterials for SERS Sensing. Adv. Mater. 2019, 31, e1803432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Ning, M.; Wang, P.; Wang, W.; Zhang, X.; Liu, Z.; Zhang, Y.; Li, S. Fast synthesis of Au nanoparticles on metal-phenolic network for sweat SERS analysis. Nanomaterials 2022, 12, 2977. [Google Scholar] [CrossRef] [PubMed]
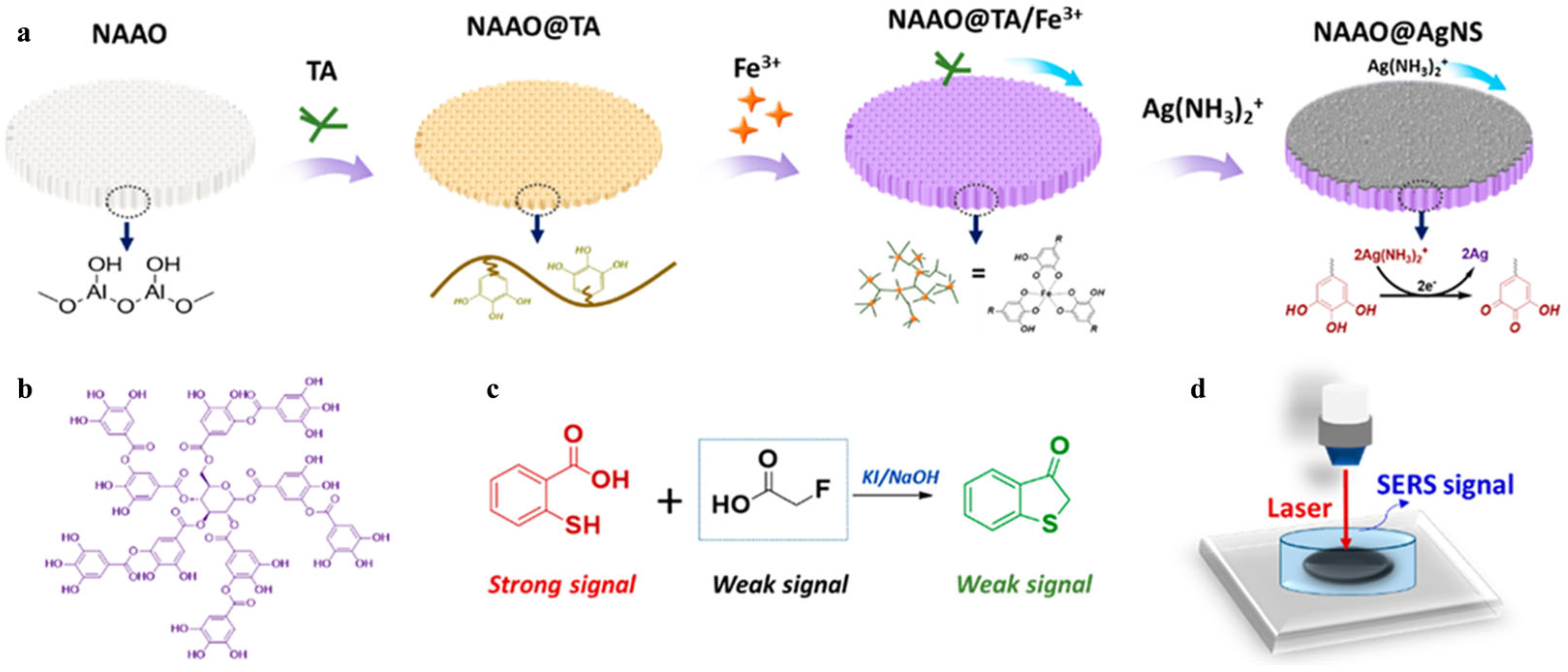
- Sun, J.; Xue, D.; Shan, W.; Liu, R.; Liu, R.; Zhao, H.; Li, T.; Wang, Z.; Zhang, J.; Shao, B. In situ growth large area silver nanostructure on metal phenolic network coated NAAO film and its SERS sensing application for monofluoroacetic acid. ACS Sens. 2021, 6, 2129–2135. [Google Scholar] [CrossRef]
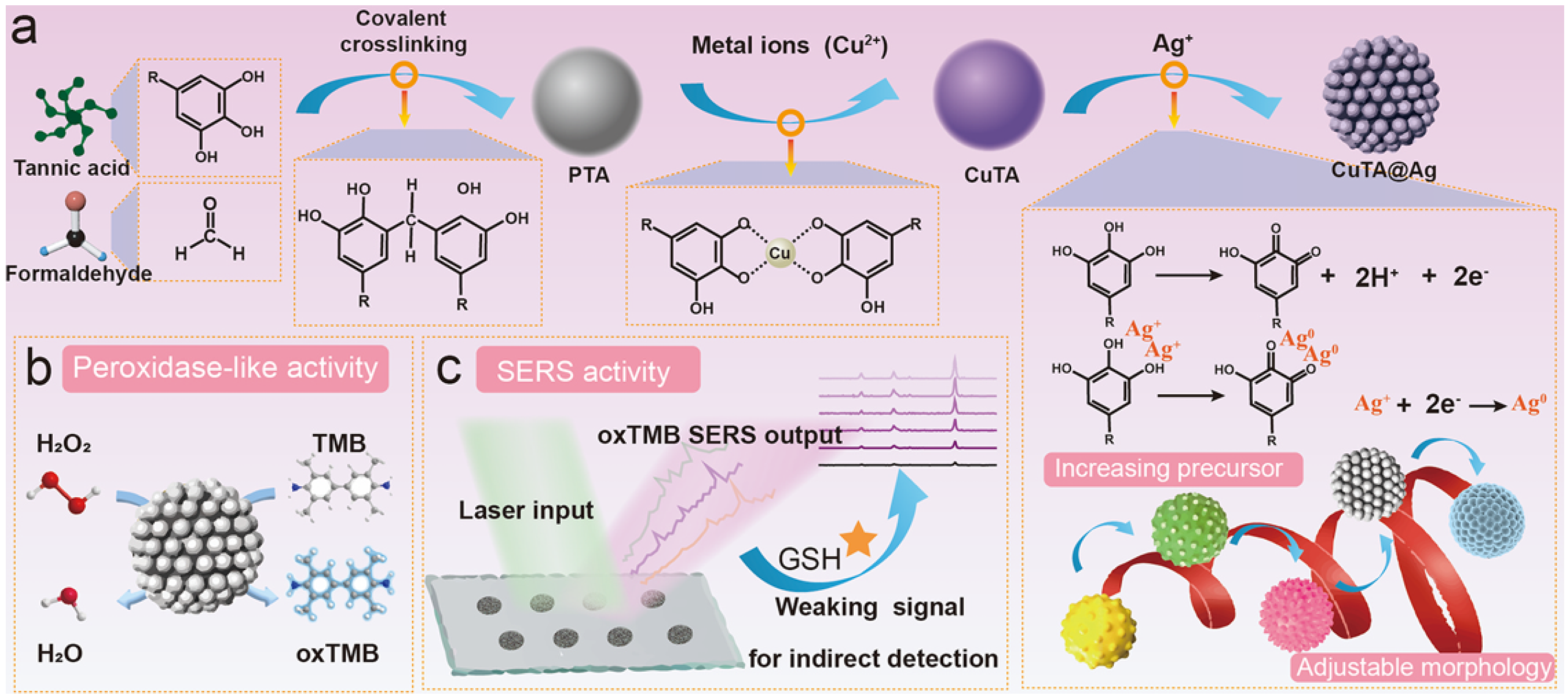
- Li, Y.; Li, P.; Chen, Y.; Wu, Y.; Wei, J. Interfacial deposition of Ag nanozyme on metal-polyphenol nanosphere for SERS detection of cellular glutathione. Biosens. Bioelectron. 2023, 228, 115200. [Google Scholar] [CrossRef]
- Chia, Z.C.; Chen, Y.L.; Chuang, C.H.; Hsieh, C.H.; Chen, Y.J.; Chen, K.H.; Huang, T.C.; Chen, M.C.; Huang, C.C. Polyphenol-assisted assembly of Au-deposited polylactic acid microneedles for SERS sensing and antibacterial photodynamic therapy. Chem. Commun. 2023, 59, 6339–6342. [Google Scholar] [CrossRef]
- Ye, H.; Jiang, S.; Yan, Y.; Zhao, B.; Grant, E.R.; Kitts, D.D.; Yada, R.Y.; Pratap-Singh, A.; Baldelli, A.; Yang, T. Integrating metal-phenolic networks-mediated separation and machine learning-aided surface-enhanced Raman spectroscopy for accurate nanoplastics quantification and classification. ACS Nano. 2024, 18, 26281–26296. [Google Scholar] [CrossRef]
- Ye, H.; Esfahani, E.B.; Chiu, I.; Mohseni, M.; Gao, G.; Yang, T. Quantitative and rapid detection of nanoplastics labeled by luminescent metal phenolic networks using surface-enhanced Raman scattering. J. Hazard. Mater. 2024, 470, 134194. [Google Scholar] [CrossRef]
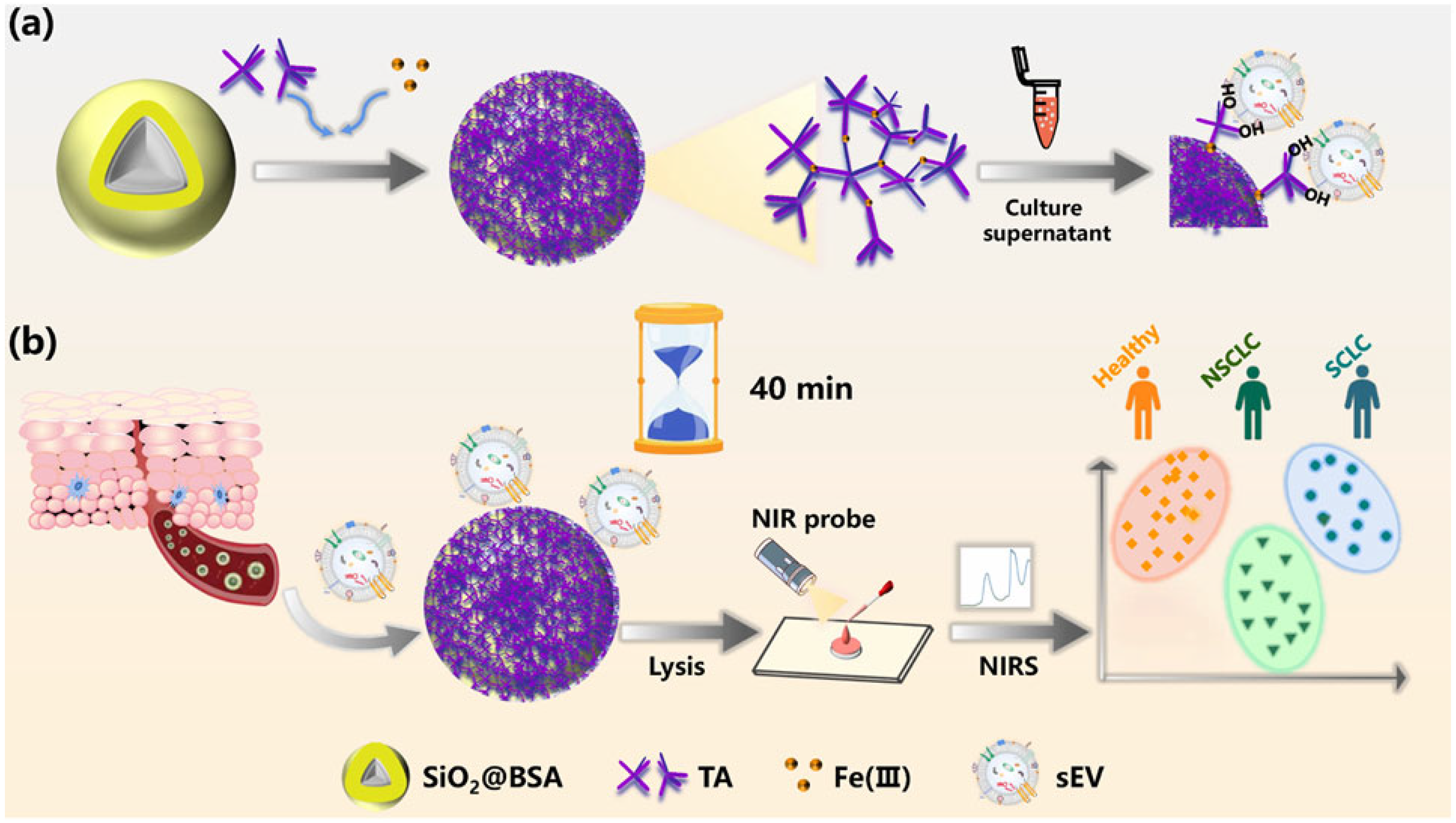
