Protecting Firefighters from Carcinogenic Exposure: Emerging Tools for PAH Detection and Decontamination
Abstract
1. Introduction
2. PAHs: Chemical Properties and Environmental Behavior
3. Firefighters and PAHs
3.1. Characteristics of Personal Protective Equipment of Firefighters
3.2. Assessment of the Toxicity of Firefighter Exposures
3.3. Challenges in PAH Removal from Firefighting Gear
3.4. PAH Exposure Assessment
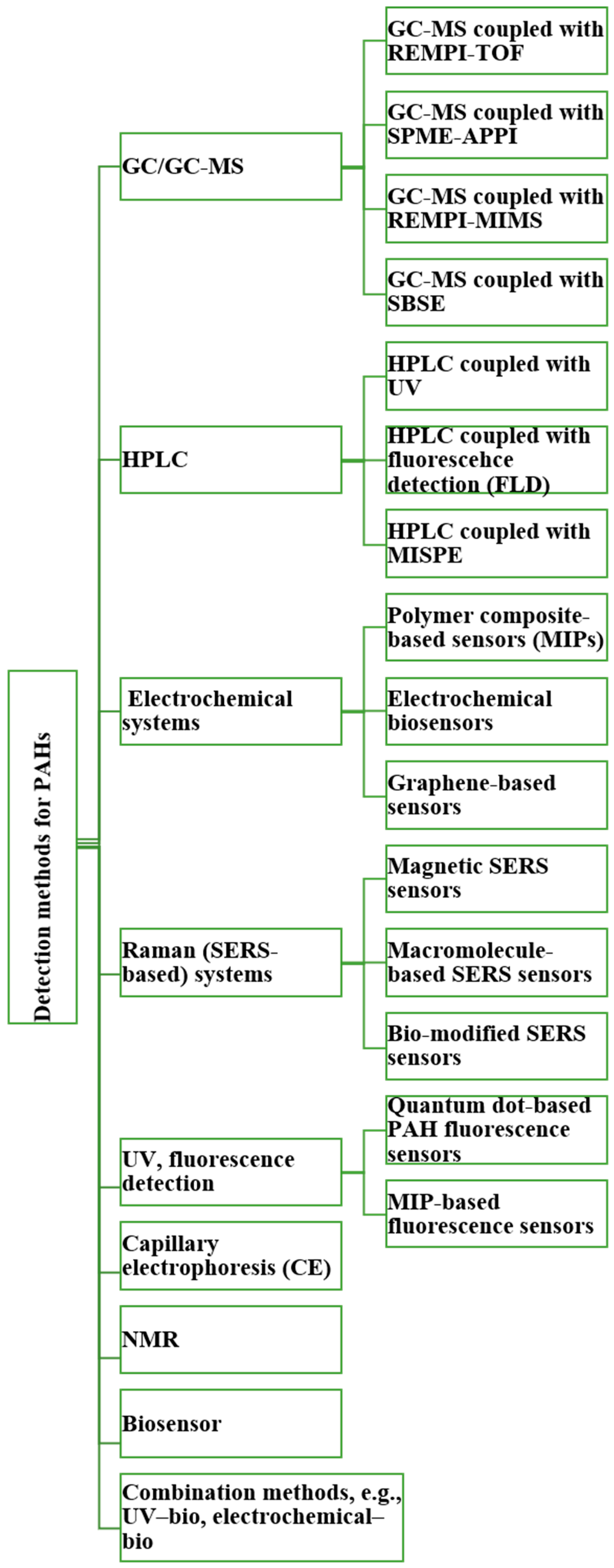
4. PAH Monitoring
4.1. Gas Chromatography–Mass Spectrometry (GC-MS)
4.2. High-Performance Liquid Chromatography (HPLC)
4.3. Electrochemistry Methods for PAH Detection
- Carboxylate-based linkers coordinated with divalent metal ions such as Zn2+, Cu2+, Ni2+, and Co2+ are commonly employed, although they may exhibit reduced stability in aqueous environments;
- Alternatively, metal cluster-based frameworks with carboxylic linkers—such as Cu4(Me3CCOO)8(teia), AuNPs/MMPF-6(Fe), and Cu3(BTC)2(H2O)—offer improved aqueous stability and enhanced sensor performance.
- HQ: linear detection range of 1–200 µM with a detection limit of 270 nM;
- CT: linear range of 1–300 µM with a detection limit of 215 nM.
Fire Scene Adaptability of Metal Oxide, Carbon-Based, and Hybrid Nanomaterial Sensors
4.4. Surface-Enhanced Raman Spectroscopy (SERS) Methods for PAH Detection
Fire Scene Adaptability of SERS and Fluorescent Nanomaterial Sensors
4.5. Fluorescence and UV Spectrometry Methods for PAH Detection


| Sensing Technology | Material | Target PAH | LOD | Preparation/Medium | Ref. |
|---|---|---|---|---|---|
| Fluorescence | Nanomaterial: M-L-cys-CdSeTe/ZnSe/ZnS-GO | Phenanthrene Anthracene | 1.07 × 10−9 mol/L 1.46 × 10−9 mol/L | PAH was in solution form and added to the sensor | [64] |
| Fluorescence | Nanomaterial: M-L-cys-CdSeTeS/ZnS-GO | Phenanthrene | 2.26 × 10−9 mol/L | PAH was in solution form and added to the sensor | [64] |
| Fluorescence | Nanomaterial: M-SWCNT-QDs | Benzo[a]pyrene Benzo[a]anthracene | - | PAH was in solution form and added to the sensor | [65] |
| Fluorescence | Pyrene-imprinted polythiophene thin film | Pyrene | 0.01 × 10−6 mol/L | - | [153] |
| Fluorescence | MIP (thymine-based co-polymers) | Benzo[a]pyrene | 39.6 × 10−12 mol/L | Dimethylsulfoxide (DMSO) | [154] |
| Fluorescence | Fe3O4-MIPs | Pyrene | 9.88 × 10−8 mol/L | Water or acetonitrile/water mixture | [155] |
| Fluorescence | Intrinsic fluorescence | Benzo[a]anthracene Chrysene | 0.016 µg/L | Dairy products | [59,156] |
| Fluorescence | Intrinsic fluorescence–constant-energy synchronous fluorescence spectroscopy | 16 PAHs in air filter | 0.058 ng/mL | 16 PAHs in atmospheric particulate matter | [157] |
4.6. Capillary Electrophoresis (CE)
4.7. NMR
4.8. Biological Methods
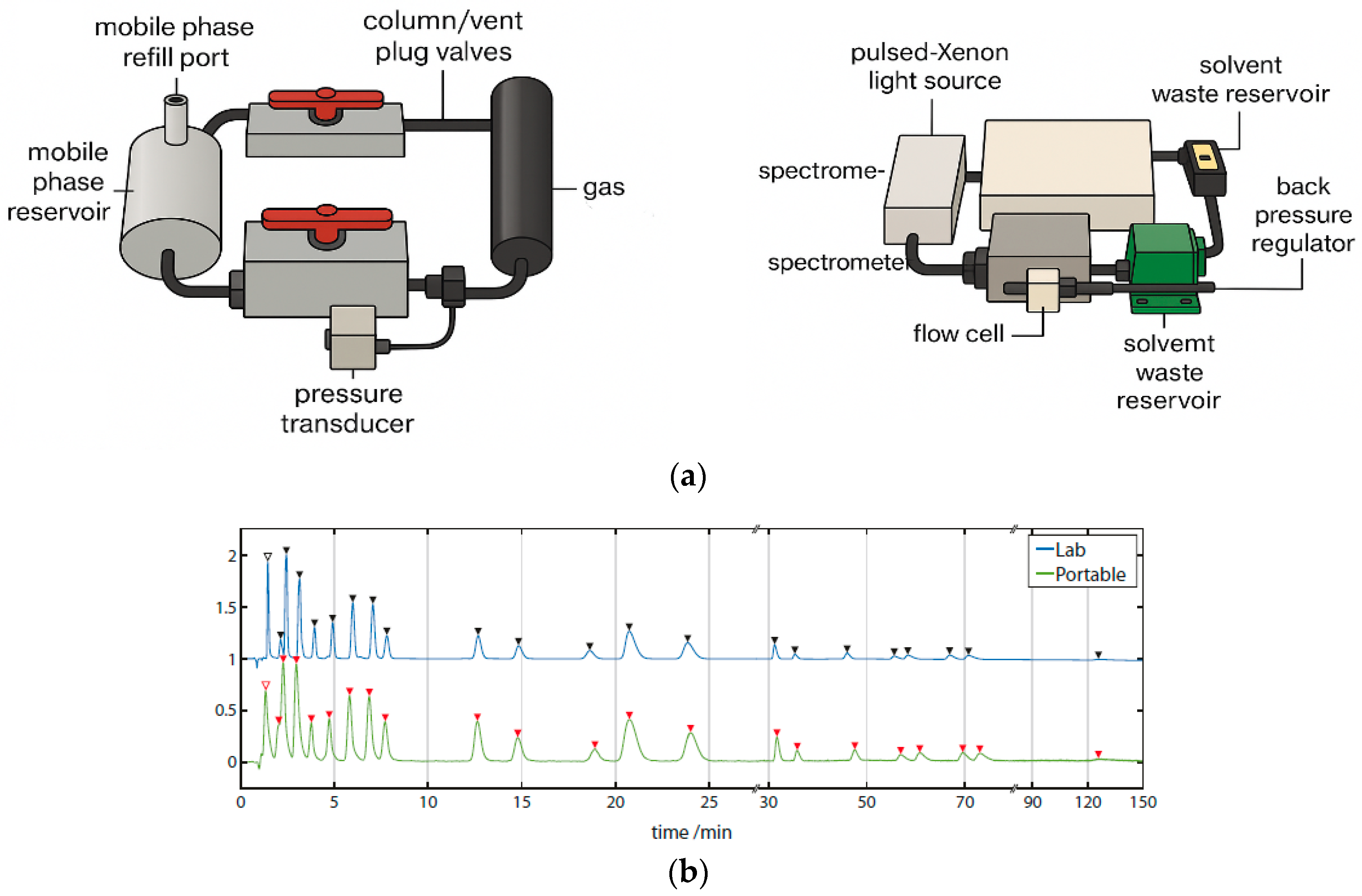
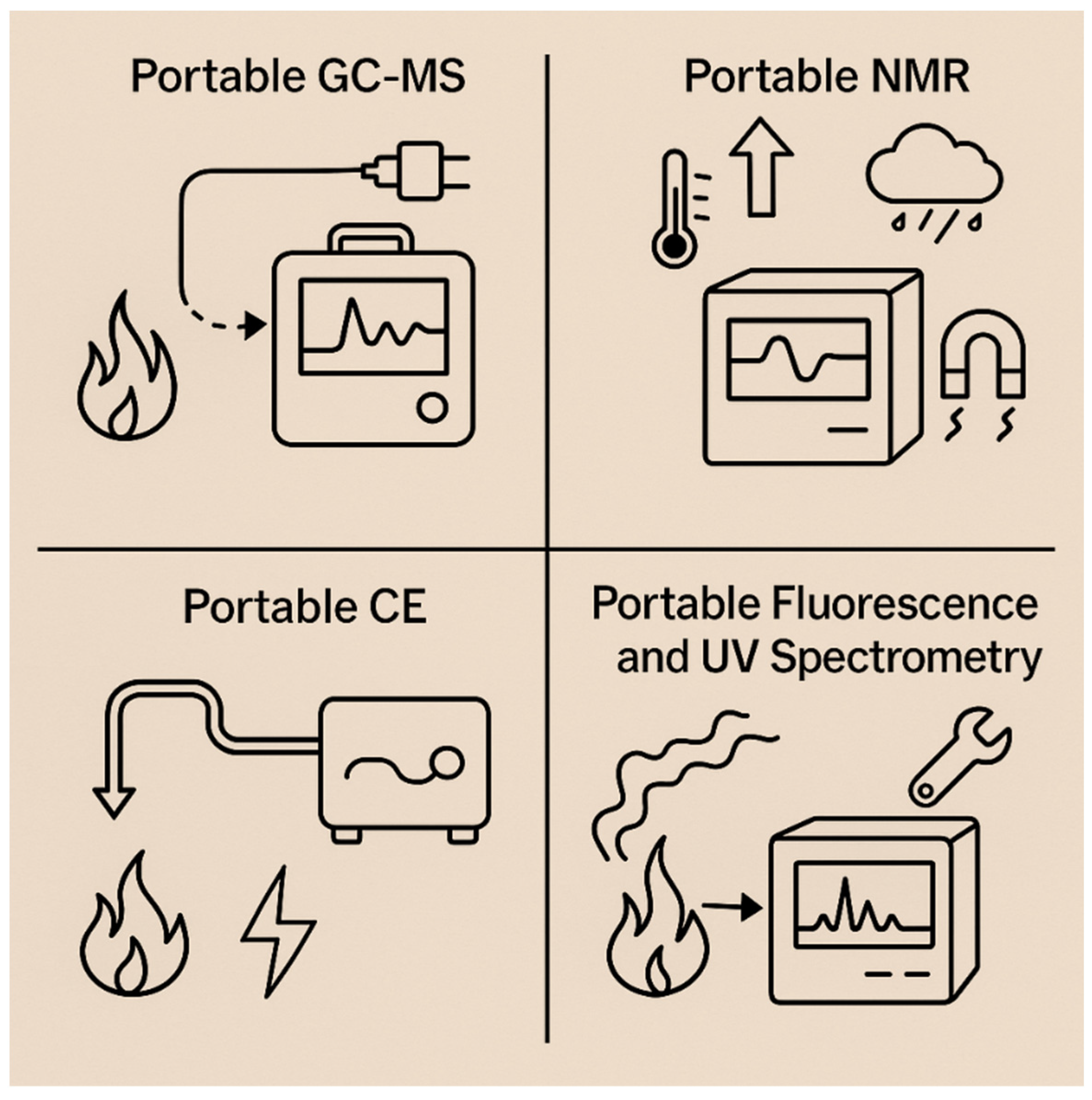
4.9. Toward Portable Methods
5. PAH Removal
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Praveenkumar, T.R.; Sekar, M.; Pasupuleti, R.R.; Gavurová, B.; Arun Kumar, G.; Vignesh Kumar, M. Current Technologies for Plastic Waste Treatment for Energy Recovery, It’s Effects on Poly Aromatic Hydrocarbons Emission and Recycling Strategies. Fuel 2024, 357, 129379. [Google Scholar] [CrossRef]
- Stellman, S.D.; Guidotti, T.L. Polycyclic Aromatic Hydrocarbons. Chemosphere 2007, 1240–1250. [Google Scholar] [CrossRef]
- Alamin, A.; Samara, F.; Al-Tamimi, A.K. Environmental Risk Assessment of Sustainable Concrete Through the Chemical Composition of Metals and Polycyclic Aromatic Hydrocarbons. Sustainability 2024, 16, 9237. [Google Scholar] [CrossRef]
- IARC—International Agency for Research on Cancer. Available online: https://www.iarc.who.int/ (accessed on 2 August 2025).
- LeMasters, G.K.; Genaidy, A.M.; Succop, P.; Deddens, J.; Sobeih, T.; Barriera-Viruet, H.; Dunning, K.; Lockey, J. Cancer Risk among Firefighters: A Review and Meta-Analysis of 32 Studies. J. Occup. Environ. Med. 2006, 48, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Feldman, S.M.; McNally, R.K.; Kannan, K. Distribution of Organohalogen and Synthetic Musk Compounds in Breast Adipose Tissue of Breast Cancer Patients in Ulster County, New York, USA. Arch. Environ. Contam. Toxicol. 2019, 77, 68–78. [Google Scholar] [CrossRef]
- Jongeneelen, F.J. Benchmark Guideline for Urinary 1-Hydroxypyrene as Biomarker of Occupational Exposure to Polycyclic Aromatic Hydrocarbons. Ann. Occup. Hyg. 2001, 45, 3–13. [Google Scholar] [CrossRef]
- Fernandez, A.R.; Treichel, A.; Myers, J.B.; Bourn, S.S.; Crowe, R.P.; Gardner, B. Evaluating Firefighter On-Scene Decontamination Practices Using a National Fire Records Management System. J. Occup. Environ. Med. 2023, 65, 931. [Google Scholar] [CrossRef]
- Keir, J.L.A. Firefighters’ Exposures to Combustion-Derived Polycyclic Aromatic Hydrocarbons and Other Mutagens. Ph.D. Dissertation, Université d’Ottawa, Ottawa, ON, Canada, 2023. [Google Scholar]
- Li, Z.; Trinidad, D.; Pittman, E.N.; Riley, E.A.; Sjodin, A.; Dills, R.L.; Paulsen, M.; Simpson, C.D. Urinary Polycyclic Aromatic Hydrocarbon Metabolites as Biomarkers to Woodsmoke Exposure—Results from a Controlled Exposure Study. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 241–248. [Google Scholar] [CrossRef]
- Andersen, M.H.G. Assessment of Polycyclic Aromatic Hydrocarbon Exposure, Lung Function, Systemic Inflammation, and Genotoxicity in Peripheral Blood Mononuclear Cells from Firefighters before and after a Work Shift. Environ. Mol. Mutagen. 2018, 59, 539–548. [Google Scholar] [CrossRef]
- Horn, G.P.; Fent, K.W.; Kerber, S.; Smith, D.L. Hierarchy of Contamination Control in the Fire Service: Review of Exposure Control Options to Reduce Cancer Risk. J. Occup. Environ. Hyg. 2022, 19, 538–557. [Google Scholar] [CrossRef]
