SERS-Driven Evolution of Lateral and Vertical Flow Assays in Medical Diagnostics
Abstract
1. Introduction
2. Conventional Flow-Based Assays: LFA and VFA
3. Recent Advances in SERS-Integrated LFA and VFA for Disease Diagnostics
3.1. SERS-LFA: Exogenous Diagnostic Case

3.2. SERS-LFA: Endogenous Diagnostic Case
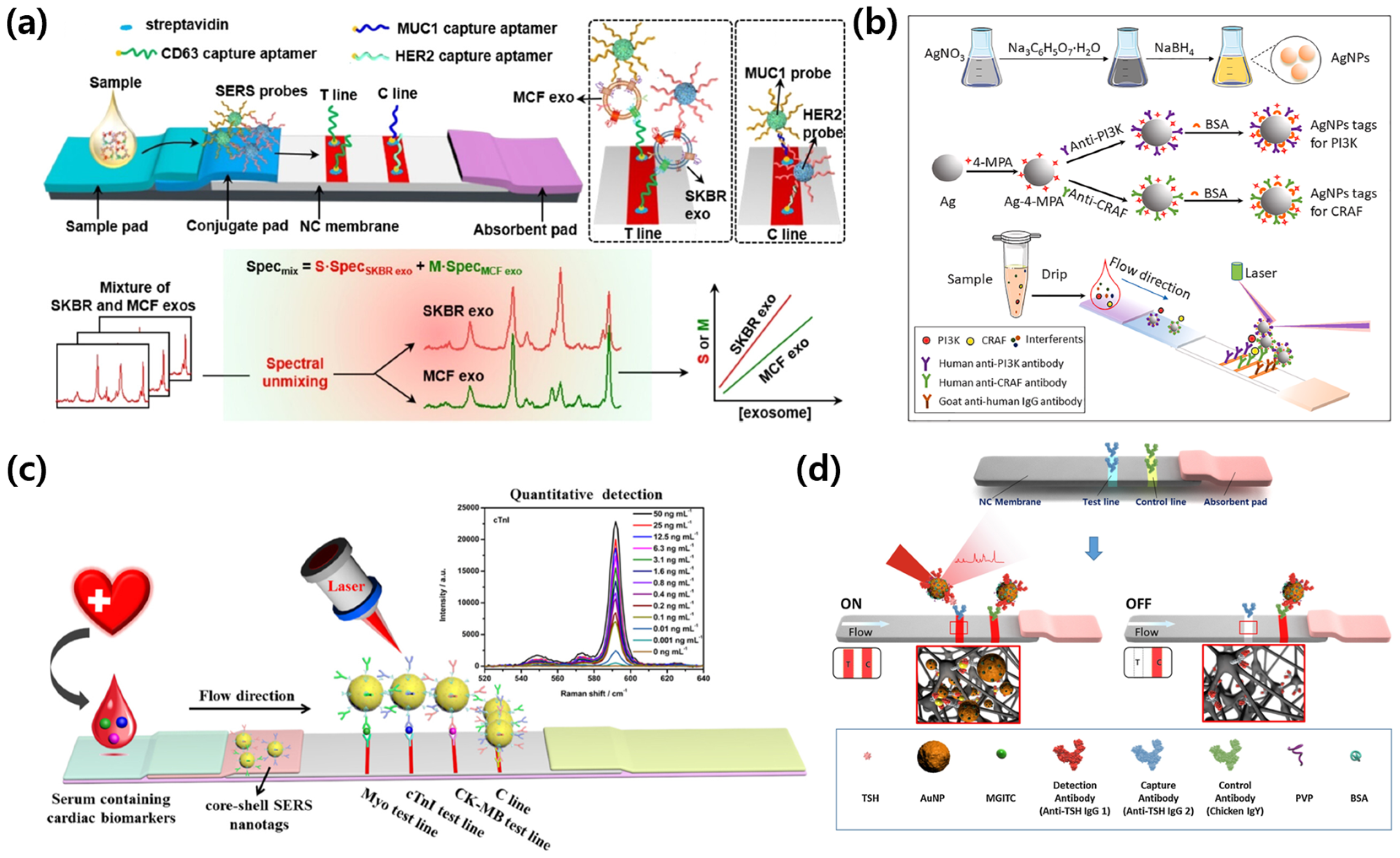
3.3. SERS-VFA: Exogenous Diagnostic Case
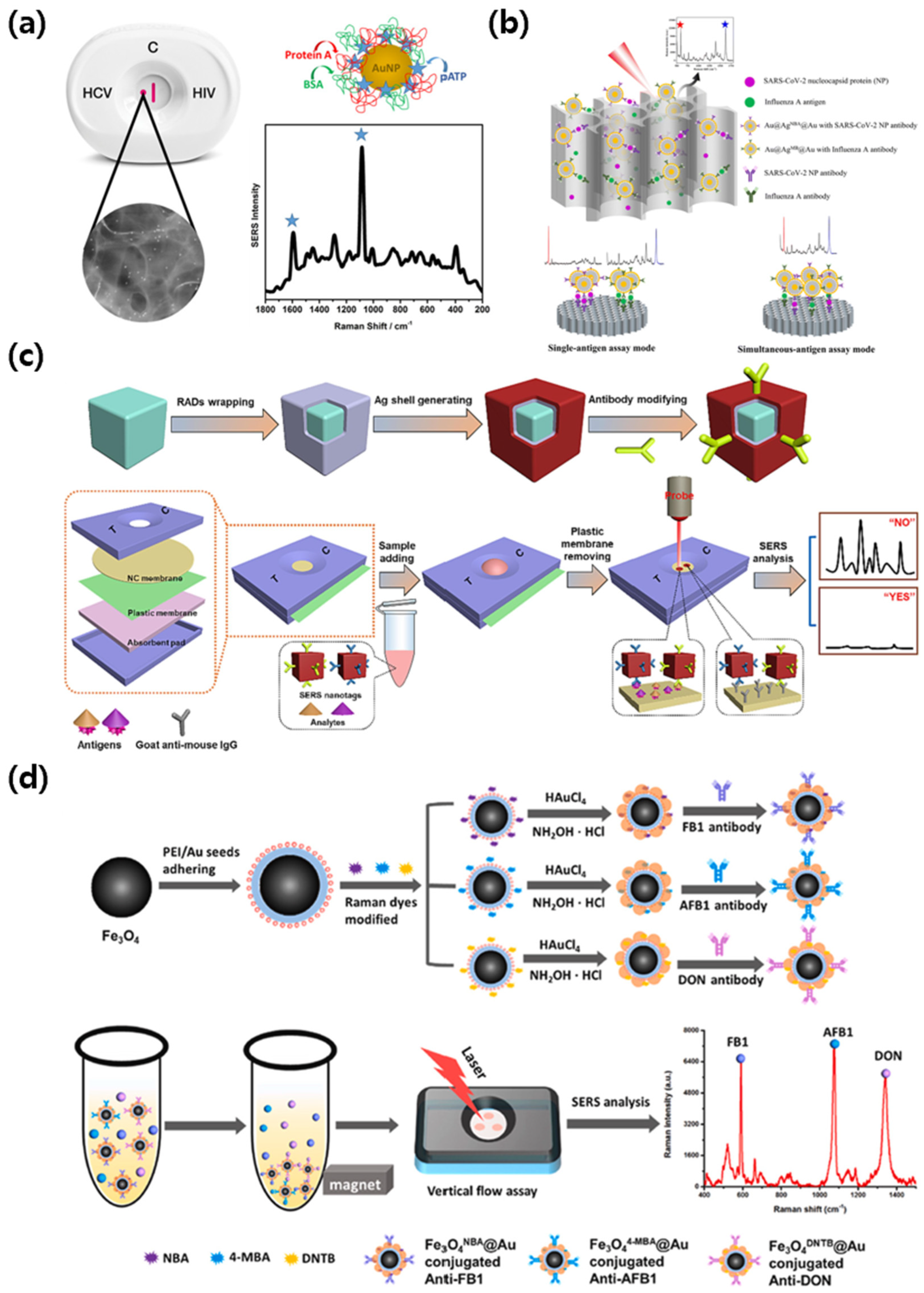
3.4. SERS-VFA: Endogenous Diagnostic Case

4. Technical Challenges and Future Prospects
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parashar, L.; Shekhar, H.; Arya, H.; Vig, S.L.; Prasad, J.; Meshram, G.G. Sociodemographic and Clinical Factors Associated with COVID-19 Mortality in India: A Retrospective Study. Acta Inform. Medica 2025, 33, 23. [Google Scholar] [CrossRef]
- Ochwoto, M.; Kuhn, S.; Schaughency, P.; Greene, B.; Hawes, K.; Koukouikila-Koussounda, F.; Elenga, R.G.; Eyenet Boussam, D.A.; Mayangue, P.I.; Schulz, J. Development and Validation of a New Mpox Virus Sequencing and Bioinformatic Analysis Pipeline. Emerg. Microbes Infect. 2025, 14, 2494733. [Google Scholar] [CrossRef] [PubMed]
- Louis, N. Oropouche virus: Critical research priorities for this re-emerging pathogen. Caribb. Med. J. 2025. [Google Scholar] [CrossRef]
- Niu, Q.; Jiang, Z.; Wang, L.; Ji, X.; Baele, G.; Qin, Y.; Lin, L.; Lai, A.; Chen, Y.; Veit, M. Prevention and control of avian influenza virus: Recent advances in diagnostic technologies and surveillance strategies. Nat. Commun. 2025, 16, 3558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ahmed, R.; Damayantharan, M.; Ünal, B.; Butt, H.; Yetisen, A.K. Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. Adv. Healthc. Mater. 2019, 8, 1900244. [Google Scholar] [CrossRef]
- Alrubayyi, A.; Fahimi, R.; Hassan, M.; Madan, J.; Uthman, O.A. Current Clinical Practices and Future Perspectives for Primary Healthcare Use of Point-of-Care Devices: A Scoping Review. Int. J. Clin. Pract. 2025, 2025, 4742851. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno) assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Lei, R.; Wang, D.; Arain, H.; Mohan, C. Design of gold nanoparticle vertical flow assays for point-of-care testing. Diagnostics 2022, 12, 1107. [Google Scholar] [CrossRef]
- Oh, Y.K.; Joung, H.-A.; Kim, S.; Kim, M.-G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab A Chip 2013, 13, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, T.; Segerink, L.I.; Nystrand, M.; Gantelius, J.; Andersson Svahn, H. Point-of-care vertical flow allergen microarray assay: Proof of concept. Clin. Chem. 2014, 60, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mu, K.; Wei, H.; Chen, H.; Wang, Y.; Zhang, W.; Rong, Z. based multiplex colorimetric vertical flow assay with smartphone readout for point-of-care detection of acute kidney injury biomarkers. Sens. Actuators B Chem. 2023, 390, 134029. [Google Scholar] [CrossRef]
