Role of Polymeric Stabilizing Agents as a Molecular Spacer in Gold Nanoparticle-Mediated FRET-Based Biosensing
Abstract
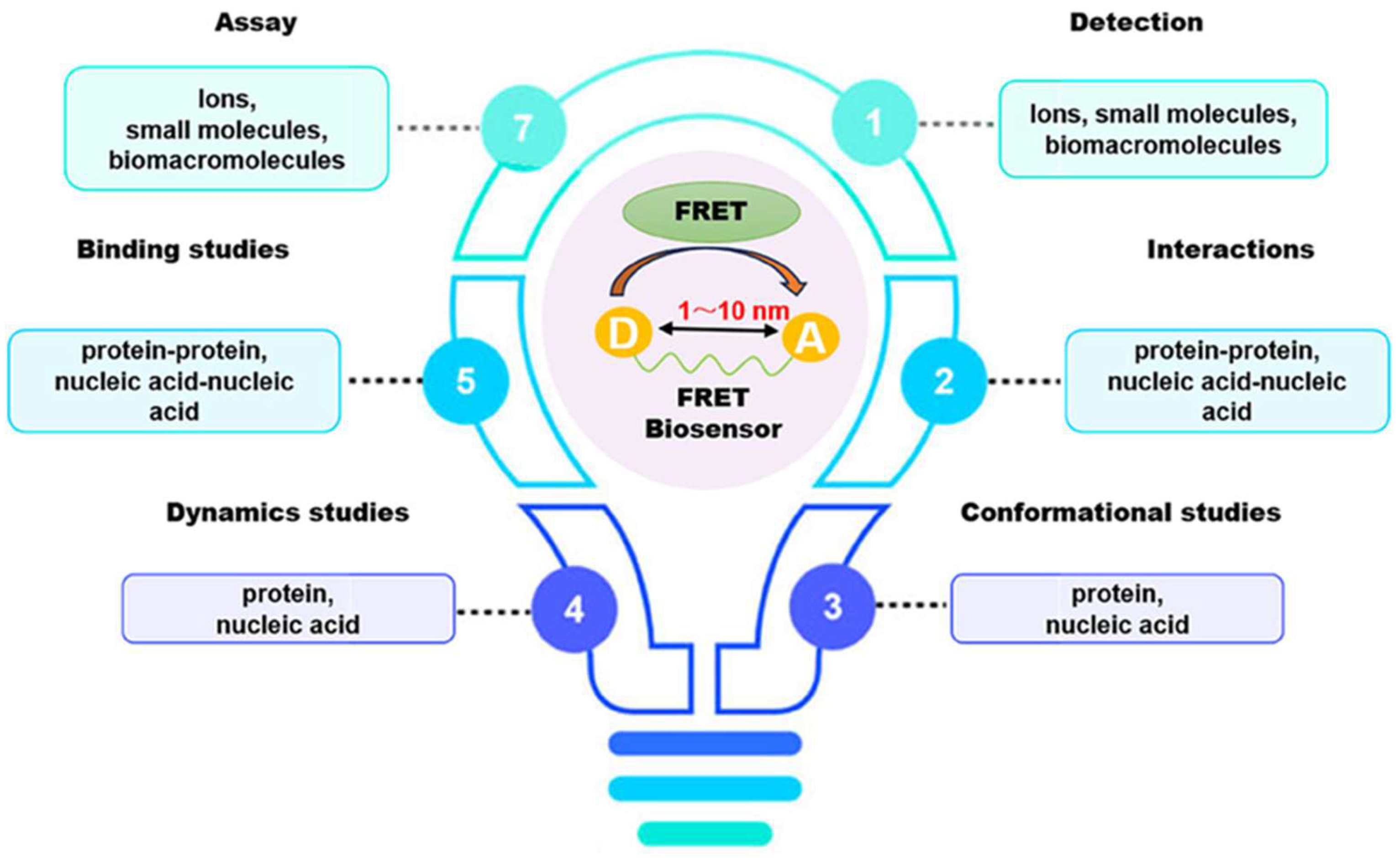
1. Introduction
2. Molecular Spacers in Fluorescence Resonance Energy Transfer
2.1. Type of Molecular Spacers, Mechanisms, Advantages, and Limitations
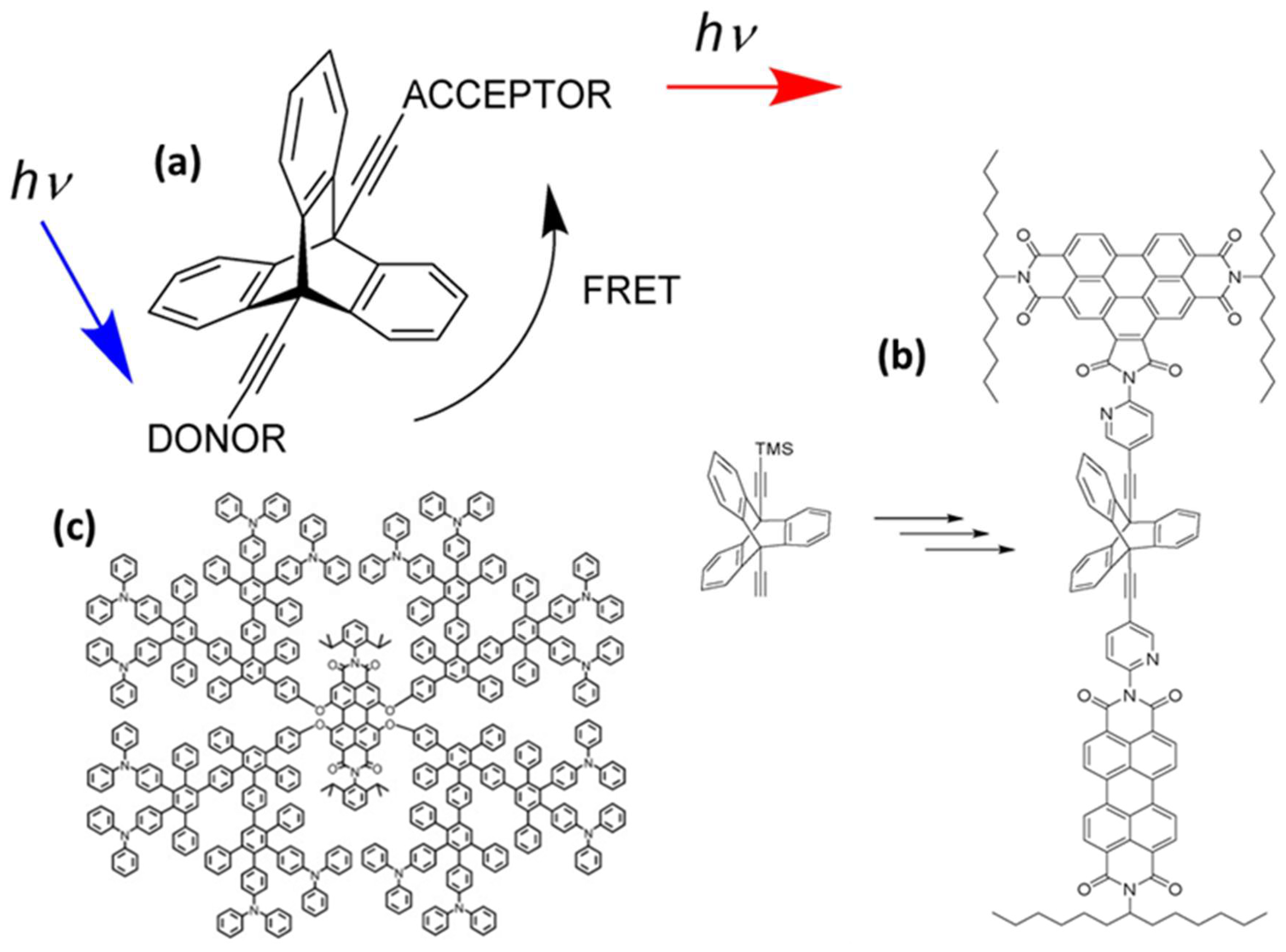
2.1.1. Rigid Spacers
2.1.2. Advantages of Inflexible Spacers
2.1.3. Drawbacks of Rigid Spacers
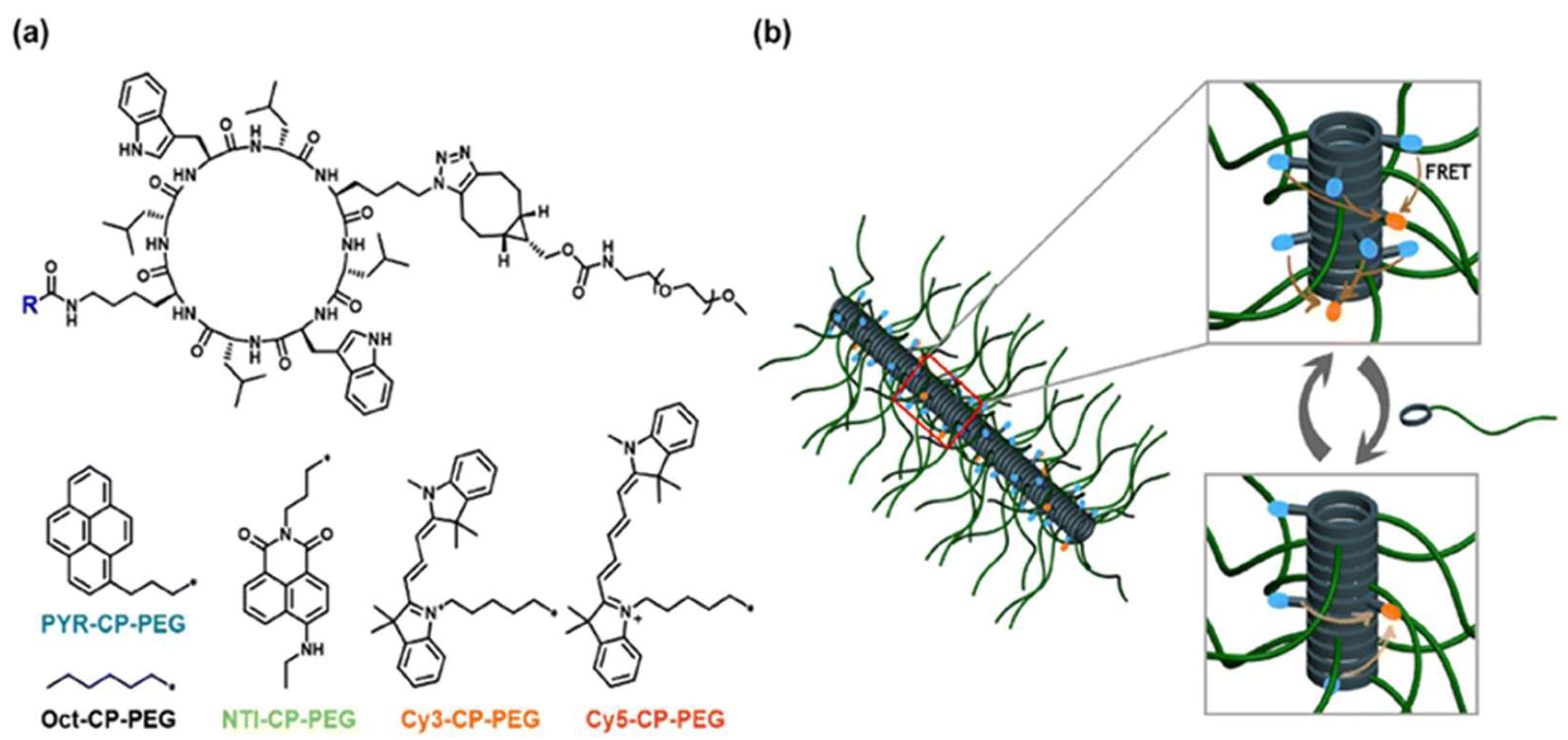
2.2. Flexible/Polymeric Spacers
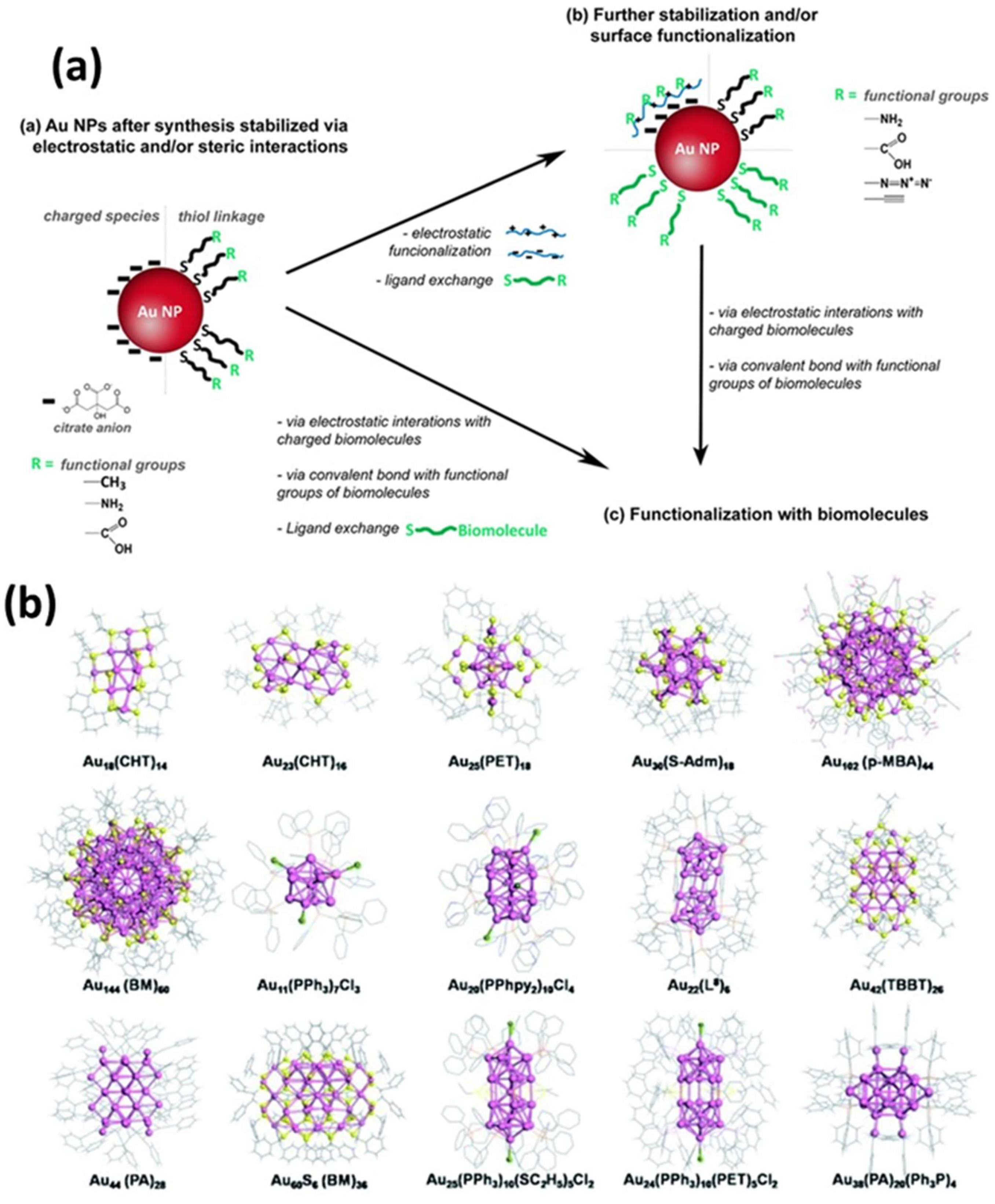
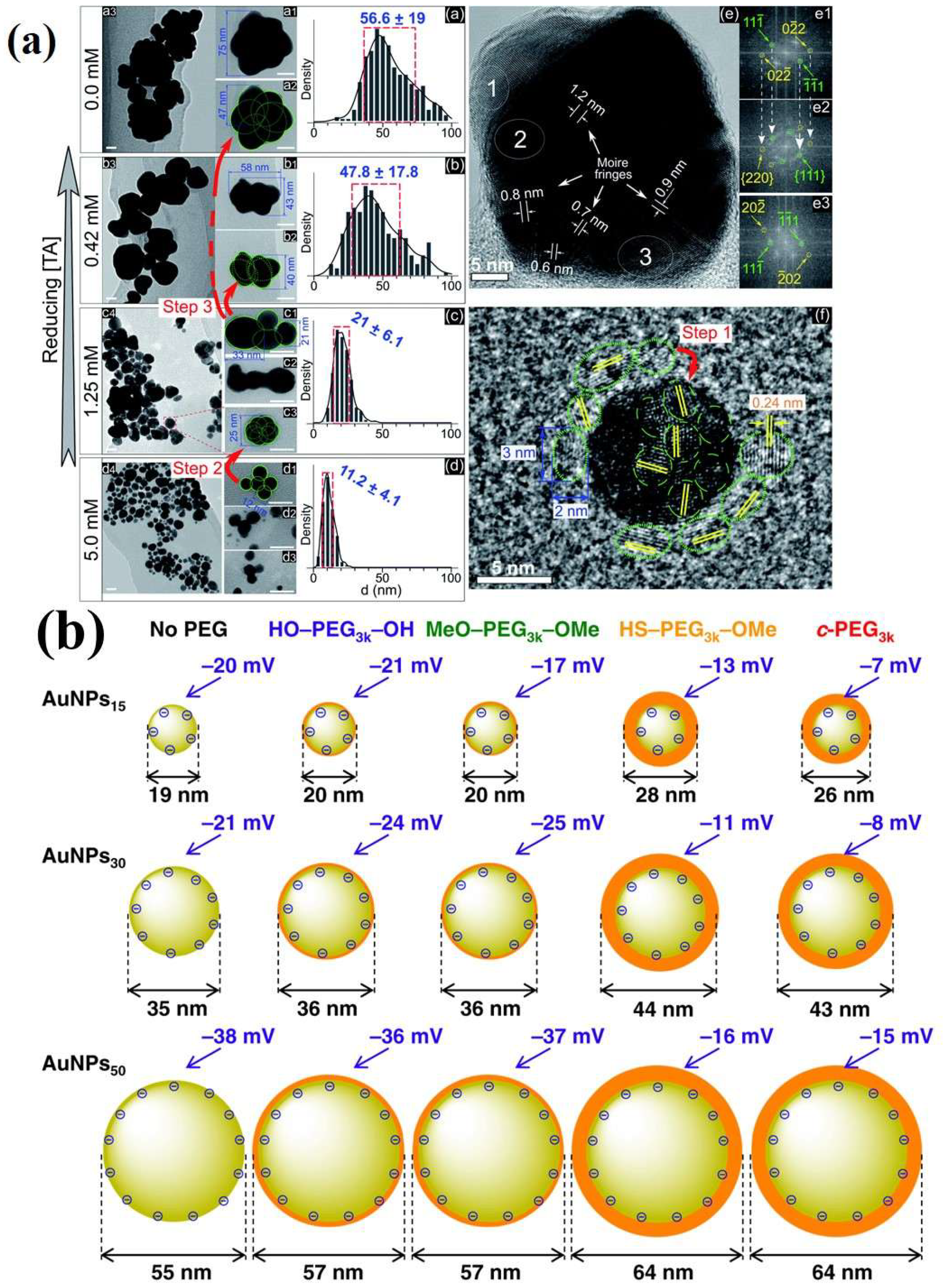
3. Surface Functionalization Strategies for Gold Nanoparticles
| Polymer | Function | Chemical Structure of Polymer | References |
|---|---|---|---|
| Poly-amidoamine (PAMAM) | Amine groups and amide groups. Dendrimer imparts a steric framework. | 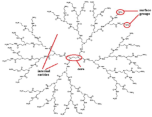 | [75,76,77,78,79,80] |
| Poly(3,4-ethylene dioxythiophene) | Reducing and immobilizing agent | 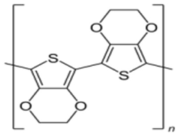 | [81,82,83] |
| Trioctylamine | Stabilizers | 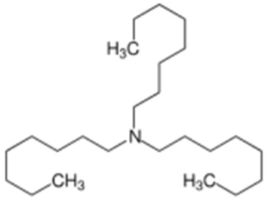 | [83] |
| Poly(N-vinylpyrrolidone) (PVP) | Steric stabilization | 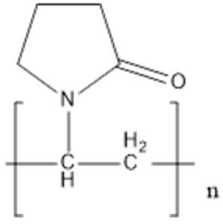 | [84,85,86,87,88,89,90] |
| Poly(ethylene) glycol (PEG) | Stabilizer | 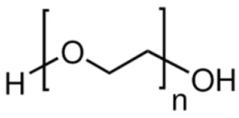 | [91,92,93,94] |
| Poly(vinylcaprolactame) (PVCL) | stabilizer | 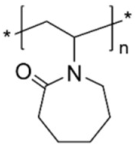 | [85] |
| Poly(diallyldimethylammonium chloride) (PDADMAC) | Polycationic stabilizer |  | [95] |
| Polyethyleneimine (PEI) | Cationic stabilizer |  | [96,97,98,99,100,101,102] |
| Polyvinyl alcohol | Reducing and stabilizing agent | 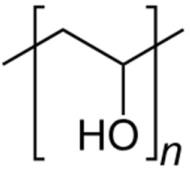 | [103,104,105,106,107] |
| Poly[2-hydroxy-3-(naphthalen-1-ylamino)propyl methacrylate] (PHNA) | Stabilizing agent | 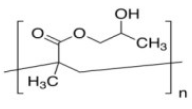 | [108] |
| Chitosan | Stabilizing agent | 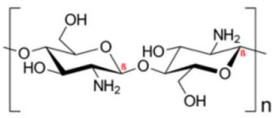 | [109,110,111,112,113,114,115,116,117] |
| 3-Aminopropyltrimethoxy silane (3 APTMS) | Stabilizing agent | 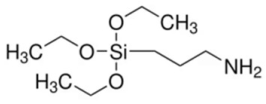 | [118,119] |
