A Bluetooth-Enabled Electrochemical Platform Based on Saccharomyces cerevisiae Yeast Cells for Copper Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrochemical Setup
2.3. Yeast Strains and Culture Conditions
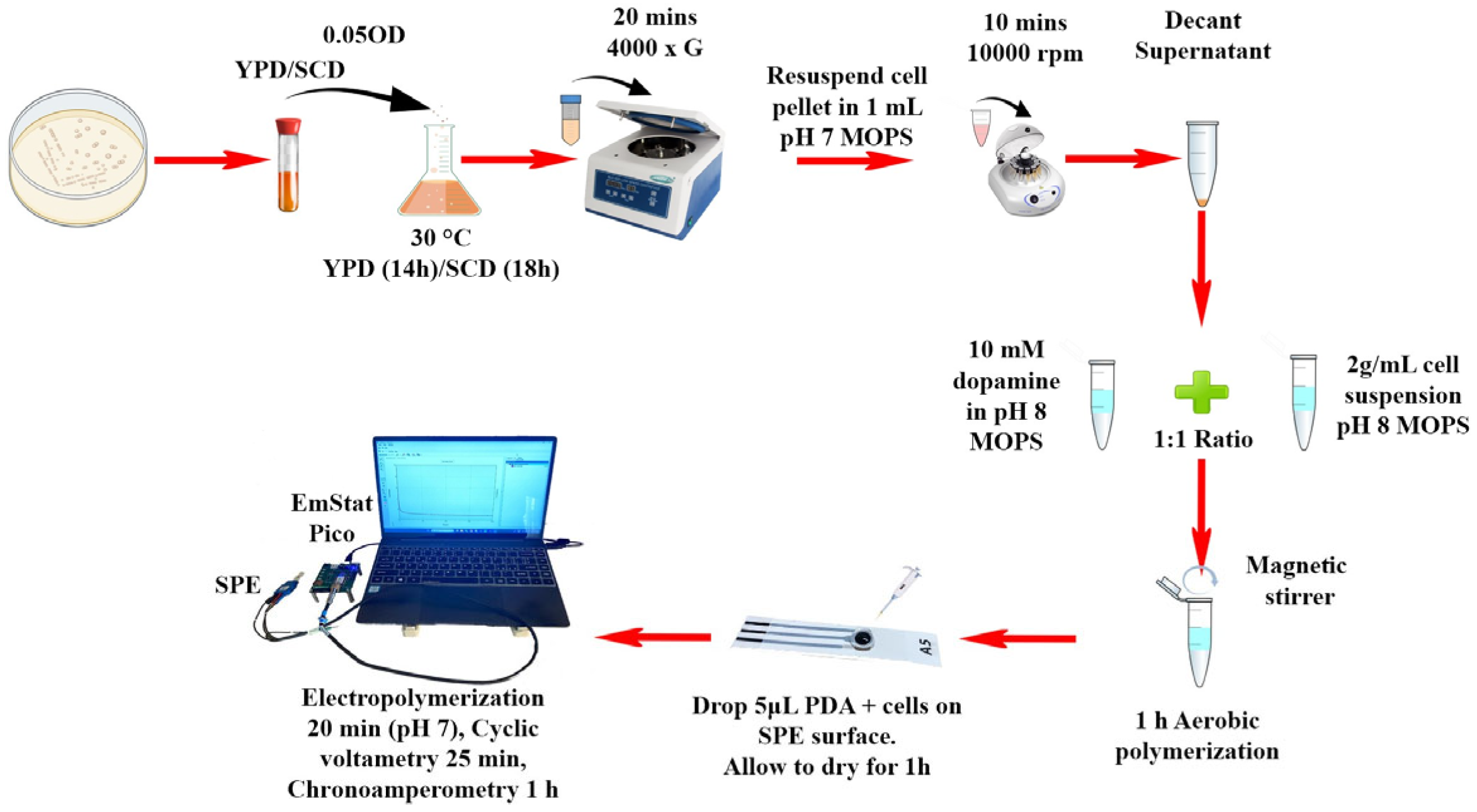
2.4. Cell Immobilization and Electrochemical Characterization
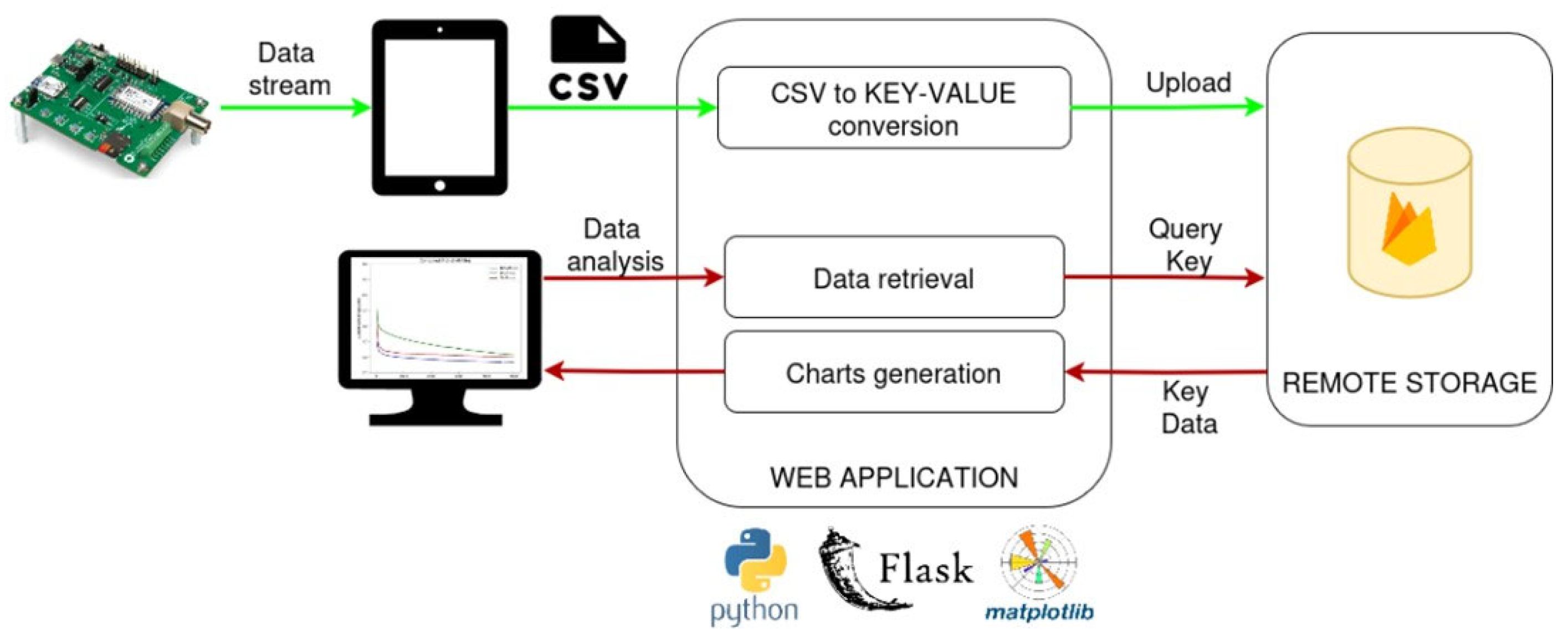
2.5. Data Analysis
3. Results and Discussion
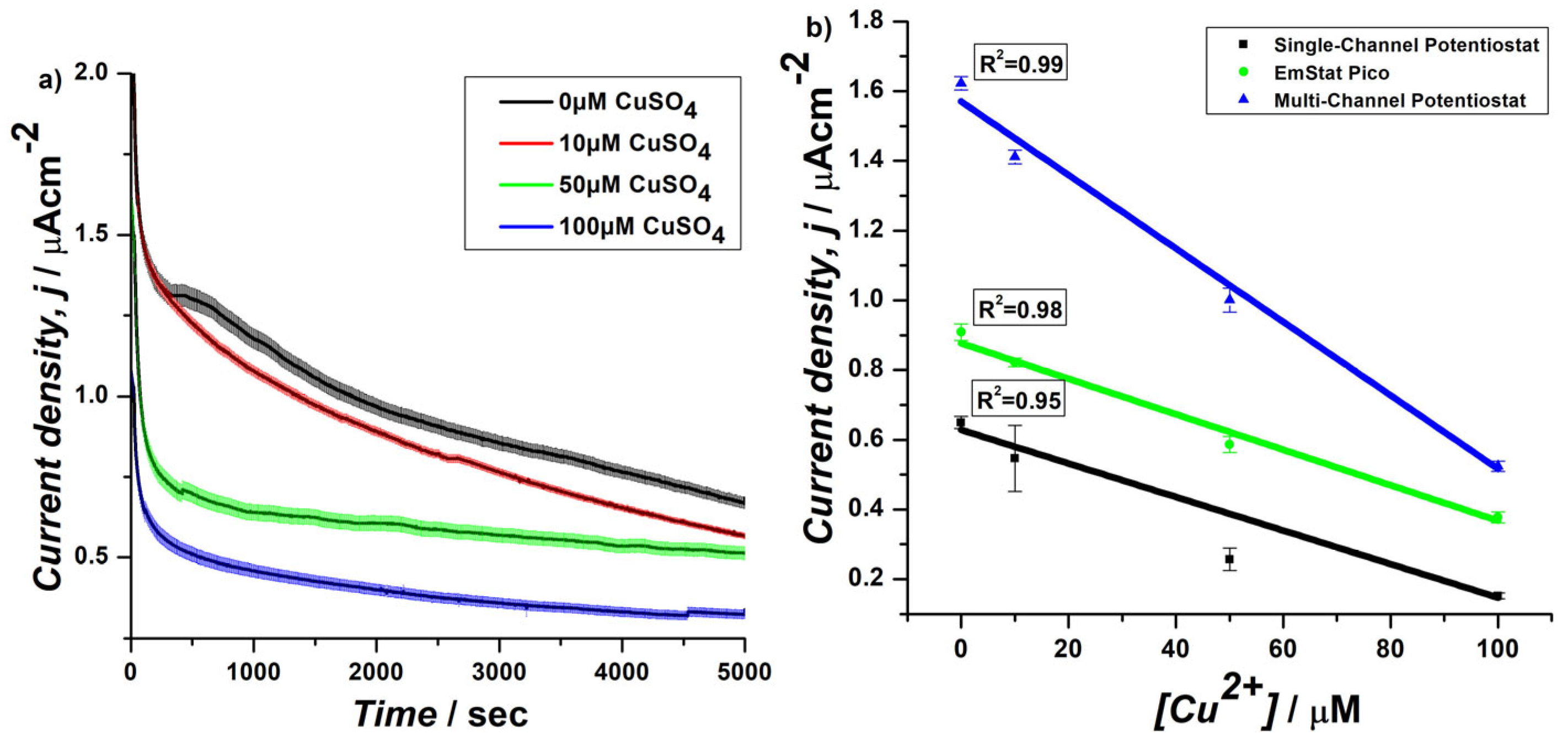
3.1. Development of EmStat Pico System for Chronoamperometric Analysis of Yeast S. cerevisiae Cells Immobilized on a PDA-Coated SPE
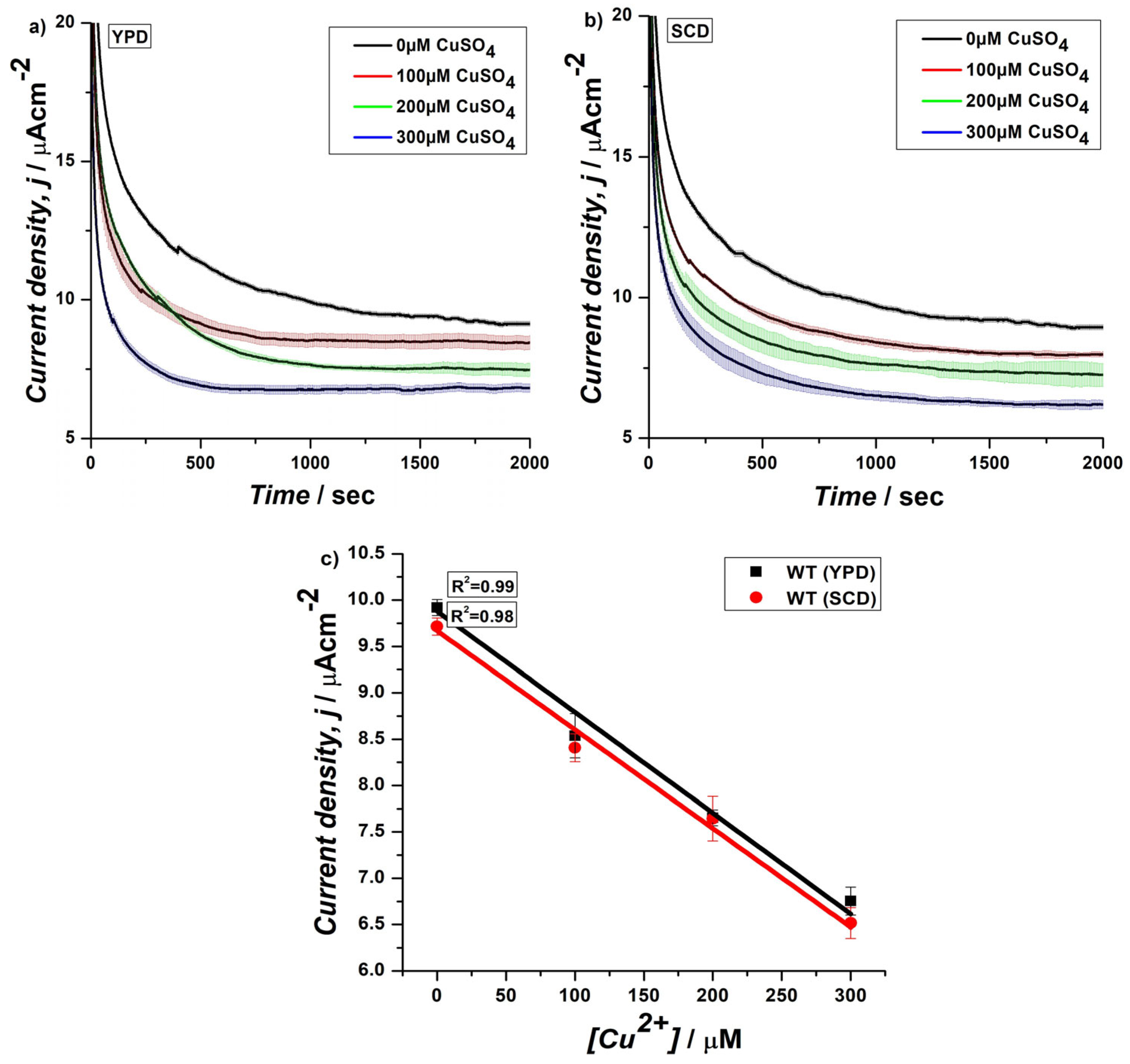
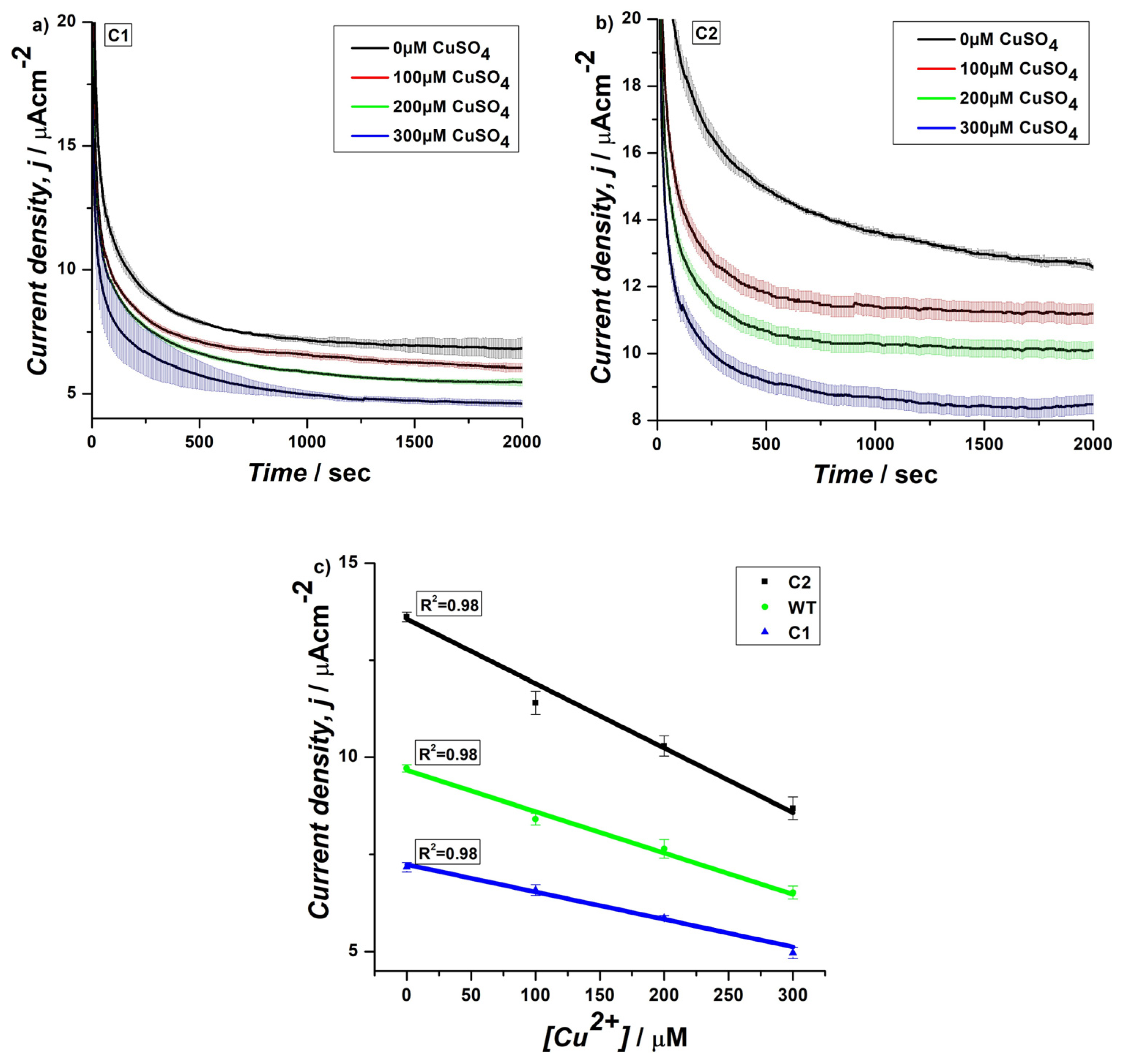
3.2. CA of Genetically Engineered Yeast Cells on SPEs Using EmStat PicoSsystem in Horizontal Assembly
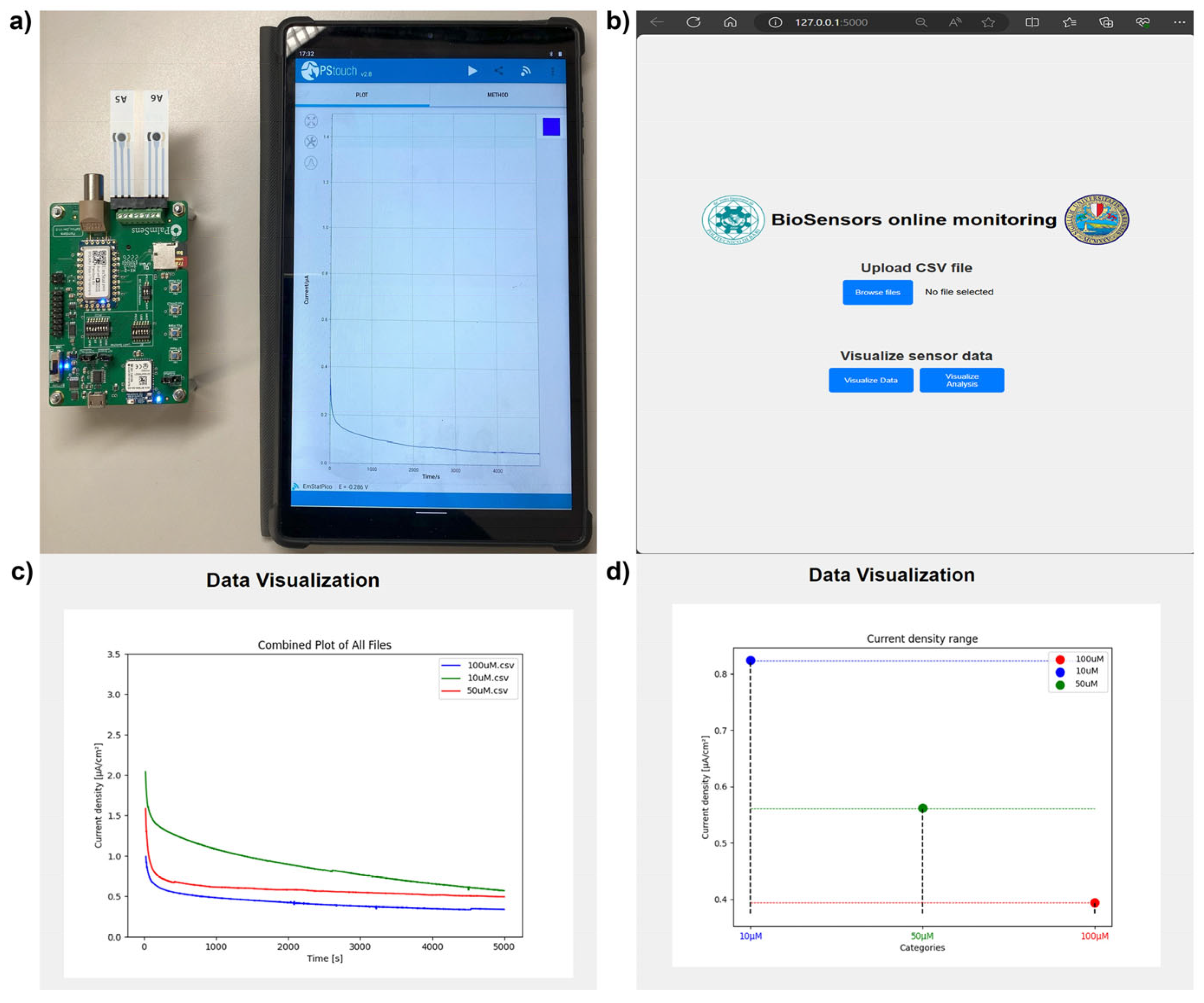
3.3. Transferability of Yeast-Based Biosensors System to Portable Microelectronic Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Rippa, M.; Battaglia, V.; Cermola, M.; Sicignano, M.; Lahoz, E.; Mormile, P. Monitoring of the copper persistence on plant leaves using pulsed thermography. Environ. Monit. Assess. 2022, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.; Sari, A.A.; Alessa, A.H.; Snari, R.M.; Alsharief, H.H.; Alatawi, I.S.; El-Zaidia, E.F.M.; El-Metwaly, N.M. High-sensitivity detection of copper ions in water via cellulose nanomaterial nano-antennas and DFT studies. Chem. Eng. J. Adv. 2024, 20, 100675. [Google Scholar] [CrossRef]