- Wang, H.; Liu, D.; Zhang, L.; Gao, X.; Nie, Y.; Liu, Y.; Jia, Y.; Yin, M.; Qiao, X. Label-free small extracellular vesicles capturing strategy for lung cancer diagnosis and typing based on a natural polyphenol-metal three-dimensional network. Anal. Chem. 2022, 94, 16103–16112. [Google Scholar] [CrossRef] [PubMed]
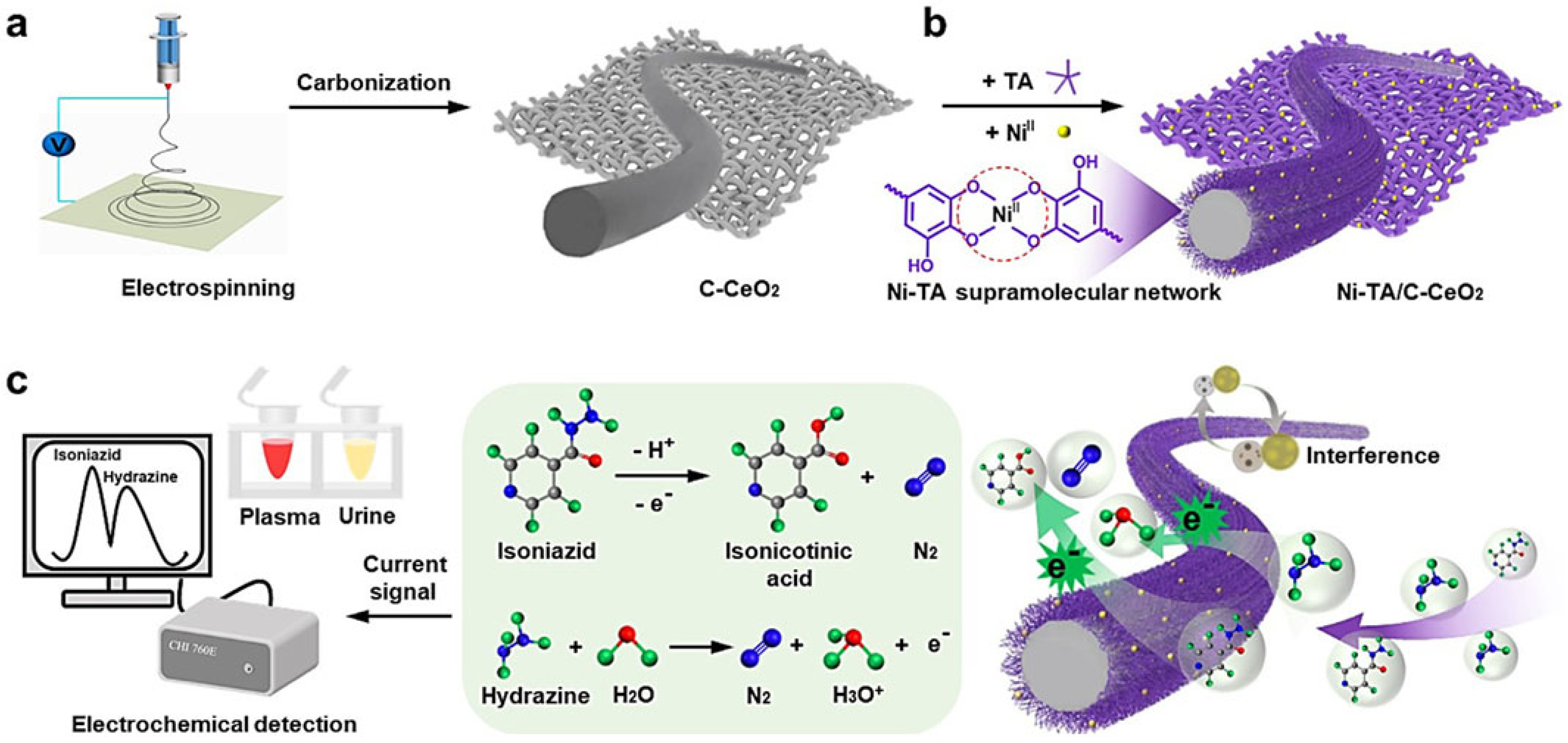
- Feng, J.; Lang, G.; Li, T.; Zhang, J.; Zhao, J.; Li, W.; Yang, W.; Jiang, Z. Enhanced electrochemical detection performance of C-Cr2O3 towards glucose and hydrazine by assembling Ni-MPN coating. Appl. Surf. Sci. 2022, 604, 154548. [Google Scholar] [CrossRef]
- Lang, G.; Feng, J.; Feng, B.; Hu, J.; Ran, Z.; Zhou, Z.; Jiang, Z.; He, Y.; Guo, J. Supramolecular phenolic network-engineered C–CeO2 nanofibers for simultaneous determination of isoniazid and hydrazine in biological fluids. Chin. Chem. Lett. 2024, 35, 109113. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Y.; Li, Y.; Su, Y.; Deng, Y.; Wei, J. Synthesis of mesoporous Ag2O/SnO2 manospheres for selective sensing of formaldehyde at a low working temperature. ACS Sens. 2022, 7, 3963–3972. [Google Scholar] [CrossRef]
- Feng, B.; Feng, Y.; Qin, J.; Wang, Z.; Zhang, Y.; Du, F.; Zhao, Y.; Wei, J. Self-template synthesis of spherical mesoporous tin dioxide from tin-polyphenol-formaldehyde polymers for conductometric ethanol gas sensing. Sens. Actuators B Chem. 2021, 341, 129965. [Google Scholar] [CrossRef]
- Feng, B.; Wu, Y.; Ren, Y.; Chen, Y.; Yuan, K.; Deng, Y.; Wei, J. Self-template synthesis of mesoporous Au-SnO2 nanospheres for low-temperature detection of triethylamine vapor. Sens. Actuators B Chem. 2022, 356, 131358. [Google Scholar] [CrossRef]
- Rahmawati, I.; Einaga, Y.; Ivandini, T.A.; Fiorani, A. Enzymatic biosensors with electrochemiluminescence transduction. ChemElectroChem 2022, 9, e202200175. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Z.; Zhang, H.; Liu, Y. A novel electrogenerated chemiluminescence biosensor for histone acetyltransferases activity analysis and inhibition based on mimetic superoxide dismutase of tannic acid assembled nanoprobes. Biosens. Bioelectron. 