A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis
Abstract
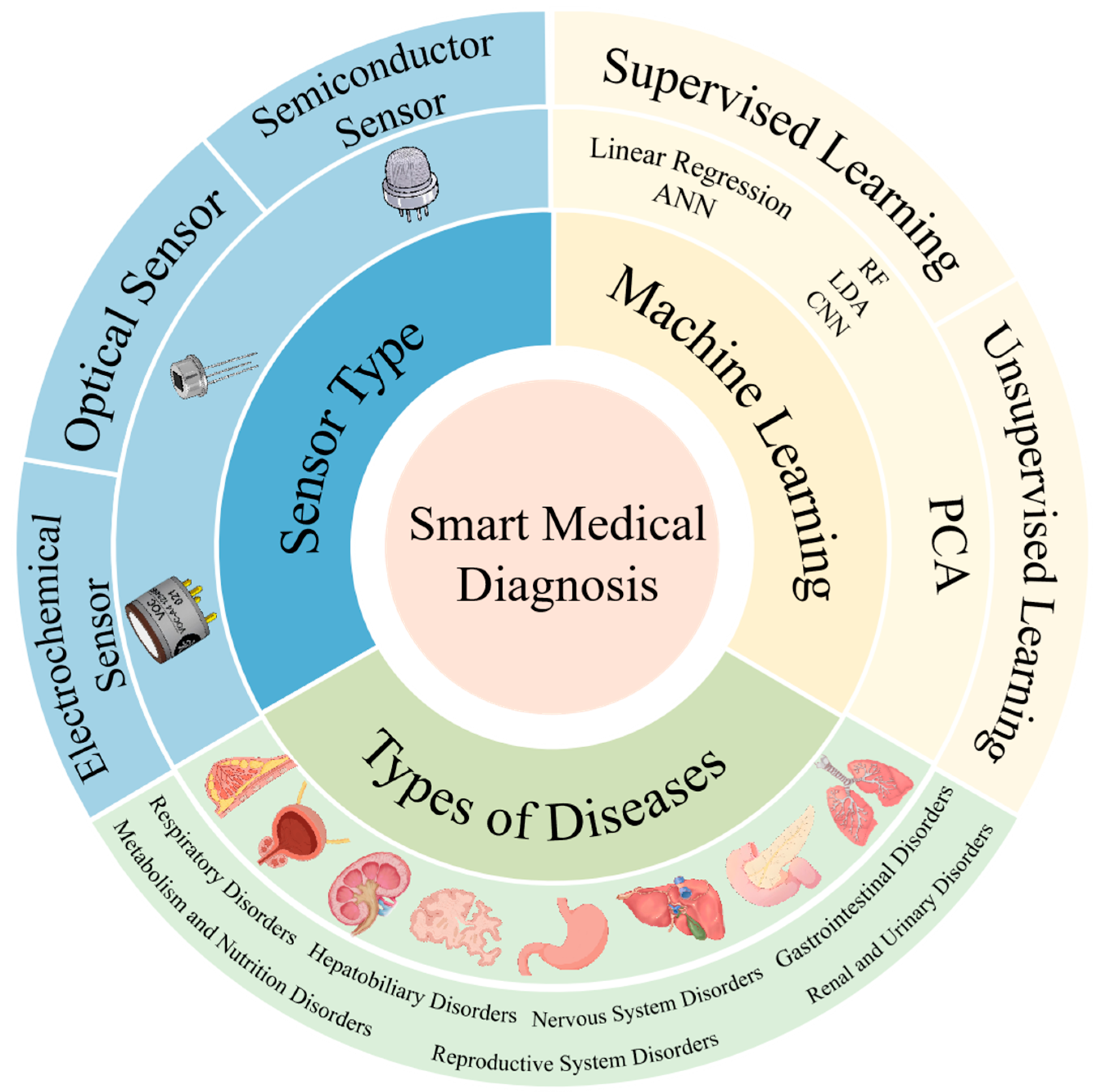
1. Introduction
2. Common Types of Gas Sensors
2.1. Electrochemical Sensor
2.2. Optical Sensor
2.3. Semiconductor Sensor

3. Machine Learning for Gas Sensors
3.1. Supervised Learning
3.1.1. Linear Regression
3.1.2. Support Vector Machine
3.1.3. Artificial Neural Network
3.1.4. Random Forest
3.1.5. Linear Discriminant Analysis

3.2. Unsupervised Learning
4. Application in Disease Diagnosis
4.1. Respiratory Disorder
4.1.1. Lung Cancer and Chronic Obstructive Pulmonary Disease
4.1.2. Asthma
4.2. Metabolism and Nutrition Disorders
4.3. Hepatobiliary Disorders

4.4. Gastrointestinal Disorders
4.5. Nervous System Disorders
4.6. Renal and Urinary Disorders
4.6.1. Nephropathy
4.6.2. Bladder Cancer

4.7. Reproductive System Disorders
4.7.1. Breast Cancer
4.7.2. Prostate Cancer
5. Challenges and Development Directions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ning, J.; Ge, T.; Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S. Early diagnosis of lung cancer: Which is the optimal choice? ? Aging 2021, 13, 6214. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si: WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef]
- Keawkim, K.; Lorjaroenphon, Y.; Vangnai, K.; Jom, K.N. Metabolite–flavor profile, phenolic content, and antioxidant activity changes in sacha inchi (Plukenetia volubilis L.) seeds during germination. Foods 2021, 10, 2476. [Google Scholar] [CrossRef] [PubMed]
- Mubinov, A.; Avdeeva, E.; Kurkin, V.; Latypova, G.; Farkhutdinov, R.; Kataev, V.; Ryazanova, T. Fatty Acid Profile and Antioxidant Activity of Nigella Sativa Fatty Oil. Pharm. Chem. J. 2021, 55, 798–802. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Isidorov, V.A.; Krauze-Baranowska, M. Characterization of Secondary Metabolites of Leaf Buds from Some Species and Hybrids of Populus by Gas Chromatography Coupled with Mass Detection and Two-Dimensional High-Performance Thin-Layer Chromatography Methods with Assessment of Their Antioxidant Activity. Int. J. Mol. Sci. 2024, 25, 3971. [Google Scholar]
- Gonçalves, F.D.; Almeida, M.L.; Martins, J.M.; Carvalho, L.H.; Rodrigues, J.A.; Ramos, R.M. Gas-diffusion microextraction combined with HPLC-DAD for the comprehensive analysis of volatile carbonyl compounds in wood-based panels. Talanta 2024, 272, 125818. [Google Scholar] [CrossRef]
- Tartaglia, A.; Romasco, T.; D’Ovidio, C.; Rosato, E.; Ulusoy, H.; Furton, K.; Kabir, A.; Locatelli, M. Determination of phenolic compounds in human saliva after oral administration of red wine by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2022, 209, 114486. [Google Scholar] [CrossRef]
- Vera, T.; Villanueva, F.; Wimmerová, L.; Tolis, E. An overview of methodologies for the determination of volatile organic compounds in indoor air. Appl. Spectrosc. Rev. 2022, 57, 625–674. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Zhong, R.; Yuan, Z.; Du, J.; Huang, J. Quality evaluation of Sojae Semen Praeparatum by HPLC combined with HS-GC-MS. Heliyon 2023, 9, e18767. [Google Scholar] [CrossRef]
- Selvaraj, R.; Vasa, N.J.; Nagendra, S.S.; Mizaikoff, B. Advances in mid-infrared spectroscopy-based sensing techniques for exhaled breath diagnostics. Molecules 2020, 25, 2227. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liao, F.; Tan, Y.; Zhang, J.; Zheng, C.; Wang, H.; Zhuang, H.; Xiong, W.; Xie, Q.; Dong, W. Application of Fourier transform infrared spectroscopy to exhaled breath analysis for detecting helicobacter pylori infection. Sci. Rep. 2024, 14, 31542. [Google Scholar] [CrossRef] [PubMed]
- Maeso-García, M.D.; Esteve-Turrillas, F.A.; Verdú-Andrés, J. Applications of the photoionization detector (PID) in occupational hygiene. estimation of air changes per hour in premises with natural ventilation. Chemosensors 2021, 9, 331. [Google Scholar] [CrossRef]
- Xu, W.; Cai, Y.; Gao, S.; Hou, S.; Yang, Y.; Duan, Y.; Fu, Q.; Chen, F.; Wu, J. New understanding of miniaturized VOCs monitoring device: PID-type sensors performance evaluations in ambient air. Sens. Actuator B-Chem. 2021, 330, 129285. [Google Scholar] [CrossRef]
- Sofia, M.; Maniscalco, M.; de Laurentiis, G.; Paris, D.; Melck, D.; Motta, A. Exploring airway diseases by NMR-based metabonomics: A review of application to exhaled breath condensate. Biomed Res. Int. 2011, 2011, 403260. [Google Scholar] [CrossRef]
- de Laurentiis, G.; Paris, D.; Melck, D.; Maniscalco, M.; Marsico, S.; Corso, G.; Motta, A.; Sofia, M. Metabonomic analysis of exhaled breath condensate in adults by nuclear magnetic resonance spectroscopy. Eur. Resp. J. 2008, 32, 1175–1183. [Google Scholar] [CrossRef]
- Maniscalco, M.; Cutignano, A.; Paris, D.; Melck, D.J.; Molino, A.; Fuschillo, S.; Motta, A. Metabolomics of exhaled breath condensate by nuclear magnetic resonance spectroscopy and mass spectrometry: A methodological approach. Curr. Med. Chem. 2020, 27, 2381–2399. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Giampetruzzi, L.; Siciliano, P.; Capone, S. Human biomonitoring of environmental and occupational exposures by GC-MS and gas sensor systems: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 10236. [Google Scholar] [CrossRef]
- Bajo-Fernández, M.; Souza-Silva, É.A.; Barbas, C.; Rey-Stolle, M.F.; García, A. GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Front. Mol. Biosci. 2024, 10, 1295955. [Google Scholar] [CrossRef]
- Xu, R.-F.; Mei, H.; Chen, L.; Tang, B.; Lu, Q.-Y.; Cai, F.-S.; Yan, X.; Zheng, J.; Shen, X.-T.; Yu, Y.-J. Development and validation of an HPLC-MS/MS method for the simultaneous analysis of volatile organic compound metabolites, hydroxylated polycyclic aromatic hydrocarbons, and 8-hydroxy-2′-deoxyguanosine in human urine. J. Chromatogr. B 2023, 1229, 123885. [Google Scholar] [CrossRef]
- Fufurin, I.L.; Anfimov, D.R.; Kareva, E.R.; Scherbakova, A.V.; Demkin, P.P.; Morozov, A.N.; Golyak, I.S. Numerical techniques for infrared spectra analysis of organic and inorganic volatile compounds for biomedical applications. Opt. Eng. 2021, 60, 082016. [Google Scholar] [CrossRef]
- Moura, P.C.; Vassilenko, V.; Ribeiro, P.A. Ion mobility spectrometry towards environmental volatile organic compounds identification and quantification: A comparative overview over infrared spectroscopy. Emission Control Sci. Tech. 2023, 9, 25–46. [Google Scholar] [CrossRef]