- Wahid, E.; Ocheja, O.B.; Marsili, E.; Guaragnella, C.; Guaragnella, N. Biological and technical challenges for implementation of yeast-based biosensors. Microb. Biotechnol. 2022, 16, 54–66. [Google Scholar] [CrossRef]
- Jarque, S.; Bittner, M.; Blaha, L.; Hilscherova, K. Yeast Biosensors for Detection of Environmental Pollutants: Current State and Limitations. Trends Biotechnol. 2016, 34, 408–419. [Google Scholar] [CrossRef]
- Wahid, E.; Ocheja, O.B.; Oguntomi, S.O.; Pan, R.; Grattieri, M.; Guaragnella, N.; Guaragnella, C.; Marsili, E. Immobilized Saccharomyces cerevisiae viable cells for electrochemical biosensing of Cu(II). Sci. Rep. 2025, 15, 1–11. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Waite, J.H. Mussel-designed Protective Coatings for Compliant Substrates. J. Dent. Res. 2008, 87, 701–709. [Google Scholar] [CrossRef]
- Papov, V.V.; Diamond, T.V.; Biemann, K.; Waite, J.H. Hydroxyarginine-containing Polyphenolic Proteins in the Adhesive Plaques of the Marine Mussel Mytilus edulis. J. Biol. Chem. 1995, 270, 20183–20192. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.-B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-Inspired Encapsulation and Functionalization of Individual Yeast Cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef]
- Fakhrullin, R.F.; Zamaleeva, A.I.; Minullina, R.T.; Konnova, S.A.; Paunov, V.N. Cyborg cells: Functionalisation of living cells with polymers and nanomaterials. Chem. Soc. Rev. 2012, 41, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Ocheja, O.B.; Wahid, E.; Franco, J.H.; Trotta, M.; Guaragnella, C.; Marsili, E.; Guaragnella, N.; Grattieri, M. Polydopamine-immobilized yeast cells for portable electrochemical biosensors applied in environmental copper sensing. Bioelectrochemistry 2024, 157, 108658. [Google Scholar] [CrossRef] [PubMed]
- Vopálenská, I.; Váchová, L.; Palková, Z. New biosensor for detection of copper ions in water based on immobilized genetically modified yeast cells. Biosens. Bioelectron. 2015, 72, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, D.; Mo, Q.; Yuan, J. Engineering Saccharomyces cerevisiae-based biosensors for copper detection. Microb. Biotechnol. 2022, 15, 2854–2860. [Google Scholar] [CrossRef]
- Franco, J.H.; Stufano, P.; Labarile, R.; Lacalamita, D.; Lasala, P.; Fanizza, E.; Trotta, M.; Farinola, G.M.; Grattieri, M. Intact photosynthetic bacteria-based electrodes for self-powered metal ions monitoring. Biosens. Bioelectron. X 2024, 21, 100552. [Google Scholar] [CrossRef]
- Adekunle, A.; Raghavan, V.; Tartakovsky, B. On-line monitoring of heavy metals-related toxicity with a microbial fuel cell biosensor. Biosens. Bioelectron. 2019, 132, 382–390. [Google Scholar] [CrossRef]
- Timoshenko, R.V.; Gorelkin, P.V.; Vaneev, A.N.; Krasnovskaya, O.O.; Akasov, R.A.; Garanina, A.S.; Khochenkov, D.A.; Iakimova, T.M.; Klyachko, N.L.; Abakumova, T.O.; et al. Electrochemical Nanopipette Sensor for In Vitro/In Vivo Detection of Cu2+ Ions. Anal. Chem. 2023, 96, 127–136. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.H. Chemo/biosensors towards effect-directed analysis: An overview of current status and future development. TrAC Trends Anal. Chemistry 2023, 158, 116824. [Google Scholar] [CrossRef]