- Daniels, R.D.; Kubale, T.L.; Yiin, J.H.; Dahm, M.M.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; Pinkerton, L.E. Mortality and Cancer Incidence in a Pooled Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2014, 71, 388–397. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/index.html (accessed on 2 August 2025).
- Mayer, A.C.; Fent, K.W.; Bertke, S.; Horn, G.P.; Smith, D.L.; Kerber, S.; La Guardia, M.J. Firefighter Hood Contamination: Efficiency of Laundering to Remove PAHs and FRs. J. Occup. Environ. Hyg. 2019, 16, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Bralewska, K. Air Pollution inside Fire Stations: State-of-the-Art and Future Challenges. Int. J. Hyg. Environ. Health 2024, 255, 114289. [Google Scholar] [CrossRef] [PubMed]
- Papas, W.; Aranda-Rodriguez, R.; Fan, X.; Kubwabo, C.; Lee, J.S.L.; Fantin, E.; Zheng, E.D.; Keir, J.L.A.; Matschke, D.; Blais, J.M.; et al. Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons. Toxics 2024, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Lakkala, J. Refurbishing the Premises of a Fire Station to a Clean Fire Station Concept. Bachelor’s Thesis, Laurea University of Applied Sciences, Vantaa, Finland, 2024. [Google Scholar]
- Hossain, M.D.T.; Girase, A.G.; Ormond, R.B. Evaluating the Performance of Surfactant and Charcoal-Based Cleaning Products to Effectively Remove PAHs from Firefighter Gear. Front. Mater. 2023, 10, 1142777. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, S.; Szewczyńska, M. Analysis and Assessment of Hazards Caused by Chemicals Contaminating Selected Items of Firefighter Personal Protective Equipment—A Literature Review. Saf. Fire Technol. 2020, 56, 92–109. [Google Scholar] [CrossRef]
- Scott, L.T. Chemistry at the Interior Atoms of Polycyclic Aromatic Hydrocarbons. Chem. Soc. Rev. 2015, 44, 6464–6471. [Google Scholar] [CrossRef]
- Mahl, M.; Niyas, M.A.; Shoyama, K.; Würthner, F. Multilayer Stacks of Polycyclic Aromatic Hydrocarbons. Nat. Chem. 2022, 14, 457–462. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Alves, M.J.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; do Carmo Pereira, M.; Morais, S. Polycyclic Aromatic Hydrocarbons at Fire Stations: Firefighters’ Exposure Monitoring and Biomonitoring, and Assessment of the Contribution to Total Internal Dose. J. Hazard. Mater. 2017, 323, 184–194. [Google Scholar] [CrossRef]
- Rossbach, B.; Wollschläger, D.; Letzel, S.; Gottschalk, W.; Muttray, A. Internal Exposure of Firefighting Instructors to Polycyclic Aromatic Hydrocarbons (PAH) during Live Fire Training. Toxicol. Lett. 2020, 331, 102–111. [Google Scholar] [CrossRef]
- Piątek, P.; Rogula-Kozłowska, W.; Walczak, A. Environmental Hazards in Firefighters’ Work. A Short Review. Environ. Prot. Eng. 2024, 50, 115–122. [Google Scholar] [CrossRef]
- Barangi, S.; Mehri, S.; Moosavi, Z.; Hayesd, A.W.; Reiter, R.J.; Cardinali, D.P.; Karimi, G. Melatonin Inhibits Benzo(a)Pyrene-Induced Apoptosis through Activation of the Mir-34a/Sirt1/Autophagy Pathway in Mouse Liver. Ecotoxicol. Environ. Saf. 2020, 196, 110556. [Google Scholar] [CrossRef]
- Sousa, G.; Teixeira, J.; Delerue-Matos, C.; Sarmento, B.; Morais, S.; Wang, X.; Rodrigues, F.; Oliveira, M. Exposure to PAHs during Firefighting Activities: A Review on Skin Levels, In Vitro/In Vivo Bioavailability, and Health Risks. Int. J. Environ. Res. Public Health 2022, 19, 12677. [Google Scholar] [CrossRef]
- Fent, K.W.; Alexander, B.; Roberts, J.; Robertson, S.; Toennis, C.; Sammons, D.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G. Contamination of Firefighter Personal Protective Equipment and Skin and the Effectiveness of Decontamination Procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef]
- Mueller, A.; Ulrich, N.; Hollmann, J.; Zapata Sanchez, C.E.; Rolle-Kampczyk, U.E.; von Bergen, M. Characterization of a Multianalyte GC-MS/MS Procedure for Detecting and Quantifying Polycyclic Aromatic Hydrocarbons (PAHs) and PAH Derivatives from Air Particulate Matter for an Improved Risk Assessment. Environ. Pollut. 2019, 255, 112967. [Google Scholar] [CrossRef] [PubMed]
- Rabajczyk, A.; Gniazdowska, J.; Stojek, P.; Bąk, Ł. Sorption Processes of Selected PAHs on Selected Fire-Resistant Materials Used in Special Firefighter Clothing. Materials 2024, 17, 1741. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Deaton, A.S.; Fang, X.; Watson, K.; DenHartog, E.A.; Barker, R. Effects of Environmental Temperature and Humidity on Evaporative Heat Loss through Firefighter Suit Materials Made with Semi-Permeable and Microporous Moisture Barriers. Text. Res. J. 2022, 92, 219–231. [Google Scholar] [CrossRef]
- Anitta, S.; Sekar, C. HAP-TiO2 Nanocomposites Based Electrochemical Sensor for Selective and Simultaneous Detection of Para-Aminohippuric Acid and Uric Acid. Microchem. J. 2022, 181, 107704. [Google Scholar] [CrossRef]
- Ravichandran, B.; Sen, S.; Dhananjayan, V.; Narayana, J. Personal Exposure to Polycyclic Aromatic Hydrocarbon and Inhalation Risk Assessment among Asphalt Hot Mixed Plant and Road Paving Workers. Aerosol Sci. Eng. 2025. [Google Scholar] [CrossRef]
- Hong, Z.; Guo, Q.; Luo, X.; Liu, L. Polycyclic Aromatic Hydrocarbons Regulate the Occurrence and Development of Nasopharyngeal Carcinoma by Regulating Aryl Hydrocarbon Receptor. Tohoku J. Exp. Med. 2025, 265, 221–228. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]Pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Desaulniers, D.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Azqueta, A.; Bisson, W.H.; Brown, D.; Brunborg, G.; Charles, A.K.; Chen, T.; et al. Causes of Genome Instability: The Effect of Low Dose Chemical Exposures in Modern Society. Carcinogenesis 2015, 36, S61. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency|US EPA. Available online: https://www.epa.gov/ (accessed on 2 August 2025).