- Sadaka, K.; Dalvand, B.; Faruqui, Z.; Aqeel, S.; Ghoohestani, M.; Goodarzi, M. Metabolomics of Volatile Organic Compounds (VOCs) in Infectious Diseases. Trac-Trends Anal. Chem. 2024, 181, 118024. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, P.; Walambe, R.; Mandaokar, S.; Chandel, P.; Kotecha, K.; Ghinea, G. Gas detection and identification using multimodal artificial intelligence based sensor fusion. Appl. Syst. Innov. 2021, 4, 3. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, Z.; Jiang, C.; Zhang, X.; Qiao, Z.; Zhang, J.; Liang, J.; Wang, S.; Duan, Z.; Wu, Y. Ultrafast Hydrogen Detection System Using Vertical Thermal Conduction Structure and Neural Network Prediction Algorithm Based on Sensor Response Process. ACS Sensors 2025, 10, 2181–2190. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Shukla, P. A review on recent developments and advances in environmental gas sensors to monitor toxic gas pollutants. Environ. Prog. Sustain. Energy 2023, 42, e14126. [Google Scholar] [CrossRef]
- Moos, R. A Brief Overview on Automotive Exhaust Gas Sensors Based on Electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar] [CrossRef]
- Peterson, P.; Aujla, A.; Grant, K.; Brundle, A.; Thompson, M.; Vande Hey, J.; Leigh, R. Practical Use of Metal Oxide Semiconductor Gas Sensors for Measuring Nitrogen Dioxide and Ozone in Urban Environments. Sensors 2017, 17, 1653. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Lee, J.-H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Nag, S.; Kimbahune, S.; Ghose, A.; Pal, A.; Guha, P.K. Selective discrimination of VOCs applying gas sensing kinetic analysis over a metal oxide-based chemiresistive gas sensor. ACS Sens. 2021, 6, 2218–2224. [Google Scholar] [CrossRef]
- Lv, W.; Shi, W.; Zhang, Z.; Ru, L.; Feng, W.; Tang, H.; Wang, X. Identification of volatile biomarkers for lung cancer from different histological sources: A comprehensive study. Anal. Biochem. 2024, 690, 115527. [Google Scholar] [CrossRef]
- Ng, K.T.; Boussaid, F.; Bermak, A. A CMOS single-chip gas recognition circuit for metal oxide gas sensor arrays. IEEE Trans. Circuits Syst. I-Regul. Pap. 2011, 58, 1569–1580. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R. Pattern analysis for machine olfaction: A review. IEEE Sens. J. 2002, 2, 189–202. [Google Scholar] [CrossRef]
- Wu, P.; Qiu, X.; Wu, Y.; Duan, Z.; Ma, Y.; Yu, H.; Yuan, Z.; Jiang, Y.; Tai, H. Linear Model for Concentration Measurement of Mixed Gases. ACS Sensors 2025, 10, 1948–1958. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Liu, B.; Duan, Z.; Zhang, Y.; Yuan, Z.; Tai, H. Enhancing the carbon dioxide sensing performance of LaFeO3 by Co doping. Sens. Actuator B-Chem. 2024, 402, 135136. [Google Scholar] [CrossRef]
- Padilla, M.; Perera, A.; Montoliu, I.; Chaudry, A.; Persaud, K.; Marco, S. Drift compensation of gas sensor array data by Orthogonal Signal Correction. Chemometrics Intell. Lab. Syst. 2010, 100, 28–35. [Google Scholar] [CrossRef]
- Barman, P.B.; Sil, A.; Hazra, S.K. Recent advancement in selective gas sensors and role of machine learning. J. Alloys Compd. 2025, 1030, 180757. [Google Scholar]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Men, H.; Shi, Y.; Jiao, Y.; Gong, F.; Liu, J. Electronic nose sensors data feature mining: A synergetic strategy for the classification of beer. Anal. Methods 2018, 10, 2016–2025. [Google Scholar] [CrossRef]
- Scott, S.M.; James, D.; Ali, Z. Data analysis for electronic nose systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Ng, X.J.K.; Mohd Khairuddin, A.S.; Liu, H.C.; Loh, T.C.; Tan, J.L.; Khor, S.M.; Leo, B.F. Artificial intelligence-assisted point-of-care devices for lung cancer. Clin. Chim. Acta 2025, 570, 120191. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Kiisk, V.; Kodu, M.; Kahro, T.; Renge, I.; Avarmaa, T.; Makaram, P.; Zurutuza, A.; Jaaniso, R. Semiquantitative classification of two oxidizing gases with graphene-based gas sensors. Chemosensors 2022, 10, 68. [Google Scholar] [CrossRef]
- Zong, B.; Wu, S.; Yang, Y.; Li, Q.; Tao, T.; Mao, S. Smart gas sensors: Recent developments and future prospective. Nano-Micro Lett. 2025, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Y.; Meng, F. Metal oxide semiconductor sensors for triethylamine detection: Sensing performance and improvements. Chemosensors 2022, 10, 231. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, Y.; Han, X.; Qin, J.; Tang, X. Recent advances in enzymeless-based electrochemical sensors to diagnose neurodegenerative diseases. J. Mat. Chem. B 2021, 9, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, X. Electrochemical sensors based on carbon nanomaterial used in diagnosing metabolic disease. Front. Chem. 2020, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Resp. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Swargiary, K.; Kongsawang, N.; Jitpratak, P.; Ajchareeyasoontorn, N.; Udomkittivorakul, J.; Viphavakit, C. Recent Advances in Sensing Materials Targeting Clinical Volatile Organic Compound (VOC) Biomarkers: A Review. Biosensors 2023, 13, 114. [Google Scholar] [CrossRef]
- Khatib, M.; Haick, H. Sensors for Volatile Organic Compounds. ACS Nano 2022, 16, 7080–7115. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Song, F.; Tittel, F.K.; Zheng, C. Mid-infrared absorption spectroscopy for gas sensing and application. In Proceedings of the 2020 IEEE 5th Optoelectronics Global Conference (OGC), Shenzhen, China, 7–11 September 2020; pp. 80–82. [Google Scholar]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Parisi, C.; Pastore, A.; Stornaiuolo, M.; Sortino, S. A fluorescent probe with an ultra-rapid response to nitric oxide. J. Mat. Chem. B 2024, 12, 5076–5084. [Google Scholar] [CrossRef]
- Ermatov, T.; Skibina, J.S.; Tuchin, V.V.; Gorin, D.A. Functionalized microstructured optical fibers: Materials, methods, applications. Materials 2020, 13, 921. [Google Scholar] [CrossRef]
- Jin, F.; Xu, Z.; Cao, D.; Ran, Y.; Guan, B.-O. Fiber-Optic Biosensors for Cancer Theranostics: From in Vitro to in Vivo. Photonic Sens. 2024, 14, 240415. [Google Scholar] [CrossRef]
