Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics
Abstract
1. Introduction
2. Fundamentals of Nanozymes for Biosensing
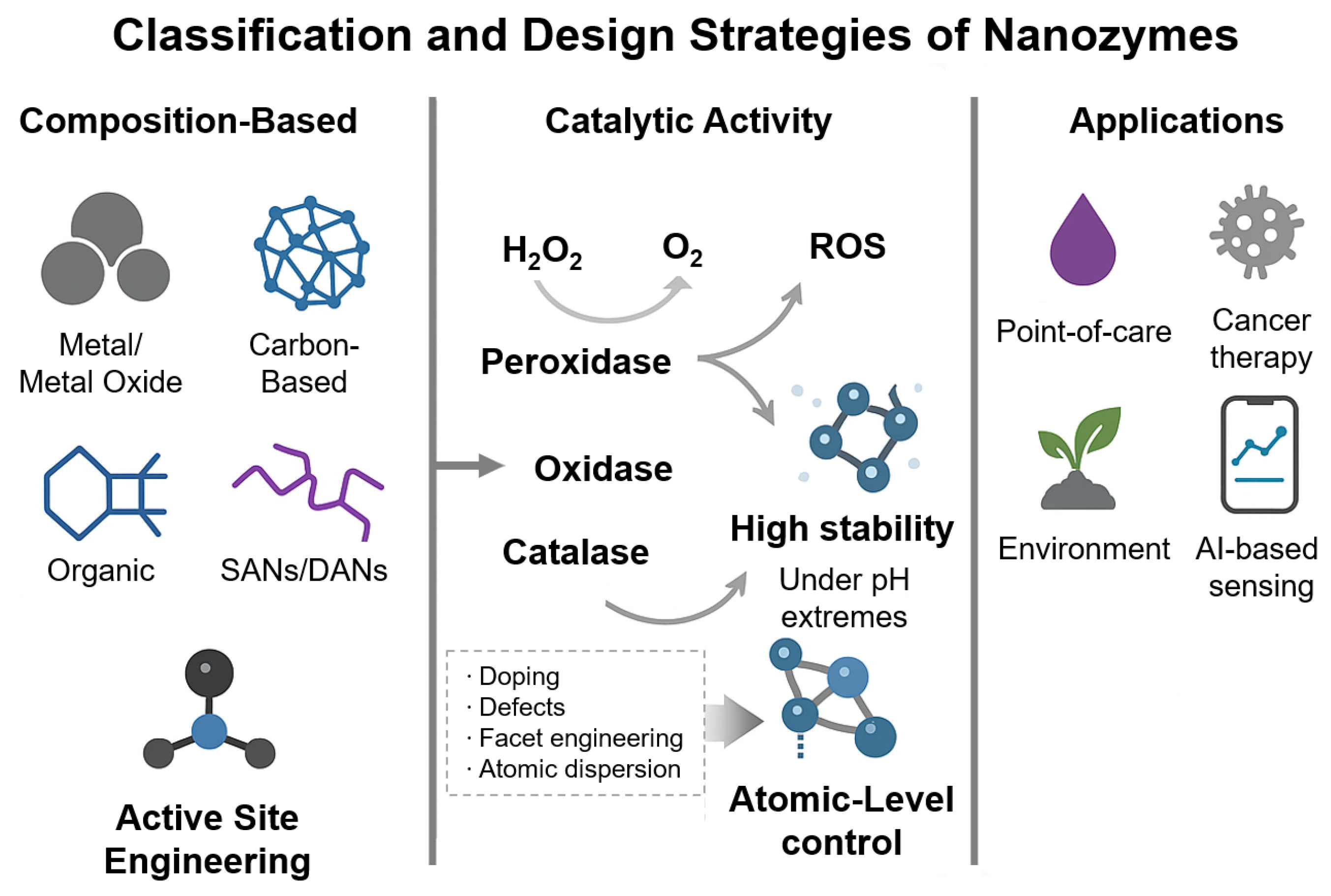
2.1. Definition and Classification of Nanozymes
2.2. Catalytic Mechanisms and Active Site Engineering
2.2.1. Catalytic Mechanisms of Nanozymes
2.2.2. Active Site Engineering for Improved Performance
2.3. Advantages and Limitations of Nanozymes in Biosensing
2.3.1. Advantages of Nanozymes in Biosensing
2.3.2. Limitations of Nanozymes in Biosensing
3. DL Architectures in Nanozyme Research
3.1. Overview of DL and CNNs
3.1.1. Understanding the DL Approach
3.1.2. Signal Collection, Filtering, and Elaboration
3.1.3. Model Training and Application
3.2. CNN Approaches Relevant to Nanozyme-Based Biosensing
4. Practical Applications of DL-Enabled Nanozyme-Based Optical Biosensors
4.1. Synergistic Opportunities
4.2. Smartphone-Integrated DL-Enhanced Nanozyme-Based Biosensors
4.2.1. DL-Powered Colorimetric Biosensors
4.2.2. Sensor Arrays for Multi-Analyte Discrimination
4.2.3. Dual-Mode Detection and Smart Sensing
4.2.4. Mechanistic Detection and Multifunctional Systems
4.2.5. Expansion to General ML Architectures
5. Challenges and Future Perspectives
5.1. Most Pressing: Data Standardization and Model Generalizability
5.2. Model Interpretability and Clinical Trust
5.3. Technical Integration: Real-Time Deployment and IoT Compatibility
5.4. Matrix Effects and Real-Sample Validation
5.5. Long-Term Materials Challenge: Synthetic Control and Biocompatibility
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid ammonium salt) |
| AI | artificial intelligence |
| ASSURED | Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable |
| CNN | convolutional neural network |
| COF | covalent organic framework |
| CQD | carbon quantum dot |
| CSAs | colorimetric sensor arrays |
| DAN | dual-atom nanozyme |
| DFT | density functional theory |
| DL | deep learning |
| DNN | deep neural network |
| FTIR | Fourier transform infrared spectroscopy |
| GO | graphene oxide |
| HCA | hierarchical clustering analysis |
| HNCs | hollow nanocubes |
| HSV | hue-saturation-value |
| IoMT | Internet of Medical Things |
| LDA | linear discriminant analysis |
| ML | machine learning |
| MOF | metal-organic framework |
| OER | oxygen evolution reaction |
| OVs | oxygen vacancies |
| POC | point-of-care |
| RGB | red-green-blue |
| ROS | reactive oxygen species |
| SAN | single-atom nanozyme |
| SERS | surface-enhanced Raman scattering |
| SPR | surface plasmon resonance |
| TMB | 3,3’,5,5’-tetramethylbenzidine |
| YOLO | You Only Look Once |
References
- Tian, Q.; Li, S.; Tang, Z.; Zhang, Z.; Du, D.; Zhang, X.; Niu, X.; Lin, Y. Nanozyme-Enabled Biomedical Diagnosis: Advances, Trends, and Challenges. Adv. Healthc. Mater. 2025, 14, 2401630. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Jiang, Y.; Li, T.; Qiu, X. Advances in Total Antioxidant Capacity Detection Based on Nanozyme. Talanta 2025, 292, 127941. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, A.; Bainik, O.; Navyatha, B.; Santhosh, C.; Banoth, E.; Balaji, R.; Chandrasekar, N.; Kumar, S.; Daima, H.K. Emerging Trends, and Prospects in Nanozyme Engineering to Enhance Dual-Mode Sensing Applications. Coord. Chem. Rev. 2025, 540, 216768. [Google Scholar] [CrossRef]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A Clear Definition with Fuzzy Edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Chang, Z.; Fu, Q.; Wang, M.; Duan, D. Advances of Nanozyme-Driven Multimodal Sensing Strategies in Point-of-Care Testing. Biosensors 2025, 15, 375. [Google Scholar] [CrossRef]
- Patil, P.D.; Karvekar, A.; Salokhe, S.; Tiwari, M.S.; Nadar, S.S. When Nanozymes Meet Enzyme: Unlocking the Dual-Activity Potential of Integrated Biocomposites. Int. J. Biol. Macromol. 2024, 271, 132357. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Su, M.; Zhu, S.; Xie, Y.; Ying, B. Smart Nanozymes for Diagnosis of Bacterial Infection: The Next Frontier from Laboratory to Bedside Testing. ACS Appl. Mater. Interfaces 2024, 16, 44361–44375. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Kurup, C.P.; Ahmed, M.U. Nanozymes towards Personalized Diagnostics: A Recent Progress in Biosensing. Biosensors 2023, 13, 461. [Google Scholar] [CrossRef]
- Xu, K.; Cui, Y.; Guan, B.; Qin, L.; Feng, D.; Abuduwayiti, A.; Wu, Y.; Li, H.; Cheng, H.; Li, Z. Nanozymes with Biomimetically Designed Properties for Cancer Treatment. Nanoscale 2024, 16, 7786–7824. [Google Scholar] [CrossRef]