2018, 122, 205–210. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Xianyu, Y. Applications of self-assembly strategies in immunoassays: A review. Coord. Chem. Rev. 2023, 478, 214974. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Darwish, I.A.; Wang, J.; Zhang, D. Gold nanomaterials-derived colorimetric signaling strategies for immunosensing and food safety. Trend. Anal. Chem. 2025, 188, 118238. [Google Scholar] [CrossRef]
- Yu, Z.J.; Yang, T.T.; Liu, G.; Deng, D.H.; Liu, L. Gold nanoparticles-based colorimetric immunoassay of carcinoembryonic antigen with metal-organic framework to load quinones for catalytic oxidation of cysteine. Sensors 2024, 24, 6701. [Google Scholar] [CrossRef]
- Liu, S.; Shu, R.; Nie, C.; Li, Y.; Luo, X.; Ji, Y.; Yin, X.; Sun, J.; Zhang, D.; Wang, J. Bioresource-derived tannic acid-supported immuno-network in lateral flow immunoassay for sensitive clenbuterol monitoring. Food Chem. 2022, 382, 132390. [Google Scholar] [CrossRef] [PubMed]
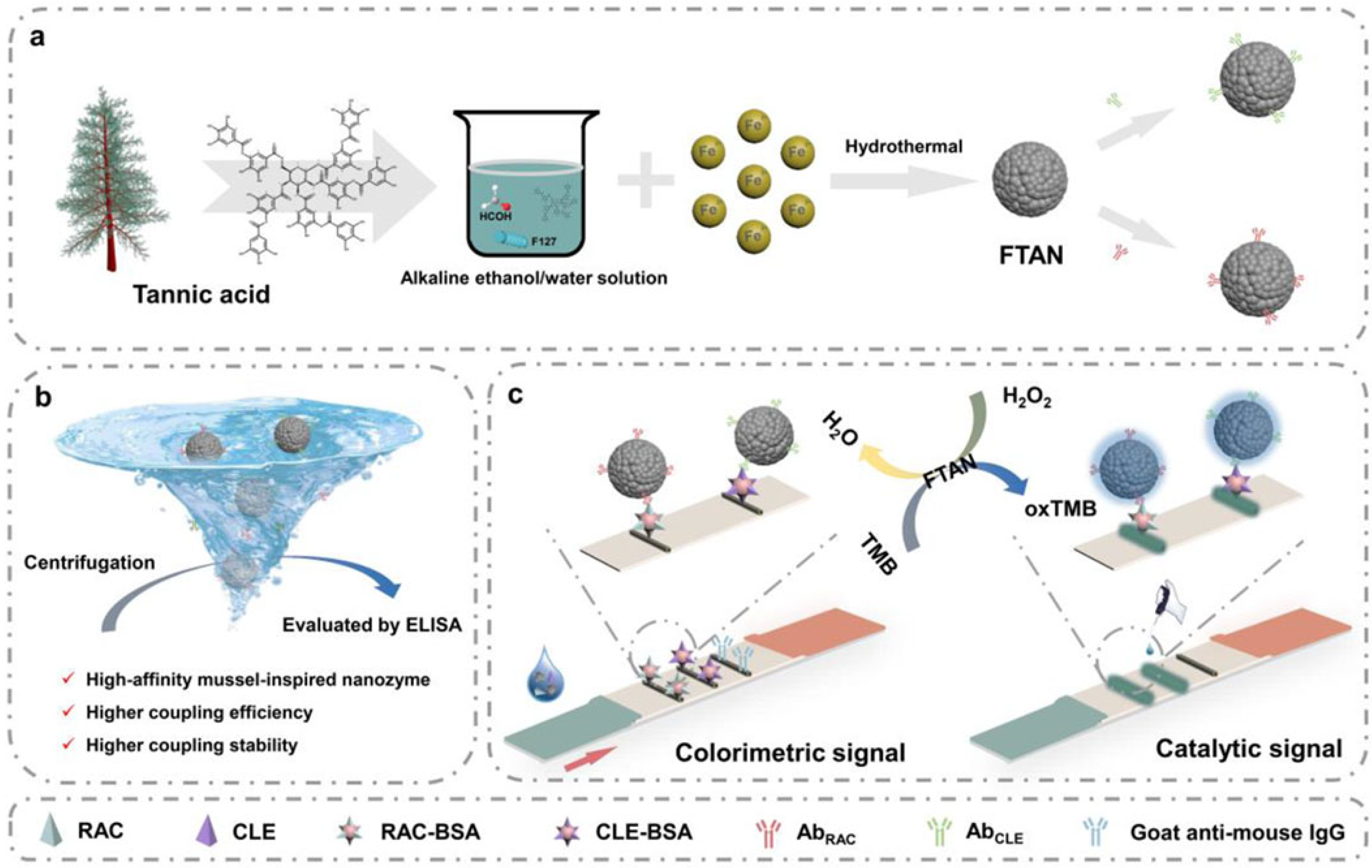
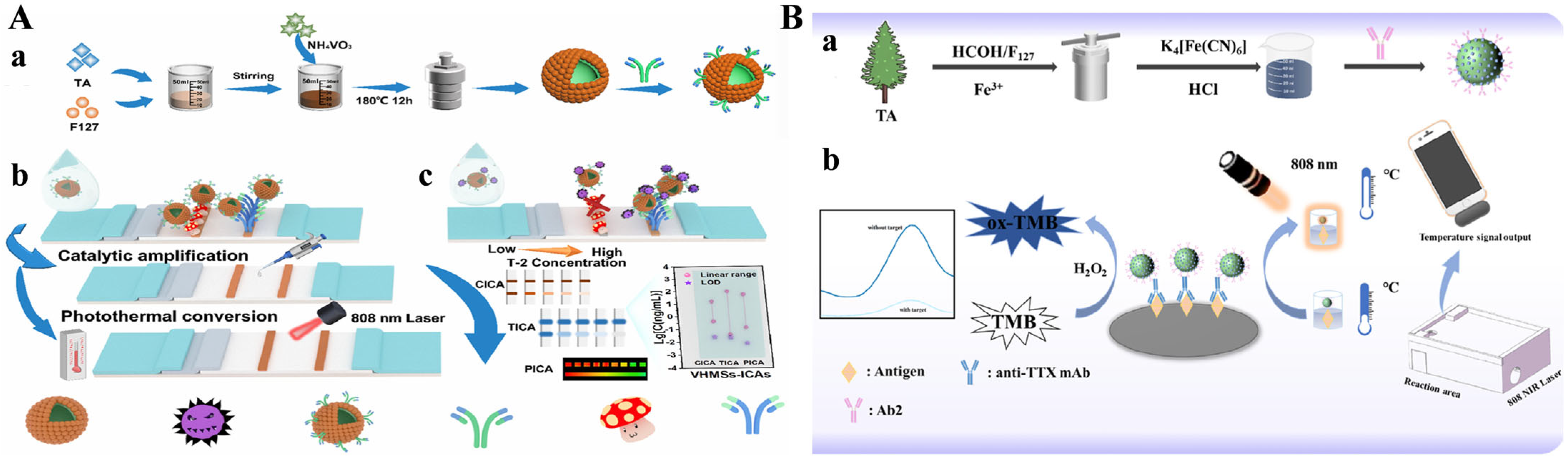
- Liu, S.; Shu, R.; Ma, J.; Dou, L.; Zhang, W.; Wang, S.; Ji, Y.; Li, Y.; Xu, J.; Zhang, D.; et al. Mussel-inspired Fe-based Tannic acid Nanozyme: A renewable bioresource-derived high-affinity signal tag for dual-readout multiplex lateral flow immunoassay. Chem. Eng. J. 2022, 446, 137382. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Yin, X.; Wang, S.; Tian, Y.; Shu, R.; Jia, C.; Chen, Y.; Sun, J.; Zhang, D.; et al. Nature-inspired nanozymes as signal markers for in-situ signal amplification strategy: A portable dual-colorimetric immunochromatographic analysis based on smartphone. Biosens. Bioelectron. 2022, 210, 114289. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ge, Y.; Xia, N. Overview of the design and application of dual-signal immunoassays. Molecules 2024, 29, 4551. [Google Scholar] [CrossRef]