- Zhu, R.; Gao, J.; Tian, Q.; Li, M.; Gao, Q.; Wu, X.; Xu, S.; Zhang, Y. Optical chemical gas sensor based on spectral autocorrelation: A method for online detection of nitric oxide and ammonia in exhaled breath. Sens. Actuator B-Chem. 2025, 422, 136694. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Schütze, A.; Baur, T.; Leidinger, M.; Reimringer, W.; Jung, R.; Conrad, T.; Sauerwald, T. Highly sensitive and selective VOC sensor systems based on semiconductor gas sensors: How to? Environments 2017, 4, 20. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor metal oxides as chemoresistive sensors for detecting volatile organic compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yue, W.; Li, Y.; Gao, S.; Zhang, C.; Kan, H.; Niu, H.; Wang, W.; Guo, Y. Carbon-based nanomaterials for the detection of volatile organic compounds: A review. Carbon 2021, 180, 274–297. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G. Breakthroughs in the design of novel carbon-based metal oxides nanocomposites for VOCs gas sensing. Nanomaterials 2020, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Šetka, M.; Vojkůvka, L.; Vallejos, S. VOCs sensing by metal oxides, conductive polymers, and carbon-based materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Wang, D.; Zhang, R.; Zhang, Y.; Zhao, L. Insight into SnO2-based gas-sensitive materials and readout circuits for semiconductor gas sensors. Nano Mater. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuator Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xiao, D.; Zhang, D.; Liu, Y.; Sun, M.; Chen, S.; Sun, M. Progress in functionalized WO3-based gas sensors for selective H2S and NH3: A review. Ceram. Int. 2024, 50, 40631–40665. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Cai, Y.; Zhao, J.; Gao, Z.; Song, Y.-Y. The Challenges and Opportunities for TiO2 Nanostructures in Gas Sensing. ACS Sens. 2024, 9, 1644–1655. [Google Scholar] [CrossRef]
- Abimaheshwari, R.; Abinaya, R.; Archana, J.; Muthamizhchelvan, C.; Navaneethan, M.; Harish, S. In-situ growth of vertically stacked 2D SnS2/SnS heterostructure for highly sensitive and rapid room temperature NO2 gas sensor. J. Alloy. Compd. 2024, 1001, 175002. [Google Scholar] [CrossRef]
- Kumar, R.; Zheng, W.; Liu, X.; Zhang, J.; Kumar, M. MoS2-Based Nanomaterials for Room-Temperature Gas Sensors. Adv. Mater. Technol. 2020, 5, 1901062. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic Gas Sensor Based on Few-Layered InSe Nanosheets for NO2 Detection with Ultrahigh Antihumidity Ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Yang, J.; Zhou, J.; Zhang, Y.; Zhang, H.; Li, Y. A Fully Integrated Flexible Tunable Chemical Sensor Based on Gold-Modified Indium Selenide Nanosheets. ACS Sens. 2022, 7, 1183–1193. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicond. Process 2021, 123, 105546. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y.; Pathak, A. NiO-Based Gas Sensors for Ethanol Detection: Recent Progress. J. Sens. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Comini, E. Low-Dimensional Nanostructures Based on Cobalt Oxide (Co3O4) in Chemical-Gas Sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Almaev, A.V.; Kushnarev, B.O.; Chernikov, E.V.; Novikov, V.A.; Korusenko, P.M.; Nesov, S.N. Structural, electrical and gas-sensitive properties of Cr2O3 thin films. Superlattices Microstruct. 2021, 151, 106835. [Google Scholar] [CrossRef]
- Liu, X.; Hu, M.; Wang, Y.; Liu, J.; Qin, Y. High sensitivity NO2 sensor based on CuO/p-porous silicon heterojunction at room temperature. J. Alloys Compd. 2016, 685, 364–369. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.-Y.; Lai, R.-P.; Dong, X.-H.; Wu, X.; Luan, Q.-T.; Wang, J.; Lin, H.-F.; Ding, W.-H.; Wu, G.-L.; Xie, W.-F. Recent advances in ethanol gas sensors based on metal oxide semiconductor heterojunctions. Rare Metals 2022, 41, 1818–1842. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, Z.; Song, J.; Zheng, C. Improvement and mechanism for the fast response of a Pt/TiO2 gas sensor. Sens. Actuator B-Chem. 2010, 148, 87–92. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Zheng, B.; Geng, X.; Debliquy, M. Hydrogen sensors based on noble metal doped metal-oxide semiconductor: A review. Int. J. Hydrogen Energy 2017, 42, 20386–20397. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Wei, L.; Chaogang, L.; Zhiwei, Z. Noble metal–semiconductor nanocomposites for optical, energy and electronics applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110106. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, K.-B.; Kim, S.H.; Park, K.-H.; Lee, M.-I.; Cho, J.W.; Lim, J.; Hwang, I.-S.; Kang, Y.C.; Hwang, J.-H. Synergistic Integration of Machine Learning with Microstructure/Composition-Designed SnO2 and WO3 Breath Sensors. ACS Sens. 2024, 9, 182–194. [Google Scholar] [CrossRef]
- Zhou, Z.-H. Machine Learning; Springer Nature: London, UK, 2021. [Google Scholar]
- El Naqa, I.; Murphy, M.J. What is machine learning. In Machine Learning in Radiation Oncology: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Dietterich, T.G. Machine learning. Annu. Rev. Comput. Sci. 1990, 4, 255–306. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine learning: Trends, perspectives, and prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef]
- Cunningham, P.; Cord, M.; Delany, S.J. Supervised Learning. In Machine Learning Techniques for Multimedia: Case Studies on Organization and Retrieval; Cord, M., Cunningham, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 21–49. [Google Scholar]
- Wang, W.-Y.; Du, W.-W.; Xu, D.; Wang, W.; Peng, W.-C. A survey on self-supervised learning for non-sequential tabular data. Mach. Learn. 2025, 114, 16. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, L.; Ge, Z. Laplacian regularization of linear regression model for semi-supervised industrial soft sensor development. Expert Syst. Appl. 2024, 254, 124459. [Google Scholar] [CrossRef]
- Etemadi, S.; Khashei, M. Etemadi multiple linear regression. Measurement 2021, 186, 110080. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, Y.; Guo, M.; Liu, L.; Chen, P.; Song, A.; Yu, W.; Hu, S.; Zhao, R.; Fang, Z.; et al. Schottky Contacts Regularized Linear Regression for Signal Inconsistency Circumvent in Resistive Gas Micro-Nanosensors. Small Methods 2021, 5, e2101194. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; Xiang, M.; Cui, D.; Li, Q. Discrimination of VOCs with e-nose consisting of a single MEMS sensor using lightweight deep learning model based on SqueezeNet. Sens. Actuator B-Chem. 2025, 422, 136640. [Google Scholar] [CrossRef]
- Hsia, J.Y.; Lin, C.J. Parameter Selection for Linear Support Vector Regression. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 5639–5644. [Google Scholar] [CrossRef]
- Peng, X.; Xu, D. Projection support vector regression algorithms for data regression. Knowl.-Based Syst. 2016, 112, 54–66. [Google Scholar] [CrossRef]
- Martínez-Merino, L.I.; Puerto, J.; Rodríguez-Chía, A.M. Ordered Weighted Average Support Vector Regression. Expert Syst. Appl. 2025, 274, 126882. [Google Scholar] [CrossRef]
- Liu, L.; Na, N.; Yu, J.; Zhao, W.; Wang, Z.; Zhu, Y.; Hu, C. Sniffing Like a Wine Taster: Multiple Overlapping Sniffs (MOSS) Strategy Enhances Electronic Nose Odor Recognition Capability. Adv. Sci. 2023, 11, e2305639. [Google Scholar] [CrossRef]