- Goncharov, A.; Joung, H.A.; Ghosh, R.; Han, G.R.; Ballard, Z.S.; Maloney, Q.; Bell, A.; Aung, C.T.Z.; Garner, O.B.; Carlo, D.D. Deep Learning-Enabled Multiplexed Point-of-Care Sensor using a Paper-Based Fluorescence Vertical Flow Assay. Small 2023, 19, 2300617. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS based lateral flow immunoassay for point-of-care detection of SARS-CoV-2 in clinical samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Cheng, L.; Ding, S.; Wang, G.; Choo, J.; Chen, L. SERS-based test strips: Principles, designs and applications. Biosens. Bioelectron. 2021, 189, 113360. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzán, L.M. SERS-based diagnosis and biodetection. Small 2010, 6, 604–610. [Google Scholar] [CrossRef]
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In vitro and in vivo SERS biosensing for disease diagnosis. Biosensors 2018, 8, 46. [Google Scholar] [CrossRef]
- Premasiri, W.; Chen, Y.; Williamson, P.; Bandarage, D.; Pyles, C.; Ziegler, L. Rapid urinary tract infection diagnostics by surface-enhanced Raman spectroscopy (SERS): Identification and antibiotic susceptibilities. Anal. Bioanal. Chem. 2017, 409, 3043–3054. [Google Scholar] [CrossRef]
- Wang, X.; Choi, N.; Cheng, Z.; Ko, J.; Chen, L.; Choo, J. Simultaneous detection of dual nucleic acids using a SERS-based lateral flow assay biosensor. Anal. Chem. 2017, 89, 1163–1169. [Google Scholar] [CrossRef]
- Ben Aissa, A.; Araújo, B.; Julián, E.; Boldrin Zanoni, M.V.; Pividori, M.I. Immunomagnetic separation improves the detection of mycobacteria by paper-based lateral and vertical flow immunochromatographic assays. Sensors 2021, 21, 5992. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Joung, H.-A.; Ballard, Z.S.; Ma, A.; Tseng, D.K.; Teshome, H.; Burakowski, S.; Garner, O.B.; Di Carlo, D.; Ozcan, A. based multiplexed vertical flow assay for point-of-care testing. Lab A Chip 2019, 19, 1027–1034. [Google Scholar] [CrossRef]
- Khelifa, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Lateral flow assays for hormone detection. Lab A Chip 2022, 22, 2451–2475. [Google Scholar] [CrossRef] [PubMed]
- Rohrman, B.A.; Leautaud, V.; Molyneux, E.; Richards-Kortum, R.R. A lateral flow assay for quantitative detection of amplified HIV-1 RNA. PLoS ONE 2012, 7, e45611. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef]
- Hering, K.; Cialla, D.; Ackermann, K.; Dörfer, T.; Möller, R.; Schneidewind, H.; Mattheis, R.; Fritzsche, W.; Rösch, P.; Popp, J. SERS: A versatile tool in chemical and biochemical diagnostics. Anal. Bioanal. Chem. 2008, 390, 113–124. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khlebtsov, N. Surface-Enhanced Raman Scattering-Based Lateral-Flow Immunoassay. Nanomaterials 2020, 10, 2228. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Lin, L.; Khlebtsov, B.N.; Ye, J. Gap-enhanced Raman tags: Fabrication, optical properties, and theranostic applications. Theranostics 2020, 10, 2067–2094. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Purrà, M.; Carré-Camps, M.; de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-Enhanced Raman Spectroscopy-Based Sandwich Immunoassays for Multiplexed Detection of Zika and Dengue Viral Biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Sivashanmugan, K.; Liao, J.-D.; Yao, C.-K.; Peng, H.-C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, O.; Gribanyov, D.; Kukushkin, V.; Kopylov, A.; Zavyalova, E. SERS-based biosensors for virus determination with oligonucleotides as recognition elements. Int. J. Mol. Sci. 2020, 21, 3373. [Google Scholar] [CrossRef]
- Ravindranath, S.P.; Wang, Y.; Irudayaraj, J. SERS driven cross-platform based multiplex pathogen detection. Sens. Actuators B Chem. 2011, 152, 183–190. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Kumar, A.; Luo, D.; Murray, J.; Jones, L.; Chen, X.; Hülck, S.; Tripp, R.A.; Zhao, Y. Multiplex Detection and Quantification of Virus Co-Infections Using Label-free Surface-Enhanced Raman Spectroscopy and Deep Learning Algorithms. ACS Sens. 2025, 10, 1298–1311. [Google Scholar] [CrossRef]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.-Y.; Tian, Z.; Shang, Y.; Xing, J.; Jiang, Y.; Zeng, J. Ag nanoparticles with ultrathin Au shell-based lateral flow immunoassay for colorimetric and SERS dual-mode detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94, 8466–8473. [Google Scholar] [CrossRef]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Maleki Dizaj, S.; Ardalan, M.; Samiei, M.; Sharifi, S.; Zununi Vahed, S.; Huseynova, I.; Khalilov, R. A comprehensive review of detection methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O.; Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Advantages, disadvantages and modifications of conventional ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA) from A to Z; Springer: Berlin/Heidelberg, Germany, 2018; pp. 67–115. [Google Scholar]
- Li, H.; Zhang, K.-L.; Kou, Y.; Xu, S.; Guo, X.-M.; Fu, S.-Y.; Li, Z.; Zhang, Y.-J.; Chen, X.; Li, J.-F. Resonance SERS probe based on the bifunctional molecule IR808 combined with SA test strips for highly sensitive detection of monkeypox virus. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 331, 125761. [Google Scholar] [CrossRef]
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of monkeypox virus–An overview. Travel Med. Infect. Dis. 2022, 50, 102459. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. Smartphone detection of antibiotic resistance using convective PCR and a lateral flow assay. Sens. Actuators B Chem. 2019, 298, 126849. [Google Scholar] [CrossRef]