- Jiang, S.; Su, G.; Wu, J.; Song, C.; Lu, Z.; Wu, C.; Wang, Y.; Wang, P.; He, M.; Zhao, Y.; et al. Co3O4/CoFe2O4 Hollow Nanocube Multifunctional Nanozyme with Oxygen Vacancies for Deep-Learning-Assisted Smartphone Biosensing and Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2023, 15, 11787–11801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Yu, X.; Zhou, B.; Xu, W.; Zhou, X.; Chen, Z. Smartphone-Based Bimetallic Single-Atom Nanozyme Sensor Array Integrated with Deep Learning for Rapid Biothiol Detection. ACS Appl. Nano Mater. 2025, 8, 5481–5493. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Z.; Chen, Z.; Guo, M.; Zhang, Y.; Wang, L.; Zhu, Z. Machine Learning in Nanozymes: From Design to Application. Biomater. Sci. 2024, 12, 2229–2243. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, M.; Fan, K. Artificial Intelligence-Driven Revolution in Nanozyme Design: From Serendipity to Rational Engineering. Mater. Horiz. 2025. [Google Scholar] [CrossRef]
- Xuan, W.; Li, X.; Gao, H.; Zhang, L.; Hu, J.; Sun, L.; Kan, H. Artificial Intelligence Driven Platform for Rapid Catalytic Performance Assessment of Nanozymes. Sci. Rep. 2025, 15, 13305. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, S.; Gao, W.; Li, N.; Zhu, P.; Zhang, L.; Yu, J. Enzyme-Nanozyme Cascade Flow Reactor Synergy with Deep Learning for Differentiation and Point-of-Care Testing of Multiple Organophosphorus Pesticides. Adv. Funct. Mater. 2025, 35, 2419499. [Google Scholar] [CrossRef]
- Acquarelli, J.; van Laarhoven, T.; Gerretzen, J.; Tran, T.N.; Buydens, L.M.C.; Marchiori, E. Convolutional Neural Networks for Vibrational Spectroscopic Data Analysis. Anal. Chim. Acta 2017, 954, 22–31. [Google Scholar] [CrossRef]
- Kautz, E.; Ma, W.; Jana, S.; Devaraj, A.; Joshi, V.; Yener, B.; Lewis, D. An Image-Driven Machine Learning Approach to Kinetic Modeling of A Discontinuous Precipitation Reaction. Mater. Charact. 2020, 166, 110379. [Google Scholar] [CrossRef]
- Rajapakse, D.; Meckstroth, J.; Jantz, D.T.; Camarda, K.V.; Yao, Z.; Leonard, K.C. Deconvoluting Kinetic Rate Constants of Catalytic Substrates from Scanning Electrochemical Approach Curves with Artificial Neural Networks. ACS Meas. Sci. Au 2023, 3, 103–112. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Cui, Z.; Shi, L.; Shang, Y.; Ji, Y.; Wang, J. Machine Learning-Assisted Nanosensor Arrays: An Efficiently High-Throughput Food Detection Analysis. Trends Food Sci. Technol. 2024, 149, 104564. [Google Scholar] [CrossRef]
- Liu, P.; Shi, C.; Liu, Y.; Yang, F.; Yang, Y. Development of AI-Integrated Smartphone Sponge-Based Sensors Utilizing His@Co-NC Nanozymes for Highly Sensitive Sarcosine Detection. Biosens. Bioelectron. 2025, 286, 117621. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Tang, Z.; Liu, S.; Peng, J.; Liang, H.; Niu, X. Machine Learning-Enabled Time-Resolved Nanozyme-Encoded Recognition of Endogenous Mercaptans for Disease Diagnosis. Anal. Chem. 2025, 97, 10463–10473. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing. Nano-Micro Lett. 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Du, Y.; Fu, Y.; Guo, Y.; Gao, X.; Guo, X.; Wei, J.; Yang, Y. Ceria-Based Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing. ACS Sens. 2023, 8, 4442–4467. [Google Scholar] [CrossRef]
- Kapoor, S.; Narayanan, A. Leakage and the Reproducibility Crisis in Machine-Learning-Based Science. Patterns 2023, 4. [Google Scholar] [CrossRef]
- Songca, S.P. Applications of Nanozymology in the Detection and Identification of Viral, Bacterial and Fungal Pathogens. Int. J. Mol. Sci. 2022, 23, 4638. [Google Scholar] [CrossRef]
- Irkham, I.; Ibrahim, A.U.; Nwekwo, C.W.; Al-Turjman, F.; Hartati, Y.W. Current Technologies for Detection of COVID-19: Biosensors, Artificial Intelligence and Internet of Medical Things (IoMT): Review. Sensors 2022, 23, 426. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, S.; Hu, Y. Recent Advances in Nanozyme Sensors Based on Metal-Organic Frameworks and Covalent-Organic Frameworks. Biosensors 2024, 14, 520. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Nanozymes: Definition, Activity, and Mechanisms. Adv. Mater. 2024, 36, 2211041. [Google Scholar] [CrossRef]
- Keum, C.; Hirschbiegel, C.-M.; Chakraborty, S.; Jin, S.; Jeong, Y.; Rotello, V.M. Biomimetic and Bioorthogonal Nanozymes for Biomedical Applications. Nano Converg. 2023, 10, 42. [Google Scholar] [CrossRef]
- Halmagyi, T.G.; Noureen, L.; Szerlauth, A.; Szilagyi, I. Engineering Inorganic Nanozyme Architectures for Decomposition of Reactive Oxygen Species. Dalton Trans. 2024, 53, 14132–14138. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Min, D.; Chen, G.; Li, M.; Tong, L.; Cao, Y. Inorganic Nanozymes: Prospects for Disease Treatments and Detection Applications. Front. Chem. 2021, 9, 773285. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.M.; Do, H.D.K.; Nam, N.N.; Tran, N.K.S.; Dan, T.T.; Trinh, K.T.L. Antioxidant Nanozymes: Mechanisms, Activity Manipulation, and Applications. Micromachines 2023, 14, 1017. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Guo, Y.; Zhang, Y.; Zhang, A.; Jia, M.; Yin, J.; Shen, G. Nanozymes: A Bibliometrics Review. J. Nanobiotechnol. 2024, 22, 704. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Lewandowska, H.; Wójciuk, K.; Karczmarczyk, U. Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Appl. Sci. 2021, 11, 9019. [Google Scholar] [CrossRef]
