Ti3C2TX MXene/Polyaniline-Modified Nylon Fabric Electrode for Wearable Non-Invasive Glucose Monitoring in Sweat
Abstract
1. Introduction
2. Materials and Methods
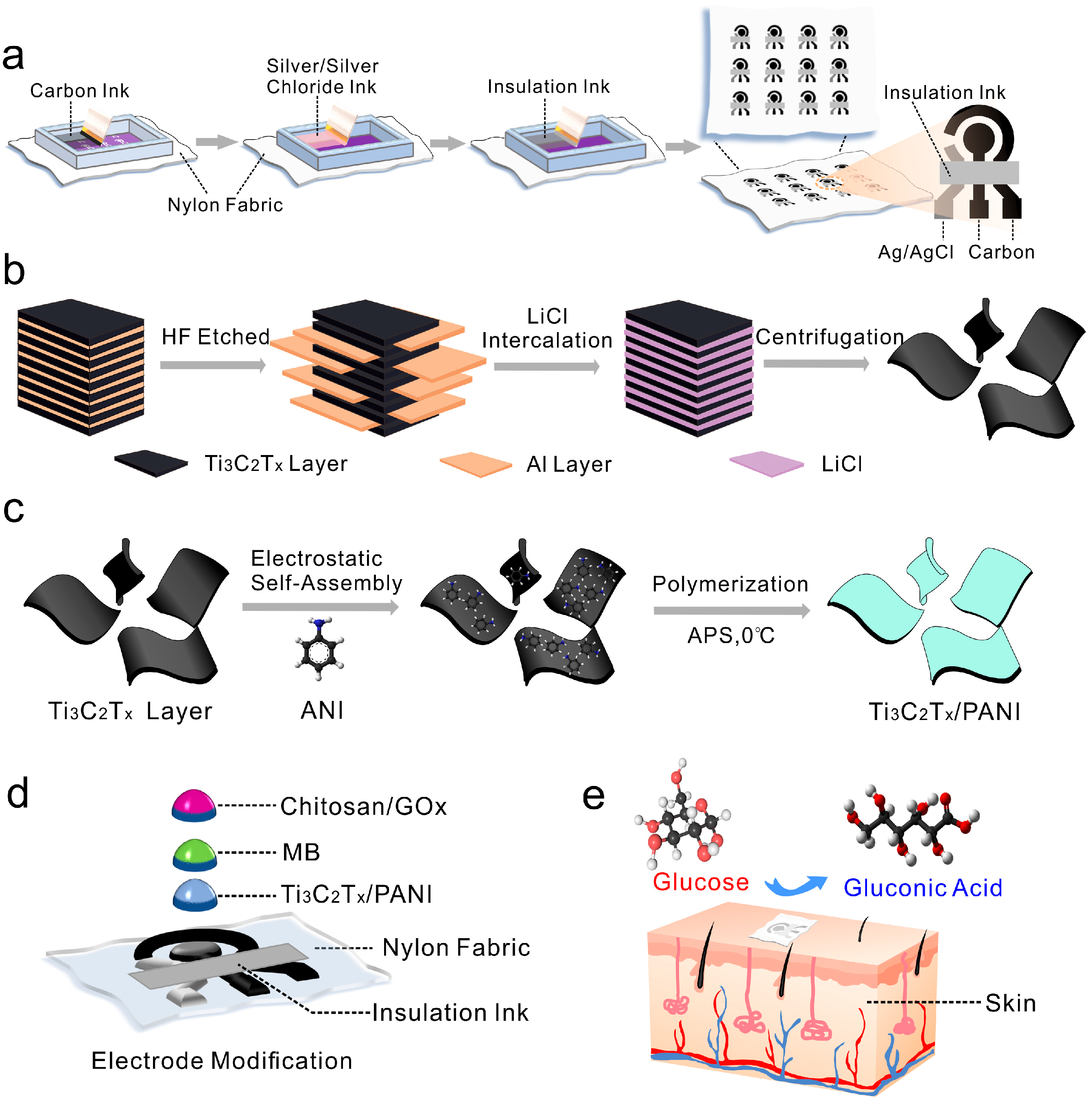
2.1. Synthesis of Ti3C2TX Dispersion
2.2. Synthesis of Ti3C2TX/PANI Nanocomposites
2.3. Electrochemical Measurement
3. Results
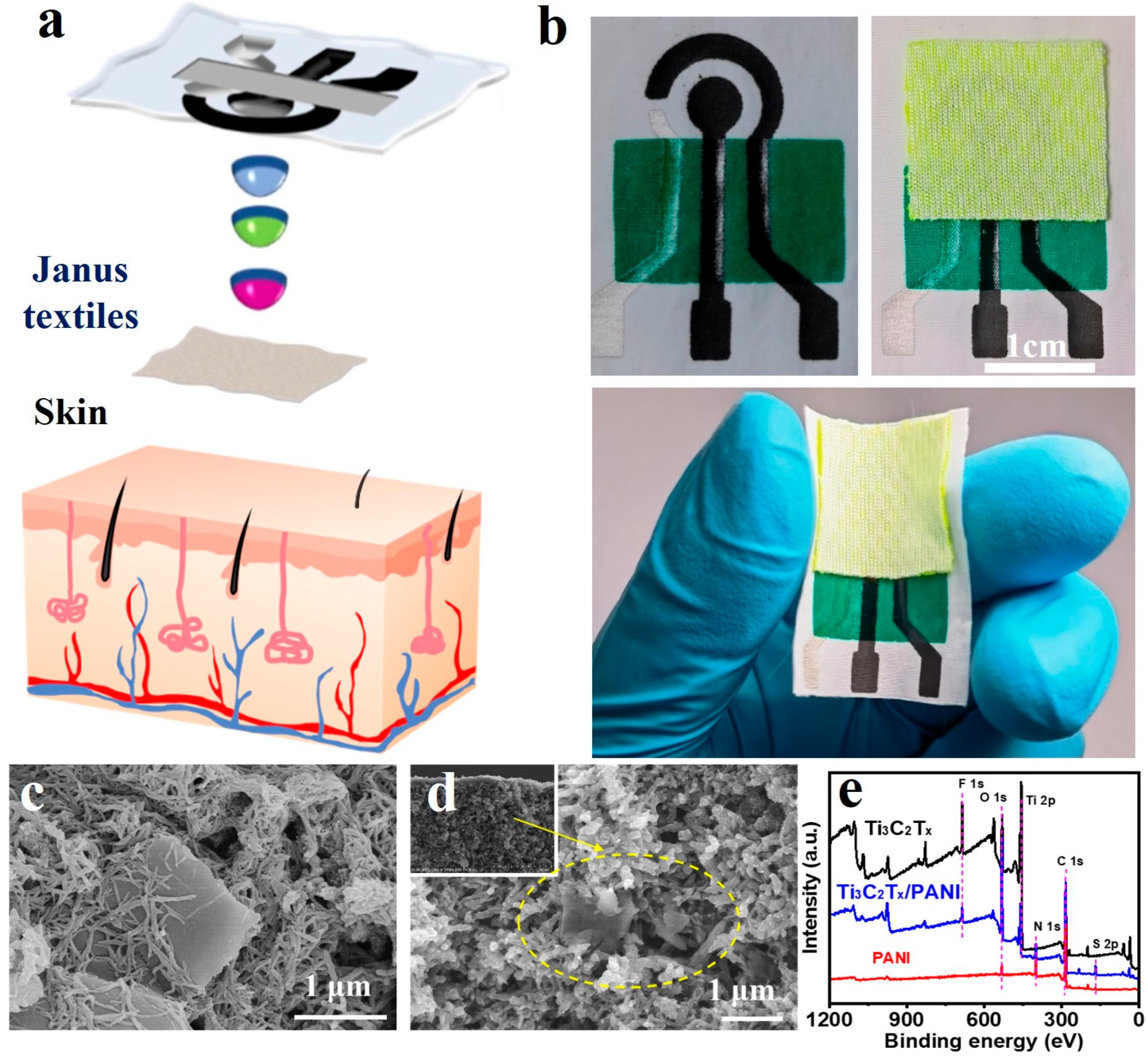
3.1. Characterization of Ti3C2TX/PANI Electrode Modification Materials
3.2. Electrochemical Performance Testing of Bare Electrodes for Fabric-Based Sensors
3.3. Electrochemical Performance Characterization of Ti3C2TX/PANI Modified Electrode
3.4. Characterization of Glucose Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPCE | Screen-printed carbon electrodes |
| GOx | Glucose oxidase |
| MB | Methylene blue |
| APS | Ammonium persulfate |
| ANI | Aniline |
| PANI | Polyaniline |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
References
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Jarnda, K.V.; Dai, H.; Ali, A.; Bestman, P.L.; Trafialek, J.; Roberts-Jarnda, G.P.; Anaman, R.; Kamara, M.G.; Wu, P.; Ding, P. A Review on Optical Biosensors for Monitoring of Uric Acid and Blood Glucose Using Portable POCT Devices: Status, Challenges, and Future Horizons. Biosensors 2025, 15, 222. [Google Scholar] [CrossRef]
- Ma, K.; Ma, L.; Li, C.; Zhu, R.; Yang, J.; Liu, S.; Tao, X. Textile-Based Mechanoreceptor Array with Tunable Pressure Thresholds for Mutli-dimensional Detection in Healthcare Monitoring. Adv. Fiber Mater. 2025, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.; Shin, J.; Park, J.; Lee, H.; Kim, D.; Liu, H. Engineering Materials for Electrochemical Sweat Sensing. Adv. Funct. Mater. 2020, 31, 2008130. [Google Scholar] [CrossRef]
- Xiao, G.; He, J.; Qiao, Y.; Wang, F.; Xia, Q.; Wang, X.; Yu, L.; Lu, Z.; Li, C.-M. Facile and Low-Cost Fabrication of a Thread/Paper-Based Wearable System for Simultaneous Detection of Lactate and pH in Human Sweat. Adv. Fiber Mater. 2020, 2, 265–278. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, X.; Zhan, W.; Fu, M.; Liu, J.; He, J.; Li, Y.; Li, Y.; Chen, X.; Yu, C. Skin-Interfaced Wearable Sensor for Long-Term Reliable Monitoring of Uric Acid and pH in Sweat. Nano Lett. 2025, 25, 1427–1435. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wang, J. Multi-Mode/Signal Biosensors: Electrochemical Integrated Sensing Techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Ying, Z.; Qiao, L.; Liu, B.; Gao, L.; Zhang, P. Development of a microfluidic wearable electrochemical sensor for the non-invasive monitoring of oxidative stress biomarkers in human sweat. Biosens. Bioelectron. 2024, 261, 116502. [Google Scholar] [CrossRef]
- Tian, H.; Ma, J.; Li, Y.; Xiao, X.; Zhang, M.; Wang, H.; Zhu, N.; Hou, C.; Ulstrup, J. Electrochemical sensing fibers for wearable health monitoring devices. Biosens. Bioelectron. 2023, 246, 115890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Z.; Wu, Y.; Bai, X.; Li, Y.; Yang, W.; Liu, Y.; Li, R.-W. A Wearable Molecularly Imprinted Electrochemical Sensor for Cortisol Stable Monitoring in Sweat. Biosensors 2025, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shi, H.; Yi, C.; Zheng, Y.; Tan, Z.; Jia, X.; Liu, Z. Recent progress of non-invasive in vitro diagnosis using electrochemical analysis strategy and wearable microfluidic devices applied to exocrine secretion sampling. TrAC Trends Anal. Chem. 2024, 172, 117561. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, Y.; Pan, J.; Li, S.; Sun, X.; Zhang, B.; Peng, H. Weaving Sensing Fibers into Electrochemical Fabric for Real-Time Health Monitoring. Adv. Funct. Mater. 2018, 28, 1804456. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.; Li, J.; Liu, L.; Yu, J.; Li, Z.; Ding, B. Wearable biosensor for sensitive detection of uric acid in artificial sweat enabled by a fiber structured sensing interface. Nano Energy 2021, 85, 106031. [Google Scholar] [CrossRef]
- Xie, M.; Yao, G.; Gan, X.; Zhang, C.; Zhang, T.; Wang, Q.; Li, X.; Zhou, C.; Zhao, K.; Gao, M.; et al. Non-Enzyme, Temperature Calibrating, and Bioactive Fiber-based Flexible Sensors for Dopamine and Lactic Acid Detection. Adv. Fiber Mater. 2024, 6, 501–511. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Li, Q.; Li, L.; He, E.; Jiao, Y.; Ye, T.; Zhang, Y. A Core–Sheath Sensing Yarn-Based Electrochemical Fabric System for Powerful Sweat Capture and Stable Sensing. Adv. Funct. Mater. 2022, 32, 2200922. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, J.; Wu, Y.; Zhang, Y.; Zhou, J.; Zhang, Y.; Huang, Q.; Zheng, Z. Porous Conductive Textiles for Wearable Electronics. Chem. Rev. 2024, 124, 1535–1648. [Google Scholar] [CrossRef]