- Aguilar-Shea, A.L.; Vera-García, M.; Güerri-Fernández, R. Rapid antigen tests for the detection of SARS-CoV-2: A narrative review. Aten. Primaria 2021, 53, 102127. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; Jeon, J.H.; Rhie, G.-e.; Choo, J. Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Skottman, T.; Piiparinen, H.; Hyytiäinen, H.; Myllys, V.; Skurnik, M.; Nikkari, S. Simultaneous real-time PCR detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 207–211. [Google Scholar] [CrossRef] [PubMed]
- McLain, J.E.; Cytryn, E.; Durso, L.M.; Young, S. Culture-based methods for detection of antibiotic resistance in agroecosystems: Advantages, challenges, and gaps in knowledge. J. Environ. Qual. 2016, 45, 432–440. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Stratton, C.W.; Persing, D.H.; Tang, Y.-W. Forty years of molecular diagnostics for infectious diseases. J. Clin. Microbiol. 2022, 60, e02446-21. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, T.; Tian, B.; Li, J.; Liu, Y.; Wu, Z.; Jin, X.; Wang, C.; Wang, C.; Gu, B. Recent advances in SERS-based immunochromatographic assay for pathogenic microorganism diagnosis: A review. Anal. Chim. Acta 2024, 1286, 341931. [Google Scholar] [CrossRef]
- Liang, J.; Wu, L.; Wang, Y.; Liang, W.; Hao, Y.; Tan, M.; He, G.; Lv, D.; Wang, Z.; Zeng, T. SERS/photothermal-based dual-modal lateral flow immunoassays for sensitive and simultaneous antigen detection of respiratory viral infections. Sens. Actuators B Chem. 2023, 389, 133875. [Google Scholar] [CrossRef]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. Influenza A virus isolation, culture and identification. Nat. Protoc. 2014, 9, 2663–2681. [Google Scholar] [CrossRef]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antivir. Res. 2021, 185, 104970. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Teymouri, M.; Mollazadeh, S.; Mortazavi, H.; Ghale-Noie, Z.N.; Keyvani, V.; Aghababaei, F.; Hamblin, M.R.; Abbaszadeh-Goudarzi, G.; Pourghadamyari, H.; Hashemian, S.M.R. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol.-Res. Pract. 2021, 221, 153443. [Google Scholar] [CrossRef] [PubMed]
- Sloan-Dennison, S.; O’Connor, E.; Dear, J.W.; Graham, D.; Faulds, K. Towards quantitative point of care detection using SERS lateral flow immunoassays. Anal. Bioanal. Chem. 2022, 414, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xie, Y.; Liu, X.; Chen, M.; Zheng, C.; Zhong, H.; Li, M. Absolute quantification of serum exosomes in patients with an sers-lateral flow strip biosensor for noninvasive clinical cancer diagnosis. ACS Appl. Mater. Interfaces 2023, 15, 37130–37142. [Google Scholar] [CrossRef]
- Song, Y.; Sun, J.; Li, C.; Lin, L.; Gao, F.; Yang, M.; Sun, B.; Wang, Y. Long-term monitoring of blood biomarkers related to intrauterine growth restriction using AgNPs SERS tags-based lateral flow assay. Talanta 2022, 241, 123128. [Google Scholar] [CrossRef] [PubMed]
- Suhag, A.; Berghella, V. Intrauterine growth restriction (IUGR): Etiology and diagnosis. Curr. Obstet. Gynecol. Rep. 2013, 2, 102–111. [Google Scholar] [CrossRef]