- Sun, H.; Bai, Y.; Zhao, D.; Wang, J.; Qiu, L. Transition-Metal-Oxide-Based Nanozymes for Antitumor Applications. Materials 2024, 17, 2896. [Google Scholar] [CrossRef]
- Zhang, N.; Du, Y.; Zhang, Z.; Zhu, L.; Jiang, L. Microbe-Mediated Synthesis of Defect-Rich CeO2 Nanoparticles with Oxidase-Like Activity for Colorimetric Detection of L-Penicillamine and Glutathione. Nanoscale 2025, 17, 4142–4151. [Google Scholar] [CrossRef]
- Li, P.; Yuan, W.; Hu, K. Porous CeO2 Nanozyme with Visible-Light-Enhanced Catalase-Mimicking Activities by Ligand-to-Metal Charge Transfer. iScience 2025, 28, 112149. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; Shen, Z.; Wu, H.; Meng, L.; Guo, X.; Jiang, B.; Fang, L. Ultrafast Synthesis of L-His-Fe3O4 Nanozymes with Enhanced Peroxidase-Like Activity for Effective Antibacterial Applications. Front. Bioeng. Biotechnol. 2025, 13, 1548025. [Google Scholar] [CrossRef]
- Jia, H.; Gong, J.; Hu, Z.; Wen, T.; Li, C.; Chen, Y.; Huang, J.; He, W. Antioxidant Carbon Dots Nanozymes Alleviate Stress-Induced Depression by Modulating Gut Microbiota. Langmuir 2024, 40, 19739–19750. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tirado, E.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon-Based Enzyme Mimetics for Electrochemical Biosensing. Micromachines 2023, 14, 1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Zhang, J.; Xu, H.; Yu, X. Recent Progress in Graphitic Carbon Nitride-Based Materials for Antibacterial Applications: Synthesis, Mechanistic Insights, and Utilization. Microstructures 2024, 4, 2024017. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Yuan, Z.; Wu, L.; Ma, J.; Tan, W.; Sun, Y.; Zhang, G.; Chai, H. MOF Nanozymes: Active Sites and Sensing Applications. Inorg. Chem. Front. 2025, 12, 400. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Gao, Y.; Ping, J.; Zhao, F. Recent Advances in Metal-Organic Framework-Based Nanozymes and Their Enabled Optical Biosensors for Food Safety Analysis. Trac-Trends Anal. Chem. 2024, 173, 117602. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Qian, W.; Lei, F.; Chen, Z.; Wu, X.; Lin, Y.; Wang, F. Recent Advances in MOF-Based Nanozymes: Synthesis, Activities, and Bioapplications. Biosens. Bioelectron. 2024, 263, 116593. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Bi, R.; Liu, J.; Cai, Y.; Zhang, S.; Lu, M.; Du, C.; Liu, M.; Ding, X.; Xiao, K.; Li, S.; et al. Dual-Atom Nanozymes: Synthesis, Characterization, Catalytic Mechanism and Biomedical Applications. Colloid Surf. B-Biointerfaces 2025, 253, 114774. [Google Scholar] [CrossRef]
- Jin, C.; Fan, S.; Zhuang, Z.; Zhou, Y. Single-Atom Nanozymes: From Bench to Bedside. Nano Res. 2023, 16, 1992–2002. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, G.; Sun, Y.; Zhang, X.; Huang, J.; Liu, S.; Lin, T.; Ye, F.; Zhao, S. Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application. Catalysts 2019, 9, 1057. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Shah, L.A.; Zhao, H.; Ye, D. Strategies for Regulating Catalytic Specificity of Nanozyme. Colloid Surf. B-Biointerfaces 2025, 255, 114925. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Y.; Tang, G.; Li, Y.; Zhao, Y.; Yu, Y.; Wang, G.; Yan, X.; Fan, K. Intelligent Nanozymes: Biomimetic Design, Mechanisms and Biomedical Applications. Fundam. Res. 2024. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Chung, S.J. Phytosynthesis of Palladium Nanoclusters: An Efficient Nanozyme for Ultrasensitive and Selective Detection of Reactive Oxygen Species. Molecules 2020, 25, 3349. [Google Scholar] [CrossRef]
- Moradi Hasan-Abad, A.; Shabankare, A.; Atapour, A.; Hamidi, G.A.; Salami Zavareh, M.; Sobhani-Nasab, A. The Application of Peroxidase Mimetic Nanozymes in Cancer Diagnosis and Therapy. Front. Pharmacol. 2024, 15, 1339580. [Google Scholar] [CrossRef]
- Wang, X.; Hou, L.; Dang, M.; Li, H.; Li, B.; Wu, J.; Gao, L. Catalytic Mechanism and Biomedical Applications of Diatomic Nanozymes. Microstructures. 2025, 5, 2025052. [Google Scholar] [CrossRef]
- Zong, X.; Xu, X.; Pang, D.-W.; Huang, X.; Liu, A.-A. Fine-Tuning Electron Transfer for Nanozyme Design. Adv. Healthc. Mater. 2025, 14, 2401836. [Google Scholar] [CrossRef]