- Li, D.; Liu, W.; Peng, T.; Liu, Y.; Zhong, L.; Wang, X. Janus Textile: Advancing Wearable Technology for Autonomous Sweat Management and Beyond. Small 2025, 21, e2409730. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, S.; Liao, C.; Du, C.; Yao, H.; Li, Y.; Zhang, Y.; Stachewicz, U.; Liu, Y. A wearable 3D nanostructured Ni-MOF electrochemical sensor integrated with Janus fabric for sweat collecting and nutrients detecting. Talanta 2025, 296, 128474. [Google Scholar] [CrossRef]
- Ren, J.; Li, Q.; Feng, K.; Gong, J.; Li, Z.; Liu, X.; Yang, L.; Zhang, J. A wearable sensor based on janus fabric upon an electrochemical analysis platform for sweat glucose detection. Talanta 2024, 284, 127236. [Google Scholar] [CrossRef]
- Tong, X.; Hua, T.; Xu, M.; Yang, D.; Xiao, G.; Li, S.; Cao, X.; Shao, Y. An Energy-Autonomous Wearable Fabric Powered by High-Power Density Sweat-Activated Batteries for Health Monitoring. Adv. Fiber Mater. 2024, 7, 254–265. [Google Scholar] [CrossRef]
- Qi, Y.; Xia, Y.; Li, P.; Wang, Z.; Ming, X.; Wang, B.; Shen, K.; Cai, G.; Li, K.; Gao, Y.; et al. Plastic-Swelling Preparation of Functional Graphene Aerogel Fiber Textiles. Adv. Fiber Mater. 2023, 5, 2016–2027. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, Y.; Zhao, J.; Hou, J.; Hou, C.; Huo, D. A sensitive electrochemical sensor based on 3D porous melamine-doped rGO/MXene composite aerogel for the detection of heavy metal ions in the environment. Talanta 2023, 256, 124294. [Google Scholar] [CrossRef]
- Cardoso, A.G.; Viltres, H.; Ortega, G.A.; Phung, V.; Grewal, R.; Mozaffari, H.; Ahmed, S.R.; Rajabzadeh, A.R.; Srinivasan, S. Electrochemical sensing of analytes in saliva: Challenges, progress, and perspectives. TrAC Trends Anal. Chem. 2023, 160, 116965. [Google Scholar] [CrossRef]
- Yuan, F.; Xia, Y.; Lu, Q.; Xu, Q.; Shu, Y.; Hu, X. Recent advances in inorganic functional nanomaterials based flexible electrochemical sensors. Talanta 2022, 244, 123419. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-L.; Zhang, Y.; Liu, H.; Gao, C. Flexible Wearable Electrochemical Sensors Based on AuNR/PEDOT:PSS for Simultaneous Monitoring of Levodopa and Uric Acid in Sweat. ACS Sens. 2024, 9, 3296–3306. [Google Scholar] [CrossRef]