- Gao, F.; Wu, Y.; Gan, C.; Hou, Y.; Deng, D.; Yi, X. Overview of the design and application of photothermal immunoassays. Sensors 2024, 24, 6458. [Google Scholar] [CrossRef]
- Sarathkumar, E.; Anjana, R.S.; Jayasree, R.S. Nanoarchitectonics of photothermal materials to enhance the sensitivity of lateral flow assays. Beilstein J. Nanotechnol. 2023, 14, 988–1003. [Google Scholar] [CrossRef]
- Wu, H.; Bu, T.; Sun, B.; Xi, J.; Cao, Y.; Wang, Y.; Xuan, C.; Feng, Q.; Yan, H.; Wang, L. “Three-in-one” multifunctional hollow nanocages with colorimetric photothermal catalytic activity for enhancing sensitivity in biosensing. Anal. Chem. 2024, 96, 4825–4834. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Jia, B.-Z.; Li, J.-M.; Yin, Q.-C.; Zhou, W.-Y.; Pant, S.D.; Lei, H.; Xu, Z.-L.; Luo, L. Prussian blue anchored Fe(III)-Tannic acid composite-mediated colorimetric and photothermal dual-mode immunosensor for the detection of tetrodotoxin. Food Control 2025, 174, 111246. [Google Scholar] [CrossRef]
- Huang, J.; Wei, F.; Cui, Y.; Hou, L.; Lin, T. Fluorescence immunosensor based on functional nanomaterials and its application in tumor biomarker detection. RSC Adv. 2022, 12, 31369–31379. [Google Scholar] [CrossRef]
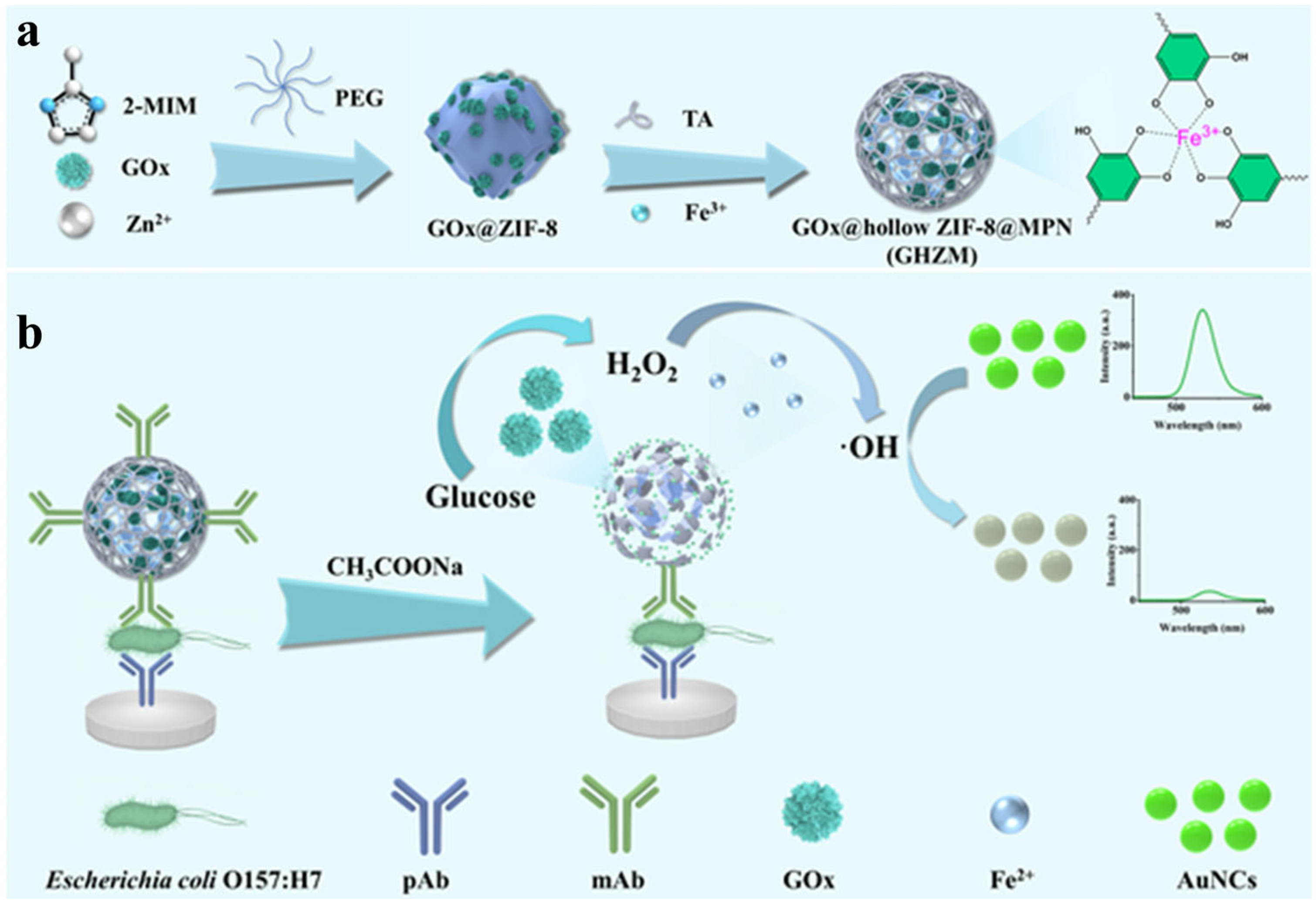
- Jing, X.; Tan, X.; Yu, Z.; Tang, Y.; Yu, S.; Zhang, G.; Xiong, Y.; Liu, D.; Lai, W. Tannic acid mediated hollow biocatalytic zeolitic imidazolate frameworks as a “three-in-one” label for enhancing the sensitivity of fluorescence immunosensor. Chem. Eng. J. 2024, 490, 151580. [Google Scholar] [CrossRef]
- Gao, F.; Liu, M.; Wang, W.; Lou, J.; Chang, Y.; Xia, N. Aggregation-induced emission-based competitive immunoassays for “signal-on” detection of proteins with multifunctional metal-organic frameworks as signal tags. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 325, 125088. [Google Scholar]