- Harakeh, A.; Mellah, S.; Ouladsine, M.; Younes, R.; Bellet, C. New approach for gas identification using supervised learning methods (SVM and LVQ). In Proceedings of the MATEC Web of Conferences, Varna, Bulgaria, 19–22 June 2019; p. 06004. [Google Scholar]
- Han, D.; Wang, Y.; Wang, Y.; Duan, Q.; Li, D.; Ge, Y.; He, X.; Zhao, L.; Wang, W.; Sang, S. Machine-learning-assisted n-GaN-Au/PANI gas sensor array for intelligent and ultra-accurate ammonia recognition. Chem. Eng. J. 2024, 495, 153705. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Bruschi, P.; Ria, A.; Magliocca, F.; Vitelli, M.; Piotto, M. An ASIC-based system-in-package MEMS gas sensor with impedance spectroscopy readout and AI-enabled identification capabilities. Sens. Actuator B-Chem. 2025, 424, 136924. [Google Scholar] [CrossRef]
- Habib, M.M.; Rodan, A.; Alazzam, A. A Classification Model for Gas Drift problem. In Proceedings of the 2019 Sixth HCT Information Technology Trends (ITT), Ras Al Khaimah, United Arab Emirates, 20–21 November 2019; pp. 172–176. [Google Scholar]
- Khan, M.A.H.; Thomson, B.; Debnath, R.; Motayed, A.; Rao, M.V. Nanowire-Based Sensor Array for Detection of Cross-Sensitive Gases Using PCA and Machine Learning Algorithms. IEEE Sens. J. 2020, 20, 6020–6028. [Google Scholar] [CrossRef]
- Zou, Q.; Itoh, T.; Shin, W.; Sawano, M. Machine-learning-assisted sensor array for detecting COVID-19 through simulated exhaled air. Sens. Actuator B-Chem. 2024, 400, 134883. [Google Scholar] [CrossRef]
- Cernat, A.; Groza, A.; Tertis, M.; Feier, B.; Hosu-Stancioiu, O.; Cristea, C. Where artificial intelligence stands in the development of electrochemical sensors for healthcare applications-A review. Trac-Trends Anal. Chem. 2024, 181, 117999. [Google Scholar] [CrossRef]
- Wiederoder, M.S.; Nallon, E.C.; Weiss, M.; McGraw, S.K.; Schnee, V.P.; Bright, C.J.; Polcha, M.P.; Paffenroth, R.; Uzarski, J.R. Graphene Nanoplatelet-Polymer Chemiresistive Sensor Arrays for the Detection and Discrimination of Chemical Warfare Agent Simulants. ACS Sens. 2017, 2, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Christinelli, W.A.; Shimizu, F.M.; Facure, M.H.M.; Cerri, R.; Oliveira Jr, O.N.; Correa, D.S.; Mattoso, L.H.C. Two-dimensional MoS2-based impedimetric electronic tongue for the discrimination of endocrine disrupting chemicals using machine learning. Sens. Actuator B-Chem. 2021, 336, 129696. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Yang, J.; Zhou, J.; Xu, Y. Linear discriminant analysis. Nat. Rev. Method. Prim. 2024, 4, 70. [Google Scholar] [CrossRef]
- Drera, G.; Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Chiesa, M.; Zanotti, M.; Pagliara, S.; Fedorov, F.S.; Nasibulin, A.G.; Montuschi, P.; et al. Exploring the performance of a functionalized CNT-based sensor array for breathomics through clustering and classification algorithms: From gas sensing of selective biomarkers to discrimination of chronic obstructive pulmonary disease. RSC Adv. 2021, 11, 30270–30282. [Google Scholar] [CrossRef]
- Thriumani, R.; Zakaria, A.; Hashim, Y.Z.H.-Y.; Jeffree, A.I.; Helmy, K.M.; Kamarudin, L.M.; Omar, M.I.; Shakaff, A.Y.M.; Adom, A.H.; Persaud, K.C. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018, 18, 362. [Google Scholar] [CrossRef]
- Kauffmann, J.; Dippel, J.; Ruff, L.; Samek, W.; Müller, K.-R.; Montavon, G. Explainable AI reveals Clever Hans effects in unsupervised learning models. Nat. Mach. Intell. 2025, 7, 412–422. [Google Scholar] [CrossRef]
- Wei, L.; Sha, R.; Shi, Y.; Wang, Q.; Shi, L.; Gao, Y. Dual consistency semi-supervised learning for 3D medical image segmentation. Biomed. Signal Process. Control 2025, 104, 107568. [Google Scholar] [CrossRef]
- Lee, B.; Kang, M.; Lee, K.; Chae, Y.; Yoon, K.-J.; Lee, D.-S.; Park, I. Multigas Identification by Temperature-Modulated Operation of a Single Anodic Aluminum Oxide Gas Sensor Platform and Deep Learning Algorithm. ACS Sens. 2025, 10, 954–964. [Google Scholar] [CrossRef]
- Danieli, M.G.; Brunetto, S.; Gammeri, L.; Palmeri, D.; Claudi, I.; Shoenfeld, Y.; Gangemi, S. Machine learning application in autoimmune diseases: State of art and future prospectives. Autoimmun. Rev. 2024, 23, 103496. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahbarpour, S.; Hosseini-Golgoo, S.M.; Jamaati, H. A single gas sensor assisted by machine learning algorithms for breath-based detection of COPD: A pilot study. Sens. Actuator A-Phys. 2024, 376, 115650. [Google Scholar] [CrossRef]
- Chang, J.-E.; Lee, D.-S.; Ban, S.-W.; Oh, J.; Jung, M.Y.; Kim, S.-H.; Park, S.; Persaud, K.; Jheon, S. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens. Actuator B-Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Binson, V.A.; Subramoniam, M.; Sunny, Y.; Mathew, L. Prediction of Pulmonary Diseases With Electronic Nose Using SVM and XGBoost. IEEE Sens. J. 2021, 21, 20886–20895. [Google Scholar] [CrossRef]
- Huang, C.-H.; Zeng, C.; Wang, Y.-C.; Peng, H.-Y.; Lin, C.-S.; Chang, C.-J.; Yang, H.-Y. A study of diagnostic accuracy using a chemical sensor array and a machine learning technique to detect lung cancer. Sensors 2018, 18, 2845. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Maki, N.; Nemoto, T.; Inada, K.; Minami, K.; Tamura, R.; Imamura, G.; Cho-Isoda, Y.; Kitazawa, S.; Kojima, H. Lung cancer detection in perioperative patients’ exhaled breath with nanomechanical sensor array. Lung Cancer 2024, 190, 107514. [Google Scholar] [CrossRef]
- Jing, Q.; Gong, C.; Bian, W.; Tian, Q.; Zhang, Y.; Chen, N.; Xu, C.; Sun, N.; Wang, X.; Li, C. Ultrasensitive chemiresistive gas sensor can diagnose asthma and monitor its severity by analyzing its biomarker H2S: An experimental, clinical, and theoretical study. ACS Sens. 2022, 7, 2243–2252. [Google Scholar] [CrossRef]
- VR, N.; Mohapatra, A.K.; Kartha, V.B.; Chidangil, S. Multiwavelength photoacoustic breath analysis sensor for the diagnosis of lung diseases: COPD and asthma. ACS Sens. 2023, 8, 4111–4120. [Google Scholar]
- Zhu, H.; Liu, C.; Zheng, Y.; Zhao, J.; Li, L. A Hybrid Machine Learning Algorithm for Detection of Simulated Expiratory Markers of Diabetic Patients Based on Gas Sensor Array. IEEE Sens. J. 2023, 23, 2940–2947. [Google Scholar] [CrossRef]
- Singh, S.; S, S.; Varma, P.; Sreelekha, G.; Adak, C.; Shukla, R.P.; Kamble, V.B. Metal oxide-based gas sensor array for VOCs determination in complex mixtures using machine learning. Microchim. Acta 2024, 191, 196. [Google Scholar] [CrossRef]