- Home—National Toxicology Program. Available online: https://ntp.niehs.nih.gov/ (accessed on 2 August 2025).
- Lin, M.; Liu, Y.; Sun, Z.; Zhang, S.; Yang, Z.; Ni, C. Electrochemical Immunoassay of Benzo[a]Pyrene Based on Dual Amplification Strategy of Electron-Accelerated Fe3O4/Polyaniline Platform and Multi-Enzyme-Functionalized Carbon Sphere Label. Anal. Chim. Acta 2012, 722, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, S.; Xu, J.; Chen, S. An Electrochemical Immune Bioassay for Naphthalene Using Prussian Blue and a Nano-Gold Particle Modified Glass Carbon Electrode. Anal. Methods 2013, 5, 6141–6146. [Google Scholar] [CrossRef]
- Kirk, K.M.; Logan, M.B. Firefighting Instructors’ Exposures to Polycyclic Aromatic Hydrocarbons During Live Fire Training Scenarios. J. Occup. Environ. Hyg. 2015, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, Q.; Yang, S.; Zheng, F.; Du, B.; Wen, S.; Wang, D. Degradation of Pyrene in Contaminated Water and Soil by Fe2+-Activated Persulfate Oxidation: Performance, Kinetics, and Background Electrolytes (Cl-, HCO3- and Humic Acid) Effects. Process Saf. Environ. Prot. 2021, 146, 686–693. [Google Scholar] [CrossRef]
- Gupta, B.S.; Afshari, M. 15—Polyacrylonitrile Fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2018; pp. 545–593. ISBN 978-0-08-101272-7. [Google Scholar]
- Wang, H.; Wang, J.; Zhao, B.; Hu, S.; Xue, G.; Xia, J. Contamination and Removal of Polycyclic Aromatic Hydrocarbons in Multilayered Assemblies of Firefighting Protective Clothing. J. Ind. Text. 2022, 52, 15280837221130772. [Google Scholar] [CrossRef]
- Wilkinson, A.F.; Fent, K.W.; Mayer, A.C.; Chen, I.C.; Kesler, R.M.; Kerber, S.; Smith, D.L.; Horn, G.P. Use of Preliminary Exposure Reduction Practices or Laundering to Mitigate Polycyclic Aromatic Hydrocarbon Contamination on Firefighter Personal Protective Equipment Ensembles. Int. J. Environ. Res. Public Health 2023, 20, 2108. [Google Scholar] [CrossRef]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of Firefighters to Particulates and Polycyclic Aromatic Hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef]
- Wingfors, H.; Nyholm, J.R.; Magnusson, R.; Wijkmark, C.H. Impact of Fire Suit Ensembles on Firefighter PAH Exposures as Assessed by Skin Deposition and Urinary Biomarkers. Ann. Work. Expo. Health 2018, 62, 221–231. [Google Scholar] [CrossRef]
- Keir, J.L.A. The Use of Urinary Biomarkers to Assess Exposures to Polycyclic Aromatic Hydrocarbons (PAHs) and Other Organic Mutagens. Ph.D. Dissertation, Université d’Ottawa, Ottawa, ON, Canada, 2017. [Google Scholar]
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; Kirkham, T.L.; Chan, H.M.; Ayotte, P.; White, P.A.; Blais, J.M. Elevated Exposures to Polycyclic Aromatic Hydrocarbons and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environ. Sci. Technol. 2017, 51, 12745–12755. [Google Scholar] [CrossRef]
- Fernando, S.; Shaw, L.; Shaw, D.; Gallea, M.; VandenEnden, L.; House, R.; Verma, D.K.; Britz-McKibbin, P.; McCarry, B.E. Evaluation of Firefighter Exposure to Wood Smoke during Training Exercises at Burn Houses. Environ. Sci. Technol. 2016, 50, 1536–1543. [Google Scholar] [CrossRef]
- Edelman, P.; Osterloh, J.; Pirkle, J.; Caudill, S.P.; Grainger, J.; Jones, R.; Blount, B.; Calafat, A.; Turner, W.; Feldman, D.; et al. Biomonitoring of Chemical Exposure among New York City Firefighters Responding to the World Trade Center Fire and Collapse. Environ. Health Perspect. 2003, 111, 1906–1911. [Google Scholar] [CrossRef]
- Fent, K.W. Firefighters’ Absorption of PAHs and VOCs during Controlled Residential Fires by Job Assignment and Fire Attack Tactic. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 338–349. [Google Scholar] [CrossRef]
- Behnisch, P.A.; Hosoe, K.; Sakai, S.-I. Bioanalytical Screening Methods for Dioxins and Dioxin-like Compounds—A Review of Bioassay/Biomarker Technology. Environ. Int. 2001, 27, 413–439. [Google Scholar] [CrossRef]
- Laitinen, J. Fire Fighting Trainers’ Exposure to Carcinogenic Agents in Smoke Diving Simulators. Toxicol. Lett. 2010, 192, 61–65. [Google Scholar] [CrossRef]
- Chang, S.; Riviere, J. Percutaneous Absorption of Parathion in Vitro in Porcine Skin: Effects of Dose, Temperature, Humidity, and Perfusate Composition on Absorptive Flux. Toxicol. Sci. 1991, 17, 494–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; He, S.; Tao, H.; Xie, W.; Zhang, X.; Ren, X.; Jiang, T.; Li, L.; Zhu, Z. Contemporary Research Progress on the Detection of Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 2790. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jing, C. Preparation of Thiol Modified Fe3O4@Ag Magnetic SERS Probe for PAHs Detection and Identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, J.; Han, C.; Xie, J. A Versatile SERS Sensor for Multiple Determinations of Polycyclic Aromatic Hydrocarbons and Its Application Potential in Analysis of Fried Foods. Int. J. Anal. Chem. 2020, 2020, 4248029. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, M.; Zheng, X.; Xu, G.; Lin, Y.; Tang, Y. Rapid Detection of Four Polycyclic Aromatic Hydrocarbons in Drinking Water by Constant-Wavelength Synchronous Fluorescence Spectrometry. Anal. Sci. 2023, 39, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Segura Carretero, A.; Martínez Galera, M.; Cruces Blanco, C.; Gil García, M.D.; Fernández Gutiérrez, A.; Martínez Vidal, J.L. Application of Partial Least-Squares Calibration to Phosphorimetric Data for Determination of Polycyclic Aromatic Hydrocarbons in Spiked Environmental Samples. J. AOAC Int. 2000, 83, 391–398. [Google Scholar] [CrossRef]
- Sánchez-Alvarado, A.B.; Zhou, J.; Jin, P.; Neumann, O.; Senftle, T.P.; Nordlander, P.; Halas, N.J. Combined Surface-Enhanced Raman and Infrared Absorption Spectroscopies for Streamlined Chemical Detection of Polycyclic Aromatic Hydrocarbon-Derived Compounds. ACS Nano 2023, 17, 25697–25706. [Google Scholar] [CrossRef]
- Girase, A.; Thompson, D.; Ormond, R.B. Comparative Analysis of the Liquid CO2 Washing with Conventional Wash on Firefighters’ Personal Protective Equipment (PPE). Textiles 2022, 2, 624–632. [Google Scholar] [CrossRef]
- Adegoke, O.; Forbes, P.B.C. L-Cysteine-Capped Core/Shell/Shell Quantum Dot–Graphene Oxide Nanocomposite Fluorescence Probe for Polycyclic Aromatic Hydrocarbon Detection. Talanta 2016, 146, 780–788. [Google Scholar] [CrossRef]
- Adegoke, O.; Montaseri, H.; Nsibande, S.A.; Forbes, P.B.C. Alloyed Quaternary/Binary Core/Shell Quantum Dot-Graphene Oxide Nanocomposite: Preparation, Characterization and Application as a Fluorescence “Switch ON” Probe for Environmental Pollutants. J. Alloys Compd. 2017, 720, 70–78. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Simonet, B.M.; Valcárcel, M. Carbon Nanotube–Quantum Dot Nanocomposites as New Fluorescence Nanoparticles for the Determination of Trace Levels of PAHs in Water. Anal. Chim. Acta 2009, 652, 278–284. [Google Scholar] [CrossRef]
- Qiao, X.; Wei, M.; Tian, D.; Xia, F.; Chen, P.; Zhou, C. One-Step Electrosynthesis of Cadmium/Aluminum Layered Double Hydroxides Composite as Electrochemical Probe for Voltammetric Detection of Anthracene. J. Electroanal. Chem. 2018, 808, 35–40. [Google Scholar] [CrossRef]
- Sehatnia, B.; Sabzi, R.E.; Kheiri, F.; Nikoo, A. Sensitive Molecular Determination of Polycyclic Aromatic Hydrocarbons Based on Thiolated Calix[4])Arene and CdSe Quantum Dots (QDs). J. Appl. Electrochem. 2014, 44, 727–733. [Google Scholar] [CrossRef]
- Del Carlo, M.; Di Marcello, M.; Giuliani, M.; Sergi, M.; Pepe, A.; Compagnone, D. Detection of Benzo(a)Pyrene Photodegradation Products Using DNA Electrochemical Sensors. Biosens. Bioelectron. 2012, 31, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Nagy, K.; Vékey, K. Chapter 5—Separation Methods. In Medical Applications of Mass Spectrometry; Vékey, K., Telekes, A., Vertes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 61–92. ISBN 978-0-444-51980-1. [Google Scholar]
- Stauffer, E.; Dolan, J.A.; Newman, R. CHAPTER 5—Detection of Ignitable Liquid Residues at Fire Scenes. In Fire Debris Analysis; Stauffer, E., Dolan, J.A., Newman, R., Eds.; Academic Press: Burlington, NJ, USA, 2008; pp. 131–161. ISBN 978-0-12-663971-1. [Google Scholar]