- Yuan, B.; Chou, H.-L.; Peng, Y.-K. Disclosing the Origin of Transition Metal Oxides as Peroxidase (and Catalase) Mimetics. ACS Appl. Mater. Interfaces 2022, 14, 22728–22736. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, H.; Fan, K. Structure-Activity Mechanism of Iron Oxide Nanozymes. In Nanozymes: Design, Synthesis, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1422, pp. 1–35. [Google Scholar]
- Chen, J.; Ma, Q.; Li, M.; Chao, D.; Huang, L.; Wu, W.; Fang, Y.; Dong, S. Glucose-Oxidase Like Catalytic Mechanism of Noble Metal Nanozymes. Nat. Commun. 2021, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.J.; Kolanthai, E.; Wei, F.; Coathup, M.; Seal, S. Surface Chemistry of Biologically Active Reducible Oxide Nanozymes. Adv. Mater. 2024, 36, 2211261. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, S.; Mo, S.-C.; Huang, H.; Liang, H.; Zhang, J.; Xu, Z.-L.; Liu, W.; Liu, Y. Dual-Active Centers Linked by Oxygen Transfer for Enhancing Proximity-Orientation Effect of Nanozyme. Adv. Funct. Mater. 2025, 35, 2418360. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Dong, X.; Mei, L.; Wu, X.; Gu, Z.; Zhao, Y. X-Ray-Facilitated Redox Cycling of Nanozyme Possessing Peroxidase-Mimicking Activity for Reactive Oxygen Species-Enhanced Cancer Therapy. Biomaterials 2021, 276, 121023. [Google Scholar] [CrossRef]
- Xia, N.; Gao, F.; Zhang, J.; Wang, J.; Huang, Y. Overview on the Development of Electrochemical Immunosensors by the Signal Amplification of Enzyme- or Nanozyme-Based Catalysis Plus Redox Cycling. Molecules 2024, 29, 2796. [Google Scholar] [CrossRef]
- ling, P.; Song, D.; Yang, P.; Tang, C.; Xu, W.; Wang, F. NIR-II-Responsive Versatile Nanozyme Based on H2O2 Cycling and Disrupting Cellular Redox Homeostasis for Enhanced Synergistic Cancer Therapy. ACS Biomater. Sci. Eng. 2024, 10, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Wu, B.; Huang, Y.; Zou, Y.; Liang, C.; Lu, Y.; Luo, H.; Chen, L.; Chen, J. Polyvalent Copper Oxide Nanozymes: Co-Delivery of ROS and NO for Inducing Cuproptosis-Like Bacterial Death Against MRSA Infections. Chem. Eng. J. 2024, 499, 156507. [Google Scholar] [CrossRef]
- Guo, J.; Han, H.; Zhao, H.; Jia, D.; Yin, L.; Sha, J. Cascade-Enhanced Based-Polyoxometalates Nanozyme for Glutathione Detection and Tumor Cell Disruption. Talanta 2025, 291, 127890. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, J.; Zhang, Y.; Li, H.; Deng, K.; Huang, H. Light-Controlled Regulation of Dual-Enzyme Properties in YbGd-Carbon Quantum Dots Nano-Hybrid for Advanced Biosensing. Anal. Chem. 2024, 96, 13455–13463. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, X.; Huo, D.; Cen, J.; Jia, Z.; Liu, Y.; Liu, J. A Hybrid Nanozymes In Situ Oxygen Supply Synergistic Photothermal/Chemotherapy of Cancer Management. Biomater. Sci. 2021, 9, 5330–5343. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Li, S.; Chen, Y.; Lv, W.; Zhang, J.; Zhang, L.; Zhang, Z.; Lu, X. Synergistic Effect Enhances the Peroxidase-Like Activity in Platinum Nanoparticle-Supported Metal—Organic Framework Hybrid Nanozymes for Ultrasensitive Detection of Glucose. Nano Res. 2021, 14, 4689–4695. [Google Scholar] [CrossRef]
- Li, J.; Yi, W.; Luo, Y.; Yang, K.; He, L.; Xu, C.; Deng, L.; He, D. Gsh-Depleting and H2o2-Self-Supplying Hybrid Nanozymes for Intensive Catalytic Antibacterial Therapy by Photothermal-Augmented Co-Catalysis. Acta Biomater. 2023, 155, 588–600. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Yang, Y.; Zhu, X.; Chen, X.; Ye, G.; Liu, J. Reactive Oxygen Species-Mediated CuRuOX@HA Hybrid Nanozymes for Multidrug-Resistant Bacterial Infections with Synergistic Photothermal Therapy. J. Mat. Chem. B 2023, 11, 5195–5206. [Google Scholar] [CrossRef]
- Zeng, X.; Ruan, Y.; Chen, Q.; Yan, S.; Huang, W. Biocatalytic Cascade in Tumor Microenvironment with a Fe2O3/Au Hybrid Nanozyme for Synergistic Treatment of Triple Negative Breast Cancer. Chem. Eng. J. 2023, 452, 138422. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent Progress in the Design Fabrication of Metal-Organic Frameworks-Based Nanozymes and Their Applications to Sensing and Cancer Therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Yang, P.; Tao, J.; Chen, F.; Chen, Y.; He, J.; Shen, K.; Zhao, P.; Li, Y. Multienzyme-Mimic Ultrafine Alloyed Nanoparticles in Metal Organic Frameworks for Enhanced Chemodynamic Therapy. Small 2021, 17, 2005865. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 Nanozyme with High Specificity for Catalysis of Catechol Oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Wang, C.; Cheng, Z.; Zheng, W.; Xu, P.; Chen, Q.; Zhao, Y. Missing-Linker-Confined Single-Atomic Pt Nanozymes for Enzymatic Theranostics of Tumor. Angew. Chem.-Int. Edit. 2023, 135, e202217995. [Google Scholar] [CrossRef]