- Ansari, H.R.; Kordrostami, Z.; Mirzaei, A.; Kraft, M. Deep-Learning-Based Blood Glucose Detection Device Using Acetone Exhaled Breath Sensing Features of α-Fe2O3-MWCNT Nanocomposites. ACS Appl. Mater. Interfaces 2024, 16, 47973–47987. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.; Zhang, Y.; Ding, X.; Zhang, H.; Cao, T.; Qu, Z.-b.; Ren, J.; Li, L.; Guo, Z.; et al. Modular Assembly of MXene Frameworks for Noninvasive Disease Diagnosis via Urinary Volatiles. ACS Nano 2022, 16, 17376–17388. [Google Scholar] [CrossRef]
- Esfahani, S.; Wicaksono, A.; Mozdiak, E.; Arasaradnam, R.P.; Covington, J.A. Non-invasive diagnosis of diabetes by volatile organic compounds in urine using FAIMS and Fox4000 electronic nose. Biosensors 2018, 8, 121. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Schulz, S.; Haueisen, J.; Vogler, S.; Horn, P.; Stallmach, A.; Reuken, P. Detection of liver dysfunction using a wearable electronic nose system based on semiconductor metal oxide sensors. Biosensors 2022, 12, 70. [Google Scholar] [CrossRef]
- Nazir, N.U.; Abbas, S.R. Identification of phenol 2, 2-methylene bis, 6 [1, 1-D] as breath biomarker of hepatocellular carcinoma (HCC) patients and its electrochemical sensing: E-nose biosensor for HCC. Anal. Chim. Acta 2023, 1242, 340752. [Google Scholar] [CrossRef]
- Zaim, O.; Diouf, A.; El Bari, N.; Lagdali, N.; Benelbarhdadi, I.; Ajana, F.Z.; Llobet, E.; Bouchikhi, B. Comparative analysis of volatile organic compounds of breath and urine for distinguishing patients with liver cirrhosis from healthy controls by using electronic nose and voltammetric electronic tongue. Anal. Chim. Acta 2021, 1184, 339028. [Google Scholar] [CrossRef]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Non-invasive detection and staging of colorectal cancer using a portable electronic nose. Sensors 2021, 21, 5440. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; Ouaret, N.; Thomas, M.G.; Quraishi, N.; Heatherington, E.; Nwokolo, C.U.; Bardhan, K.D.; Covington, J.A. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 999–1003. [Google Scholar] [CrossRef]
- Bach, J.-P.; Gold, M.; Mengel, D.; Hattesohl, A.; Lubbe, D.; Schmid, S.; Tackenberg, B.; Rieke, J.; Maddula, S.; Baumbach, J.I. Measuring compounds in exhaled air to detect Alzheimer’s disease and Parkinson’s disease. PLoS ONE 2015, 10, e0132227. [Google Scholar] [CrossRef]
- Finberg, J.P.; Schwartz, M.; Jeries, R.; Badarny, S.; Nakhleh, M.K.; Abu Daoud, E.; Ayubkhanov, Y.; Aboud-Hawa, M.; Broza, Y.Y.; Haick, H. Sensor array for detection of early stage Parkinson’s disease before medication. ACS Chem. Neurosci. 2018, 9, 2548–2553. [Google Scholar] [CrossRef]
- Day, B.A.; Wilmer, C.E. Computational design of MOF-based electronic noses for dilute gas species detection: Application to kidney disease detection. ACS Sens. 2021, 6, 4425–4434. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Li, H.; Chen, J.; Lv, C.; Yang, X.; Liu, F.; Chen, X.; Dong, H. Artificial intelligence-assisted wearable porous eutectogel with high-performance NH3 enrichment and visual sensing enables non-invasive monitoring of chronic kidney disease. Chem. Eng. J. 2025, 507, 160678. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuator B-Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, N.; Liu, T.; Zhu, Y.; Wang, D.; Dong, H.; Guo, L.; Qu, D.; Jiang, X.; Du, T. Artificially intelligent olfaction for fast and noninvasive diagnosis of bladder cancer from urine. ACS Sens. 2022, 7, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Han, Y.; Sun, Y.; Zhu, Z. Detection and recognition of urinary VOCs marker gases for bladder cancer based on electronic nose technology. Int. J. Biomed. Eng. Technol. 2024, 47, 115–122. [Google Scholar]
- Yang, H.-Y.; Wang, Y.-C.; Peng, H.-Y.; Huang, C.-H. Breath biopsy of breast cancer using sensor array signals and machine learning analysis. Sci Rep 2021, 11, 103. [Google Scholar] [CrossRef]
- Gómez, J.K.C.; Vásquez, C.A.C.; Acevedo, C.M.D.; Llecha, J.B. Assessing data fusion in sensory devices for enhanced prostate cancer detection accuracy. Chemosensors 2024, 12, 228. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Liu, S.; Chen, X.; Li, Z. Rapid diagnosis of urinary tract cancers on a LEGO-inspired detection platform via chemiresistive profiling of volatile metabolites. Anal. Chem. 2023, 95, 14822–14829. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Roointan, A.; Mir, T.A.; Wani, S.I.; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; Chinnappan, R. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef]
- Chen, X.; Muhammad, K.G.; Madeeha, C.; Fu, W.; Xu, L.; Hu, Y.; Liu, J.; Ying, K.; Chen, L.; Yurievna, G.O. Calculated indices of volatile organic compounds (VOCs) in exhalation for lung cancer screening and early detection. Lung Cancer 2021, 154, 197–205. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2021, 10, 32. [Google Scholar] [CrossRef]
- Gutiérrez Villegas, C.; Paz-Zulueta, M.; Herrero-Montes, M.; Parás-Bravo, P.; Madrazo Pérez, M. Cost analysis of chronic obstructive pulmonary disease (COPD): A systematic review. Health Econ. Rev. 2021, 11, 31. [Google Scholar] [CrossRef]
- Kahnert, K.; Jörres, R.A.; Behr, J.; Welte, T. The diagnosis and treatment of COPD and its comorbidities. Dtsch. Arztebl. Int. 2023, 120, 434. [Google Scholar] [CrossRef]
- Lange, P.; Ahmed, E.; Lahmar, Z.M.; Martinez, F.J.; Bourdin, A. Natural history and mechanisms of COPD. Respirology 2021, 26, 298–321. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hannon, A.; Yu, G.; Idziak, L.A.; Sahasrabhojanee, A.; Govindarajan, P.; Maldonado, Y.A.; Ngo, K.; Abdou, J.P.; Mai, N.; et al. Electronic Nose Development and Preliminary Human Breath Testing for Rapid, Non-Invasive COVID-19 Detection. ACS Sens. 2023, 8, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography–mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef]
- Fan, X.; Zhong, R.; Liang, H.; Zhong, Q.; Huang, H.; He, J.; Chen, Y.; Wang, Z.; Xie, S.; Jiang, Y. Exhaled VOC detection in lung cancer screening: A comprehensive meta-analysis. BMC Cancer 2024, 24, 775. [Google Scholar] [CrossRef]
- Anand, A.; Castiglia, E.; Zamora, M.L. The Association between Personal Air Pollution exposures and Fractional exhaled nitric oxide (FeNO): A systematic review. Curr. Environ. Health Rep. 2024, 11, 210–224. [Google Scholar] [CrossRef]
- Lv, J.-j.; Li, X.-y.; Shen, Y.-c.; You, J.-x.; Wen, M.-z.; Wang, J.-b.; Yang, X.-t. Assessing volatile organic compounds exposure and chronic obstructive pulmonary diseases in US adults. Front. Public Health 2023, 11, 1210136. [Google Scholar] [CrossRef]
