Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Benchtop Sensor Fabrication
2.3. In Vivo Sensor Array Design and Fabrication
2.4. Hydrogel Preparation and Coating
2.5. Sensor Interrogation
2.6. Intestinal Mucosa Sample Collection
2.7. In Vitro Sensitivity, Selectivity and Stability Experiments
2.8. In Vivo Pilot Feasibility Study
2.9. X-Ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Validation of Sensor Quality
3.2. Optimisation of Aptamer Concentration and Sensitivity
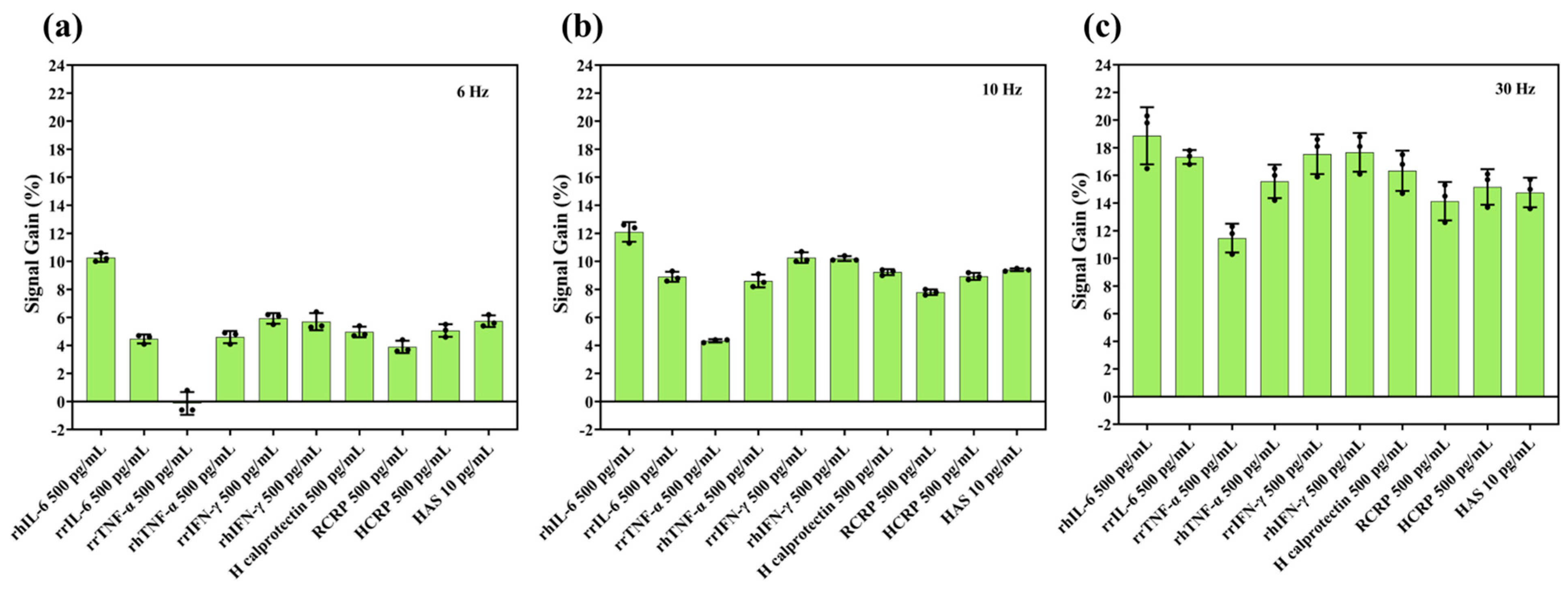
3.3. Selectivity of IL-6 Sensors
3.4. IL-6 Sensor Stability
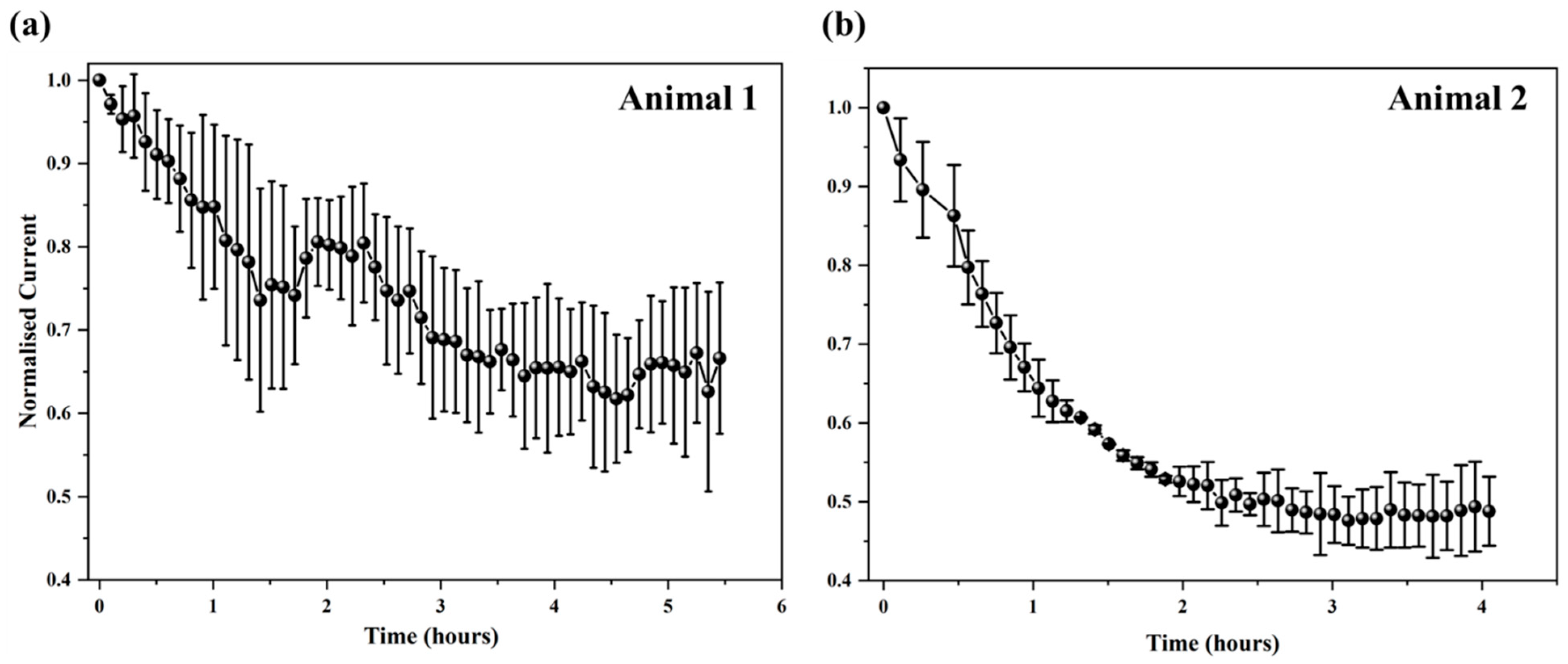
3.5. Determining Sensor Degradation Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Gore, R.M.; Levine, M.S. 41—Crohn’s Disease of the Small Bowel. In Textbook of Gastrointestinal Radiology, 2-Volume Set, 4th ed.; Richard, M.G., Marc, S.L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 725–755. [Google Scholar]
- Joana, T.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar]
- Daniel, C.B.; Simon, R.C. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Dhyani, M.; Joshi, N.; Bemelman, W.A.; Gee, M.S.; Yajnik, V.; D’Hoore, A.; Traverso, G.; Donowitz, M.; Mostoslavsky, G.; Lu, T.K.; et al. Challenges in IBD Research: Novel Technologies. Inflamm. Bowel Dis. 2019, 25 (Suppl. S2), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.M.; Verstockt, B.; Parkes, M.; Lee, J.C. Personalised medicine in Crohn’s disease. Lancet Gastroenterol. Hepatol. 2020, 5, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Chevaux, J.-B.; Bommelaer, G.; Peyrin-Biroulet, L. Diagnosis, prevention and treatment of postoperative Crohn’s disease recurrence. Dig. Liver Dis. 2012, 44, 453–460. [Google Scholar] [CrossRef]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2020, 39, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Clara, A.; Dulai, P.S.; Vermeire, S.; Sandborn, W.J. Lessons Learned From Trials Targeting Cytokine Pathways in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 374–388.e4. [Google Scholar] [CrossRef]
- Loo, S.W.; Pui, T.-S. Cytokine and Cancer Biomarkers Detection: The Dawn of Electrochemical Paper-Based Biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef]
- Przybylski, C.; Gonnet, F.; Saesen, E.; Lortat-Jacob, H.; Daniel, R. Surface plasmon resonance imaging coupled to on-chip mass spectrometry: A new tool to probe protein-GAG interactions. Anal. Bioanal. Chem. 2020, 412, 507–519. [Google Scholar] [CrossRef]