- Guerby, P.; Bujold, E. Early detection and prevention of intrauterine growth restriction and its consequences. JAMA Pediatr. 2020, 174, 749–750. [Google Scholar] [CrossRef]
- Figueras, F.; Gardosi, J. Intrauterine growth restriction: New concepts in antenatal surveillance, diagnosis, and management. Am. J. Obstet. Gynecol. 2011, 204, 288–300. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and ultrasensitive detection of multiplex cardiac biomarkers in lateral flow assay with core-shell SERS nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef]
- McCann, C.J.; Glover, B.M.; Menown, I.B.; Moore, M.J.; McEneny, J.; Owens, C.G.; Smith, B.; Sharpe, P.C.; Young, I.S.; Adgey, J.A. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur. Heart J. 2008, 29, 2843–2850. [Google Scholar] [CrossRef]
- Mirzaeizadeh, Z.; Sadrabadi, E.A.; Naseri, N.; Golmohammadi, H.; Omidfar, K. Smart early diagnosis of acute myocardial infarction: A ZIF-based nanofluorescence lateral flow immunoassay for point-of-care detection of cTnI. Mater. Adv. 2025, 6, 839–848. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, J.; Lee, S.; Lim, D.W.; Joo, H.; Choo, J. Quantitative analysis of thyroid-stimulating hormone (TSH) using SERS-based lateral flow immunoassay. Sens. Actuators B Chem. 2017, 240, 358–364. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Bhana, S.; Mrabeti, S. Challenges in interpreting thyroid stimulating hormone results in the diagnosis of thyroid dysfunction. J. Thyroid Res. 2019, 2019, 4106816. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; McNamee, S.E.; Vance, P.; McLaughlin, J. High-sensitive detection and quantitative analysis of thyroid-stimulating hormone using gold-nanoshell-based lateral flow immunoassay device. Biosensors 2022, 12, 182. [Google Scholar] [CrossRef]
- Peng, S.; Fan, M.; Xiao, C.; Chen, Y.; You, R.; Xu, Y.; Chen, Y.; Liu, Y.; Xiao, X.; Feng, S. Portable SERS-based lateral flow immunoassay strips with self-calibration for detection of a prostate cancer biomarker. Sens. Actuators B Chem. 2024, 401, 135012. [Google Scholar] [CrossRef]
- Clarke, O.; Goodall, B.; Hui, H.; Vats, N.; Brosseau, C. Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal. Chem. 2017, 89, 1405–1410. [Google Scholar] [CrossRef]
- Verucchi, G.; Calza, L.; Manfredi, R.; Chiodo, F. Human immunodeficiency virus and hepatitis C virus coinfection: Epidemiology, natural history, therapeutic options and clinical management. Infection 2004, 32, 33–46. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, P.; Wang, S.; Melnykov, A.; Jiang, Q.; Seth, A.; Wang, Z.; Morrissey, J.J.; George, I.; Gandra, S. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 2023, 7, 1556–1570. [Google Scholar] [CrossRef]
- Lu, Y.; Fei, R.; Zhang, J.; Zhu, G.; Mo, X.; Wan, Y.; Huang, Y.; Sun, Q.; Meng, D.; Zhao, X. Rapid and simultaneous detection of SARS-CoV-2 and influenza A using vertical flow assay based on AAO and SERS nanotags. Sens. Diagn. 2023, 2, 1292–1301. [Google Scholar] [CrossRef]