| Pullulan | Stabilizing agent | 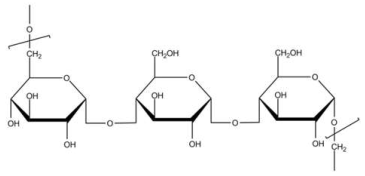 | [120] |
| Pectin | Stabilizing agent | 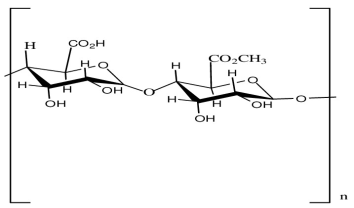 | [121] |
| Polyaniline | Stabilizing agent (Conducting polymer) |  | [122] |
| Poly-Indole | Stabilizing agent (Conducting polymer) | 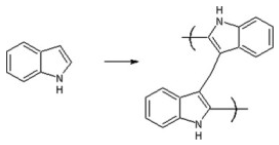 | [123] |
4. Polymer-Stabilized Gold Nanoparticles in Fluorometric Sensing
| Polymeric Stabilizer | Sensing Mechanism | Analyte | Detection Limit | Quenching/ Enhancement | Quenching/ Enhancement Efficiency | pH/Temperature | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polyethyleneimine (PEI) | FRET | Glutathione | 7.8 nM | Enhancement | 95% | 7.2/RT | Selectivity | [100] |
| PEI | FRET | Polymyxin B | 8.5 nM | Enhancement | 82% | 7.2/RT | Interaction with other biological molecules, such as BSA | [99] |
| β-cyclodextrin | FRET/PET | Dopamine | 20.0 nM | Quenching | Significant | 8.0 | NA | [157] |
| Bovine Serum Albumin | FRET | Dopamine | 1.8 nM | Quenching | Significant | 7.5 | Interference | [158] |
| Peptides | FRET | Histone deacetylase and protein tyrosine phosphatase 1B | 1 nM to 28 pM and 0.015 to 0.3 nM/0.8 pM | Quenching | Significant | 8.0 | NA | [159] |
| Poly(9,9-bis(4′-sulfnoatobutyl) fluorene-co-alt-1,4-phenylene) | FRET | Cysteine | 25 nM | Quenching | Significant | 6.0 | NA | [160] |
| PEI | FRET | Doxorubicin | 5 pM | Quenching | Significant | 7.0 | NA | [161] |
| Polydiallyldimethylammonium | FRET | Ascorbic acid | 50 nM | Enhancement | Significant | 7.0 | NA | [161] |
| mCherryProtein | FRET | Melamine | 28 µM | Quenching | 95–97% | 8.0/RT | Explored only for bio-thiols | [162] |
| Poly N, N-dimethylacrylamide | FRET | L-cysteine | 20.0–80.0 µM | Quenching | 85% | 7.5/RT | In vitro application | [163] |
| poly(N-isopropylacrylamide-co-2-(dimethylamino)ethylmethacrylate) (P(NIPAM-co-DMA)) | FRET | Hg2+ | 31 nM | Quenching | 92% | 6.0 | NA | [164] |
| Polyethylene glycol (PEG) | FRET | Hg2+ | 2.24 nM | Enhancement | High | 5.65/RT | NA | [165] |
| Poly(N-isopropylacrylamide) | Photoluminescence quenching | Hg2+ | 1.9 and 1.7 nM | Quenching | Moderate | 7.0/RT | Not explored in biological condition | [166] |