- Ahmad, M.; Shakouri, S.K.; Dolati, S. Biosensors: A novel approach to and recent discovery in detection of cytokines. Cytokine 2020, 136, 155272. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.H.; Lin, K.-C.; Whang, J.; Shahub, S.; Churcher, N.K.M.; Helmus, D.; Muthukumar, S.; Sands, B.; Prasad, S. Longitudinal monitoring of IL-6 and CRP in inflammatory bowel disease using IBD-AWARE. Biosens. Bioelectron. X 2024, 16, 100435. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Lillehoj, P.B.; Estrela, P.; Dutta, G. Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors 2021, 11, 94. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-Los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Corrigan, D.K. Comparing nanobody and aptamer-based capacitive sensing for detection of interleukin-6 (IL-6) at physiologically relevant levels. Anal. Bioanal. Chem. 2023, 415, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.J.; Patiño, E.B.; Orozco, J. Electrochemical Nanobiosensors as Point-of-Care Testing Solution to Cytokines Measurement Limitations. Electroanalysis 2022, 34, 184–211. [Google Scholar] [CrossRef]
- Sheraz, M.; Sun, X.-F.; Wang, Y.; Chen, J.; Sun, L. Recent Developments in Aptamer-Based Sensors for Diagnostics. Sensors 2024, 24, 7432. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; He, S.; Deng, L.; Wang, H.; Cao, Z.; Feng, Z.; Xiong, B.; Yin, Y. A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors 2025, 15, 120. [Google Scholar] [CrossRef]
- Aebisher, D.; Bartusik-Aebisher, D.; Przygórzewska, A.; Oleś, P.; Woźnicki, P.; Kawczyk-Krupka, A. Key Interleukins in Inflammatory Bowel Disease-A Review of Recent Studies. Int. J. Mol. Sci. 2024, 26, 121. [Google Scholar] [CrossRef]
- Atreya, R.; Neurath, M.F. New therapeutic strategies for treatment of inflammatory bowel disease. Mucosal. Immunol. 2008, 1, 175–182. [Google Scholar] [CrossRef]
- Gesiorowski, A.; Ettich, J.; Werner, J.; Wittich, C.; Pieper, S.; Padrini, G.; Behnke, K.; Floss, D.M.; Lang, P.A.; Moll, J.M.; et al. Bispecific soluble cytokine receptor-nanobody fusions inhibit Interleukin (IL-)6 trans-signaling and IL-12/23 or tumor necrosis factor (TNF) signaling. J. Biol. Chem. 2023, 299, 105343. [Google Scholar] [CrossRef]
- Garbers, C.; Lokau, J. Cytokines of the interleukin-6 family as emerging targets in inflammatory bowel disease. Expert Opin. Ther. Targets 2024, 28, 57–65. [Google Scholar] [CrossRef]