- Havasi, A.; Visan, S.; Cainap, C.; Cainap, S.S.; Mihaila, A.A.; Pop, L.-A. Influenza A, influenza B, and SARS-CoV-2 similarities and differences—A focus on diagnosis. Front. Microbiol. 2022, 13, 908525. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Ishikane, M.; Ueda, P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA 2020, 323, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Peck, K.R. Early diagnosis and rapid isolation: Response to COVID-19 outbreak in Korea. Clin. Microbiol. Infect. 2020, 26, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Q.; Zhong, K.; Wu, W.; Zheng, S.; Yao, W.; Gao, B.; Sun, F. Enhanced SERS-based vertical flow assay for high sensitivity multiplex analysis of antibiotics. Microchem. J. 2024, 199, 110008. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Deng, W.-J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Qiao, L.; Xu, J.; Yang, Z.; Li, X.; Chen, L.; Sun, H.; Mu, Y. Residual risk of avermectins in food products of animal origin and their research progress on toxicity and determination. Food Rev. Int. 2023, 39, 7019–7047. [Google Scholar] [CrossRef]
- Ramatla, T.; Ngoma, L.; Adetunji, M.; Mwanza, M. Evaluation of antibiotic residues in raw meat using different analytical methods. Antibiotics 2017, 6, 34. [Google Scholar] [CrossRef]
- Chen, R.; Wu, J.; Wang, H.; Nisar, M.S.; Li, Y.; Chen, Y.; Mao, Y.; Nan, X.; Zhang, F.; Yang, L. Fe3O4@ Au magnetic SERS nanotags-based vertical flow immunoassay for simultaneous detection of fumonisin B1, aflatoxin B1 and deoxnivalenol. Anal. Chim. Acta 2025, 1348, 343837. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Elliott, C.T.; Sooksimuang, T.; Charlermroj, R.; Petchkongkaew, A.; Karoonuthaisiri, N. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J. Hazard. Mater. 2022, 432, 128706. [Google Scholar] [CrossRef]
- Gambacorta, S.; Solfrizzo, H.; Visconti, A.; Powers, S.; Cossalter, A.M.; Pinton, P.; Oswald, I.P. Validation study on urinary biomarkers of exposure for aflatoxin B1, ochratoxin A, fumonisin B1, deoxynivalenol and zearalenone in piglets. World Mycotoxin J. 2013, 6, 299–308. [Google Scholar] [CrossRef]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Xie, J.; Li, X.; Huang, Z. Rapid simultaneous detection of fumonisin B1 and deoxynivalenol in grain by immunochromatographic test strip. Anal. Biochem. 2020, 606, 113878. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass mycotoxin analysis in food, environmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges; a review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Berger, A.G.; Restaino, S.M.; White, I.M. Vertical-flow paper SERS system for therapeutic drug monitoring of flucytosine in serum. Anal. Chim. Acta 2017, 949, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-S.; Lee, M.-H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Sigera, L.S.M.; Denning, D.W. Flucytosine and its clinical usage. Ther. Adv. Infect. Dis. 2023, 10, 20499361231161387. [Google Scholar] [CrossRef]