| BSA | IFE | Uric acid | 0.39 μM | Quenching | High | NA | NA | [167] |
| Antibody-tagged bovine serum albumin | IFE | Troponin I | 0.51 ng/mL | Quenching | 90% | 7.2/RT | NA | [168] |
| 6-Deoxy-6-mercapto-β-cyclodextrin | IFE | Chloretetracyclin | 2.7 nM | Quenching | High | 8.0/RT | NA | [169] |
| PEI-OVA-AuNCs | IFE | tetracycline | 0.563 μM | Quenching | High | RT | Binding of phenolic hydroxyl and ketone carbonyl groups | [170] |
5. Challenges That Need to Be Addressed While Choosing a Suitable Polymeric Spacer for Efficient FRET, IFE, and NSET-Based Sensors
- (1)
- Quenching effects: For example, AuNPs can diminish fluorescence, affecting probe efficiency. Balancing the distance between the fluorophore and the AuNPs is important for mitigating quenching while enhancing the signal.
- (2)
- Stability: Establishing a stable bond between AuNPs and the polymeric spacer is difficult, necessitating strong conjugation techniques.
- (3)
- Uniformity: Achieving monodispersity and preparing a polymer coating are challenging, which impacts probe performance.
- (4)
- Biocompatibility: Materials must be nontoxic and biocompatible, particularly for in vivo applications, which limit polymer options.
- (5)
- Spacer length control: Precisely designing the length of the polymeric spacers is essential for optimizing distance-dependent interactions.
- (6)
- Surface functionalization: Attaching recognition elements while maintaining optical properties and stability is a complex task.
- (7)
- Characterization: Accurately analyzing the structure and properties of the conjugate is challenging because of system complexity.
- (8)
- Scalability: Developing cost-effective production processes is difficult, given the need for high-quality materials.
- (9)
- Environmental sensitivity: Probe performance may be influenced by changes in pH, temperature, or ionic strength.
- (10)
- Photo stability: Maintaining long-term stability under continuous illumination is challenging because the components may undergo photo-induced changes.