- Xu, C.; Peng, C.; Yang, X.; Zhang, R.; Zhao, Z.; Yan, B.; Zhang, J.; Gong, J.; He, X.; Kwok, R.T.K.; et al. One-pot synthesis of customized metal-phenolic-network-coated AIE dots for in vivo bioimaging. Adv. Sci. 2022, 9, e2104997. [Google Scholar]
- Zhang, G.; Yu, S.; Peng, J.; Xiong, Y.; Hu, L.; Lai, W. Novel reporter based on aggregation-induced emission luminogens for lateral flow immunoassay: A mini review. TrAC-Trend. Anal. Chem. 2025, 183, 118098. [Google Scholar]
- Chen, Z.; Tang, Y.; Guo, P.; Zhang, W.; Peng, J.; Xiong, Y.; Ma, B.; Lai, W. Integration of a biocompatible metal-phenolic network and fluorescence microspheres as labels for sensitive and stable detection of carbendazim with a lateral flow immunoassay. Food Chem. 2024, 450, 139260. [Google Scholar] [CrossRef]
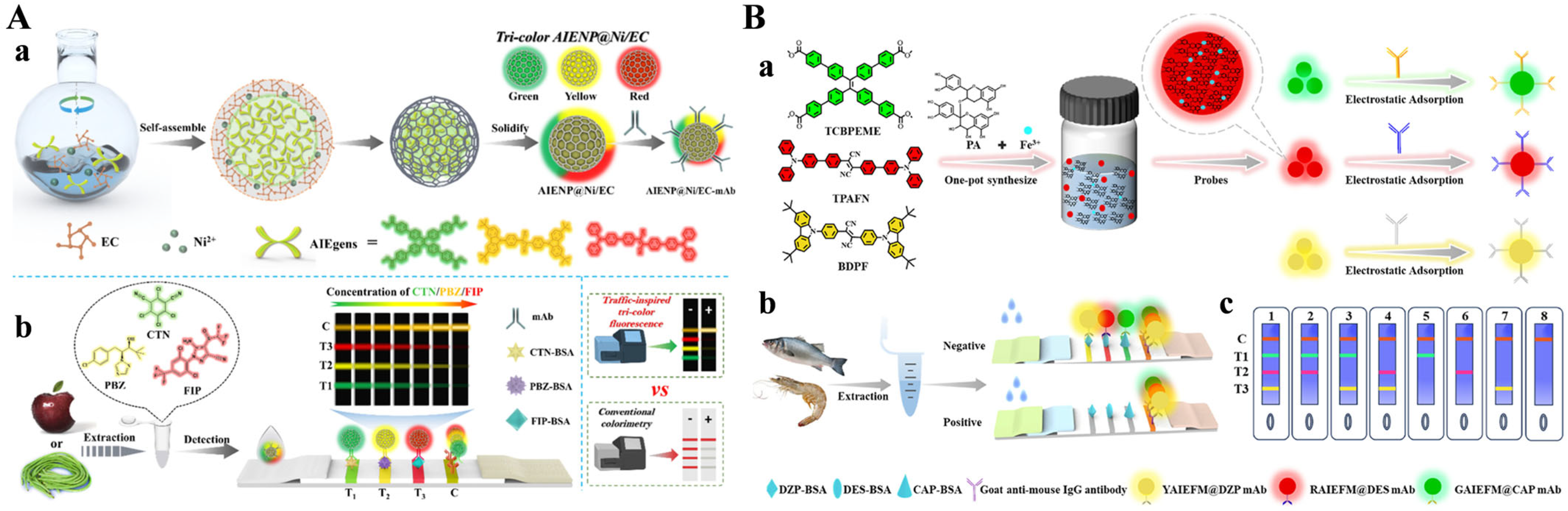
- Lai, X.; Cao, W.; Zhang, G.; Ang, E.H.; Su, L.; Liu, C.; He, W.; Lai, W.; Deng, S. Traffic signal-inspired fluorescence lateral flow immunoassay utilizing self-assembled AIENP@Ni/EC for simultaneous multi-pesticide residue detection. Chem. Eng. J. 2024, 501, 157565. [Google Scholar] [CrossRef]
- Su, L.; Lai, X.; Zhang, G.; Liu, C.; He, W.; Zhang, G.; Lai, W.; Deng, S. Multiplex immunochromatographic assay based on triple-color aggregation-induced emission fluorescent microspheres with good biocompatibility for the simultaneous detection of veterinary drugs in aquatic products. Anal. Chim. Acta 2025, 1345, 343700. [Google Scholar] [CrossRef]
- Ochoa-Ruiz, A.G.; Parra, G.; López-Espinoza, D.; Astudillo, P.; Galyamin, D.; Sabaté, N.; Esquivel, J.P.; Vallejo-Cardona, A.A. Electrochemical Immunosensors: The Evolution from ELISA to EμPADs. Electroanalysis 2023, 35, 2200053–2200067. [Google Scholar] [CrossRef]
- Puiu, M.; Nativi, C.; Bala, C. Early detection of tumour-associated antigens: Assessment of point-of-care electrochemical immunoassays. Trend. Anal. Chem. 2023, 160, 116981. [Google Scholar] [CrossRef]
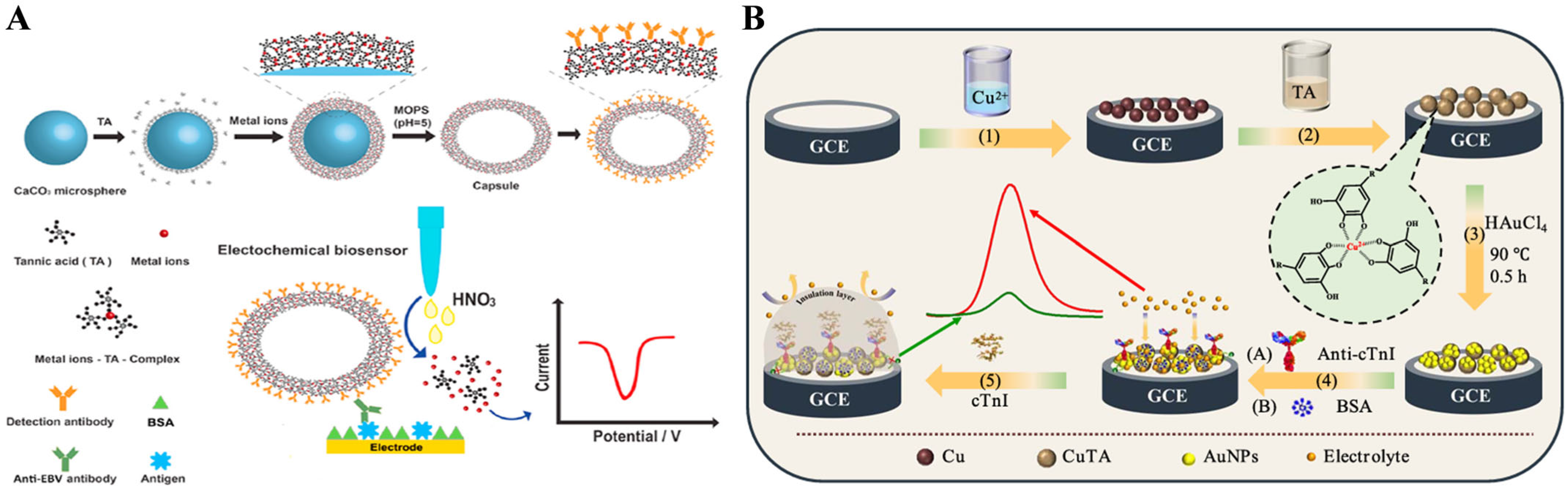