- Schmidt, H.; Ha, N.B.; Pfannkuche, J.; Amann, H.; Kronfeldt, H.-D.; Kowalewska, G. Detection of PAHs in Seawater Using Surface-Enhanced Raman Scattering (SERS). Mar. Pollut. Bull. 2004, 49, 229–234. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic Aromatic Hydrocarbons. A Review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical Reactivity and Long-Range Transport Potential of Polycyclic Aromatic Hydrocarbons—A Review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Marchel, M.; Boczkaj, G.; Eng Gdańsk, S. Advanced Oxidation Processes for the Removal of Mono and Polycyclic Aromatic Hydrocarbons—A Review. Sci. Total Environ. 2023, 857, 159043. [Google Scholar] [CrossRef]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic Aromatic Hydrocarbons: Soil Pollution and Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Zainal, P.N.S.; Shahrul Ainliah Alang, A.; Siti Fatimah Nur Abdul, A.; Rosly, N.Z. Polycyclic Aromatic Hydrocarbons: Occurrence, Electroanalysis, Challenges, and Future Outlooks. Crit. Rev. Anal. Chem. 2022, 52, 878–896. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
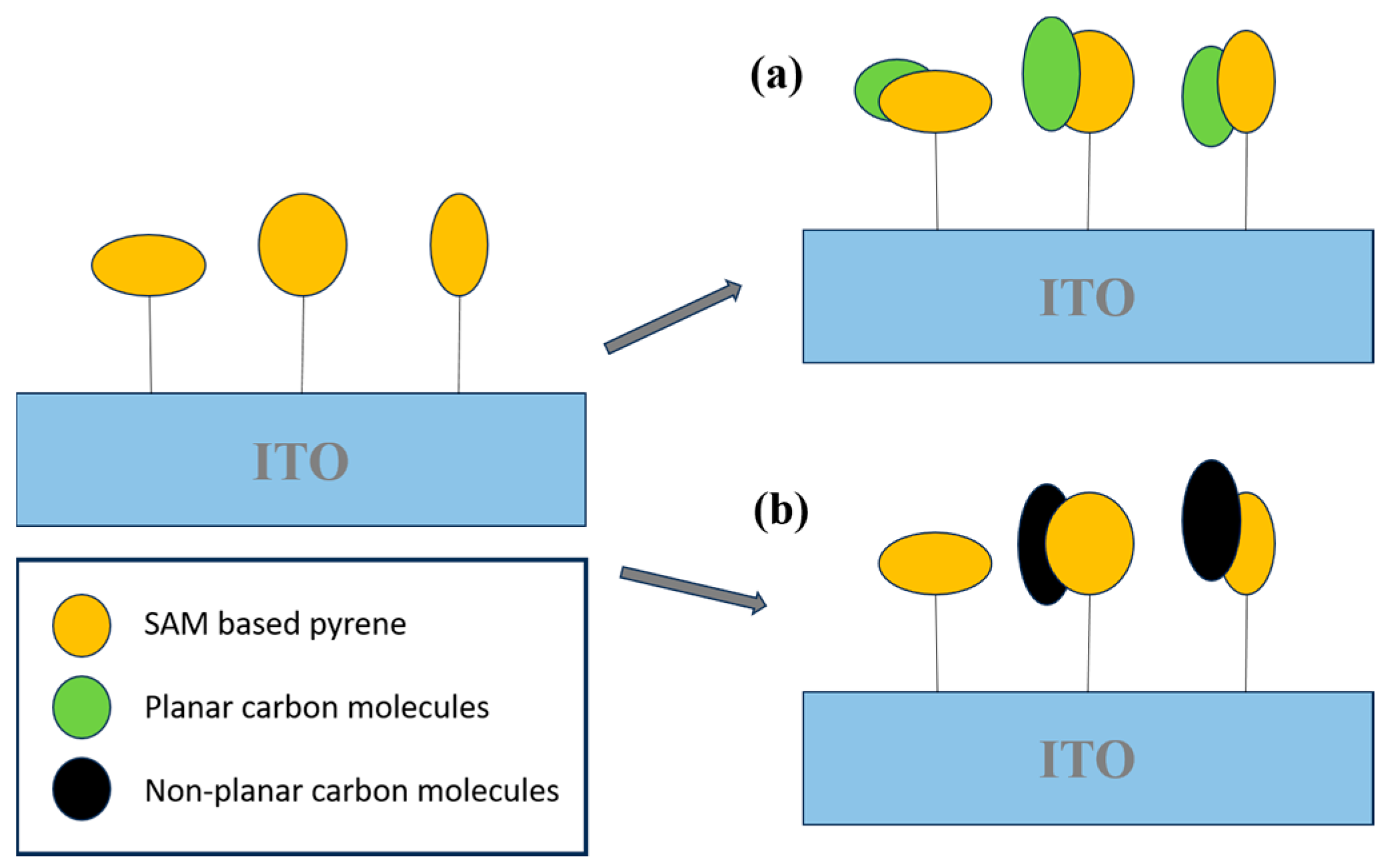
- Muñoz, J.; Crivillers, N.; Mas-Torrent, M. Carbon-Rich Monolayers on ITO as Highly Sensitive Platforms for Detecting Polycyclic Aromatic Hydrocarbons in Water: The Case of Pyrene. Chem.—A Eur. J. 2017, 23, 15289–15293. [Google Scholar] [CrossRef]
- Latif, U.; Ping, L.; Dickert, F.L. Conductometric Sensor for PAH Detection with Molecularly Imprinted Polymer as Recognition Layer. Sensors 2018, 18, 767. [Google Scholar] [CrossRef]
- Chen, M.; Cui, D.; Haick, H.; Tang, N. Artificial Intelligence-Based Medical Sensors for Healthcare System. Adv. Sens. Res. 2024, 3, 2300009. [Google Scholar] [CrossRef]
- Han, Y.; Han, A.; Qin, Y.; Tian, Y.; Peng, B.; He, L.; Zhang, W.; Zhao, W.; Zhang, S. Simple and Sensitive Monitoring of Polycyclic Aromatic Hydrocarbons in Edible Oils by Polydimethylsiloxane/Pyrazine-Based Hyper-Crosslinked Polymer Coated Stir Bar Sorptive Extraction Followed by Gas Chromatography-Mass Spectrometry Detection. Anal. Chim. Acta 2025, 1337, 343554. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Heger, H.J.; Dorfner, R.; Boesl, U.; Blumenstock, M.; Lenoir, D.; Kettrup, A. A Mobile Laser Mass Spectrometer (REMPI-TOFMS) for Continuous Monitoring of Toxic Combustion Byproducts: Real-Time on-Line Analysis of PAH in Waste Incineration Flue Gases. Combust. Sci. Technol. 1998, 134, 87–101. [Google Scholar] [CrossRef]
- Gehm, C.; Streibel, T.; Ehlert, S.; Schulz-Bull, D.; Zimmermann, R. Development and Optimization of an External-Membrane Introduction Photoionization Mass Spectrometer for the Fast Analysis of (Polycyclic)Aromatic Compounds in Environmental and Process Waters. Anal. Chem. 2019, 91, 15547–15554. [Google Scholar] [CrossRef]
- Mitschke, S.; Adam, T.; Streibel, T.; Baker, R.R.; Zimmermann, R. Application of Time-of-Flight Mass Spectrometry with Laser-Based Photoionization Methods for Time-Resolved on-Line Analysis of Mainstream Cigarette Smoke. Anal. Chem. 2005, 77, 2288–2296. [Google Scholar] [CrossRef]
- Ketola, R.A.; Kotiaho, T.; Cisper, M.E.; Allen, T.M. Environmental Applications of Membrane Introduction Mass Spectrometry. J. Mass. Spectrom. 2002, 37, 457–476. [Google Scholar] [CrossRef]
- Lacroix, C.; Le Cuff, N.; Receveur, J.; Moraga, D.; Auffret, M.; Guyomarch, J. Development of an Innovative and “Green” Stir Bar Sorptive Extraction–Thermal Desorption–Gas Chromatography–Tandem Mass Spectrometry Method for Quantification of Polycyclic Aromatic Hydrocarbons in Marine Biota. J. Chromatogr. A 2014, 1349, 1–10. [Google Scholar] [CrossRef]
- Garcıa-Falcón, M.S.; Cancho-Grande, B.; Simal-Gándara, J. Stirring Bar Sorptive Extraction in the Determination of PAHs in Drinking Waters. Water Res. 2004, 38, 1679–1684. [Google Scholar] [CrossRef]
- Zuin, V.G.; Montero, L.; Bauer, C.; Popp, P. Stir Bar Sorptive Extraction and High-Performance Liquid Chromatography–Fluorescence Detection for the Determination of Polycyclic Aromatic Hydrocarbons in Mate Teas. J. Chromatogr. A 2005, 1091, 2–10. [Google Scholar] [CrossRef]
- Wicker, A.P.; Carlton, D.D.; Tanaka, K.; Nishimura, M.; Chen, V.; Ogura, T.; Hedgepeth, W.; Schug, K.A. On-Line Supercritical Fluid Extraction—Supercritical Fluid Chromatography-Mass Spectrometry of Polycyclic Aromatic Hydrocarbons in Soil. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical Fluid Extraction: Recent Advances and Applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical Fluid Extraction in Herbal and Natural Product Studies—A Practical Review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, N.; Ying, Y.; Tian, C.; Feng, L.; Wu, P.; Wang, Z.; Han, J. Simultaneous Determination of 16 Polycyclic Aromatic Hydrocarbons in Source Water and Tap Water by Performance Liquid Chromatography with Ultraviolet Detector Tandem Fluorescence Detector Combined with Solid Phase Extraction. Wei Sheng Yan Jiu 2020, 49, 480–485. [Google Scholar]
- Wang, X.D.; Zhang, H.H.; Wang, L.; Guo, X.F. Study of Effects of Ionic Strength and PH on PAHs Removal by Nanofiltration. In Proceedings of the 2nd Annual Congress on Advanced Engineering and Technology II (CAET 2015), Hong Kong, China, 4–5 April 2015; pp. 4–5. [Google Scholar]
- Chatzimichail, S.; Rahimi, F.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. Hand-Portable HPLC with Broadband Spectral Detection Enables Analysis of Complex Polycyclic Aromatic Hydrocarbon Mixtures. Commun. Chem. 2021, 4, 17. [Google Scholar] [CrossRef]
- Panahi, A.; Abbasian, F.; Ayala-Charca, G.; Tabrizi, H.O.; Roshanfar, A.; Ghafar-Zadeh, M.; Movahed, M.; Tahernezhad, Y.; Magierowski, S.; Ghafar-Zadeh, E. A Portable and Cost-Effective System for Electronic Nucleic Acid Mass Measurement. Sci. Rep. 2025, 15, 5387. [Google Scholar] [CrossRef]
- Ramesh, A.; Walker, S.A.; Hood, D.B.; Guillén, M.D.; Schneider, K.; Weyand, E.H. Bioavailability and Risk Assessment of Orally Ingested Polycyclic Aromatic Hydrocarbons. Int. J. Toxicol. 2004, 23, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Nsibande, S.A.; Montaseri, H.; Forbes, P.B.C. Advances in the Application of Nanomaterial-Based Sensors for Detection of Polycyclic Aromatic Hydrocarbons in Aquatic Systems. TrAC—Trends Anal. Chem. 2019, 115, 52–69. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, L.; Wu, R.; Zhu, Y.; Sheng, Y.; Nie, P.; Liu, P.; Xu, L.; Wen, Y. Portable Wireless Intelligent Sensing of Ultra-Trace Phytoregulator α-Naphthalene Acetic Acid Using Self-Assembled Phosphorene/Ti3C2-MXene Nanohybrid with High Ambient Stability on Laser Induced Porous Graphene as Nanozyme Flexible Electrode. Biosens. Bioelectron. 2021, 179, 113062. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Platinum Nanoparticles/Ti3C2Tx (MXene) Composite for the Effectual Electrochemical Sensing of Bisphenol A in Aqueous Media. J. Electroanal. Chem. 2021, 880, 114934. [Google Scholar] [CrossRef]
- Huang, R.; Liao, D.; Liu, Z.; Yu, J.; Jiang, X. Electrostatically Assembling 2D Hierarchical Nb2CTx and Zifs-Derivatives into Zn-Co-NC Nanocage for the Electrochemical Detection of 4-Nitrophenol. Sens. Actuators B Chem. 2021, 338, 129828. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R. Electrochemical Synthesis of Nanostructured Copper-Curcumin Complex and Its Electrocatalytic Application towards Reduction of 4-Nitrophenol. Sens. Actuators B Chem. 2017, 253, 502–512. [Google Scholar] [CrossRef]
- Huang, R.; Chen, S.; Yu, J.; Jiang, X. Self-Assembled Ti3C2/MWCNTs Nanocomposites Modified Glassy Carbon Electrode for Electrochemical Simultaneous Detection of Hydroquinone and Catechol. Ecotoxicol. Environ. Saf. 2019, 184, 109619. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Yu, H.; Zheng, F.; Lin, L.; Chen, J.; Li, Y.; Lin, Y. In Situ Construction of Hollow Carbon Spheres with N, Co, and Fe Co-Doping as Electrochemical Sensors for Simultaneous Determination of Dihydroxybenzene Isomers. Nanoscale 2019, 11, 8950–8958. [Google Scholar] [CrossRef]
- Beitollahi, H.; Dourandish, Z.; Tajik, S.; Sharifi, F.; Jahani, P.M. Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen. Biosensor 2022, 12, 872. [Google Scholar] [CrossRef]