- (11)
- Multiplexing: Creating probes that can detect multiple targets while retaining their functionality is a complex process.
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, D.L.; Bradshaw, D.S.; Dinshaw, R.; Scholes, G.D. Resonance energy transfer. In Photonics: Biomedical Photonics, Spectroscopy, and Microscopy, IV; Wiley: Hoboken, NJ, USA, 2015; pp. 101–127. [Google Scholar]
- Van Der Meer, B.W. Resonance Energy Transfer: Theory and Data; Van Der Meer, B.W., Coker, G., III, Simon Chen, S.Y., Eds.; Wiley: Hoboken, NJ, USA, 1994; Available online: https://www.amazon.com/Resonance-Energy-Transfer-Theory-Data/dp/0471185892 (accessed on 22 February 2025).
- Andrews, D.L.; Bradshaw, D.S. Virtual photons, dipole fields and energy transfer: A quantum electrodynamical approach. Eur. J. Phys. 2004, 25, 845. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Fernández-Ramos, M.D.; Capitán-Vallvey, L.F. Fluorescence resonance energy transfer disposable sensor for copper (II). Anal. Chim. Acta 2006, 555, 299–307. [Google Scholar] [CrossRef]
- Andrews, D.L. A unified theory of radiative and radiation less molecular energy transfer. Chem. Phys. 1989, 135, 195–201. [Google Scholar] [CrossRef]
- Mooney, A.M. CC BY-SA 3.0. Available online: https://commons.wikimedia.org/w/index.php?curid=23197114 (accessed on 12 May 2025).
- Clegg, R.M. Fluorescence resonance energy transfer. Curr. Opin. Biotechnol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000, 7, 730–734. [Google Scholar] [CrossRef]
- Forster, T. Energiewanderung und fluoreszenz. Naturwissenschaften 1946, 33, 166–175. [Google Scholar] [CrossRef]
- Ha, T.; Enderle, T.; Ogletree, D.F.; Chemla, D.S.; Selvin, P.R.; Weiss, S. Probing the interaction between two single molecules: Fluorescence resonance energy transfer between a single donor and a single acceptor. Proc. Natl. Acad. Sci. USA 1996, 93, 6264–6268. [Google Scholar] [CrossRef]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Yang, W.C.; Li, S.Y.; Ni, S.; Liu, G. Advances in FRET-based biosensors from donor-acceptor design to applications. Aggregate 2024, 5, e460. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor–acceptor combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, B. Effect of Polymer Spacer Length in FRET-Based Fluorescent Donor-Acceptor Sensing System. APS March Meet. 2019, C50.005. Available online: https://ui.adsabs.harvard.edu/abs/2019APS..MARC50005P/abstract (accessed on 12 May 2025).
- Yu, H.; Xiao, Y.; Guo, H.; Qian, X. Convenient and Efficient FRET Platform Featuring a Rigid Biphenyl Spacer between Rhodamine and BODIPY: Transformation of ‘Turn-On’Sensors into Ratiometric Ones with Dual Emission. Chem. A Eur. J. 2011, 17, 3179–3191. [Google Scholar] [CrossRef]
- Deniz, A.A.; Maxime, D.; Jocelyn, R.G.; Taekjip, H.; Ann, E.F.; Daniel, S.C.; Shimon, W.; Peter, G.S. Single-pair fluorescence resonance energy transfer on freely diffusing molecules: Observation of Förster distance dependence and subpopulations. Proc. Natl. Acad. Sci. USA 1999, 96, 3670–3675. [Google Scholar] [CrossRef]
- Sekar, R.B.; Periasamy, A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 2003, 160, 629. [Google Scholar] [CrossRef] [PubMed]
- Schuler, B. Single-molecule FRET of protein structure and dynamics—A primer. J. Nanobiotechnol. 2013, 11 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Sahoo, H. Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Langhals, H.; Dietl, C.; Mayer, P. FRET in Orthogonal, Increasingly Strain-Rigidified Systems. Isr. J. Chem. 2022, 62, e202100021. [Google Scholar] [CrossRef]
- Qu, J.; Pschirer, N.G.; Liu, D.; Stefan, A.; De Schryver, F.C.; Müllen, K. Dendronized perylenetetracarboxdiimides with peripheral triphenylamines for intramolecular energy and electron transfer. Chem. A Eur. J. 2004, 10, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.H.; Luo, J.; Mao, Y.L.; Lai, S.; Gong, Y.N.; Zhong, D.C.; Lu, T.B. π-π stacking interactions: Non-negligible forces for stabilizing porous supramolecular frameworks. Sci. Adv. 2020, 6, eaax9976. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Stryer, L.; Richard, P.H. Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef]
- Tang, F.K.; Chen, Y.; Nnaemaka Tritton, D.; Cai, Z.; Cham-Fai Leung, K. A Piperazine Linked Rhodamine-BODIPY FRET-based Fluorescent Sensor for Highly Selective Pd2+ and Biothiol Detection. Chem. Asian J. 2023, 18, e202300477. [Google Scholar] [CrossRef]
- Zhu, K.; Lu, H.; Xue, Q.; Zhou, F.; Guo, W.; Sun, C.; Duan, X. A FET-based flexible biosensor system for dynamic behavior observation of lipid membrane with nanoparticles in vitro. Lab Chip 2025, 25, 393–402. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wu, W.; Liu, H.; Xu, C.; Zhang, T. DNA Crossover Flexibilities upon Discrete Spacers Revealed by Single-Molecule FRET. Soft Matter 2024, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.S.; Yashmeen, A. FRET Events in Fluorescent Pentapeptides Containing Aliphatic Triazolo Amino Acid Scaffolds: Role of Spacer Lengths. J. Photochem. Photobiol. A Chem. 2019, 378, 171–183. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, J.; Yu, X.; Cheng, Z.; Yang, J.; Hall, S.C.L.; Perrier, S. Tailoring the Luminescence of FRET Systems Built Using Supramolecular Polymeric Nanotubes. Polym. Chem. 2022, 13, 4366–4371. [Google Scholar] [CrossRef]
- Wu, Y.; Tianyu, J. Developments in FRET-and BRET-based biosensors. Micromachines 2022, 13, 1789. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Li, G.; Jin, R. Atomically Precise Gold Nanoclusters as New Model Catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef]
- Jin, R. Atomically Precise Metal Nanoclusters: Stable Sizes and Optical Properties. Nanoscale 2014, 7, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Huston, A.L.; Shabaev, A.; Efros, A.; Currie, M.; Susumu, K.; Bussmann, K.; Goswami, R.; Fatemi, F.K.; Medintz, I.L. Energy transfer sensitization of luminescent gold nanoclusters: More than just the classical Förster mechanism. Sci. Rep. 2016, 6, 35538. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Donkers, R.L.; Wang, G.; Harper, A.S.; Murray, R.W. Electrochemistry and optical absorbance and luminescence of molecule-like Au38 nanoparticles. J. Am. Chem. Soc. 2004, 126, 6193–6199. [Google Scholar] [CrossRef]
- Wang, G.; Huang, T.; Murray, R.W.; Menard, L.; Nuzzo, R.G. Near-IR Luminescence of Monolayer-Protected Metal Clusters. J. Am. Chem. Soc. 2004, 127, 812–813. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Z.; Lee, K.; Chang, H. Synthesis of Highly Fluorescent Gold Nanoparticles for Sensing Mercury (II). Angew. Chem. Int. Ed. 2007, 46, 6824–6828. [Google Scholar] [CrossRef]
- Shang, L.; Azadfar, N.; Stockmar, F.; Send, W.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. One-Pot Synthesis of Near-Infrared Fluorescent Gold Clusters for Cellular Fluorescence Lifetime Imaging. Small 2011, 7, 2614–2620. [Google Scholar] [CrossRef]