- Huang, J.; Meng, J.; Chen, S.; Zhang, S.; Liu, T.; Li, C.; Wang, F. A soft metal-polyphenol capsule-based ultrasensitive immunoassay for electrochemical detection of Epstein-Barr (EB) virus infection. Biosens. Bioelectron. 2020, 164, 112310. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Ni, J.; Zheng, X.; Song, Z.; Gao, F.; Wang, Q. Copper(II)-tannic acid@Cu with in situ grown gold nanoparticles as a bifunctional matrix for facile construction of label-free and ultrasensitive electrochemical cTnI immunosensor. ACS Appl. Bio. Mater. 2024, 7, 5258–5267. [Google Scholar]
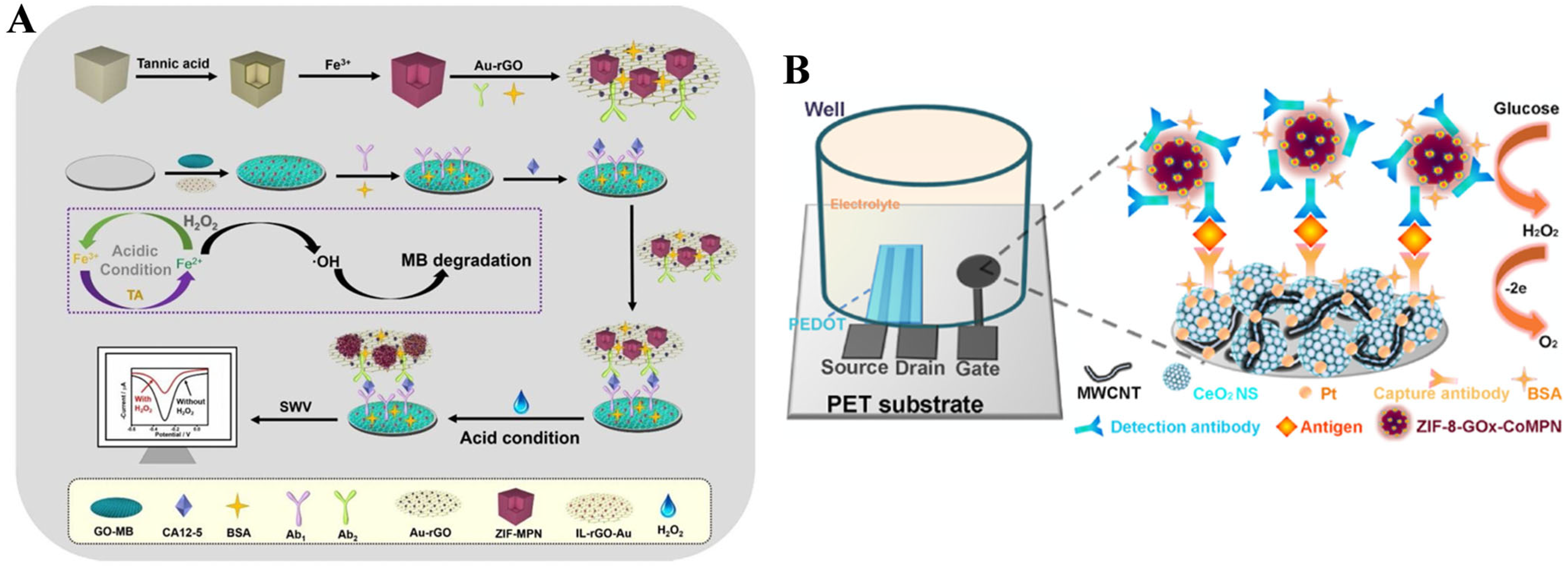
- Zhang, N.; Xu, Y.; Ma, Z. Signal amplification based on tannic acid-assisted cyclic conversion of Fe(III)/Fe(II) for ultrasensitive electrochemical immunoassay of CA 12-5. Sens. Actuators B: Chem. 2020, 317, 128244. [Google Scholar] [CrossRef]
- Liu, J.; Kong, T.; Xiao, Y.; Bai, L.; Chen, N.; Tang, H. Organic electrochemical transistor-based immuno-sensor using platinum loaded CeO2 nanosphere-carbon nanotube and zeolitic imidazolate framework-enzyme-metal polyphenol network. Biosens. Bioelectron. 2023, 230, 115236. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Qi, H.; Zhang, C. Recent advances and challenges in developing electrochemiluminescence biosensors for health analysis. Chem. Commun. 2023, 59, 3507–3522. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cheng, W.; Liu, C.; Zhao, M.; Ding, S.; Deng, Z. Coordination-induced self-assembly based carbon dot dendrimers as efficient signal labels for electrochemiluminescent immunosensor construction. Talanta 2023, 254, 124101. [Google Scholar] [CrossRef]
- Wang, L.; Song, K.; Jiang, C.; Liu, S.; Huang, S.; Yang, H.; Li, X.; Zhao, F. Metal-coordinated polydopamine structures for tumor imaging and therapy. Adv. Healthc. Mater. 2024, 13, e2401451. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-containing nanoparticles: Synthesis, properties, and therapeutic delivery. Adv. Mater. 2021, 33, e2007356. [Google Scholar]