- Murtada, K. Trends in Nanomaterial-Based Solid-Phase Microextraction with a Focus on Environmental Applications-a Review. Trends Environ. Anal. Chem. 2020, 25, e00077. [Google Scholar] [CrossRef]
- Subhan, M.A.; Chandra Saha, P.; Sumon, S.A.; Ahmed, J.; Asiri, A.M.; Rahman, M.M.; Al-Mamun, M. Enhanced Photocatalytic Activity and Ultra-Sensitive Benzaldehyde Sensing Performance of a SnO2·ZnO·TiO2 Nanomaterial. RSC Adv. 2018, 8, 33048–33058. [Google Scholar] [CrossRef]
- Kamil Reza, K.; Azahar Ali, M.; Singh, M.K.; Agrawal, V.V.; Biradar, A.M. Amperometric Enzymatic Determination of Bisphenol A Using an ITO Electrode Modified with Reduced Graphene Oxide and Mn3O4 Nanoparticles in a Chitosan Matrix. Microchim. Acta 2017, 184, 1809–1816. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Electrochemical Detection of Phenanthrene Using Nickel Oxide Doped PANI Nanofiber Based Modified Electrodes. J. Nanomater. 2016, 2016, 9614897. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Servant, L.; Toupance, T.; Li, S.; Klein, A.; Jaegermann, W. Improved Photocatalytic Activity in RuO 2–ZnO Nanoparticulate Heterostructures Due to Inhomogeneous Space Charge Effects. Phys. Chem. Chem. Phys. 2015, 17, 5090–5102. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Sivakumar, M.; Huh, Y.S.; Han, Y.-K. Facile Synthesis of MnO2/Carbon Nanotubes Decorated with a Nanocomposite of Pt Nanoparticles as a New Platform for the Electrochemical Detection of Catechin in Red Wine and Green Tea Samples. J. Mater. Chem. B 2015, 3, 6285–6292. [Google Scholar] [CrossRef] [PubMed]
- Nikahd, B.; Khalilzadeh, M.A. Liquid Phase Determination of Bisphenol A in Food Samples Using Novel Nanostructure Ionic Liquid Modified Sensor. J. Mol. Liq. 2016, 215, 253–257. [Google Scholar] [CrossRef]
- Hu, L.; Fong, C.-C.; Zhang, X.; Chan, L.L.; Lam, P.K.S.; Chu, P.K.; Wong, K.-Y.; Yang, M. Au Nanoparticles Decorated TiO2 Nanotube Arrays as a Recyclable Sensor for Photoenhanced Electrochemical Detection of Bisphenol A. Environ. Sci. Technol. 2016, 50, 4430–4438. [Google Scholar] [CrossRef]
- Mahshid, S.S.; Luo, S.; Yang, L.; Mahshid, S.; Dolati, A.; Ghorbani, M.; Cai, Q. A Well-Dispersed Pt/Ni/TiO2 Nanotubes Modified Electrode as an Amperometric Non-Enzymatic Glucose Biosensor. Sens. Lett. 2011, 9, 1598–1605. [Google Scholar] [CrossRef]
- Liu, S.; Wei, M.; Zheng, X.; Xu, S.; Xia, F.; Zhou, C. Alizarin Red S Functionalized Mesoporous Silica Modified Glassy Carbon Electrode for Electrochemical Determination of Anthracene. Electrochim. Acta 2015, 160, 108–113. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M. Simultaneous Electrochemical Determination of Acetaminophen, Caffeine and Ascorbic Acid Using a New Electrochemical Sensor Based on CuO–Graphene Nanocomposite. RSC Adv. 2015, 5, 95140–95148. [Google Scholar] [CrossRef]
- Ganesamurthi, J.; Shanmugam, R.; Chen, S.-M.; Alagumalai, K.; Balamurugan, M.; Fan, C.-H. A Portable Electrochemical Sensor Based on Binary Transition Metal Oxide (CoO/ZnO) for the Evaluation of Eugenol in Real-Time Samples. Surf. Interfaces 2023, 38, 102845. [Google Scholar] [CrossRef]
- Caetano, F.R.; Felippe, L.B.; Zarbin, A.J.G.; Bergamini, M.F.; Marcolino-Junior, L.H. Gold Nanoparticles Supported on Multi-Walled Carbon Nanotubes Produced by Biphasic Modified Method and Dopamine Sensing Application. Sens. Actuators B Chem. 2017, 243, 43–50. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, Y.; Zhou, T.; Shi, G. A Novel Composite of Reduced Graphene Oxide and Molecularly Imprinted Polymer for Electrochemical Sensing 4-Nitrophenol. Electrochim. Acta 2014, 130, 504–511. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Recent Advances in Electrochemical Sensors Based on Molecularly Imprinted Polymers and Nanomaterials. Electroanalysis 2019, 31, 188–201. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Electrochemical Sensors Based on Magnetic Molecularly Imprinted Polymers: A Review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, M.; Yao, X.; Fa, H.; Wang, Y.; Hou, C. A Zeolitic Imidazolate Framework/Carbon Nanofiber Nanocomposite Based Electrochemical Sensor for Simultaneous Detection of Co-Existing Dihydroxybenzene Isomers. Sens. Actuators B Chem. 2020, 320, 128294. [Google Scholar] [CrossRef]
- Pop, A.; Manea, F.; Baciu, A.; Motoc, S. Simultaneous Voltammetric Detection of Benzene, Naphthalene and Anthracene from Water Using Boron-Doped Diamond Electrode. Sens. Biosensing Res. 2024, 44, 100641. [Google Scholar] [CrossRef]
- Chang, S.-K.; Zainal, Z.; Tan, K.-B.; Yusof, N.A.; Yusoff, W.M.D.W.; Prabaharan, S.R.S. Recent Development in Spinel Cobaltites for Supercapacitor Application. Ceram. Int. 2015, 41, 1–14. [Google Scholar] [CrossRef]
- Makelane, H.; John, S.V.; Yonkeu, A.L.D.; Waryo, T.; Tovide, O.; Iwuoha, E. Phase Selective Alternating Current Voltammetric Signalling Protocol: Application in Dendritic Co-polymer Sensor for Anthracene. Electroanalysis 2017, 29, 1887–1893. [Google Scholar] [CrossRef]
- Hamnca, S.; Ward, M.; Ngema, X.T.; Iwuoha, E.; Baker, P.G.L. Development of Graphenated Polyamic Acid Sensors for Electroanalytical Detection of Anthracene. J. Nano Res. 2016, 43, 11–22. [Google Scholar] [CrossRef]
- Hamnca, S. Polyamic Acid-Graphene Oxide Nanocomposite for Electrochemical Screening of Antibiotic Residues in Water. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2015. [Google Scholar]
- Hamnca, S.; Phelane, L.; Iwuoha, E.; Baker, P. Electrochemical Determination of Neomycin and Norfloxacin at a Novel Polymer Nanocomposite Electrode in Aqueous Solution. Anal. Lett. 2017, 50, 1887–1896. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, N.; Huang, C.; Duan, X.; Ren, D.; Huo, Z. The Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Ozone-Based Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2024, 46, 26–42. [Google Scholar] [CrossRef]
- Scanlon, M.D.; Smirnov, E.; Stockmann, T.J.; Peljo, P. Gold Nanofilms at Liquid–Liquid Interfaces: An Emerging Platform for Redox Electrocatalysis, Nanoplasmonic Sensors, and Electrovariable Optics. Chem. Rev. 2018, 118, 3722–3751. [Google Scholar] [CrossRef] [PubMed]
- Mosier-Boss, P.A.; Lieberman, S.H. Surface-Enhanced Raman Spectroscopy Substrate Composed of Chemically Modified Gold Colloid Particles Immobilized on Magnetic Microparticles. Anal. Chem. 2005, 77, 1031–1037. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, H.; Yu, Q.; Li, Q.; Lu, X.; Kong, X. On-Site Separation and Identification of Polycyclic Aromatic Hydrocarbons from Edible Oil by TLC-SERS on Diatomite Photonic Biosilica Plate. Microchem. J. 2021, 160, 105672. [Google Scholar] [CrossRef]
- Hahm, E.; Jeong, D.; Cha, M.G.; Choi, J.M.; Pham, X.-H.; Kim, H.-M.; Kim, H.; Lee, Y.-S.; Jeong, D.H.; Jung, S.; et al. β-CD Dimer-Immobilized Ag Assembly Embedded Silica Nanoparticles for Sensitive Detection of Polycyclic Aromatic Hydrocarbons. Sci. Rep. 2016, 6, 26082. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Han, X.; Song, W.; Ruan, W.; Liu, J.; Zhao, B.; Ozaki, Y. Selective SERS Detection of Each Polycyclic Aromatic Hydrocarbon (PAH) in a Mixture of Five Kinds of PAHs. J. Raman Spectrosc. 2011, 42, 945–950. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, T. SERS Detection of Polyaromatic Hydrocarbons on a Β-cyclodextrin Containing Polymer Brush. J. Raman Spectrosc. 2018, 49, 452–461. [Google Scholar] [CrossRef]
- Dribek, M.; Rinnert, E.; Colas, F.; Crassous, M.-P.; Thioune, N.; David, C.; de la Chapelle, M.; Compère, C. Organometallic Nanoprobe to Enhance Optical Response on the Polycyclic Aromatic Hydrocarbon Benzo [a] Pyrene Immunoassay Using SERS Technology. Environ. Sci. Pollut. Res. 2017, 24, 27070–27076. [Google Scholar] [CrossRef]
- Bao, L.; Sheng, P.; Li, J.; Wu, S.; Cai, Q.; Yao, S. Surface Enhanced Raman Spectroscopic Detection of Polycyclic Aromatic Hydrocarbons (PAHs) Using a Gold Nanoparticles-Modified Alginate Gel Network. Analyst 2012, 137, 4010–4015. [Google Scholar] [CrossRef]
- Shi, X.; Kwon, Y.; Ma, J.; Zheng, R.; Wang, C.; Kronfeldt, H. Trace Analysis of Polycyclic Aromatic Hydrocarbons Using Calixarene Layered Gold Colloid Film as Substrates for Surface-enhanced Raman Scattering. J. Raman Spectrosc. 2013, 44, 41–46. [Google Scholar] [CrossRef]