- Bigioni, T.P.; Whetten, R.L.; Dag, Ö. Near-Infrared Luminescence from Small Gold Nanocrystals. J. Phys. Chem. B 2000, 104, 6983–6986. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected gold clusters revisited: Bridging the gap between gold (I)− thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Muhammed, M.A.H.; Verma, P.K.; Pal, S.K.; Retnakumari, A.; Koyakutty, M.; Nair, S.; Pradeep, T. Luminescent Quantum Clusters of Gold in Bulk by Albumin-Induced Core Etching of Nanoparticles: Metal Ion Sensing, Metal-Enhanced Luminescence, and Biolabeling. Chem. Eur. J. 2010, 16, 10103–10112. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Nie, S. Etching Colloidal Gold Nanocrystals with Hyperbranched and Multivalent Polymers: A New Route to Fluorescent and Water-Soluble Atomic Clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Muhammed, M.a.H.; Palui, G.; Zhan, N.; Mattoussi, H. Growth of Highly Fluorescent Polyethylene Glycol- and Zwitterion-Functionalized Gold Nanoclusters. ACS Nano 2013, 7, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Fatemi, F.K.; Currie, M.; Delehanty, J.B.; Pons, T.; Fragola, A.; Lévêque-Fort, S.; Goswami, R.; Susumu, K.; Huston, A.L.; et al. PEGYlated Luminescent Gold Nanoclusters: Synthesis, Characterization, Bioconjugation, and Application to One- and Two-Photon Cellular Imaging. Part. Part. Syst. Charact. 2013, 30, 453–466. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Li, L.; Zhang, G.; Hussain, I.; Li, Z.; Tan, B. Photoreductive Synthesis of Water-Soluble Fluorescent Metal Nanoclusters. Chem. Commun. 2011, 48, 567–569. [Google Scholar] [CrossRef]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. Polymer@ gold nanoparticles prepared via RAFT polymerization for opto-biodetection. Polymers 2018, 10, 189. [Google Scholar] [CrossRef]
- Xia, N.; Wu, Z. Controlling ultrasmall gold nanoparticles with atomic precision. Chem. Sci. 2021, 12, 2368–2380. [Google Scholar] [CrossRef]
- Wilcoxon, J.P.; Martin, J.E.; Schaefer, D.W. Aggregation in Colloidal Gold. Phys. Review. Gen. Phys. 1989, 39, 2675–2688. [Google Scholar] [CrossRef]
- Chow, M.K.; Zukoski, C.F. Gold Sol Formation Mechanisms: Role of Colloidal Stability. J. Colloid Interface Sci. 1994, 165, 97–109. [Google Scholar] [CrossRef]
- Thai, V.-P.; Nguyen, H.D.; Saito, N.; Takahashi, K.; Sasaki, T.; Kikuchi, T. Precise Size-Control and Functionalization of Gold Nanoparticles Synthesized by Plasma–Liquid Interactions: Using Carboxylic, Amino, and Thiol Ligands. Nanoscale Adv. 2022, 4, 4490–4501. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Xu, L.; Li, J.; Yang, W. Conformational Change Induced Reversible Assembly/Disassembly of Poly-L-Lysine-Functionalized Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 9172–9176. [Google Scholar] [CrossRef]
- Horovitz, O.; Mocanu, A.; Tomoaia, G.; Bobos, L.; Dubert, D.; Daian, I.; Yusanis, T.; Tomoaia-Cotisel, M. Lysine mediated assembly of gold nanoparticles. Stud. Univ. Babes-Bolyai Chem. 2007, 52, 97–108. [Google Scholar]
- Murthy, V.S.; Cha, J.N.; Stucky, G.D.; Wong, M.S. Charge-driven flocculation of poly (L-lysine) gold nanoparticle assemblies leading to hollow microspheres. J. Am. Chem. Soc. 2004, 126, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Y.; Xie, R.; Zhuang, J.; Yang, W.; Li, T. Three-Dimensional Assembly of Au Nanoparticles Using Dipeptides. Nanotechnology 2002, 13, 725–728. [Google Scholar] [CrossRef]
- Lim, I.-I.S.; Ip, W.; Crew, E.; Njoki, P.N.; Mott, D.; Zhong, C.-J.; Pan, Y.; Zhou, S. Homocysteine-Mediated Reactivity and Assembly of Gold Nanoparticles. Langmuir 2006, 23, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Luhrs, C.C.; Pérez-Luna, V.H. Controlled and Reversible Aggregation of Biotinylated Gold Nanoparticles with Streptavidin. J. Phys. Chem. B 2004, 108, 15631–15639. [Google Scholar] [CrossRef]
- Lazarides, A.A.; Schatz, G.C. DNA-Linked Metal Nanosphere Materials: Structural Basis for the Optical Properties. J. Phys. Chem. B 1999, 104, 460–467. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-Based Method for Rationally Assembling Nanoparticles into Macroscopic Materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Thanh, N.T.; Green, L.A. Functionalization of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Wang, Y.; Quinsaat, J.E.Q.; Ono, T.; Maeki, M.; Tokeshi, M.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.-I.; Miura, Y.; et al. Enhanced Dispersion Stability of Gold Nanoparticles by the Physisorption of Cyclic Poly (Ethylene Glycol). Nat. Commun. 2020, 11, 6089. [Google Scholar] [CrossRef]
- Lévy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.S.; Krack, M.; Aleksandrovic, V.; Kornowski, A.; Förster, S.; Weller, H. Tailor-Made Ligands for Biocompatible Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 6577–6580. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Chairam, S.; Somsook, E. Starch Vermicelli Template for Synthesis of Magnetic Iron Oxide Nanoclusters. J. Magn. Magn. Mater. 2008, 320, 2039–2043. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and Albumin Derivatised Iron Oxide Nanoparticles: Influence on Fibroblasts in Vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Park, J.-H.; Im, K.-H.; Lee, S.-H.; Kim, D.-H.; Lee, D.-Y.; Lee, Y.-K.; Kim, K.-M.; Kim, K.-N. Preparation and Characterization of Magnetic Chitosan Particles for Hyperthermia Application. J. Magn. Magn. Mater. 2005, 293, 328–333. [Google Scholar] [CrossRef]
- Bao, Y.; Zhong, C.; Vu, D.M.; Temirov, J.P.; Dyer, R.B.; Martinez, J.S. Nanoparticle-Free Synthesis of Fluorescent Gold Nanoclusters at Physiological Temperature. J. Phys. Chem. C 2007, 111, 12194–12198. [Google Scholar] [CrossRef]
- Oh, E.; Hong, M.-Y.; Lee, D.; Nam, S.-H.; Yoon, H.C.; Kim, H.-S. Inhibition Assay of Biomolecules Based on Fluorescence Resonance Energy Transfer (FRET) between Quantum Dots and Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 3270–3271. [Google Scholar] [CrossRef]
- Mohammadi, S.; Salimi, A.; Hamd-Ghadareh, S.; Fathi, F.; Soleimani, F. A FRET Immunosensor for Sensitive Detection of CA 15-3 Tumor Marker in Human Serum Sample and Breast Cancer Cells Using Antibody Functionalized Luminescent Carbon-Dots and AuNPs-Dendrimer Aptamer as Donor-Acceptor Pair. Anal. Biochem. 2018, 557, 18–26. [Google Scholar] [CrossRef]
- Mardani, H.; Roghani-Mamaqani, H.; Shahi, S.; Roustanavi, D. Anti-Counterfeiting Inks Based on Förster Resonance Energy Transfer in Microcrystalline Cellulose-Grafted Poly (Amidoamine) for Artificial Industries. ACS Appl. Polym. Mater. 2023, 5, 1092–1102. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.-Q.; Li, L.; Li, Y.-X.; Yu, L.-P.; Chen, Y. Ratiometric Fluorescence Sensor for Point-of-Care Testing of Bilirubin Based on Tetraphenylethylene Functionalized Polymer Nanoaggregate and Rhodamine B. Sens. Actuators B Chem. 2022, 369, 132392. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.-T.; Wang, Y.-T.; Zhang, T.-J.; Zheng, X.; Chen, L.; Li, S.; Tian, X.; Zhang, D.; Wang, Y. A Hydrogel-Based Ratiometric Fluorescent Sensor Relying on Rhodamine B Labelled AIE-Featured Hyperbranched Poly (Amido Amine) for Heparin Detection. Anal. Chim. Acta 2024, 1300, 342466. [Google Scholar] [CrossRef]