- Mendes, F.; Miranda, E.; Amaral, L.; Carvalho, C.; Castro, B.B.; Sousa, M.J.; Chaves, S.R. Novel yeast-based biosensor for environmental monitoring of tebuconazole. Appl. Microbiol. Biotechnol. 2024, 108, 1–12. [Google Scholar] [CrossRef]
- Maria, S.R.S.; Marina, D.B.; Tieze, S.M.; Liddell, L.C.; Bhattacharya, S. BioSentinel: Long-Term Saccharomyces cerevisiae Preservation for a Deep Space Biosensor Mission. Astrobiology 2023, 23, 617–630. [Google Scholar] [CrossRef]
- Žunar, B.; Mosrin, C.; Bénédetti, H.; Vallée, B. Re-engineering of CUP1 promoter and Cup2/Ace1 transactivator to convert Saccharomyces cerevisiae into a whole-cell eukaryotic biosensor capable of detecting 10 nM of bioavailable copper. Biosens. Bioelectron. 2022, 214, 114502. [Google Scholar] [CrossRef]
- Ecker, D.J.; Butt, T.R.; Sternberg, E.J.; Neeper, M.P.; Debouck, C.A.; Gorman, J.; Crooke, S.T. Yeast metallothionein function in metal ion detoxification. J. Biol. Chem. 1986, 261, 16895–16900. [Google Scholar] [CrossRef]
- Shen, C.-H.; Leblanc, B.P.; Alfieri, J.A.; Clark, D.J. Remodeling of Yeast CUP1 Chromatin Involves Activator-Dependent Repositioning of Nucleosomes over the Entire Gene and Flanking Sequences. Mol. Cell. Biol. 2001, 21, 534–547. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, R.K.; Tomar, R.S. The N-Terminal Tail of Histone H3 Regulates Copper Homeostasis in Saccharomyces cerevisiae. Mol. Cell. Biol. 2021, 41, e00210-20. [Google Scholar] [CrossRef]