- Wang, X.; Hao, W.; Zhang, H.; Pan, Y.; Kang, Y.; Zhang, X.; Zou, M.; Tong, P.; Du, Y. Analysis of Polycyclic Aromatic Hydrocarbons in Water with Gold Nanoparticles Decorated Hydrophobic Porous Polymer as Surface-Enhanced Raman Spectroscopy Substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 214–221. [Google Scholar] [CrossRef]
- Taniya, O.S.; Khasanov, A.F.; Sadieva, L.K.; Santra, S.; Nikonov, I.L.; Al-Ithawi, W.K.A.; Kovalev, I.S.; Kopchuk, D.S.; Zyryanov, G.V.; Ranu, B.C. Polymers and polymer-based materials for the detection of (nitro-) explosives. Materials 2023, 16, 6333. [Google Scholar]
- Gu, H.-X.; Xue, L.; Zhang, Y.-F.; Li, D.-W.; Long, Y.-T. Facile Fabrication of a Silver Dendrite-Integrated Chip for Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2015, 7, 2931–2936. [Google Scholar] [CrossRef]
- Fu, S.; Guo, X.; Wang, H.; Yang, T.; Wen, Y.; Yang, H. Functionalized Au Nanoparticles for Label-Free Raman Determination of Ppb Level Benzopyrene in Edible Oil. Sens. Actuators B Chem. 2015, 212, 200–206. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Hossain, M.K.; Al-Saadi, A.A. Hydroxylamine Reduced Silver Colloid for Naphthalene and Phenanthrene Detection Using Surface-Enhanced Raman Spectroscopy. Chem. Eng. J. 2016, 304, 141–148. [Google Scholar] [CrossRef]
- Xu, J.; Du, J.; Jing, C.; Zhang, Y.; Cui, J. Facile Detection of Polycyclic Aromatic Hydrocarbons by a Surface-Enhanced Raman Scattering Sensor Based on the Au Coffee Ring Effect. ACS Appl. Mater. Interfaces 2014, 6, 6891–6897. [Google Scholar] [CrossRef] [PubMed]
- López-Tocón, I.; Otero, J.C.; Arenas, J.F.; García-Ramos, J.V.; Sánchez-Cortés, S. Multicomponent Direct Detection of Polycyclic Aromatic Hydrocarbons by Surface-Enhanced Raman Spectroscopy Using Silver Nanoparticles Functionalized with the Viologen Host Lucigenin. Anal. Chem. 2011, 83, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Tijunelyte, I.; Betelu, S.; Moreau, J.; Ignatiadis, I.; Berho, C.; Lidgi-Guigui, N.; Guénin, E.; David, C.; Vergnole, S.; Rinnert, E. Diazonium Salt-Based Surface-Enhanced Raman Spectroscopy Nanosensor: Detection and Quantitation of Aromatic Hydrocarbons in Water Samples. Sensors 2017, 17, 1198. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.-L.; Li, Y.-T.; Li, D.-W.; Xue, J.-Q.; Fossey, J.S.; Long, Y.-T. Humic Acids-Based One-Step Fabrication of SERS Substrates for Detection of Polycyclic Aromatic Hydrocarbons. Analyst 2013, 138, 1523–1528. [Google Scholar] [CrossRef]
- Tang, K.H.D. Phytoremediation of Petroleum Hydrocarbons: An Update of Its Recent Progress. Trop. Environ. Biol. Technol. 2024, 2, 106–123. [Google Scholar] [CrossRef]
- Tropp, J.; Ihde, M.H.; Williams, A.K.; White, N.J.; Eedugurala, N.; Bell, N.C.; Azoulay, J.D.; Bonizzoni, M. A sensor array for the discrimination of polycyclic aromatic hydrocarbons using conjugated polymers and the inner filter effect. Chem. Sci. 2019, 10, 10247–10255. [Google Scholar]
- Sunuwar, S.; Haddad, A.; Acheson, A.; Manzanares, C.E. Synchronous Fluorescence as a Sensor of Trace Amounts of Polycyclic Aromatic Hydrocarbons. Sensors 2024, 24, 3800. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Krupadam, R.J.; Advincula, R.C. Pyrene-Imprinted Polythiophene Sensors for Detection of Polycyclic Aromatic Hydrocarbons. Sens. Actuators B Chem. 2016, 228, 693–701. [Google Scholar] [CrossRef]
- Ledesma, J.; Pisano, P.L.; Martino, D.M.; Boschetti, C.E.; Bortolato, S.A. Thymine Based Copolymers: Feasible Sensors for the Detection of Persistent Organic Pollutants in Water. RSC Adv. 2017, 7, 49066–49073. [Google Scholar] [CrossRef]
- Medina-Castillo, A.L.; Mistlberger, G.; Fernandez-Sanchez, J.F.; Segura-Carretero, A.; Klimant, I.; Fernandez-Gutierrez, A. Novel Strategy to Design Magnetic, Molecular Imprinted Polymers with Well-Controlled Structure for the Application in Optical Sensors. Macromolecules 2010, 43, 55–61. [Google Scholar] [CrossRef]
- Cai, Q.; Zhao, C.; Zhu, H.; Shen, Y.; Hou, H.; Tang, Y. Constant-wavelength Synchronous Fluorescence Spectrometry for Simultaneous and Rapid Determination of Five Polycyclic Aromatic Hydrocarbon Residues in Dairy Products. Luminescence 2021, 36, 353–359. [Google Scholar] [CrossRef]
- Wang, G.Y.; Tian, H.Q.; Niu, X.L.; Jia, S.M.; Liu, Y.R.; Chen, X.F.; Xie, Z.; Yang, D.Z.; Li, L.; Shi, G.F. Constant-Energy Synchronous Fluorescence Spectrum Characteristics of 15 Polycyclic Aromatic Hydrocarbons from Atmospheric Particulate Matters. Acta Sci. Circumstantiae 2019, 39, 44–52. [Google Scholar]
- Benhabib, M.; Chiesl, T.N.; Stockton, A.M.; Scherer, J.R.; Mathies, R.A. Multichannel Capillary Electrophoresis Microdevice and Instrumentation for in Situ Planetary Analysis of Organic Molecules and Biomarkers. Anal. Chem. 2010, 82, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.T.; Fleming, A.M.; Johnson, R.P.; Burrows, C.J.; White, H.S. Detection of Benzo[a]Pyrene-Guanine Adducts in Single-Stranded DNA Using the α-Hemolysin Nanopore. Nanotechnology 2015, 26, 074002. [Google Scholar] [CrossRef]
- Lauterbur, P.C. C13 Nuclear Magnetic Resonance Spectroscopy. I. Aromatic Hydrocarbons. J. Am. Chem. Soc. 1961, 83, 1838–1846. [Google Scholar] [CrossRef]
- Guiteras, J.; Beltran, J.L.; Ferrer, R. Quantitative Multicomponent Analysis of Polycyclic Aromatic Hydrocarbons in Water Samples. Anal. Chim. Acta 1998, 361, 233–240. [Google Scholar] [CrossRef]
- Penalver, A.; Pocurull, E.; Borrull, F.; Marce, R.M. Trends in Solid-Phase Microextraction for Determining Organic Pollutants in Environmental Samples. TrAC Trends Anal. Chem. 1999, 18, 557–568. [Google Scholar] [CrossRef]
- Wagner, L.; Kalli, C.; Fridjonsson, E.O.; May, E.F.; Zhen, J.; Johns, M.L. Simultaneous Quantification of Aliphatic and Aromatic Hydrocarbons in Produced Water Analysis Using Mobile 1H NMR. Meas. Sci. Technol. 2018, 29, 085501. [Google Scholar] [CrossRef]
- Wagner, L.; Kalli, C.; Fridjonsson, E.O.; May, E.F.; Stanwix, P.L.; Graham, B.F.; Carroll, M.R.J.; Johns, M.L. Quantitative Produced Water Analysis Using Mobile 1H NMR. Meas. Sci. Technol. 2016, 27, 105501. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhang, L.; Wang, C. An Amperometric Biosensor Based on Rat Cytochrome P450 1A1 for Benzo[a]Pyrene Determination. Biosens. Bioelectron. 2011, 26, 2177–2182. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Ki, M.-R.; Yoon, H.J.; Pack, S.P. Microfluidic Sensors for Micropollutant Detection in Environmental Matrices: Recent Advances and Prospects. Biosensors 2025, 15, 474. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M. Frontiers in Nanoparticles Redefining Enzyme Immobilization: A Review Addressing Challenges, Innovations, and Unlocking Sustainable Future Potentials. Micro Nano Syst. Lett. 2025, 13, 7. [Google Scholar] [CrossRef]
- Sakshi; Haritash, A.K. A Comprehensive Review of Metabolic and Genomic Aspects of PAH-Degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- Sengupta, J.; Mustansar Hussain, C. Sensitive and Selective Detection of Heavy Metal Ions and Organic Pollutants with Graphene-Integrated Sensing Platforms. Nanoscale 2024, 16, 14195–14212. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Zhang, L.; Yang, J.; Yao, Z.P.; He, Y.; Li, Y. Integrated Hand-Held Electrochemical Sensor for Multicomponent Detection in Urine. Biosens. Bioelectron. 2021, 193, 113534. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, J.; Xu, K.; Zhu, H.; Zhang, S.; Lu, X.; Li, X. Development of a Portable Gas Chromatograph–Mass Spectrometer Embedded with a Low-Temperature Adsorption Thermal Desorption Module for Enhanced Detection of Volatile Organic Compounds. Analyst 2025, 150, 470–480. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, S.; Lin, J.; Zhu, X.; Gao, W.; Li, J.; Wang, C.; Wu, Y.; Han, R.; Tang, K.; et al. Determination of Ethanol and Aromatics in Blood by Headspace Portable Gas Chromatography-Mass Spectrometry (HS-PGC-MS). Anal. Lett. 2025, 58, 724–735. [Google Scholar] [CrossRef]