- Li, Y.; Hua, X.; Wang, J.; Jin, B. cMWCNT/CoHCF/AuNPs nanocomposites aptasensor for electrochemical detection of interleukin-6. Talanta Open 2023, 7, 100188. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Bin Lim, S.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef] [PubMed]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Shaver, A.; Curtis, S.D.; Arroyo-Currás, N. Alkanethiol Monolayer End Groups Affect the Long-Term Operational Stability and Signaling of Electrochemical, Aptamer-Based Sensors in Biological Fluids. ACS Appl. Mater. Interfaces 2020, 12, 11214–11223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lai, R.Y.; Plaxco, K.W. Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat. Protoc. 2007, 2, 2875–2880. [Google Scholar] [CrossRef]
- Bryant, S.J.; Davis-Arehart, K.A.; Luo, N.; Shoemaker, R.K.; Arthur, J.A.; Anseth, K.S. Synthesis and Characterization of Photopolymerized Multifunctional Hydrogels: Water-Soluble Poly(Vinyl Alcohol) and Chondroitin Sulfate Macromers for Chondrocyte Encapsulation. Macromolecules 2004, 37, 6726–6733. [Google Scholar] [CrossRef]
- Chia, S.; Guo, T.; Goldys, E.M.; Payne, S.C.; Duan, W.; Lovell, N.H.; Shivdasani, M.N.; Deng, F. A CRISPR mediated point-of-care assay for the detection of mucosal calprotectin in an animal model of ulcerative colitis. Bioeng. Transl. Med. 2025, 10, e10725. [Google Scholar] [CrossRef]
- Carty, E.; De Brabander, M.; Feakins, R.M.; Rampton, D.S. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 2000, 46, 487–492. [Google Scholar] [CrossRef]
- Clark, V.; Pellitero, M.A.; Arroyo-Currás, N. Explaining the Decay of Nucleic Acid-Based Sensors under Continuous Voltammetric Interrogation. Anal. Chem. 2023, 95, 4974–4983. [Google Scholar] [CrossRef]
- Leung, K.K.; Downs, A.M.; Ortega, G.; Kurnik, M.; Plaxco, K.W. Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood. ACS Sens. 2021, 6, 3340–3347. [Google Scholar] [CrossRef]
- Haffar, H.; Djamila, A.; Hania, A. Optical, electrical and photoelectrochemical characterization of electropolymerized poly methylene blue on fluorine doped tin oxide conducting glass. Electrochim. Acta. 2013, 106, 69–74. [Google Scholar]
- Liu, Y.; Tuleouva, N.; Ramanculov, E.; Revzin, A. Aptamer-Based Electrochemical Biosensor for Interferon Gamma Detection. Anal. Chem. 2010, 82, 8131–8136. [Google Scholar] [CrossRef]