- Lai, C.-M.; Xiao, X.-S.; Chen, J.-Y.; He, W.-Y.; Wang, S.-S.; Qin, Y.; He, S.-H. Revolutionizing nanozymes: The Synthesis, Enzyme-Mimicking Capabilities of Carbon Dots, and Advancements in Catalytic Mechanisms. Int. J. Biol. Macromol. 2025, 293, 139284. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Falcao, S.; Méndez-Arriaga, J.M.; García-Almodóvar, V.; García-Valdivia, A.A.; Gómez-Ruiz, S. Gold Nanozymes: Smart Hybrids with Outstanding Applications. Catalysts 2023, 13, 13. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.-N.; Liang, X.; Sun, H.-b.; Xin, H.; Liang, Q. Recent Advances in Nanozymes: From Matters to Bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, X.; Wang, Y.; Shen, B.; Yang, Y.; Huang, H. Heteroatom-Doped Nanozyme Progress and Perspectives: From Synthesis Strategies to Biomedical Applications. Chem. Eng. J. 2023, 468, 143703. [Google Scholar] [CrossRef]
- Yan, H.; Wang, L.; Chen, Y.; Jiao, L.; Wu, Y.; Xu, W.; Gu, W.; Song, W.; Du, D.; Zhu, C. Fine-Tuning Pyridinic Nitrogen in Nitrogen-Doped Porous Carbon Nanostructures for Boosted Peroxidase-Like Activity and Sensitive Biosensing. Research 2020, 2020, 8202584. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhou, S. Progress and Perspective on Carbon-Based Nanozymes for Peroxidase-like Applications. J. Phys. Chem. Lett. 2021, 12, 11751–11760. [Google Scholar] [CrossRef]
- Fan, K.; Xi, J.; Fan, L.; Wang, P.; Zhu, C.; Tang, Y.; Xu, X.; Liang, M.; Jiang, B.; Yan, X.; et al. In Vivo Guiding Nitrogen-Doped Carbon Nanozyme for Tumor Catalytic Therapy. Nat. Commun. 2018, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Batool, I.; Anwar, A.; Imran, M.; Alvi, Z.I. Prospecting Carbon-Based Nanomaterials for Harnessing Multienzyme-Like Activities. Top. Catal. 2025, 68, 823–855. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Jiao, L.; Tang, Y.; Chen, Y.; Gu, W.; Zhu, C. Defect Engineering in Nanozymes. Mater. Today 2022, 52, 327–347. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, B.; Tao, Y.; Zhao, H.; Zhang, Z.; Wang, X.; Zhou, X.; Jiang, B.; Yang, Z.; Yan, X.; et al. Edge-Site Engineering of Defective Fe-N4 Nanozymes with Boosted Catalase-Like Performance for Retinal Vasculopathies. Adv. Mater. 2022, 34, 2205324. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Abdullah, W.; Alanezi, S.T.; Kasasbeh, W.H.; Fohely, F.; Khaniabadi, P.M.; Jameel, M.S.; Oladzadabbasabadi, N.; Ghasemlou, M. Engineering Colloidal Nanozymes for Cancer Diagnosis and Therapy: From Surface Chemistry to Catalytic Mechanisms and Precision Medicine. ACS Appl. Bio Mater. 2025, 8, 4514–4548. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Gong, X.; Wang, Y.; Li, Y.F.; Huang, C.Z. Engineering Metal-Organic Frameworks-Based Nanozymes for Enhanced Biomimetic Catalytic Sensing. Trac-Trends Anal. Chem. 2024, 178, 117862. [Google Scholar] [CrossRef]
- Lu, W.; Chen, J.; Kong, L.; Zhu, F.; Feng, Z.; Zhan, J. Oxygen Vacancies Modulation Mn3O4 Nanozyme with Enhanced Oxidase-Mimicking Performance for l-Cysteine Detection. Sens. Actuator B-Chem. 2021, 333, 129560. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, S.; Wang, M.; Xie, Q.; Zhang, L.; Gong, M.; Zhang, D.; Zhou, C. Reactive Oxygen Species Scavenging Nanozymes: Emerging Therapeutics for Acute Liver Injury Alleviation. Int. J. Nanomed. 2023, 18, 7901–7922. [Google Scholar] [CrossRef]
- Li, F.; Qiu, Y.; Xia, F.; Sun, H.; Liao, H.; Xie, A.; Lee, J.; Lin, P.; Wei, M.; Shao, Y.; et al. Dual Detoxification and Inflammatory Regulation by Ceria Nanozymes for Drug-Induced Liver Injury Therapy. Nano Today 2020, 35, 100925. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-Atom Nanozymes. Sci. Adv. 2019, 5, eaav5490. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Teffo, M.L.; Sadiku, E.R. Cancer Therapy with Engineered Nanozymes: From Molecular Design to Tumour-Responsive Catalysis. Nanomedicine 2025, 20, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, C.A.S.; Mercante, L.A.; Alvarenga, A.D.; Facure, M.H.M.; Schneider, R.; Correa, D.S. Recent Trends in Nanozymes Design: From Materials and Structures to Environmental Applications. Mat. Chem. Front. 2021, 5, 7419–7451. [Google Scholar] [CrossRef]
- Wang, Y.; Du, R.; Lee, L.Y.S.; Wong, K.-Y. Rational Design and Structural Engineering of Heterogeneous Single-Atom Nanozyme for Biosensing. Biosens. Bioelectron. 2022, 216, 114662. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, X.; Li, X.; Yu, J.; Peng, L.; Wei, Z. Advances in Spin Regulation of M-N-C Single-Atom Catalysts and Their Applications in Electrocatalysis. Chin. J. Catal. 2025, 69, 17–34. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, J.; Zhou, H.; Hu, C.; Dalangood, S.; Li, H.; Yang, D.; Yang, Y.; Gui, J. A Single-Atom Manganese Nanozyme Mn-N/C Promotes Anti-Tumor Immune Response via Eliciting Type I Interferon Signaling. Adv. Sci. 2024, 11, 2305979. [Google Scholar] [CrossRef]