- Ates, H.C.; Roberts, J.A.; Lipman, J.; Cass, A.E.; Urban, G.A.; Dincer, C. On-site therapeutic drug monitoring. Trends Biotechnol. 2020, 38, 1262–1277. [Google Scholar] [CrossRef]
- Chen, R.; Du, X.; Cui, Y.; Zhang, X.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assay for inflammatory biomarkers based on nanofluidic channel array and SERS nanotags. Small 2020, 16, 2002801. [Google Scholar] [CrossRef]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and chronic systemic inflammation: Features and differences in the pathogenesis, and integral criteria for verification and differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef]
- Qu, J.; Lü, X.; Liu, Y.; Wang, X. Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J. Med. Res. 2015, 141, 315–321. [Google Scholar] [PubMed]
- Zhu, S.; Zeng, C.; Zou, Y.; Hu, Y.; Tang, C.; Liu, C. The clinical diagnostic values of SAA, PCT, CRP, and IL-6 in children with bacterial, viral, or co-infections. Int. J. Gen. Med. 2021, 14, 7107–7113. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lei, W.; Dawei, Y.; Mengyu, Z.; Siyu, W. Diagnostic Value of Interleukin 6, Interleukin 12P70, Serum Amyloid A, and Procalcitonin for Rheumatoid Arthritis and Their Relationship With the Disease Activity. J. Sichuan Univ. (Med. Sci.) 2024, 55, 995–1000. [Google Scholar]
- Wylezinski, L.S.; Gray, J.D.; Polk, J.B.; Harmata, A.J.; Spurlock, C.F., III. Illuminating an invisible epidemic: A systemic review of the clinical and economic benefits of early diagnosis and treatment in inflammatory disease and related syndromes. J. Clin. Med. 2019, 8, 493. [Google Scholar] [CrossRef]
- Pfäfflin, A.; Schleicher, E. Inflammation markers in point-of-care testing (POCT). Anal. Bioanal. Chem. 2009, 393, 1473–1480. [Google Scholar] [CrossRef]
- Macovei, D.-G.; Irimes, M.-B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033–1063. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assays based on core–shell SERS nanotags for multiplex prostate cancer biomarker detection. Analyst 2019, 144, 4051–4059. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Ren, H.; Chen, T.; Gao, L.; Di, L.; Song, Z.; Zhang, Y.; Yang, T.; Thakur, A. Combining multiple serum biomarkers in tumor diagnosis: A clinical assessment. Mol. Clin. Oncol. 2013, 1, 153–160. [Google Scholar] [CrossRef]
- Pezaro, C.; Woo, H.H.; Davis, I.D. Prostate cancer: Measuring PSA. Intern. Med. J. 2014, 44, 433–440. [Google Scholar] [CrossRef]
- Yang, A.-P.; Liu, J.; Lei, H.-Y.; Zhang, Q.-W.; Zhao, L.; Yang, G.-H. CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin. Chim. Acta 2014, 437, 183–186. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Singal, A.G. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators B Chem. 2013, 188, 988–998. [Google Scholar] [CrossRef]