- White, R.J.; Plaxco, K.W. Exploiting Binding-Induced Changes in Probe Flexibility for the Optimization of Electrochemical Biosensors. Anal. Chem. 2010, 82, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Kundu, N.; Young, B.E.; Vieira, P.A.; Sczepanski, J.T.; Arroyo-Currás, N. Nuclease Hydrolysis Does Not Drive the Rapid Signaling Decay of DNA Aptamer-Based Electrochemical Sensors in Biological Fluids. Langmuir 2021, 37, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef]
- Xu, X.; Makaraviciute, A.; Kumar, S.; Wen, C.; Sjödin, M.; Abdurakhmanov, E.; Danielson, U.H.; Nyholm, L.; Zhang, Z. Structural Changes of Mercaptohexanol Self-Assembled Monolayers on Gold and Their Influence on Impedimetric Aptamer Sensors. Anal. Chem. 2019, 91, 14697–14704. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Zhu, M.; Arroyo-Currás, N.; Li, H.; Wang, Y.; Wang, Q.; Lou, X.; Kippin, T.E.; Wang, S.; et al. Implantable Hydrogel-Protective DNA Aptamer-Based Sensor Supports Accurate, Continuous Electrochemical Analysis of Drugs at Multiple Sites in Living Rats. ACS Nano 2023, 17, 18525–18538. [Google Scholar] [CrossRef] [PubMed]
- Watkins, Z.; Karajic, A.; Young, T.; White, R.; Heikenfeld, J. Week-Long Operation of Electrochemical Aptamer Sensors: New Insights into Self-Assembled Monolayer Degradation Mechanisms and Solutions for Stability in Serum at Body Temperature. ACS Sens. 2023, 8, 1119–1131. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Hoggarth, D.A.; Maliniak, D.; Ploense, K.; White, R.J.; Woodward, N.; Hsieh, K.; Bonham, A.J.; Eisenstein, M.; Kippin, T.; et al. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci. Transl. Med. 2013, 5, 213ra165. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Arroyo-Currás, N.; Kang, D.; Ricci, F.; Plaxco, K.W. Dual-Reporter Drift Correction To Enhance the Performance of Electrochemical Aptamer-Based Sensors in Whole Blood. J. Am. Chem. Soc. 2016, 138, 15809–15812. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Currás, N.; Dauphin-Ducharme, O.; Ortega, O.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. Subsecond-Resolved Molecular Measurements in the Living Body Using Chronoamperometrically Interrogated Aptamer-Based Sensors. ACS Sens. 2018, 3, 360–366. [Google Scholar] [CrossRef]