- Ma, D.; Tang, Z.; Guan, X.; Liang, Z.; Liang, Q.; Jiao, Y.; Wang, L.; Ye, L.; Huang, H.; He, C.; et al. Unraveling Valence Electron Number Dependent Excitonic Effects over M1-N3C1 Sites in Single-Atom Catalysts. ACS Nano 2024, 18, 6579–6590. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, K.; Yue, Q.; Yin, K.; Xu, X.; Li, Y.; Gao, Y.; Gao, B. Coordination Number Regulation of Iron Single-Atom Nanozyme for Enhancing H2O2 Activation and Selectively Eliminating Cephalosporin Antibiotics. Adv. Funct. Mater. 2024, 34, 2406790. [Google Scholar] [CrossRef]
- Wong, K.-Y.; Wong, M.-S.; Liu, J. Nanozymes for Treating Ocular Diseases. Adv. Healthc. Mater. 2025, 14, 2401309. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Yang, M.; Wang, R.; Xie, Y.; Zhu, L.; Huang, J.; Xu, W. Applications of DNA Functionalized Gold Nanozymes in Biosensing. Biosens. Bioelectron. 2025, 271, 116987. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The Nanozyme Revolution: Enhancing the Performance of Medical Biosensing Platforms. Adv. Mater. 2024, 36, 2300184. [Google Scholar] [CrossRef]
- Lee, J.; Liao, H.; Wang, Q.; Han, J.; Han, J.-H.; Shin, H.E.; Ge, M.; Park, W.; Li, F. Exploration of Nanozymes In Viral Diagnosis and Therapy. Exploration 2022, 2, 20210086. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, B.; Zhang, Y.; Shang, X.; Ma, C.; Gao, W.; Zhu, Z. Bioinspired Rational Design of Nanozymes. Mater. Horiz. 2025. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Mienye, I.D.; Swart, T.G. A Comprehensive Review of Deep Learning: Architectures, Recent Advances, and Applications. Information 2024, 15, 755. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep Learning in Neural Networks: An Overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Alzahrani, M.; Usman, M.; Jarraya, S.K.; Anwar, S.; Helmy, T. Deep Models for Multi-view 3D Object Recognition: A Review. Artif. Intell. Rev. 2024, 57, 323. [Google Scholar] [CrossRef]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Hasan, M.; Van Essen, B.C.; Awwal, A.A.S.; Asari, V.K. A State-of-the-Art Survey on Deep Learning Theory and Architectures. Electronics 2019, 8, 292. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of Deep Learning: Concepts, CNN Architectures, Challenges, Applications, Future Directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Singhal, C.M.; Kaushik, V.; Awasthi, A.; Zalke, J.B.; Palekar, S.; Rewatkar, P.; Srivastava, S.K.; Kulkarni, M.B.; Bhaiyya, M.L. Deep Learning-Enhanced Portable Chemiluminescence Biosensor: 3D-Printed, Smartphone-Integrated Platform for Glucose Detection. Bioengineering 2025, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyya, M.; Rewatkar, P.; Pimpalkar, A.; Jain, D.; Srivastava, S.K.; Zalke, J.; Kalambe, J.; Balpande, S.; Kale, P.; Kalantri, Y.; et al. Deep Learning-Assisted Smartphone-Based Electrochemiluminescence Visual Monitoring Biosensor: A Fully Integrated Portable Platform. Micromachines 2024, 15, 1059. [Google Scholar] [CrossRef] [PubMed]
- Berezsky, O.; Liashchynskyi, P.; Pitsun, O.; Izonin, I. Synthesis of Convolutional Neural Network Architectures for Biomedical Image Classification. Biomed. Signal Process. Control 2024, 95, 106325. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Yang, J.; Chen, L.; Zhou, H.; Liu, X.; Li, H.; Lin, T.; Ying, Y. Understanding the Learning Mechanism of Convolutional Neural Networks in Spectral Analysis. Anal. Chim. Acta 2020, 1119, 41–51. [Google Scholar] [CrossRef]
- Chin, C.-L.; Chang, C.-E.; Chao, L. Interpretable Multiscale Convolutional Neural Network for Classification and Feature Visualization of Weak Raman Spectra of Biomolecules at Cell Membranes. ACS Sens. 2025, 10, 2652–2666. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, T.; Xu, J.; Luo, X.; Ying, Y. DeepSpectra: An End-to-End Deep Learning Approach for Quantitative Spectral Analysis. Anal. Chim. Acta 2019, 1058, 48–57. [Google Scholar] [CrossRef]
- Jin, K.-X.; Shen, J.; Wang, Y.-J.; Yang, Y.; Cao, S.-H. Enhanced Nanoparticle Recognition via Deep Learning-Accelerated Plasmonic Sensing. Biosensors 2024, 14, 363. [Google Scholar] [CrossRef]
- Oiticica, P.R.A.; Angelim, M.K.S.C.; Soares, J.C.; Soares, A.C.; Proença-Módena, J.L.; Bruno, O.M.; Oliveira, O.N., Jr. Using Machine Learning and Optical Microscopy Image Analysis of Immunosensors Made on Plasmonic Substrates: Application to Detect the SARS-CoV-2 Virus. ACS Sens. 2025, 10, 1407–1418. [Google Scholar] [CrossRef]
- Thadson, K.; Sasivimolkul, S.; Suvarnaphaet, P.; Visitsattapongse, S.; Pechprasarn, S. Measurement Precision Enhancement of Surface Plasmon Resonance Based Angular Scanning Detection Using Deep Learning. Sci. Rep. 2022, 12, 2052. [Google Scholar] [CrossRef]