- Phillips, C.; Mac Parthaláin, N.; Syed, Y.; Deganello, D.; Claypole, T.; Lewis, K. Short-Term Intra-Subject Variation in Exhaled Volatile Organic Compounds (VOCs) in COPD Patients and Healthy Controls and Its Effect on Disease Classification. Metabolites 2014, 4, 300–318. [Google Scholar] [CrossRef]
- Van Berkel, J.J.B.N.; Dallinga, J.W.; Möller, G.M.; Godschalk, R.W.L.; Moonen, E.J.; Wouters, E.F.M.; Van Schooten, F.J. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef]
- Shiba, K.; Sugiyama, T.; Takei, T.; Yoshikawa, G. Controlled growth of silica–titania hybrid functional nanoparticles through a multistep microfluidic approach. Chem. Commun. 2015, 51, 15854–15857. [Google Scholar] [CrossRef]
- Saglani, S.; Menzie-Gow, A.N. Approaches to asthma diagnosis in children and adults. Front. Pediatr. 2019, 7, 148. [Google Scholar] [CrossRef]
- Savito, L.; Scarlata, S.; Bikov, A.; Carratù, P.; Carpagnano, G.E.; Dragonieri, S. Exhaled volatile organic compounds for diagnosis and monitoring of asthma. World J. Clin. Cases 2023, 11, 4996. [Google Scholar] [CrossRef]
- Dragonieri, S.; Schot, R.; Mertens, B.J.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef]
- Sharma, R.; Zang, W.; Zhou, M.; Schafer, N.; Begley, L.A.; Huang, Y.J.; Fan, X. Real time breath analysis using portable gas chromatography for adult asthma phenotypes. Metabolites 2021, 11, 265. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandya, H.C.; Thomas, C.L.P. Metabolomics Pilot Study to Identify Volatile Organic Compound Markers of Childhood Asthma in Exhaled Breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Robroeks, C.M.H.H.T.; Van Berkel, J.J.B.N.; Moonen, E.J.C.; Godschalk, R.W.L.; Jöbsis, Q.; Dompeling, E.; Wouters, E.F.M.; Van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2010, 40, 68–76. [Google Scholar] [CrossRef]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current understanding of asthma pathogenesis and biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef]
- Turner, S.W.; Chang, A.B.; Yang, I.A. Clinical utility of exhaled nitric oxide fraction in the management of asthma and COPD. Breathe 2019, 15, 306–316. [Google Scholar] [CrossRef]
- Toombs, C.F.; Insko, M.A.; Wintner, E.A.; Deckwerth, T.L.; Usansky, H.; Jamil, K.; Goldstein, B.; Cooreman, M.; Szabo, C. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br. J. Clin. Pharmacol. 2010, 69, 626–636. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Chen, Y.; Yao, W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology 2014, 19, 1165–1169. [Google Scholar] [CrossRef]
- Yu, G.; Li, Z.; Li, S.; Liu, J.; Sun, M.; Liu, X.; Sun, F.; Zheng, J.; Li, Y.; Yu, Y.; et al. The role of artificial intelligence in identifying asthma in pediatric inpatient setting. Ann. Transl. Med. 2020, 8, 1367. [Google Scholar] [CrossRef]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of potential biomarkers in exhaled breath for diagnosis of type 2 diabetes mellitus based on GC-MS with metabolomics. RSC Adv. 2014, 4, 25430–25439. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef]
- Das, S.; Pal, S.; Mitra, M. Significance of exhaled breath test in clinical diagnosis: A special focus on the detection of diabetes mellitus. J. Med. Biol. Eng. 2016, 36, 605–624. [Google Scholar] [CrossRef]
- King, J.; Unterkofler, K.; Teschl, G.; Teschl, S.; Koc, H.; Hinterhuber, H.; Amann, A. A mathematical model for breath gas analysis of volatile organic compounds with special emphasis on acetone. J. Math. Biol. 2011, 63, 959–999. [Google Scholar] [CrossRef]
- Kalapos, M.P. On the mammalian acetone metabolism: From chemistry to clinical implications. Biochim. Biophys. Acta-Gen. Subj. 2003, 1621, 122–139. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.-J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef]
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured gas sensors: From air quality and environmental monitoring to healthcare and medical applications. Nanomaterials 2021, 11, 1927. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured gas sensors for medical and health applications: Low to high dimensional materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Sandrini, A.; Taylor, D.R.; Thomas, P.S.; Yates, D.H. Fractional exhaled nitric oxide in asthma: An update. Respirology 2010, 15, 57–70. [Google Scholar] [CrossRef]
- Fan, G.-T.; Yang, C.-L.; Lin, C.-H.; Chen, C.-C.; Shih, C.-H. Applications of Hadamard transform-gas chromatography/mass spectrometry to the detection of acetone in healthy human and diabetes mellitus patient breath. Talanta 2014, 120, 386–390. [Google Scholar] [CrossRef]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Siraj, S.; Bansal, G.; Hasita, B.; Srungaram, S.; K. S, S.; Rybicki, F.J.; Sonkusale, S.; Sahatiya, P. MXene/MoS2 Piezotronic Acetone Gas Sensor for Management of Diabetes. ACS Appl. Nano Mater. 2024, 7, 11350–11361. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Z.; Xia, C.; Zhong, R.; Liu, L.; Long, L. Increased levels of urine volatile organic compounds are associated with diabetes risk and impaired glucose homeostasis. J. Clin. Endocrinol. Metab. 2024, 109, e531–e542. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Wu, X.; Song, X.; Yang, X.; Zhang, G.; Niu, P.; Chen, T. Exposure to volatile organic compounds is a risk factor for diabetes: A cross-sectional study. Chemosphere 2023, 338, 139424. [Google Scholar] [CrossRef]
- Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled breath analysis for diabetes diagnosis and monitoring: Relevance, challenges and possibilities. Biosensors 2021, 11, 476. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Singal, A.G. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 947–949. [Google Scholar] [CrossRef]
- Aasi, A.; Aasi, E.; Aghaei, S.M.; Panchapakesan, B. CNT biodevices for early liver cancer diagnosis based on biomarkers detection-a promising platform. J. Mol. Graph. 2022, 114, 108208. [Google Scholar] [CrossRef]
- Stavropoulos, G.; van Munster, K.; Ferrandino, G.; Sauca, M.; Ponsioen, C.; van Schooten, F.-J.; Smolinska, A. Liver impairment—The potential application of volatile organic compounds in hepatology. Metabolites 2021, 11, 618. [Google Scholar] [CrossRef]
- Wilson, A.D. Recent applications of electronic-nose technologies for the noninvasive early diagnosis of gastrointestinal diseases. Proceedings 2018, 2, 147. [Google Scholar]
- Buijck, M.; Berkhout, D.J.; de Groot, E.F.; Benninga, M.A.; van der Schee, M.P.; Kneepkens, C.M.F.; de Boer, N.K.; de Meij, T.G. Sniffing out paediatric gastrointestinal diseases: The potential of volatile organic compounds as biomarkers for disease. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perdoni, F.; Infantino, V.; Faliva, M.A.; Peroni, G.; Iannello, G.; Nichetti, M.; Alalwan, T.A.; Perna, S.; Cocuzza, C. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J. Anal. Methods Chem. 2019, 2019, 7247802. [Google Scholar] [CrossRef]