- Kim, M.; Iezzi, R., Jr.; Shim, B.S.; Martin, D.C. Impedimetric biosensors for detecting vascular endothelial growth factor (VEGF) based on poly (3, 4-ethylene dioxythiophene) (PEDOT)/gold nanoparticle (Au NP) composites. Front. Chem. 2019, 7, 234. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Sun, D.; Zhang, R.; Lin, W.-F.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold Nanoparticle-Polymer Nanocomposites Synthesized by Room Temperature Atmospheric Pressure Plasma and Their Potential for Fuel Cell Electrocatalytic Application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Tan, Y.; Yang, C.; Li, Y. Self-Assembly of Gold Nanoparticles Prepared with 3,4-Ethylenedioxythiophene as Reductant. J. Phys. Chem. B 2004, 108, 5192–5199. [Google Scholar] [CrossRef]
- Ortega-Córdova, R.; Sánchez-Carillo, K.; Carrasco-Saavedra, S.; Ramírez-García, G.; Pérez-García, M.G.; Soltero-Martínez, J.F.A.; Mota-Morales, J.D. Polyvinylpyrrolidone-Mediated Synthesis of Ultra-Stable Gold Nanoparticles in a Nonaqueous Choline Chloride–Urea Deep Eutectic Solvent. RSC Appl. Interfaces 2024, 1, 600–611. [Google Scholar] [CrossRef]
- Nurakhmetova, Z.A.; Azhkeyeva, A.N.; Klassen, I.A.; Tatykhanova, G.S. Synthesis and Stabilization of Gold Nanoparticles Using Water-Soluble Synthetic and Natural Polymers. Polymers 2020, 12, 2625. [Google Scholar] [CrossRef] [PubMed]
- Trotsiuk, L.; Antanovich, A.; Lizunova, A.; Kulakovich, O. Direct Synthesis of Amphiphilic Polyvinylpyrrolidone-Capped Gold Nanoparticles in Chloroform. Colloids Interface Sci. Commun. 2020, 37, 100289. [Google Scholar] [CrossRef]
- Malmir, M.; Shemirani, F. Gold Nanoparticles Coated with PVP as a Novel Colorimetric Sensor for Sensitive and Selective Determination of Atenolol. Heliyon 2023, 9, e22675. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target Delivery of Doxorubicin Tethered with PVP Stabilized Gold Nanoparticles for Effective Treatment of Lung Cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef]
- Santos-Santos, I.J.; Zamora-Justo, J.A.; Vázquez-Martínez, G.R.; Cabrera-Sierra, R.; Balderas-López, J.A. Synthesis of Gold Nanoparticles Coated with Glucose Oxidase Using PVP as Passive Adsorption Linkage. Front. Nanotechnol. 2024, 6, 1419239. [Google Scholar] [CrossRef]
- Laxmi, R.; Anshuman, N.; Behere, R.P.; Manna, A.; Kuila, B.K. UV Cross-Linked Polymer Stabilized Gold Nanoparticles as a Reusable Dip-Catalyst for Aerobic Oxidation of Alcohols and Cross-Aldol Reactions. ACS Appl. Nano Mater. 2023, 6, 19061–19072. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene Glycol Functionalized Gold Nanoparticles: The Influence of Capping Density on Stability in Various Media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Wang, W.; Wei, Q.-Q.; Wang, J.; Wang, B.-C.; Zhang, S.-H.; Yuan, Z. Role of Thiol-Containing Polyethylene Glycol (Thiol-PEG) in the Modification Process of Gold Nanoparticles (AuNPs): Stabilizer or Coagulant? J. Colloid Interface Sci. 2013, 404, 223–229. [Google Scholar] [CrossRef]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the role and impact of poly (ethylene glycol) (PEG) on nanoparticle formulation: Implications for COVID-19 vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold nanoparticles based optical biosensors for cancer biomarker proteins: A review of the current practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef]
- Nguyen, Q.K.; Hoang, T.H.; Bui, X.T.; Nguyen, T.A.H.; Pham, T.D.; Pham, T.N.M. Synthesis and Application of Polycation-Stabilized Gold Nanoparticles as a Highly Sensitive Sensor for Molecular Cysteine Determination. Microchem. J. 2021, 168, 106481. [Google Scholar] [CrossRef]
- Note, C.; Kosmella, S.; Koetz, J. Poly (Ethyleneimine) as Reducing and Stabilizing Agent for the Formation of Gold Nanoparticles in w/o Microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 150–156. [Google Scholar] [CrossRef]
- Philip, A.; Ankudze, B.; Pakkanen, T.T. Polyethylenimine-Assisted Seed-Mediated Synthesis of Gold Nanoparticles for Surface-Enhanced Raman Scattering Studies. Appl. Surf. Sci. 2018, 444, 243–252. [Google Scholar] [CrossRef]
- Cavuslar, O.; Nakay, E.; Kazakoglu, U.; Abkenar, S.K.; Ow-Yang, C.W.; Acar, H.Y. Synthesis of Stable Gold Nanoparticles Using Linear Polyethyleneimines and Catalysis of Both Anionic and Cationic Azo Dye Degradation. Mater. Adv. 2020, 1, 2407–2417. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gupta, M.K.; Meena, R.; Pandey, P.C.; Narayan, R.J. Molecular Weights of Polyethyleneimine-Dependent Physicochemical Tuning of Gold Nanoparticles and FRET-Based Turn-On Sensing of Polymyxin B. Sensors 2024, 24, 2169. [Google Scholar] [CrossRef]
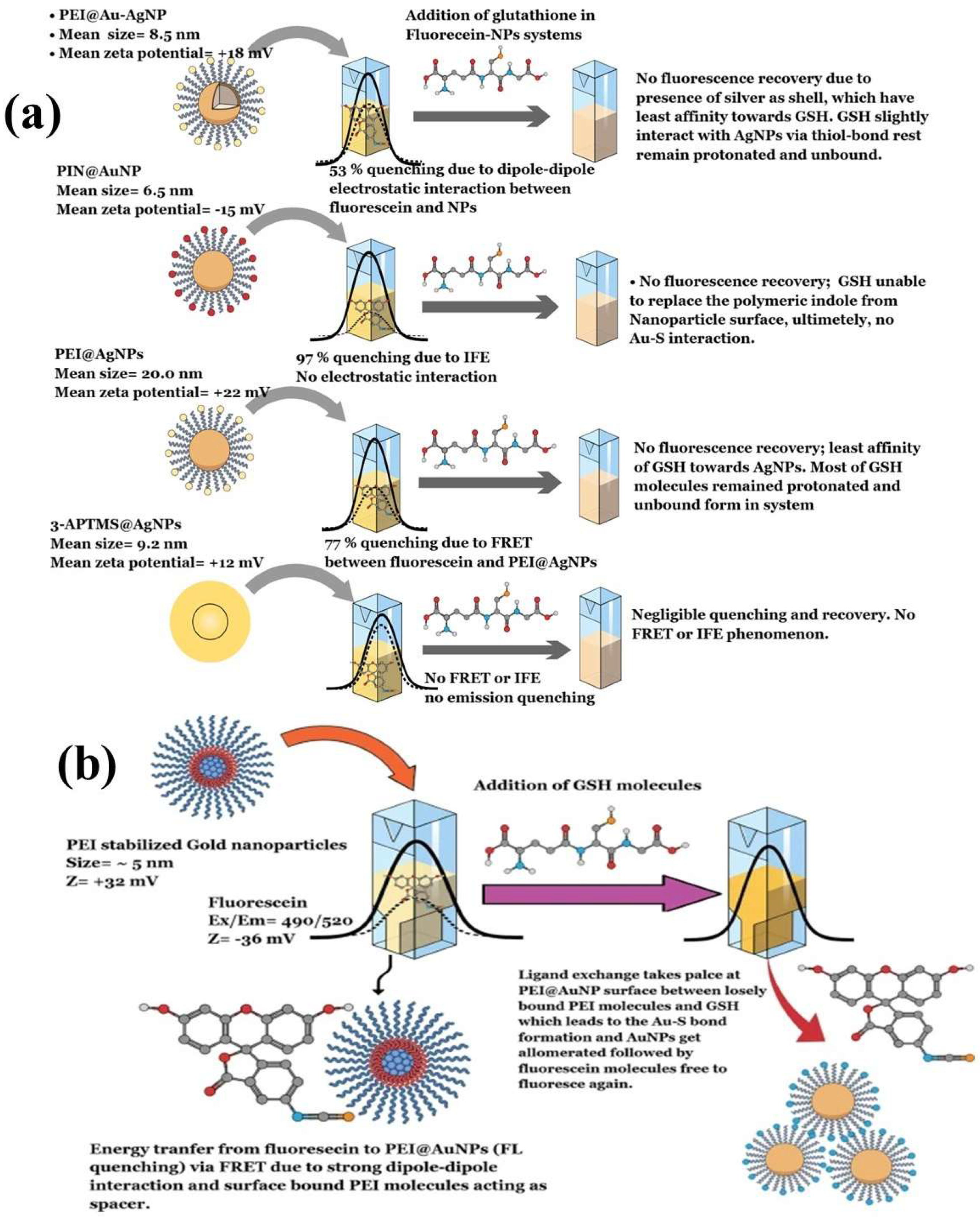
- Tiwari, A.K.; Pandey, P.C.; Narayan, R.J. Role of Different Polymer-Stabilized Metal Nanoparticles in Fluorescein Reporter–Based Turn-off-on Glutathione Detection. MRS Commun. 2025, 15, 227–234. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Gupta, M.K.; Yadav, H.P.; Narayan, R.J.; Pandey, P.C. Aggregation-Resistant, Turn-On-Off Fluorometric Sensing of Glutathione and Nickel (II) Using Vancomycin-Conjugated Gold Nanoparticles. Biosensors 2024, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Yadav, H.P.; Gupta, M.K.; Narayan, R.J.; Pandey, P.C. Synthesis of Vancomycin Functionalized Fluorescent Gold Nanoparticles and Selective Sensing of Mercury (II). Front. Chem. 2023, 11, 1238631. [Google Scholar] [CrossRef]
- Kim, E.J.; Yeum, J.H.; Choi, J.H. Effects of Polymeric Stabilizers on the Synthesis of Gold Nanoparticles. J. Mater. Sci. Technol. 2013, 30, 107–111. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Yaseen, A.I.B.; Kailani, M.H. Synthesis of Monodispersed Gold Nanoparticles with Exceptional Colloidal Stability with Grafted Polyethylene Glycol-g-polyvinyl Alcohol. J. Nanomater. 2015, 2015, 712359. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, N.; Kumar, S.; Gandhi, S.; Zussman, E.; Arun, R.K. A Paper-Based Point-of-Care Device for the Detection of Cysteine Using Gold Nanoparticles from Whole Blood. Anal. Methods 2024, 16, 3007–3019. [Google Scholar] [CrossRef] [PubMed]
- Jayeoye, T.J.; Muangsin, N. Sustainable fabrication of gold nanoparticles in poly (aminobenzene boronic acid)/(Poly vinyl alcohol-Hydroxypropyl methyl cellulose) matrix, for hazardous cyanide ion detection and its recyclable catalytic reduction activities. Mater. Today Sustain. 2024, 27, 100829. [Google Scholar] [CrossRef]
- Kalashgrani, M.Y.; Mousavi, S.M.; Akmal, M.H.; Gholami, A.; Omidifar, N.; Chiang, W.; Althomali, R.H.; Lai, C.W.; Rahman, M.M. Gold Fluorescence Nanoparticles for Enhanced SERS Detection in Biomedical Sensor Applications: Current Trends and Future Directions. Chem. Rec. 2024, e202300303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fan, L.; Yang, B.; Du, J. Multifunctional Homopolymer Vesicles for Facile Immobilization of Gold Nanoparticles and Effective Water Remediation. ACS Nano 2014, 8, 5022–5031. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, X. Synthesis of Chitosan-Stabilized Gold Nanoparticles in the Absence/Presence of Tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-Capped Gold Nanoparticles for Indicating Temperature Abuse in Frozen Stored Products. npj Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef]
- Kuno, A.; Hama, N.; Wattanasin, P.; Rujiralai, T. Chitosan-Stabilized Gold Nanoparticles Decorated with a Thiodiacetic Acid Nanoprobe for Selective Detection of Arsenic (Iii) in Rice and Water Samples. RSC Adv. 2024, 14, 26648–26658. [Google Scholar] [CrossRef]