- Stratmann, L.; Heery, B.; Coffey, B. EmStat Pico: Embedded Electrochemistry with a Miniaturized Software-Enabled Potentiostat System on Module. Available online: https://www.analog.com/en/resources/technical-articles/emstat-pico-embedded-electrochemistry-with-a-miniaturized-software-enabled-potentiostat-system-on-mo.html?gated=1755509085133 (accessed on 22 May 2025).
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Wahid, E. Design and Characterization of Yeast-based Electrochemical Biosensors for Application in Precision Agriculture. Ph.D. Thesis, Politecnico di Bari, Bari, Italy, 2024. [Google Scholar]
- Buscemi, G.; Vona, D.; Stufano, P.; Labarile, R.; Cosma, P.; Agostiano, A.; Trotta, M.; Farinola, G.M.; Grattieri, M. Bio-Inspired Redox-Adhesive Polydopamine Matrix for Intact Bacteria Biohybrid Photoanodes. ACS Appl. Mater. Interfaces 2022, 14, 26631–26641. [Google Scholar] [CrossRef] [PubMed]
- Rella, S.; Mazzotta, E.; Caroli, A.; De Luca, M.; Bucci, C.; Malitesta, C. Investigation of polydopamine coatings by X-ray Photoelectron Spectroscopy as an effective tool for improving biomolecule conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, Y.; Yang, Y.; Peng, Y.; Li, C. Copper metabolism in Saccharomyces cerevisiae: An update. BioMetals 2020, 34, 3–14. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Ferrari, A.G.M.; Dempsey, N.C.; Banks, C.E. Electroanalytical overview: Screen-printed electrochemical sensing platforms for the detection of vital cardiac, cancer and inflammatory biomarkers. Sens. Diagn. 2022, 1, 405–428. [Google Scholar] [CrossRef]
- Eliodório, K.P.; Cunha, G.C.; Lino, F.S.; Sommer, M.O.A.; Gombert, A.K.; Giudici, R.; Basso, T.O. Physiology of Saccharomyces cerevisiae during growth on industrial sugar cane molasses can be reproduced in a tailor-made defined synthetic medium. Sci. Rep. 2023, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, P.; Kuberský, P. The Effect of the Orientation Towards Analyte Flow on Electrochemical Sensor Performance and Current Fluctuations. Sensors 2020, 20, 1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, K.; Liu, Y.; Zhao, F.; He, J.; Wu, H.; Wu, J.; Liang, H.; Huang, C. Constructing an ion-oriented channel on a zinc electrode through surface engineering. Carbon Energy 2023, 5, e343. [Google Scholar] [CrossRef]
- Welch, J.; Fogel, S.; Buchman, C.; Karin, M. The CUP2 gene product regulates the expression of the CUP1 gene, coding for yeast metallothionein. EMBO J. 1989, 8, 255–260. [Google Scholar] [CrossRef]
- Anshori, I.; Harimurti, S.; Rama, M.B.; Langelo, R.E.; Jessika; Yulianti, L.P.; Gumilar, G.; Yusuf, M.; Prastriyanti, S.; Yuliarto, B.; et al. Web-based surface plasmon resonance signal processing system for fast analyte analysis. SoftwareX 2022, 18, 101057. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Wang, J.; Wang, E.; Fang, X. Development of a two component system based biosensor with high sensitivity for the detection of copper ions. Commun. Biol. 2024, 7, 1–10. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.S.M.M.F.; Zhou, T.; Yue, W. Enzyme Method-Based Microfluidic Chip for the Rapid Detection of Copper Ions. Micromachines 2021, 12, 1380. [Google Scholar] [CrossRef]
- Lucci, T.J.; Neufarth, A.; Gaillard, J.-F.; Lucks, J.B. A Sensor for Detecting Aqueous Cu2+ That Functions in a Just-Add-Water Format. ACS Omega 2024, 10, 1188–1197. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I.; Pena-Pereira, F.; de la Calle, I.; Romero, V. Nanomaterial-Integrated Cellulose Platforms for Optical Sensing of Trace Metals and Anionic Species in the Environment. Sensors 2021, 21, 604. [Google Scholar] [CrossRef]
- Nidhisha; Kizhakayil, R.N. Onsite naked-eye detection and quantification of Cu(ii) ions in drinking water using N-doped carbon nanodots. Mater. Adv. 2025, 6, 3678–3685. [Google Scholar] [CrossRef]
- LaMotte Europe. Insta Test Copper and Iron Test Strip. Available online: https://www.lamotte-europe.com/products/pool-and-spa/test-strips/insta-test-copper-and-iron-test-strip-kit/en (accessed on 22 July 2025).
- FStest. Heavy Metal Copper(Cu) Test Strip for Water. Available online: https://www.fstestcorp.com/products-detail/id-118.html (accessed on 22 July 2025).