- Eckenrode, B.A. Environmental and Forensic Applications of Field-Portable GC-MS: An Overview. J. Am. Soc. Mass. Spectrom. 2001, 12, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, G. Development and Characterization of a Portable NMR Probe for the Monitoring of Water Quality and for the Study of the Kinetics of Chemical Reactions. Ph.D. Dissertation, Université de Strasbourg, Strasbourg, France, 2024; p. 255. [Google Scholar] [CrossRef]
- Sakellariou, D.; Le Goff, G.; Jacquinot, J.-F. High-Resolution, High-Sensitivity NMR of Nanolitre Anisotropic Samples by Coil Spinning. Nature 2007, 447, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Elmorsi, E.T.; Lai, E.P.C. Optimization of Capillary Electrophoresis by Central Composite Design for Separation of Pharmaceutical Contaminants in Water Quality Testing. Environments 2025, 12, 22. [Google Scholar] [CrossRef]
- Sovocool, G.W.; Brumley, W.C.; Donnelly, J.R. Capillary Electrophoresis and Capillary Electrochromatography of Organic Pollutants. Electrophoresis 1999, 20, 3297–3310. [Google Scholar] [CrossRef]
- Könnel, E.J.; Di Nonno, S.; Ulber, R. Low-Cost and Easy-to-Use: A Portable Photometer for Simple and Comprehensive Analysis of Critical Water Quality Parameters. Microchem. J. 2025, 214, 113946. [Google Scholar] [CrossRef]
- Saavedra-Ruiz, A.; Resto-Irizarry, P.J. A Portable UV-LED/RGB Sensor for Real-Time Bacteriological Water Quality Monitoring Using ML-Based MPN Estimation. Biosensors 2025, 15, 284. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Dai, B.; Xue, Z.; Kang, Y.; Liu, S.; Hou, L.; Zhuang, S.; Zhang, D. Integrated System for Rapid Enrichment and Detection of Airborne Polycyclic Aromatic Hydrocarbons. Sci. Total Environ. 2023, 864, 161057. [Google Scholar] [CrossRef]
- Munawar, H.; Mankar, J.S.; Sharma, M.D.; Garcia-Cruz, A.; Fernandes, L.A.L.; Peacock, M.; Krupadam, R.J. Highly Selective Electrochemical Nanofilm Sensor for Detection of Carcinogenic PAHs in Environmental Samples. Talanta 2020, 219, 121273. [Google Scholar] [CrossRef]
- Barathi, S.; Gitanjali, J.; Rathinasamy, G.; Sabapathi, N.; Aruljothi, K.N.; Lee, J.; Kandasamy, S. Recent Trends in Polycyclic Aromatic Hydrocarbons Pollution Distribution and Counteracting Bio-Remediation Strategies. Chemosphere 2023, 337, 139396. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Comfort, S.; Chokejaroenrat, C.; Harris, C.; Li, X. A Combined Chemical and Biological Approach to Transforming and Mineralizing PAHs in Runoff Water. Chemosphere 2014, 117, 1–9. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Li, S.; Zhu, G. Air-Assisted Liquid-Liquid Microextraction Based on the Solidification of Floating Deep Eutectic Solvents for the Simultaneous Determination of Bisphenols and Polycyclic Aromatic Hydrocarbons in Tea Infusions via HPLC. Food Chem. 2021, 348, 129106. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Huang, C.-P.; Lam, S.S.; Chen, C.-W.; Dong, C.-D. The Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Marine Sediments Using Persulfate over a Nano-Sized Iron Composite of Magnetite and Carbon Black Activator. J. Environ. Chem. Eng. 2020, 8, 104440. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Song, X.; Zeng, G.; Lee, K. Photocatalytic Ozonation of Offshore Produced Water by TiO2 Nanotube Arrays Coupled with UV-LED Irradiation. J. Hazard. Mater. 2021, 402, 123456. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Wang, L.; Zhao, J.; Song, T.; Chu, M. Removal of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by a Microbial Consortium Immobilized in Magnetic Floating Biochar Gel Beads. Mar. Pollut. Bull. 2020, 159, 111489. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Chen, B.; Zhang, B.; Zheng, J.; Liu, B. Naphthalene Degradation in Seawater by UV Irradiation: The Effects of Fluence Rate, Salinity, Temperature and Initial Concentration. Mar. Pollut. Bull. 2014, 81, 149–156. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Dong, J.; Zhao, J.; Gong, X.; Liu, S. Preparation of Biochar from Enteromorpha Prolifera and Its Use for the Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Aqueous Solution. Ecotoxicol. Environ. Saf. 2018, 149, 80–87. [Google Scholar] [CrossRef]
- Qi, Y.-B.; Wang, C.-Y.; Lv, C.-Y.; Lun, Z.-M.; Zheng, C.-G. Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. Int. J. Environ. Res. Public Health 2017, 14, 215. [Google Scholar] [CrossRef]
- Mortazavi, M.; Baghdadi, M.; Javadi, N.H.S.; Torabian, A. The Black Beads Produced by Simultaneous Thermal Reducing and Chemical Bonding of Graphene Oxide on the Surface of Amino-Functionalized Sand Particles: Application for PAHs Removal from Contaminated Waters. J. Water Process Eng. 2019, 31, 100798. [Google Scholar] [CrossRef]
- Lai, X.; Ning, X.; Zhang, Y.; Li, Y.; Li, R.; Chen, J.; Wu, S. Treatment of Simulated Textile Sludge Using the Fenton/Cl− System: The Roles of Chlorine Radicals and Superoxide Anions on PAHs Removal. Environ. Res. 2021, 197, 110997. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Wick, L.Y.; Duran, R. Fungi in PAH-Contaminated Marine Sediments: Cultivable Diversity and Tolerance Capacity towards PAH. Mar. Pollut. Bull. 2021, 164, 112082. [Google Scholar] [CrossRef]
- Sbani, N.H.A.L.; Abdullah, S.R.S.; Idris, M.; Hasan, H.A.; Halmi, M.I.E.; Jehawi, O.H.; Ismail, N. PAH-Degrading Rhizobacteria of Lepironia Articulata for Phytoremediation Enhancement. J. Water Process Eng. 2021, 39, 101688. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhang, D.; Feng, T.; Zhang, L. Nitrate-Assisted Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in the Water-Level-Fluctuation Zone of the Three Gorges Reservoir, China: Insights from in Situ Microbial Interaction Analyses and a Microcosmic Experiment. Environ. Pollut. 2021, 268, 115693. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Y.; Wang, X.; Wei, C. Efficient Adsorption of Phenanthrene by Simply Synthesized Hydrophobic MCM-41 Molecular Sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Chowdhury, Z.Z.; Shemsi, A.M.; Saleh, T.A. Effect of the Oxidation Process on the Molecular Interaction of Polyaromatic Hydrocarbons (PAH) with Carbon Nanotubes: Adsorption Kinetic and Isotherm Study. J. Mol. Liq. 2019, 289, 111107. [Google Scholar] [CrossRef]
- Ates, H.; Argun, M.E. Fate of PAHs under Subcritical and Supercritical Conditions in Landfill Leachate: Removal or Formation? Chem. Eng. J. 2021, 414, 128762. [Google Scholar] [CrossRef]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Investigating the Degradation of NC12 to NC23 Alkanes and PAHs in Petroleum- Contaminated Water by Electrochemical Advanced Oxidation Process Using an Inexpensive Ti/Sb-SnO2/PbO2 Anode. Chem. Eng. J. 2021, 404, 125268. [Google Scholar] [CrossRef]
- Sher, S.; Waseem, M.; Leta, M.K. Review of techniques for the removal of polycyclic aromatic hydrocarbons from produced water. Environments 2023, 10, 40. [Google Scholar] [CrossRef]






| Sample Condition * | Compound | Theoretical (µg/L) | GC-MS (µg/L) | REMPI-eMIMS (µg/L) |
|---|---|---|---|---|
| Stored—Sample 1 | Naphthalene | 2.91 | 3.8 | 3.9 |
| Stored—Sample 2 | Naphthalene | 5.82 | 4.6 | 4.8 |
| Fresh—Rep 1 | Naphthalene | 5.82 | 4.7 | 5.1 |
| Stored—Sample 1 | Phenanthrene | 2.37 | 1.3 | 1.4 |
| Stored—Sample 2 | Phenanthrene | 4.74 | 1.5 | 1.6 |
| Fresh—Rep 1 | Phenanthrene | 4.74 | 1.7 | 1.9 |
| Pollutant | Sensing Material | Technique | LOD (nM) | Detection Range | Ref. |
|---|---|---|---|---|---|
| Naphthalene | 2D BP:Ti3C2Tx | LSV | 1.6 | 0.02–40 µM | [101] |
| BPA | PtNPs/Ti3C2Tx | CV | 32 | 50 nM–5 µM | [102] |
| 4-NP | Nb2CTx/Zn–Co–NC | DPV | 70 | 1–500 µM | [103] |
| 4-NP | Cu–curcumin | DPV | 68.2 | 0.1–1030 µM | [104] |
| HQ | MWCNTs–Ti3C2 | DPV | 6.6 | 2–150 µM | [105] |
| CT | N–Co–Fe–HCS | DPV | 75 | 0.5–500 µM | [106] |
| HQ | CT | DPV | 80 | 0.5–1500 µM | |
| Naproxen | Ni–Co LDHs | DPV | 2 | 0.01–435 µM | [107] |
| Pollutant | Sensing Material | Technique | LOD (nM) | Detection Range | Ref. |
|---|---|---|---|---|---|
| BPA | Tyrs-rGO/Mn3O4/ITO | CV | 10 | 0.05–100 µM | [110] |
| PHE | GCE–PANI–NiO | CV | 0.732 (pM) | 7.6–14 µM | [111] |
| 2-NP | ZnO/RuO2 | SWV | 52.2 (pM) | 0.1 nM–0.01 mM | [112] |
| Catechin | Pv/MnO2/f-MWCNT/GCE | SWV | 2 | 2–950 µM | [113] |
| BPA | ZnO/CNT/IL | SWV | 9 | 0.002–700 µM | [114] |
| BPA | TiO2/AuNTAS | Amperometry | 6.2 | 100 nM–38.9 µM | [115] |
| CT | Ag–TiO2 electrode | Amperometry | 24.9 | 1–15 µM | [116] |