- Tian, K.; Siegel, G.; Tiwari, A. A Simple and Selective Colorimetric Mercury (II) Sensing System Based on Chitosan Stabilized Gold Nanoparticles and 2,6-Pyridinedicarboxylic Acid. Mater. Sci. Eng. C 2016, 71, 195–199. [Google Scholar] [CrossRef]
- Li, F.; He, T.; Wu, S.; Peng, Z.; Qiu, P.; Tang, X. Visual and Colorimetric Detection of Uric Acid in Human Serum and Urine Using Chitosan Stabilized Gold Nanoparticles. Microchem. J. 2021, 164, 105987. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Chen, X.; Xu, H.; Liu, J. Chitosan-Capped Gold Nanoparticles for Selective and Colorimetric Sensing of Heparin. J. Nanopart. Res. 2013, 15, 1930. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–Gold Nanoparticles as Peroxidase Mimic and Their Application in Glucose Detection in Serum. RSC Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Pourhassan-Moghaddam, M.; Majdi, H.; Maleki, M.J. Chitosan gold nanoparticle-based dot-blot assay for sensitive visual detection of histidine-tagged recombinant proteins. bioRxiv 2025, 2025–02. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Li, H.; Tao, H.; Zou, H.; Zeng, W.; Huang, H. Functional Evolution of Gold Nanoclusters under Light-Induction: From Fluorescence to Multi-Enzyme Mimetic Transition. Small 2025, 21, 2502890. [Google Scholar] [CrossRef]
- Pandey, P.C.; Shukla, S.; Pandey, G.; Narayan, R.J. Organotrialkoxysilane-Mediated Controlled Synthesis of Noble Metal Nanoparticles and Their Impact on Selective Fluorescence Enhancement and Quenching. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2020, 38, 052801. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, G.; Walcarius, A. 3-Aminopropyltrimethoxysilane Mediated Solvent Induced Synthesis of Gold Nanoparticles for Biomedical Applications. Mater. Sci. Eng. C 2017, 79, 45–54. [Google Scholar] [CrossRef]
- Ghaffarlou, M.; İlk, S.; Hammamchi, H.; Kıraç, F.; Okan, M.; Güven, O.; Barsbay, M. Green and Facile Synthesis of Pullulan-Stabilized Silver and Gold Nanoparticles for the Inhibition of Quorum Sensing. ACS Appl. Bio Mater. 2022, 5, 517–527. [Google Scholar] [CrossRef]
- Park, H.; Kim, W.; Kim, M.; Lee, G.; Lee, W.; Park, J. Eco-Friendly and Enhanced Colorimetric Detection of Aluminum Ions Using Pectin-Rich Apple Extract-Based Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 245, 118880. [Google Scholar] [CrossRef]
- Mazeiko, V.; Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Balevicius, Z.; Ramanavicius, A. Gold Nanoparticle and Conducting Polymer-Polyaniline-Based Nanocomposites for Glucose Biosensor Design. Sens. Actuators B Chem. 2013, 189, 187–193. [Google Scholar] [CrossRef]
- Thadathil, A.; Pradeep, H.; Joshy, D.; Ismail, Y.A.; Periyat, P. Polyindole and Polypyrrole as a Sustainable Platform for Environmental Remediation and Sensor Applications. Mater. Adv. 2022, 3, 2990–3022. [Google Scholar] [CrossRef]
- Beija, M.; Marty, J.D.; Destarac, M. RAFT/MADIX polymers for the preparation of polymer/inorganic nanohybrids. Prog. Polym. Sci. 2011, 36, 845–886. [Google Scholar] [CrossRef]
- Ieong, N.S.; Biggs, C.I.; Walker, M.; Gibson, M.I. Comparison of RAFT-derived Poly (Vinylpyrrolidone) Verses Poly (Oligoethyleneglycol Methacrylate) for the Stabilization of Glycosylated Gold Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Kusolkamabot, K.; Sae-Ung, P.; Niamnont, N.; Wongravee, K.; Sukwattanasinitt, M.; Hoven, V.P. Poly(N-Isopropylacrylamide)-Stabilized Gold Nanoparticles in Combination with Tricationic Branched Phenylene-Ethynylene Fluorophore for Protein Identification. Langmuir 2013, 29, 12317–12327. [Google Scholar]
- Zhang, Z.; Maji, S.; Da Fonseca Antunes, A.B.; De Rycke, R.; Zhang, Q.; Hoogenboom, R.; De Geest, B.G. Salt Plays a Pivotal Role in the Temperature-Responsive Aggregation and Layer-by-Layer Assembly of Polymer-Decorated Gold Nanoparticles. Chem. Mater. 2013, 25, 4297–4303. [Google Scholar]
- Durand-Gasselin, C.; Koerin, R.; Rieger, J.; Lequeux, N.; Sanson, N. Colloidal Stability of Zwitterionic Polymer-Grafted Gold Nanoparticles in Water. J. Colloid Interface Sci. 2014, 434, 188–194. [Google Scholar] [CrossRef]
- Chen, N.; Xiang, X.; Heiden, P.A. Tuning Thermoresponsive Behavior of Diblock Copolymers and Their Gold Core Hybrids. Part 2. How Properties Change Depending on Block Attachment to Gold Nanoparticles. J. Colloid Interface Sci. 2013, 396, 39–46. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Chuah, K.; Liu, J.; Davis, T.P. Modulation of the Surface Charge on Polymer-Stabilized Gold Nanoparticles by the Application of an External Stimulus. Langmuir 2009, 26, 2721–2730. [Google Scholar] [CrossRef]
- Beija, M.; Palleau, E.; Sistach, S.; Zhao, X.; Ressier, L.; Mingotaud, C.; Destarac, M.; Marty, J.-D. Control of the Catalytic Properties and Directed Assembly on Surfaces of MADIX/RAFT Polymer-Coated Gold Nanoparticles by Tuning Polymeric Shell Charge. J. Mater. Chem. 2010, 20, 9433. [Google Scholar]
- Beija, M.; Marty, J.-D.; Destarac, M. Thermoresponsive Poly (N-Vinyl Caprolactam)-Coated Gold Nanoparticles: Sharp Reversible Response and Easy Tunability. Chem. Commun. 2011, 47, 2826. [Google Scholar] [CrossRef]
- Gibson, M.I.; Paripovic, D.; Klok, H. Size-Dependent LCST Transitions of Polymer-Coated Gold Nanoparticles: Cooperative Aggregation and Surface Assembly. Adv. Mater. 2010, 22, 4721–4725. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.I.; Danial, M.; Klok, H.-A. Sequentially Modified, Polymer-Stabilized Gold Nanoparticle Libraries: Convergent Synthesis and Aggregation Behavior. ACS Comb. Sci. 2011, 13, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, B.; Vana, P. RAFT-Polymers with Single and Multiple Trithiocarbonate Groups as Uniform Gold-Nanoparticle Coatings. Macromolecules 2013, 46, 4862–4871. [Google Scholar] [CrossRef]
- Rossner, C.; Ebeling, B.; Vana, P. Spherical Gold-Nanoparticle Assemblies with Tunable Interparticle Distances Mediated by Multifunctional RAFT Polymers. ACS Macro Lett. 2013, 2, 1073–1076. [Google Scholar] [CrossRef]
- Rossner, C.; Glatter, O.; Saldanha, O.; Köster, S.; Vana, P. The Structure of Gold-Nanoparticle Networks Cross-Linked by Di- and Multifunctional RAFT Oligomers. Langmuir 2015, 31, 10573–10582. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Z.; Su, X.; Tang, Z. Application of Au Based Nanomaterials in Analytical Science. Nano Today 2016, 12, 64–97. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Ray, P.C.; Fan, Z.; Crouch, R.A.; Sinha, S.S.; Pramanik, A. Nanoscopic optical rulers beyond the FRET distance limit: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 6370–6404. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Patra, A. Recent Advances in Energy Transfer Processes in Gold-Nanoparticle-Based Assemblies. J. Phys. Chem. C 2012, 116, 17307–17317. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Yang, L.; Wang, F.; Liu, A. Recent Advances in Gold Nanostructures Based Biosensing and Bioimaging. Coord. Chem. Rev. 2018, 370, 1–21. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, D.; Li, Y.; Achilefu, S.; Gu, Y. Gold Nanoparticles Based Molecular Beacons for in Vitro and in Vivo Detection of the Matriptase Expression on Tumor. Biosens. Bioelectron. 2013, 49, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wei, Y.; Dai, Z.; Chen, G.; Chu, Y.; Zhao, Y. Nanostructure and Corresponding Quenching Efficiency of Fluorescent DNA Probes. Materials 2018, 11, 272. [Google Scholar] [CrossRef]