- Wang, X.; Fan, Y.; Yan, J.; Yang, M. Engineering polyphenol-based polymeric nanoparticles for drug delivery and bioimaging. Chem. Eng. J. 2022, 439, 135661. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; He, M.; Wang, G.; Zhao, S. Remodeling the tumor microenvironment via metal-phenolic network-coated poly (lactic acid-co-glycolic acid) nanoparticles for inducing multimodal combination therapy in non-small cell lung cancer. ACS Appl. Bio. Mater. 2025, 8, 6066–6078. [Google Scholar]
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal ion/tannic acid assembly as a versatile photothermal platform in engineering multimodal nanotheranostics for advanced applications. ACS Nano. 2018, 12, 3917–3927. [Google Scholar] [CrossRef]
- Yang, L.; Han, L.; Jia, L. A novel platelet-repellent polyphenolic surface and its micropattern for platelet adhesion detection. ACS Appl. Mater. Interfaces 2016, 8, 26570–26577. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, J.; Nkanga, C.I.; Jin, Z.; He, T.; Borum, R.M.; Yim, W.; Zhou, J.; Cheng, Y.; Xu, M.; et al. One-step supramolecular multifunctional coating on plant virus nanoparticles for bioimaging and therapeutic applications. ACS Appl. Mater. Interfaces 2022, 14, 13692–13702. [Google Scholar] [CrossRef]
- Wang, R.; Kim, S.H.; Sun, F.; Zheng, X.; Jiang, F.; Wang, X.; Diao, B.; Zhang, H.; Li, X.; Li, R.; et al. Bio-inspired hydrogen bonding cross-linking strategy for DIW-printed carbon-based conductive hydrogels in wearable self-powered sensing systems. ACS Appl. Electron. Mater. 2025, 7, 1217–1229. [Google Scholar] [CrossRef]
- Liu, T.; Wu, Q.; Liu, H.; Zhao, X.; Yi, X.; Liu, J.; Nong, Z.; Zhou, B.; Wang, Q.; Liu, Z. A crosslinked eutectogel for ultrasensitive pressure and temperature monitoring from nostril airflow. Nat. Commun. 2025, 16, 3334. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Yin, M.; Yang, K.; Wang, S.; Pei, Y.; Luo, R.; Zhou, S.; Li, H. Ultrafast polymerization of a self-adhesive and strain sensitive hydrogel-based flexible sensor for human motion monitoring and handwriting recognition. Polymers 2024, 16, 1595. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, G.; Wang, H.; Du, K.; Guo, J.; Shang, Z.; Guo, R.; Zhou, F.; Liu, W. Ultra-stretchable composite organohydrogels polymerized based on MXene@tannic acid-Ag autocatalytic system for highly sensitive wearable sensors. Small 2024, 20, e2404435. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-healing, wet-adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Fu, Y.; Wang, N.N.; Zhan, S.Q.; Sheng, L.; Yang, H.Y.; Liu, C. Healable and transparent ionic conductive hydrogels based on PNATF as multiple-signal sensors. ACS Appl. Polym. Mater. 2025, 7, 2529–2540. [Google Scholar] [CrossRef]
- Mi, Y.; Wu, T.; Lu, Y.; Wang, Y.; Ma, Z.; Cao, X.; Wang, N. Multifunctional ionic hydrogels with biomimetic stability: Synergistic design and versatile self-powered sensing applications. Chem. Eng. J. 2025, 518, 164460. [Google Scholar] [CrossRef]
- Khan, A.; Kisannagar, R.R.; Mahmood, S.; Chuang, W.T.; Katiyar, M.; Gupta, D.; Lin, H.C. Intrinsically stretchable conductive self-healable organogels for strain, pressure, temperature, and humidity sensing. ACS Appl. Mater. Interfaces 2023, 15, 42954–42964. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Liang, S.; Deng, D.; Chang, Y.; Yi, X. Metal–Phenolic Networks for Sensing Applications. Biosensors 2025, 15, 600. https://doi.org/10.3390/bios15090600
Xia N, Liang S, Deng D, Chang Y, Yi X. Metal–Phenolic Networks for Sensing Applications. Biosensors. 2025; 15(9):600. https://doi.org/10.3390/bios15090600
Chicago/Turabian StyleXia, Ning, Sirui Liang, Dehua Deng, Yong Chang, and Xinyao Yi. 2025. "Metal–Phenolic Networks for Sensing Applications" Biosensors 15, no. 9: 600. https://doi.org/10.3390/bios15090600
APA StyleXia, N., Liang, S., Deng, D., Chang, Y., & Yi, X. (2025). Metal–Phenolic Networks for Sensing Applications. Biosensors, 15(9), 600. https://doi.org/10.3390/bios15090600