- Su, X.; Liu, X.; Xie, Y.; Chen, M.; Zheng, C.; Zhong, H.; Li, M. Integrated SERS-vertical flow biosensor enabling multiplexed quantitative profiling of serological exosomal proteins in patients for accurate breast cancer subtyping. ACS Nano 2023, 17, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-based cancer biomarkers in liquid biopsy: A promising non-invasive alternative to tissue biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Bu, H.; He, D.; He, X.; Wang, K. Exosomes: Isolation, analysis, and applications in cancer detection and therapy. Chembiochem 2019, 20, 451–461. [Google Scholar] [CrossRef]
- Crow, J.; Samuel, G.; Godwin, A.K. Beyond tumor mutational burden: Potential and limitations in using exosomes to predict response to immunotherapy. Expert Rev. Mol. Diagn. 2019, 19, 1079–1088. [Google Scholar] [CrossRef]
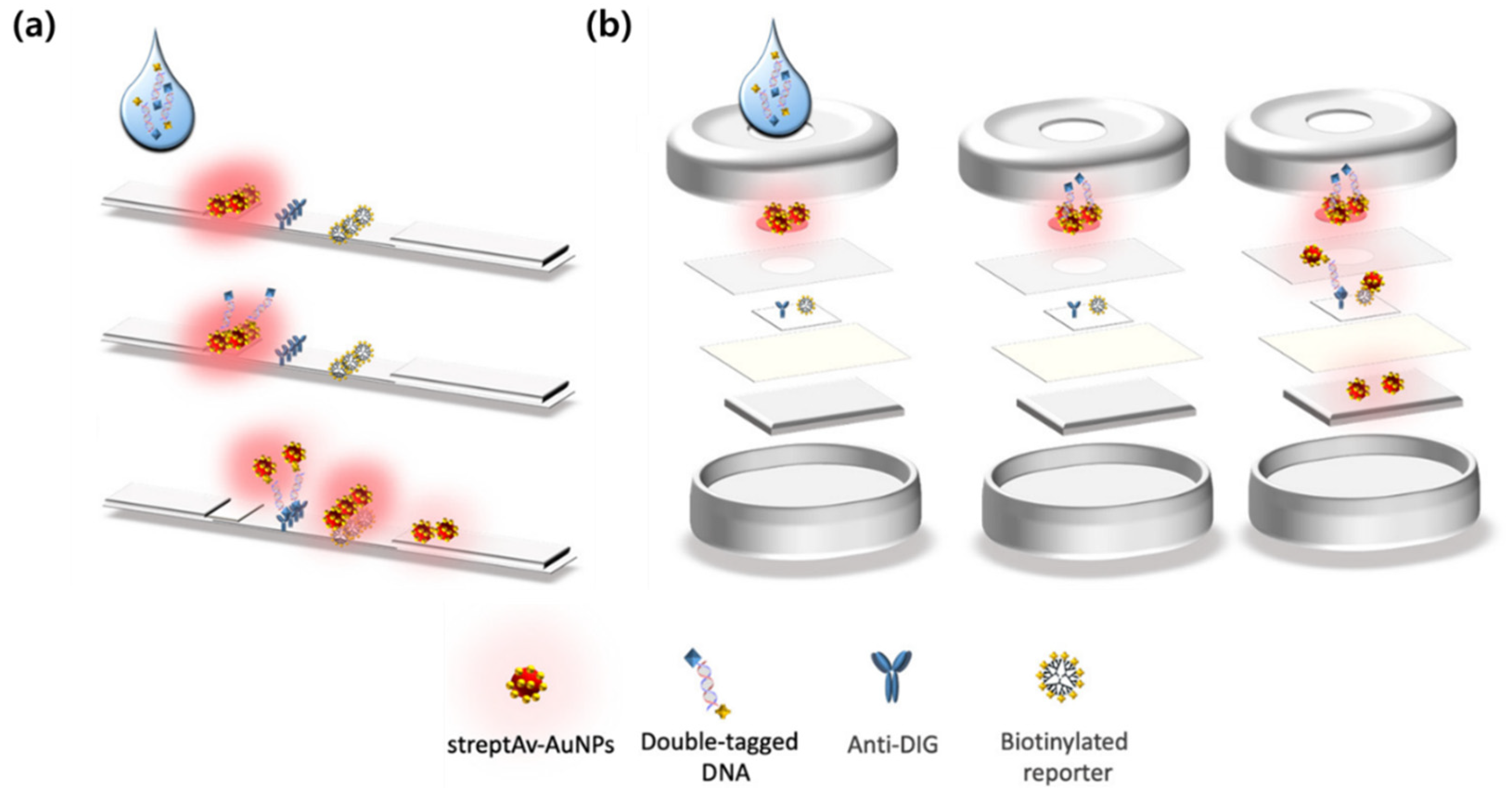
| Format | LFA | LFA | LFA | LFA | VFA | VFA | VFA | VFA |
|---|---|---|---|---|---|---|---|---|
| Target type | Exogenous | Exogenous | Endogenous | Endogenous | Exogenous | Exogenous | Endogenous | Endogenous |
| Analyte(s) | SARS-CoV-2 IgG | IAV, IBV, SARS-CoV-2 N | Myoglobin, cTnI, CK-MB | TSH | SARS-CoV-2 NP, Influenza A HA | FB1, AFB1, DON | CRP, IL-6, SAA, PCT | Exosomal HER2, CEA, MUC1 |
| Probe or reporter | Ag@Au, 4-MBA | Au@Ag, p-ATP dual modal | AuNP, MGITC | Au@Ag@Au | Fe3O4@Au, NBA; 4-MBA; etc. | Au@Ag tags, NBA; 4-MBA; DTNB; MB | Au@Ag tags, NBA; 4-MBA; DTNB; MB | AuNP tags, NBA; 4-MBA; DTNB |
| LoD | 0.52 pg mL−1 | 31.25 pg mL−1 SERS; 15.63 pg mL−1 ΔT | 0.10; 0.01; 0.02 ng mL−1 | 0.025 μIU mL−1 | 0.47; 0.62 pg mL−1 | 0.053; 0.028; 0.079 pg mL−1 | 0.32; 0.30; 0.37; 0.35 ng mL−1 | 6.25 × 105; 1.25 × 105; 2.5 × 104 particles μL−1 |
| Matrix | Serum | Nasopharyngeal swab | Serum | Not specified | Throat swab | Wheat extract | Serum | Serum |
| Assay time | Not specified | Not specified | Not specified | Less than 10 min | Not specified | Less than 10 min | Less than 10 min | Less than 10 min |
| Multiplex | No | 3-plex | 3-plex | No | 2-plex | 3-plex | 4-plex | 3-plex |
| Ref. | [37] | [49] | [61] | [65] | [75] | [83] | [70] | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, B.; Jung, H.S. SERS-Driven Evolution of Lateral and Vertical Flow Assays in Medical Diagnostics. Biosensors 2025, 15, 573. https://doi.org/10.3390/bios15090573
Heo B, Jung HS. SERS-Driven Evolution of Lateral and Vertical Flow Assays in Medical Diagnostics. Biosensors. 2025; 15(9):573. https://doi.org/10.3390/bios15090573
Chicago/Turabian StyleHeo, Boyou, and Ho Sang Jung. 2025. "SERS-Driven Evolution of Lateral and Vertical Flow Assays in Medical Diagnostics" Biosensors 15, no. 9: 573. https://doi.org/10.3390/bios15090573
APA StyleHeo, B., & Jung, H. S. (2025). SERS-Driven Evolution of Lateral and Vertical Flow Assays in Medical Diagnostics. Biosensors, 15(9), 573. https://doi.org/10.3390/bios15090573