| ANT | ARS–SBA15/GCE | DPV | 0.5 (pM) | 1 pM–10 nM | [117] |
| Acetaminophen | CuO–Gr/CPE | DPV | 8 | 0.025–5.3 µM | [118] |
| Eugenol | CoO/ZnO/GCE | DPV | 4 | 0.049–179.8 µM | [119] |
| Sensor | Detection Method | PAH Analyte | LOD | Matrix | Ref. |
|---|---|---|---|---|---|
| Cd/Al-LDHS/GCE | DPV | Anthracene | 0.5 × 10−15 mol/L | Electrolyte solution | [125] |
| Fe3O4–Calix[4]arene @ CdSe | SWV | Anthracene | 0.11 × 10−6 mol/L | Tap water | [67] |
| Fe3O4–Calix[4]arene @ CdSe | SWV | Naphthalene | 4.29 × 10−6 mol/L | Tap water | [67] |
| ARS-SBA15/GCE | DPV | Anthracene | 0.5 × 10−12 mol/L | Wastewater | [117] |
| AQS/PDDA/ITO | CV | Phenanthrene | 0.50 × 10−12 mol/L | Cloud water | [126] |
| AQS/PDDA/ITO | CV | Phenanthrene | - | Rain water | [126] |
| Au(G3PPT-co-P3HT) | PSACV | Anthracene | 2.62 × 10−9 mol/L | Oil-polluted wastewater | [127] |
| Au(G3PPT-co-P3HT) | ACV | Phenanthrene | 1.42 × 10−9 mol/L | Oil-polluted wastewater | [127] |
| Au(G3PPT-co-P3HT) | CV | Phenanthrene | 12.62 × 10−9 mol/L | Tap water | [128] |
| PAA/GO/SPCE | SWV | Anthracene | 6.7 × 10−7 mol/L | Electrolyte solution | [129] |
| GO/SPCE | SWV | - | 7.42 × 10−7 mol/L | Electrolyte solution | [128,130] |
| ITO/PAA films | LSV | Anthracene | 3.79 × 10−6 mol/L | Electrolyte solution | [130] |
| Carbon-rich monolayer on ITO | EIS | Pyrene | - | Water | [80] |
| Affinity Agent Type | Sensor/Substrate | Analyte | LOD | Matrix | Ref. |
|---|---|---|---|---|---|
| Macromolecule | b-CD dimer@Ag@SiO2 NPs | Perylene | 0.1 × 10−6 mol/L | DCM | [135] |
| Macromolecule | b-CD-AgNPs | Anthracene | 10 × 10−6 mol/L | Water | [136] |
| Pyrene | 7.5 × 10−6 mol/L | ||||
| Macromolecule | b-CD-SH-AuNPs/PGMA-b-CD | Pyrene | 0.8 × 10−9 mol/L | - | [137] |
| Anthracene | 2.4 × 10−9 mol/L | ||||
| Macromolecule | GNPS-DSNB | Benzo[a]pyrene | 2 × 10−9 mol/L | Sea water | [138] |
| Macromolecule | AuNP–alginate gel network | Benzo[a]pyrene | 0.485 × 10−9 mol/L | River, spring, tap water | [139] |
| Macromolecule | AuNPs-DMCX | Pyrene | 0.5 × 10−9 mol/L | Artificial sea water | [140] |
| Macromolecule | AuNPs-DMCX | Anthracene | 0.5 × 10−9 mol/L | Artificial sea water | [140] |
| Polymers | AuNPs-GMA-EDMA | Anthracene | 0.93 × 10−7 mol/L | Water | [141] |
| Polymers | AuNPs-GMA-EDMA | Phenanthrene | 4.5 × 10−7 mol/L | Water | [141] |
| Pyrene | 1.1 × 10−7 mol/L | ||||
| Polymers | pNIPAM-coated nanostars | Pyrene | - | Gas phase | [142] |
| Polymers | AgNO3-PVP dendrites | Fluoranthene | 0.45 × 10−9 mol/L | - | [143] |
| Polymers | IP6-AuNPs | Benzo[a]pyrene | 1 mg/L | EtOH | [144] |
| Polymers | Hydroxylamine-reduced AgNPs | Naphthalene | 1 × 10−12 mol/L | Water | [145] |
| Phenanthrene | 0.1 × 10−9 mol/L | ||||
| Ligands | Citrate–AuNPs | Benzo[a]pyrene | 0.5 × 10−6 mol/L | River water | [146] |
| Benzo[g,h,i]perylene | 0.25 × 10−6 mol/L | ||||
| Ligands | AgNPs—LG | Anthracene | 1 × 10−6 mol/L | - | [147] |
| Benzo[c]phenanthrene | 1 × 10−7 mol/L | ||||
| Ligands | GNS-DS-C10H21 | Benzo[a]pyrene | 0.1 × 10−6 mol/L | Water–MeOH | [148] |
| Fluoranthene | 0.32 × 10−6 mol/L | ||||
| Naphthalene | 31 × 10−6 mol/L | ||||
| Ligands | HAs-AgNPs | Fluoranthene | 1.3 × 10−7 mol/L | Acetone | [149] |
| 3,4-Benzopyrene | 1.3 × 10−7 mol/L | ||||
| Magnetic NPs | Fe3O4@Ag Fe3O4@AuNR assemblies Fe3O4@Au core–satellite MNPs | Perylene | 0.8 × 10−6 mol/L | - | [150] |
| Benzo[a]pyrene | 0.8 × 10−6 mol/L | ||||
| Pyrene | 1 × 10−6 mol/L | ||||
| Anthracene | 5 × 10−6 mol/L | ||||
| Phenanthrene | 20 × 10−6 mol/L |
| Technique | Cost (USD) | Response Time | Environmental Robustness | Accuracy/Sensitivity | Sample Preparation | Ref. |
|---|---|---|---|---|---|---|
| Portable GC-MS | 150,000–300,000 | ~10 min | Moderate (field-usable but limited high-temperature resilience) | High sensitivity (sub-ppb), lab-grade separation | Requires solid/liquid/vapor sampling (e.g., SPME, probes) | [169] |
| Portable NMR | ~100,000 | Tens of minutes to hours | Low (sensitive to EMI, temperature, humidity) | Low sensitivity for airborne ppb-level pollutants | Requires liquid samples; not suited for gas-phase analysis | [170] |
| Portable CE | ~50,000–100,000 (estimated) | Minutes to tens of minutes | Low (not stable in rugged environments) | Moderate in clean aqueous matrices; poor for complex smoke mixtures | Requires precise injection; clean aqueous samples; capillary conditioning | [181] |
| Fluorescence/UV | Few thousands–tens of thousands | Seconds to a few minutes | Low–moderate (optical interference; some ruggedized models exist) | Moderate (ppm–ppb in clear matrices); low specificity in smoky air | Minimal preparation; requires filtered/clear presentation to reduce light scattering | [182] |
| Phase | Strategy |
|---|---|
| Short-Term (1–2 years) | Translate validated lab sensors (electrochemical, SERS, aptamer-based) into handheld field devices |
| Optimize sensors for aqueous-phase samples (e.g., rinse water, saliva) | |
| Integrate modular sample preparation units (e.g., SPE, microfluidics) | |
| Develop environmental protection features (optical shielding, thermal insulation, calibration protocols) | |
| Long-Term (3–7 years) | Develop gas-phase PAH sensors with aerosol pre-concentration and capture membranes |
| Embed sensors into wearable firefighter gear (helmets, jackets, masks) | |
| Enable wireless data transmission to centralized monitoring systems | |
| Advance biosensors for multi-analyte detection and regenerative capabilities | |
| Integrate with occupational health surveillance platforms for real-time exposure tracking |
| PAH | Method | Efficiency of Removal | Sample | Ref. |
|---|---|---|---|---|
| Naphthalene | UV–Vis | 62% | Sea water | [189] |
| Pyrene and benzo[a]pyrene | Adsorption | 40% and 48% | Synthetic wastewater | [190] |
| 16 PAHs | Photocatalyst ozonation and UV | 57% | Offshore-produced water | [187] |
| Naphthalene, phenanthrene, anthracene | Bioremediation | 100%, 95.4%, 73.8% | Oilfield-produced water | [191] |
| Naphthalene and acenaphthene | Adsorption | 100% to 97% | Water treatment plant | [192] |
| Anthracene, phenanthrene | Fenton process | 85.47%, 63.16% | Textile dying sludge | [193] |
| 16 PAHs | Phytoremediation | 89% | Wastewater | [194] |
| 16 PAHs | Biodegradation | 67.27% | River | [195] |
| Pyrene, benzo[a]pyrene | Magnetic floatation | 89.9%, 66.9% | Sea water | [196] |
| Phenanthrene, naphthalene | Oxidation | 90.1%, 97.5% | Soil | [188] |
| Naphthalene, phenanthrene | Air-assisted liquid–liquid microextraction | 82.0% to 116.6% | Water | [185] |
| Phenanthrene | Adsorption | 90% | Wastewater | [197] |
| Naphthalene and fluorene | Oxidation adsorption | 92% to 100% | Produced water | [198] |
| Pyrene, fluoranthene, chrysene | Precipitation method | 99%, 98%, 87% | Marine sediments | [186] |
| Naphthalene, anthracene | Oxidation | 97%, 95% | Landfill leachate | [199] |
| 16 PAHs | Electrochemical advanced oxidation | 99.9% | Petroleum-contaminated water | [200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafar-Zadeh, M.; Biyouki, A.A.; Heidari, N.; Delfan, N.; Norouzi, P.; Magierowski, S.; Ghafar-Zadeh, E. Protecting Firefighters from Carcinogenic Exposure: Emerging Tools for PAH Detection and Decontamination. Biosensors 2025, 15, 547. https://doi.org/10.3390/bios15080547
Ghafar-Zadeh M, Biyouki AA, Heidari N, Delfan N, Norouzi P, Magierowski S, Ghafar-Zadeh E. Protecting Firefighters from Carcinogenic Exposure: Emerging Tools for PAH Detection and Decontamination. Biosensors. 2025; 15(8):547. https://doi.org/10.3390/bios15080547
Chicago/Turabian StyleGhafar-Zadeh, Morteza, Azadeh Amrollahi Biyouki, Negar Heidari, Niloufar Delfan, Parviz Norouzi, Sebastian Magierowski, and Ebrahim Ghafar-Zadeh. 2025. "Protecting Firefighters from Carcinogenic Exposure: Emerging Tools for PAH Detection and Decontamination" Biosensors 15, no. 8: 547. https://doi.org/10.3390/bios15080547
APA StyleGhafar-Zadeh, M., Biyouki, A. A., Heidari, N., Delfan, N., Norouzi, P., Magierowski, S., & Ghafar-Zadeh, E. (2025). Protecting Firefighters from Carcinogenic Exposure: Emerging Tools for PAH Detection and Decontamination. Biosensors, 15(8), 547. https://doi.org/10.3390/bios15080547