- Wilson, A. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Vassilenko, V.; Moura, P.C.; Raposo, M. Diagnosis of Carcinogenic Pathologies through Breath Biomarkers: Present and Future Trends. Biomedicines 2023, 11, 3029. [Google Scholar] [CrossRef]
- Dalis, C.; Mesfin, F.M.; Manohar, K.; Liu, J.; Shelley, W.C.; Brokaw, J.P.; Markel, T.A. Volatile organic compound assessment as a screening tool for early detection of gastrointestinal diseases. Microorganisms 2023, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mou, S.; Zhang, X.; Sun, J.; Xue, Y.; Xiong, H.; Hsia, K.J.; Wan, H.; Wang, P. Application of Sensing Devices in the Detection of Oral, Pulmonary, and Gastrointestinal Diseases. Chemosensors 2024, 12, 57. [Google Scholar] [CrossRef]
- Stine, J.M.; Ruland, K.L.; Levy, J.A.; Beardslee, L.A.; Ghodssi, R. Electrochemical Sensor for Ingestible Capsule-Based In-Vivo Detection of Hydrogen Sulfide. In Proceedings of the 2023 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kyoto, Japan, 25–29 June 2023; pp. 2026–2029. [Google Scholar]
- Kafili-Hajlari, T.; Naseri, A.; Ansarin, A.; Rasoulzadeh, F. Technical Approaches for Breath Aldehyde Biomarker Detection and Disease Diagnosis: A Review. Anal. Biochem. 2025, 702, 115841. [Google Scholar] [CrossRef]
- de la Fuente-Fernández, R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology 2012, 78, 696–701. [Google Scholar] [CrossRef]
- Assady, S.; Marom, O.; Hemli, M.; Ionescu, R.; Jeries, R.; Tisch, U.; Abassi, Z.; Haick, H. Impact of hemodialysis on exhaled volatile organic compounds in end-stage renal disease: A pilot study. Nanomedicine 2014, 9, 1035–1045. [Google Scholar] [CrossRef]
- Haick, H.; Hakim, M.; Patrascu, M.; Levenberg, C.; Shehada, N.; Nakhoul, F.; Abassi, Z. Sniffing chronic renal failure in rat model by an array of random networks of single-walled carbon nanotubes. ACS Nano 2009, 3, 1258–1266. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic kidney disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Collins, A.J.; Kasiske, B.; Herzog, C.; Chavers, B.; Foley, R.; Gilbertson, D.; Grimm, R.; Liu, J.; Louis, T.; Manning, W. United States renal data system 2005 annual data report abstract. Am. J. Kidney Dis. 2006, 47, A5–A6. [Google Scholar] [CrossRef]
- Usman, F.; Ghazali, K.H.; Muda, R.; Dennis, J.O.; Ibnaouf, K.H.; Aldaghri, O.A.; Alsadig, A.; Johari, N.H.; Jose, R. Detection of Kidney Complications Relevant Concentrations of Ammonia Gas Using Plasmonic Biosensors: A Review. Chemosensors 2023, 11, 119. [Google Scholar] [CrossRef]
- Bevc, S.; Mohorko, E.; Kolar, M.; Brglez, P.; Holobar, A.; Kniepeiss, D.; Podbregar, M.; Piko, N.; Hojs, N.; Knehtl, M. Measurement of breath ammonia for detection of patients with chronic kidney disease. Clin. Nephrol. 2017, 88, 14. [Google Scholar] [CrossRef]
- Liyanage, T.; Toyama, T.; Hockham, C.; Ninomiya, T.; Perkovic, V.; Woodward, M.; Fukagawa, M.; Matsushita, K.; Praditpornsilpa, K.; Hooi, L.S. Prevalence of chronic kidney disease in Asia: A systematic review and analysis. BMJ Glob. Health 2022, 7, e007525. [Google Scholar] [CrossRef]
- Cheng, K.; Wan, S.; Yang, J.-W.; Chen, S.-Y.; Wang, H.-L.; Xu, C.-H.; Qiao, S.-H.; Li, X.-r.; Li, Y. Applications of biosensors in bladder cancer. Crit. Rev. Anal. Chem. 2024, 16, 309–342. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M. Bladder cancer: A review. JAMA-J. Am. Med. Assoc. 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 67. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA-J. Am. Med. Assoc. 2019, 321, 316. [Google Scholar]
- Hendrick, R.E.; Helvie, M.A. Mammography Screening: A New Estimate of Number Needed to Screen to Prevent One Breast Cancer Death. Am. J. Roentgenol. 2012, 198, 723–728. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research progress of electronic nose technology in exhaled breath disease analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahab, M.R.; Palaniyandi, T.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Sugumaran, A.; Sivaji, A.; Kaliamoorthy, S.; Kumarasamy, S. Biomarker-specific biosensors revolutionise breast cancer diagnosis. Clin. Chim. Acta 2024, 555, 117792. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Duan, Y. Diagnosis of breast cancer based on breath analysis: An emerging method. Crit. Rev. Oncol./Hematol. 2013, 87, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wan, Q. Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef] [PubMed]
- Koç, H.; King, J.; Teschl, G.; Unterkofler, K.; Teschl, S.; Mochalski, P.; Hinterhuber, H.; Amann, A. The role of mathematical modeling in VOC analysis using isoprene as a prototypic example. J. Breath Res. 2011, 5, 037102. [Google Scholar] [CrossRef]
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Fan, L.; Xu, N.; Chen, H.; Zhou, J.; Deng, S. A millisecond response and microwatt power-consumption gas sensor: Realization based on cross-stacked individual Pt-coated WO3 nanorods. Sens. Actuator B-Chem. 2021, 346, 130545. [Google Scholar] [CrossRef]
- Lee, B.; Lee, J.; Lee, J.-O.; Hwang, Y.; Bahn, H.-K.; Park, I.; Jheon, S.; Lee, D.-S. Breath analysis system with convolutional neural network (CNN) for early detection of lung cancer. Sens. Actuator B-Chem. 2024, 409, 135578. [Google Scholar] [CrossRef]
- Gudiño-Ochoa, A.; García-Rodríguez, J.A.; Ochoa-Ornelas, R.; Cuevas-Chávez, J.I.; Sánchez-Arias, D.A. Noninvasive diabetes detection through human breath using TinyML-Powered E-Nose. Sensors 2024, 24, 1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, D.; Chang, Z.; Zhang, R.; Dai, J.; Fang, Y. Wearable bioelectronic masks for wireless detection of respiratory infectious diseases by gaseous media. Matter 2022, 5, 4347–4362. [Google Scholar] [CrossRef] [PubMed]





| Disease | Sensor Array | Sensor Type | Material | Algorithm | Gas Markers | Parameter | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung cancer and COPD | 8 independent sensors form a heterogeneous sensor array | Electrochemical | SnO2-based sensitive film, Pt filament carrier + Pd/Al2O3 catalyst, precious metal electrode (Pt/Au) + liquid electrolyte | KPCA + XGBoost/AdaBoost/RF | Lung cancer | Formaldehyde | 0–1000 ppm | Accuracy: 84.75% Sensitivity: 81.36% Specificity: 88.14% | [123] | |||
| Benzene series substances | 0–100 ppm | |||||||||||
| Alkanes | 1–10,000 ppm | |||||||||||