- EZ Series Copper Analysers. EZ1010 Copper. Available online: https://my.hach.com/ez-series-analysers/ez-series-copper-analysers/family?productCategoryId=59429629671&utm (accessed on 22 July 2025).
- MeRcK. Copper Test. Available online: https://www.sigmaaldrich.com/IT/en/product/mm/110003?utm= (accessed on 22 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahid, E.; Ocheja, O.B.; Longo, A.; Marsili, E.; Trotta, M.; Grattieri, M.; Guaragnella, C.; Guaragnella, N. A Bluetooth-Enabled Electrochemical Platform Based on Saccharomyces cerevisiae Yeast Cells for Copper Detection. Biosensors 2025, 15, 583. https://doi.org/10.3390/bios15090583
Wahid E, Ocheja OB, Longo A, Marsili E, Trotta M, Grattieri M, Guaragnella C, Guaragnella N. A Bluetooth-Enabled Electrochemical Platform Based on Saccharomyces cerevisiae Yeast Cells for Copper Detection. Biosensors. 2025; 15(9):583. https://doi.org/10.3390/bios15090583
Chicago/Turabian StyleWahid, Ehtisham, Ohiemi Benjamin Ocheja, Antonello Longo, Enrico Marsili, Massimo Trotta, Matteo Grattieri, Cataldo Guaragnella, and Nicoletta Guaragnella. 2025. "A Bluetooth-Enabled Electrochemical Platform Based on Saccharomyces cerevisiae Yeast Cells for Copper Detection" Biosensors 15, no. 9: 583. https://doi.org/10.3390/bios15090583
APA StyleWahid, E., Ocheja, O. B., Longo, A., Marsili, E., Trotta, M., Grattieri, M., Guaragnella, C., & Guaragnella, N. (2025). A Bluetooth-Enabled Electrochemical Platform Based on Saccharomyces cerevisiae Yeast Cells for Copper Detection. Biosensors, 15(9), 583. https://doi.org/10.3390/bios15090583