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Hyakumura, T.; Aregueta-Robles, U.; Duan, W.; Villalobos, J.; Adams, W.K.; Poole-Warren, L.; Fallon, J.B. Improving Deep Brain Stimulation Electrode Performance in vivo Through Use of Conductive Hydrogel Coatings. Front. Neurosci. 2021, 15, 761525. [Google Scholar] [CrossRef]
- Fetter, L.C.; McDonough, M.H.; Kippin, T.E.; Plaxco, K.W. Effects of Physiological-Scale Variation in Cations, pH, and Temperature on the Calibration of Electrochemical Aptamer-Based Sensors. ACS Sens. 2024, 9, 6675–6684. [Google Scholar] [CrossRef]
- Arroyo-Curras, N. Beyond the Gold-Thiol Paradigm: Exploring Alternative Interfaces for Electrochemical Nucleic Acid-Based Sensing. ACS Sens. 2024, 9, 2228–2236. [Google Scholar] [CrossRef] [PubMed]




| Protein Name | Host Species | Abbreviation | Purpose | Supplier |
|---|---|---|---|---|
| Recombinant IL-6 | Human | rhIL-6 | Target | [1] |
| Recombinant IL-6 | Rat | rrIL-6 | Target | [2] |
| Recombinant Interferon-gamma (IFN-γ) | Human | rhIFN-γ | Non-target | [1] |
| Recombinant IFN-γ | Rat | rrIFN- γ | Non-target | [1] |
| Recombinant Tumour Necrosis Factor-Alpha (TNF-α) | Human | rhTNF-α | Non-target | [1] |
| Recombinant TNF-α | Rat | rrTNF-α | Non-target | [2] |
| IL-6 ELISA Kit | Rat | N/A | Target | [3] |
| Calprotectin | Human | N/A | Non-target | [4] |
| C-Reactive Protein | Human | hCRP | Non-target | [1] |
| C-Reactive Protein | Rat | rCRP | Non-target | [2] |
| Human albumin serum | Human | HAS | Non-target | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Duan, W.; Deng, F.; Tang, W.; Payne, S.C.; Guo, T.; Goldys, E.M.; Lovell, N.H.; Shivdasani, M.N. Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease. Biosensors 2025, 15, 546. https://doi.org/10.3390/bios15080546
Huang Y, Duan W, Deng F, Tang W, Payne SC, Guo T, Goldys EM, Lovell NH, Shivdasani MN. Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease. Biosensors. 2025; 15(8):546. https://doi.org/10.3390/bios15080546
Chicago/Turabian StyleHuang, Yanan, Wenlu Duan, Fei Deng, Wenxian Tang, Sophie C. Payne, Tianruo Guo, Ewa M. Goldys, Nigel H. Lovell, and Mohit N. Shivdasani. 2025. "Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease" Biosensors 15, no. 8: 546. https://doi.org/10.3390/bios15080546
APA StyleHuang, Y., Duan, W., Deng, F., Tang, W., Payne, S. C., Guo, T., Goldys, E. M., Lovell, N. H., & Shivdasani, M. N. (2025). Towards an Implantable Aptamer Biosensor for Monitoring in Inflammatory Bowel Disease. Biosensors, 15(8), 546. https://doi.org/10.3390/bios15080546