- Madsen, J.Ø.; Topalian, S.O.N.; Jacobsen, M.F.; Skovby, T.; Gernaey, K.V.; Myerson, A.S.; Woodley, J. Raman Spectroscopy and One-Dimensional Convolutional Neural Network Modeling as A Real-Time Monitoring Tool for In Vitro Transaminase-Catalyzed Synthesis of A Pharmaceutically Relevant Amine Precursor. Biotechnol. Prog. 2024, 40, e3444. [Google Scholar] [CrossRef]
- Nitika, N.; Keerthiveena, B.; Thakur, G.; Rathore, A.S. Convolutional Neural Networks Guided Raman Spectroscopy as a Process Analytical Technology (PAT) Tool for Monitoring and Simultaneous Prediction of Monoclonal Antibody Charge Variants. Pharm. Res. 2024, 41, 463–479. [Google Scholar] [CrossRef]
- Fu, J. Research on Meat Adulteration Based on One-Dimensional Convolutional Neural Network (1DCNN) Combined with Electrochemical Technology. J. Food Meas. Charact. 2024, 18, 5720–5728. [Google Scholar] [CrossRef]
- Gecgel, O.; Ramanujam, A.; Botte, G.G. Selective Electrochemical Detection of SARS-CoV-2 Using Deep Learning. Viruses 2022, 14, 1930. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Thik, J.; Yamaguchi, T.; Ling, C. Computer Vision Enabled High-Quality Electrochemical Experimentation. Digit. Discov. 2024, 3, 2183–2191. [Google Scholar] [CrossRef]
- Hoar, B.B.; Zhang, W.; Chen, Y.; Sun, J.; Sheng, H.; Zhang, Y.; Chen, Y.; Yang, J.Y.; Costentin, C.; Gu, Q.; et al. Redox-Detecting Deep Learning for Mechanism Discernment in Cyclic Voltammograms of Multiple Redox Events. ACS Electrochem. 2025, 1, 52–62. [Google Scholar] [CrossRef]
- Hoar, B.B.; Zhang, W.; Xu, S.; Deeba, R.; Costentin, C.; Gu, Q.; Liu, C. Electrochemical Mechanistic Analysis from Cyclic Voltammograms Based on Deep Learning. ACS Meas. Sci. Au 2022, 2, 595–604. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Meng, B.; Zhang, Y.; Wei, Y.; Dai, X.; An, D.; Zhao, Y.; Fang, X. ProteoNet: A CNN-Based Framework for Analyzing Proteomics MS-RGB Images. iScience 2024, 27, 111362. [Google Scholar] [CrossRef]
- Zompola, A.; Korfiati, A.; Theofilatos, K.; Mavroudi, S. Omics-CNN: A Comprehensive Pipeline for Predictive Analytics in Quantitative Omics Using One-Dimensional Convolutional Neural Networks. Heliyon 2023, 9, e21165. [Google Scholar] [CrossRef]
- Kopylov, A.T.; Petrovsky, D.V.; Stepanov, A.A.; Rudnev, V.R.; Malsagova, K.A.; Butkova, T.V.; Zakharova, N.V.; Kostyuk, G.P.; Kulikova, L.I.; Enikeev, D.V.; et al. Convolutional Neural Network in Proteomics and Metabolomics for Determination of Comorbidity between Cancer And Schizophrenia. J. Biomed. Inform. 2021, 122, 103890. [Google Scholar] [CrossRef]
- Li, R.; Zhang, D.; Han, Y.; Liu, Z.; Zhang, Q.; Zhang, Q.; Wang, X.; Pan, S.; Sun, J.; Wang, K. Hybrid Deep Learning Approaches for Improved Genomic Prediction in Crop Breeding. Agriculture 2025, 15, 1171. [Google Scholar] [CrossRef]
- Samukhina, Y.V.; Matyushin, D.D.; Grinevich, O.I.; Buryak, A.K. A Deep Convolutional Neural Network for Prediction of Peptide Collision Cross Sections in Ion Mobility Spectrometry. Biomolecules 2021, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Park, B.U.; Jeon, H.-J. Advances in Machine Learning-Enhanced Nanozymes. Front. Chem. 2024, 12, 1483986. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhong, X.; Liang, C.; Liang, Z.; Nong, Y.; Deng, L.; Guo, Y.; Li, J.; Zhang, M.; Tang, S.; et al. Nanozyme-Based Colorimetric Sensor Arrays Coupling with Smartphone for Discrimination And “Segmentation-Extraction-Regression” Deep Learning Assisted Quantification of Flavonoids. Biosens. Bioelectron. 2024, 263, 116604. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Diao, Q.; Tang, Z.; Niu, X. Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications. Biosensors 2025, 15, 344. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Ran, X.; Liu, C.; Ren, J.; Qu, X. Nanozyme as Artificial Receptor with Multiple Readouts for Pattern Recognition. Anal. Chem. 2018, 90, 11775–11779. [Google Scholar] [CrossRef]
- Gao, J.; Pu, K.; Lin, H.; Zhao, X. The Perovskite-Like La0.96Sr0.04NiO3-δ Nanozyme Based on Deep Learning Assisted Colorimetric and Intelligent Detection for Epinephrine. J. Phys. Chem. Solids, 2025; 200, 112563. [Google Scholar] [CrossRef]
- Sun, M.; Gao, J.; Pu, K.; Liu, T.; Su, G.; Wu, C.; Lu, Z.; Zhao, X. SrCoNiO3-δ Perovskite Nanozyme for Deep-Learning-Assisted Intelligent Detection of Dopamine Hydrochloride and Cd2+. Mater. Chem. Phys. 2025, 329, 129970. [Google Scholar] [CrossRef]
- Zhong, X.; Qin, Y.; Liang, C.; Liang, Z.; Nong, Y.; Luo, S.; Guo, Y.; Yang, Y.; Wei, L.; Li, J.; et al. Smartphone-Assisted Nanozyme Colorimetric Sensor Array Combined “Image Segmentation-Feature Extraction” Deep Learning for Detecting Unsaturated Fatty Acids. ACS Sens. 2024, 9, 5167–5178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Shao, C.; Liu, T.; Sun, M.; Wu, C.; Su, G.; Wang, Y.; Ye, J.; Hu, H.; et al. Nanozyme-Induced Deep Learning-Assisted Smartphone Integrated Colorimetric and Fluorometric Dual-Mode for Detection of Tetracycline Analogs. Anal. Chim. Acta. 2024, 1297, 342373. [Google Scholar] [CrossRef]