- Pons, T.; Medintz, I.L.; Sapsford, K.E.; Higashiya, S.; Grimes, A.F.; English, D.S.; Mattoussi, H. On the Quenching of Semiconductor Quantum Dot Photoluminescence by Proximal Gold Nanoparticles. Nano Lett. 2007, 7, 3157–3164. [Google Scholar] [CrossRef]
- Rakshit, S.; Moulik, S.P.; Bhattacharya, S.C. Deciphering the Role of the Length of the Corona in Controlled NSET within Triblock Copolymers. J. Phys. Chem. B 2015, 119, 8457–8467. [Google Scholar] [CrossRef]
- Sutradhar, S.; Patnaik, A. Structure and dynamics of a N-methylfulleropyrrolidine-mediated gold nanocomposite: A spectroscopic ruler. ACS Appl. Mater. Interfaces 2017, 9, 21921–21932. [Google Scholar] [CrossRef]
- Vaishnav, J.K.; Mukherjee, T.K. Long-Range Resonance Coupling-Induced Surface Energy Transfer from CdTe Quantum Dot to Plasmonic Nanoparticle. J. Phys. Chem. C 2018, 122, 28324–28336. [Google Scholar] [CrossRef]
- Breshike, C.J.; Riskowski, R.A.; Strouse, G.F. Leaving Forster resonance energy transfer behind: Nanometal surface energy transfer predicts the size-enhanced energy coupling between a metal nanoparticle and an emitting dipole. J. Phys. Chem. C 2013, 117, 23942–23949. [Google Scholar] [CrossRef]
- Liu, M.; Zhuang, H.; Zhang, Y.; Jia, Y. A Sandwich FRET Biosensor for Lysozyme Detection Based on Peptide-Functionalized Gold Nanoparticles and FAM-Labeled Aptamer. Talanta 2024, 276, 126226. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Y.; Yan, C.; Lu, Y. DNA-Functionalization Gold Nanoparticles Based Fluorescence Sensor for Sensitive Detection of Hg2+ in Aqueous Solution. Sens. Actuators B Chem. 2015, 211, 1–6. [Google Scholar] [CrossRef]
- Jin, Z.; Ling, C.; Li, Y.; Zhou, J.; Li, K.; Yim, W.; Yeung, J.; Chang, Y.-C.; He, T.; Cheng, Y.; et al. Spacer Matters: All-Peptide-Based Ligand for Promoting Interfacial Proteolysis and Plasmonic Coupling. Nano Lett. 2022, 22, 8932–8940. [Google Scholar] [CrossRef] [PubMed]
- Bener, M.; Şen, F.B.; Apak, R. Protamine Gold Nanoclusters − Based Fluorescence Turn-on Sensor for Rapid Determination of Trinitrotoluene (TNT). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121462. [Google Scholar] [CrossRef] [PubMed]
- Cepraga, C. Two-Photon Chromophore-Polymer Conjugates Grafted onto Gold Nanoparticles as Fluorescent Probes for Bioimaging and Photodynamic Therapy Applications. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 2012. [Google Scholar]
- Zhang, S.; Wang, Z.; Miao, T. Tryptophan-Functionalized Gold Nanoclusters Used as a Fluorescence Sensor for Alizarin Determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 335, 125963. [Google Scholar] [CrossRef]
- Halawa, M.I.; Wu, F.; Fereja, T.H.; Lou, B.; Xu, G. One-Pot Green Synthesis of Supramolecular β-Cyclodextrin Functionalized Gold Nanoclusters and Their Application for Highly Selective and Sensitive Fluorescent Detection of Dopamine. Sens. Actuators B Chem. 2017, 254, 1017–1024. [Google Scholar] [CrossRef]
- Guo, X.; Wu, F.; Ni, Y.; Kokot, S. Synthesizing a Nano-Composite of BSA-Capped Au Nanoclusters/Graphitic Carbon Nitride Nanosheets as a New Fluorescent Probe for Dopamine Detection. Anal. Chim. Acta 2016, 942, 112–120. [Google Scholar] [CrossRef]
- Zhang, D.; Meng, Y.-R.; Zhang, C.-Y. Peptide-Templated Gold Nanoparticle Nanosensor for Simultaneous Detection of Multiple Posttranslational Modification Enzymes. Chem. Commun. 2019, 56, 213–216. [Google Scholar] [CrossRef]
- Shang, L.; Qin, C.; Wang, T.; Wang, M.; Wang, L.; Dong, S. Fluorescent Conjugated Polymer-Stabilized Gold Nanoparticles for Sensitive and Selective Detection of Cysteine. J. Phys. Chem. C 2007, 111, 13414–13417. [Google Scholar] [CrossRef]
- Jahan, S.; Mansoor, F.; Kanwal, S. Polymers Effects on Synthesis of AuNPs, and Au/Ag Nanoalloys: Indirectly Generated AuNPs and Versatile Sensing Applications Including Anti-Leukemic Agent. Biosens. Bioelectron. 2013, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Aldeek, F.; Ji, X.; Safi, M.; Wang, W.; Del Cid, A.; Steinbock, O.; Mattoussi, H. Self-Assembled Gold Nanoparticle–Fluorescent Protein Conjugates as Platforms for Sensing Thiolate Compounds via Modulation of Energy Transfer Quenching. Bioconjug. Chem. 2017, 28, 678–687. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, J.; Li, N.; Qi, L.; Zhang, S. Fluorescent Probe for Turn-on Sensing of l-Cysteine by Ensemble of AuNCs and Polymer Protected AuNPs. Anal. Chim. Acta 2015, 879, 97–103. [Google Scholar] [CrossRef]
- Tang, Y.; Ding, Y.; Wu, T.; Lv, L.; Yan, Z. A Turn-on Fluorescent Probe for Hg2+ Detection by Using Gold Nanoparticle-Based Hybrid Microgels. Sens. Actuators B Chem. 2016, 228, 767–773. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Z.; Zhang, Z.; Qu, C.; Chen, L.; Shen, D. Fluorescent Sensing of Mercury (II) Based on Formation of Catalytic Gold Nanoparticles. Analyst 2013, 138, 4280. [Google Scholar] [CrossRef]
- Chen, L.Y.; Ou, C.M.; Chen, W.Y.; Huang, C.C.; Chang, H.T. Synthesis of photoluminescent Au ND–PNIPAM hybrid microgel for the detection of Hg2+. ACS Appl. Mater. Interfaces 2013, 5, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, N.; Wen, J.; Yang, D.; Chen, H.; Long, Y.; Zheng, H. Detecting Uric Acid Base on the Dual Inner Filter Effect Using BSA@Au Nanoclusters as Both Peroxidase Mimics and Fluorescent Reporters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122504. [Google Scholar] [CrossRef]
- Anju, S.M.; Merin, K.A.; Varghese, S.; Shkhair, A.I.; Rajeevan, G.; Indongo, G.; George, S. Antibody-Functionalized Gold Nanoclusters/Gold Nanoparticle Platform for the Fluorescence Turn-on Detection of Cardiac Troponin I. Microchim. Acta 2024, 191, 124. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, S.; Ye, N.; Zhang, L.; Xiang, Y. Ratiometric Fluorescence for Ultrasensitive Detection of Chlortetracycline in Milk Matrix Based on Its Blue Alkaline Degradation Product and Red-Emitting Cyclodextrin Stabilized Gold Nanocluster. Dye. Pigment. 2022, 206, 110660. [Google Scholar] [CrossRef]
- Li, M.; Zhu, N.; Zhu, W.; Zhang, S.; Li, F.; Wu, P.; Li, X. Enhanced emission and higher stability ovalbumin-stabilized gold nanoclusters (OVA-AuNCs) modified by polyethyleneimine for the fluorescence detection of tetracyclines. Microchem. J. 2021, 169, 106560. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.K.; Narayan, R.J. Role of Polymeric Stabilizing Agents as a Molecular Spacer in Gold Nanoparticle-Mediated FRET-Based Biosensing. Biosensors 2025, 15, 593. https://doi.org/10.3390/bios15090593
Tiwari AK, Narayan RJ. Role of Polymeric Stabilizing Agents as a Molecular Spacer in Gold Nanoparticle-Mediated FRET-Based Biosensing. Biosensors. 2025; 15(9):593. https://doi.org/10.3390/bios15090593
Chicago/Turabian StyleTiwari, Atul Kumar, and Roger J. Narayan. 2025. "Role of Polymeric Stabilizing Agents as a Molecular Spacer in Gold Nanoparticle-Mediated FRET-Based Biosensing" Biosensors 15, no. 9: 593. https://doi.org/10.3390/bios15090593
APA StyleTiwari, A. K., & Narayan, R. J. (2025). Role of Polymeric Stabilizing Agents as a Molecular Spacer in Gold Nanoparticle-Mediated FRET-Based Biosensing. Biosensors, 15(9), 593. https://doi.org/10.3390/bios15090593