| CO | 1–100 ppm | |||||||||||
| Ethanol | 30–5000 ppm | |||||||||||
| Methane | 1–100 ppm | |||||||||||
| COPD | Isobutane | 300–10,000 ppm | ||||||||||
| Ethanol | 30–5000 ppm | |||||||||||
| CO | 1–100 ppm | |||||||||||
| Ammonia | 30–300 ppm | |||||||||||
| 32-channel sensor array | Resistive | Carbon nanotubes, polymer substrates | LDA + SVM | Ethanol | ppb level | AUC = 0.90, 0.91 Sensitivity: 75.0%, 83.3% Specificity: 96.6%, 85.4% | [124] | |||||
| Isopropyl alcohol | ||||||||||||
| Lipid peroxide-related VOCs | ||||||||||||
| 12-channel sensor array | Mechanical | Silica/titanium dioxide-based hybrid nanoparticles, commercial polymers | RF classifier | VOCs | Accuracy: 80.9% | [125] | ||||||
| Asthma | Single-channel heterojunction | Resistive | N-type γ-Bi2MoO6 microspheres | PCA | H2S | 5 ppb–100 ppm | The response value of 5 ppb H2S = 1.5 100 ppb H2S is = 4.9 | [126] | ||||
| NO | >50 ppb | |||||||||||
| Virtual multi-wavelength array | Optical | Quartz, UV-grade fused quartz, metal | PCA + Match/No-match | NO | >40 ppb | Sensitivity 88% AUC-ROC 0.948 Specificity: 89% | [127] | |||||
| Diabetes | Cross-response model array | Resistive | MOS | Baseline correction + KPCA + AdaBoost + MVRVM + GS/PSO | Acetone | 0.1–19.8 ppm | [128] | |||||
| A heterogeneous array composed of three independent sensors | Resistive | NiO, CuO, ZnO thin films | KNN, RF, DT, logistic regression, naive Bayes, LDA, ANN, SVM | Acetone | 100–2400 ppm | classification accuracy > 99% | [129] | |||||
| Ethanol | 100–2400 ppm | |||||||||||
| Interdigital electrode structure | Resistive | α-Fe2O3-MWCNT nanocomposites, Pt interdigital electrodes, Al2O3, Pt microheaters | CNN, Adam | Acetone | 0.5–50 ppm | Accuracy: 85% | [130] | |||||
| 8-channel sensor array | Resistive | Porous MXene framework | PCA, t-SNE, SVM | C4-C7 aldehydes, ketones, alcohols | 5–50 ppm | Accuracy: 91.7% Sensitivity: 88.9% Specificity: 96.8% | [131] | |||||
| Scan parameters to obtain multi-dimensional data | Electrochemical | Ni-63 ionization source, parallel metal plate electrode | Two-dimensional wavelet transform + PCA + sparse logistic regression/RF/Gaussian process/SVM | VOCs | <1 year | Specificity: 100% Sensitivity: 92% | [132] | |||||
| 18 sensor arrays | Resistive | Composite metal oxide | One to four years | Specificity: 82% Sensitivity: 87% | ||||||||
| Hepatic disease | 3 sensor modules | Resistive | SnO2, WO3, Pd | LDA | Alkanes, NO | ppb level | Accuracy: 95–100% | [133] | ||||
| Three-electrode system | Electrochemical | Gold nanoparticles, glassy carbon electrodes | PCA, heat map analysis | MBMBP | 0.15–0.38 M | —— | [134] | |||||
| 5 commercial MQ sensors + 6 interdigitated sensors | Resistive | Composite metal oxides, WO3 nanowire-based | PCA, DFA, SVM | Methanol, dimethyl sulfide, ethanol, toluene | Accuracy: 98.33% AUC = 0.965 | [135] | ||||||
| Five-electrode voltammetry array | Electrochemical | Au, Pd, Pt, glassy carbon (GC), Cu | Cu2+ and other electroactive substances | Accuracy: 97.50% AUC = 0.950 | ||||||||
| Gastrointestinal disorders | 10 thick film MOS sensors | Resistive | Composite metal oxides | RF, neural networks | Aldehydes, acetone, 2-heptanone, p-xylene | AUC = 0.81 | [136] | |||||
| Single channel ion separation system | Electrochemical | Metal electrode plates | FDA, wavelet transform | VOCs | Accuracy: >75% | [137] | ||||||
| 18 sensor arrays | Resistive | Composite metal oxides | PCA, DFA | |||||||||
| Nervous system disorders | 32 chemical sensor array | Resistive | Carbon black-polymer composite materials | PCA, LDA | 1-butanol, 2-methylfuran | Sensitivity: 50–70% Accuracy: 68–77% | [138] | |||||
| Cross-reactive array consisting of 40 sensors | Resistive | GNPs, SWCNTs | DFA | Benzaldehyde, phenylacetone | ppb to ppm level | Accuracy: 81% Sensitivity: 79% Specificity: 84% | [139] | |||||
| Nephropathy | 23-unit metal–organic frameworks (MOFs) array | Resonant | Metal–organic framework | CLAC calculation, SVD, iterative numerical model | NH3 | 3.32 ± 2.19 ppm | Accuracy: 100% | [140] | ||||
| The spiral porous structure printed by DLP 3D printing | Optical | NAGA, Gly, choline chloride, BCG | CNN | NH3 | 0.5–10 ppm | Accuracy: 96.5% | [141] | |||||
| 6 sensor array | Resistive | Composite metal oxide | PCA, HCA, SVM, PLS | Dichloromethane, 6-nitro-2-picoline, 4-amino-4H-1,2, 4-triazole, styrene, limonene | 5–20,000 ppm | Accuracy: 100% | [142] | |||||
| Bladder cancer | 10-sensor array | Resistive | Polyaniline (substrate material) Fluorine-doped tin oxide (electrode) | PCA, SVM, Kmeans | Benzaldehyde, 2-pentanone, butylbenzene, etc. | 25–200 ppm | Accuracy: 96.67% Sensitivity: 100% Specificity: 83.33% | [143] | ||||
| piperone | 7–50 ppm | |||||||||||
| 8 types of metal oxide gas sensor arrays | Resistive | Composite metal oxides | PCA, LDA, SVM, RF, KNN | VOCs | Accuracy: PCA + SVM: 97%; LDA + KNN: 97%; LDA + RF: 94% | [144] | ||||||
| Breast cancer | 32-sensor array | Resistive | Carbon nanotubes | KNN, SVM, DT, neural network | VOCs | Accuracy: 91% Sensitivity: 86% Specificity: 97% | [145] | |||||
| Prostate cancer | Various MEMS gas sensor arrays | Resistive | Composite metal oxides | PCA, SVM, KNN, RF, decision tree, naive Bayes | Ethanol | 0.3–100 ppm | Accuracy: 100% Sensitivity: 100% | [146] | ||||
| Screen-printed electrode array | Electrochemical | Carbon-based electrodes, gold-based electrodes | Formaldehyde | 0.1–208 ppm | ||||||||
| 8 types of MXene-TMDC nanocomposite sensor arrays | Resistive | MXene-TMDC nanocomposites, transition metal dichalcogenides | PCA, hierarchical clustering analysis, CNN, LDA, logistic regression, RF, SVM | Glyoxal 4-Heptanone 2-Pentanone | 10–100 ppm | Accuracy: 90% Sensitivity: 98% Specificity: 97% | [147] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Cao, X.; Li, C.; Zhou, M.; Liu, T.; Liu, J.; Zhang, L. A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis. Biosensors 2025, 15, 548. https://doi.org/10.3390/bios15080548
Yu Y, Cao X, Li C, Zhou M, Liu T, Liu J, Zhang L. A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis. Biosensors. 2025; 15(8):548. https://doi.org/10.3390/bios15080548
Chicago/Turabian StyleYu, Yueting, Xin Cao, Chenxi Li, Mingyue Zhou, Tianyu Liu, Jiang Liu, and Lu Zhang. 2025. "A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis" Biosensors 15, no. 8: 548. https://doi.org/10.3390/bios15080548
APA StyleYu, Y., Cao, X., Li, C., Zhou, M., Liu, T., Liu, J., & Zhang, L. (2025). A Review of Machine Learning-Assisted Gas Sensor Arrays in Medical Diagnosis. Biosensors, 15(8), 548. https://doi.org/10.3390/bios15080548