- Huang, S.; Xiang, H.; Lv, J.; Zhu, D.; Yu, L.; Guo, Y.; Xu, L. Au Nanozyme-Based Colorimetric Sensor Array Integrates Machine Learning to Identify and Discriminate Monosaccharides. J. Colloid Interface Sci. 2024, 672, 200–208. [Google Scholar] [CrossRef]







| Category | Subtypes/Examples | Typical Enzyme Mimicry | Key Features | Representative Applications |
|---|---|---|---|---|
| Metal/Metal Oxide | Fe3O4, MnO2, CeO2, Au, Pt, Pd | Peroxidase, Oxidase, Catalase | High catalytic activity, redox cycling, robust in harsh media | Colorimetric biosensing, ROS modulation |
| Carbon-Based | Graphene, GO *, CNTs *, CQDs *, g-C3N4 | Peroxidase, Oxidase | Tunable surface chemistry, high conductivity | Electrochemical sensing, antibacterial agents |
| MOF/COF-Based | MOF-545 *, ZIF-8, COF *-TpPa | Peroxidase, Oxidase | High porosity, modular structure, controllable active sites | Multiplex biosensing, environmental sensing |
| Polymer/Organic | Imidazole-functionalized polymers, micelles | Oxidase, Peroxidase | Biomimetic microenvironment, low toxicity | In vivo detection, drug delivery |
| Single-/Dual-Atom Nanozymes | Fe-N4, Cu-N, Fe-Fe SANs*/DANs | Peroxidase, Catalase | Maximum atom utilization, high selectivity | Precision biosensors, enzyme replacement |
| Strategy | Mechanism/Target | Effects on Catalysis | Representative Materials | Applications |
|---|---|---|---|---|
| Surface Doping/ Elemental Substitution | Introduction of heteroatoms (e.g., N, P, and S) into carbon or metal oxide lattices | Modifies electronic structure; creates or enhances active sites; tunes d-band center | N-doped carbon dots; S-doped CeO2; P-doped MnO2 | ORR/OER catalysis, ROS scavenging, biosensing |
| Defect Engineering | Generation of OVs, edge defects, and dislocations | Provides unsaturated coordination sites; enhances electron transfer and adsorption | Oxygen-deficient CeO2, MoS2 edge-defected nanosheets | Peroxidase-like catalysis, H2O2 sensing |
| Facet Exposure Control | Synthesis-directed exposure of specific crystal planes (e.g., {111}, {100}) | Alters surface atom arrangement; modulates substrate binding and reaction pathways | Fe3O4 (111), CeO2 (100) | Selective biomarker detection, ROS modulation |
| Single-/Dual-Atom Dispersion | Isolated atoms or atom pairs anchored on supports (e.g., Fe-N4, Cu-N, Fe-Fe) | Maximizes atom utilization; unique reactivity due to quantum and support interactions | SANs on graphene, Pt-Fe DANs on N-doped carbon | Enzyme-mimetic biosensors, drug monitoring |
| Surface Functionalization | Attachment of ligands, polymers, antibodies, or aptamers | Improves biocompatibility; enhances substrate specificity and matrix stability | Ab-functionalized Au nanozymes, PEGylated Fe3O4 | Targeted diagnostics, in vivo sensing, immunoassays |
| Target Analyte | Nanozyme Type | DL Architecture | Smartphone Function | LOD | Notable Features | Ref |
|---|---|---|---|---|---|---|
| Epinephrine | La0.96Sr0.04NiO3−δ (perovskite) | Custom DL with RGB/HSV analysis | Real-time quantification via RGB/HSV image analysis | 0.1821 μM | High linearity (R2 > 0.99); multi-color model use | [138] |
| Glyphosate, Omethoate, Paraoxon (OPs) | C60@MOF-545-Fe + AChE cascade | YOLOv5-OPs | Image segmentation, RGB/HSV feature extraction, linear fitting | Not explicitly listed; multiplex confirmed | Multiplexed OP detection; YOLOv5 WeChat app | [16] |
| Cys, GSH, Hcy (biothiols) | CuZn-N bimetallic SAzyme | YOLOv5 + PCA *, HCA * | “ThiolSense” app; RGB data + clustering | 1.17 nM | Dual-channel array; serum quantification | [12] |
| Dopamine hydrochloride (DAH), Cd2+ | SrCo NiO3−δ (perovskite) | HSV/RGB + DL regression | On-off-on color change detection + app-based regression | DAH: 0.098 μM Cd2+: 0.343 μM | Environmental sensing; on-site detection | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Moussa, N.A.M.; Kang, S.H. Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics. Biosensors 2025, 15, 571. https://doi.org/10.3390/bios15090571
Lee S, Moussa NAM, Kang SH. Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics. Biosensors. 2025; 15(9):571. https://doi.org/10.3390/bios15090571
Chicago/Turabian StyleLee, Seungah, Nayra A. M. Moussa, and Seong Ho Kang. 2025. "Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics" Biosensors 15, no. 9: 571. https://doi.org/10.3390/bios15090571
APA StyleLee, S., Moussa, N. A. M., & Kang, S. H. (2025). Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics. Biosensors, 15(9), 571. https://doi.org/10.3390/bios15090571