- Cai, X.; Xia, R.-Z.; Liu, Z.-H.; Dai, H.-H.; Zhao, Y.-H.; Chen, S.-H.; Yang, M.; Li, P.-H.; Huang, X.-J. Fully Integrated Multiplexed Wristwatch for Real-Time Monitoring of Electrolyte Ions in Sweat. ACS Nano 2024, 18, 12808–12819. [Google Scholar] [CrossRef]
- Qiao, X.; Cai, Y.; Kong, Z.; Xu, Z.; Luo, X. A Wearable Electrochemical Sensor Based on Anti-Fouling and Self-Healing Polypeptide Complex Hydrogels for Sweat Monitoring. ACS Sens. 2023, 8, 2834–2842. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, W.; Zeng, J.; He, Z.; Wang, X.; Zhu, Z.; Hu, R.; Liu, C.; Wang, Q. Wearable non-invasive glucose sensors based on metallic nanomaterials. Mater. Today Bio 2023, 20, 100638. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.K.; Batcha, M.I.K.; Mahalingam, S.; Raj, B.; Kim, J. Recent Advances in Two-Dimensional Nanomaterials for Healthcare Monitoring. ACS Sens. 2024, 9, 1706–1734. [Google Scholar] [CrossRef]
- Lin, X.; Song, D.; Shao, T.; Xue, T.; Hu, W.; Jiang, W.; Zou, X.; Liu, N. A Multifunctional Biosensor via MXene Assisted by Conductive Metal–Organic Framework for Healthcare Monitoring. Adv. Funct. Mater. 2023, 34, 2311637. [Google Scholar] [CrossRef]
- Chen, R.; Jia, X.; Zhou, H.; Ren, S.; Han, D.; Li, S.; Gao, Z. Applications of MXenes in wearable sensing: Advances, challenges, and prospects. Mater. Today 2024, 75, 359–385. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Chen, L.; Lin, H.; Han, D.; Bao, Y.; Wang, W.; Niu, L. A Wearable Electrochemical Biosensor Utilizing Functionalized Ti3C2Tx MXene for the Real-Time Monitoring of Uric Acid Metabolite. Anal. Chem. 2024, 96, 3914–3924. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef] [PubMed]
- Iakunkov, A.; Lienert, U.; Sun, J.; Talyzin, A.V. Swelling of Ti3C2Tx MXene in Water and Methanol at Extreme Pressure Conditions. Adv. Sci. 2023, 11, e2307067. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, D.; Liu, F.; Li, W.; Lin, J. Synthesis of an MXene/polyaniline composite with excellent electrochemical properties. J. Mater. Chem. A 2020, 8, 5853–5858. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Chen, C.; Liu, L.; Lu, Z. Multifunctional enhanced energy density of flexible wide-temperature supercapacitors based on MXene/PANI conductive hydrogel. Chem. Eng. J. 2024, 485, 149951. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Q.; Xu, S.; Li, X.; Liu, C.; Ni, L.; Chen, N.; Lu, C.; Wang, X.; Peng, L.; et al. Ultrahigh rate capability of 1D/2D polyaniline/titanium carbide (MXene) nanohybrid for advanced asymmetric supercapacitors. Nano Res. 2021, 15, 285–295. [Google Scholar] [CrossRef]
- Boeva, Z.; Mousavi, Z.; Sokalski, T.; Bobacka, J. Recent trends in non-invasive on-body chemical sensing. TrAC Trends Anal. Chem. 2024, 172, 117542. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wei, W.; Wang, L.; Han, W. High-performance flexible sensing devices based on polyaniline/MXene nanocomposites. InfoMat 2019, 1, 407–416. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Moncada, J.; Chen, H.; Kayali, E.; Orangi, J.; Carrero, C.A.; Beidaghi, M. Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J. Mater. Chem. A 2018, 6, 22123–22133. [Google Scholar] [CrossRef]
- Li, J.; Levitt, A.; Kurra, N.; Juan, K.; Noriega, N.; Xiao, X.; Wang, X.; Wang, H.; Alshareef, H.N.; Gogotsi, Y. MXene-conducting polymer electrochromic microsupercapacitors. Energy Storage Mater. 2019, 20, 455–461. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, L.; Guan, F.; Gao, Y.; Hedin, N.E.; Zhu, L.; Fong, H. Crystalline Morphology and Polymorphic Phase Transitions in Electrospun Nylon-6 Nanofibers. Macromolecules 2007, 40, 6283–6290. [Google Scholar] [CrossRef]
- Shakiba, M.; Ghomi, E.R.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Wu, C.; Zhang, X.; Jiang, Z.; Liu, S.; Li, N. Plasma-promoted surface regulation of a novel integrative carbon network for boosting the long-cycle capability of sodium-ion storage. Carbon 2022, 191, 112–121. [Google Scholar] [CrossRef]
- Yuan, F.; Lei, Y.; Wang, H.; Li, X.; Hu, J.; Wei, Y.; Zhao, R.; Li, B.; Kang, F.; Zhai, D. Pseudo-capacitance reinforced modified graphite for fast-charging potassium-ion batteries. Carbon 2021, 185, 48–56. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Li, M.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. MXene-Coated Air-Permeable Pressure-Sensing Fabric for Smart Wear. ACS Appl. Mater. Interfaces 2020, 12, 46446–46454. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tan, Y.; Li, Y. Conducting polyaniline nanofiber networks prepared by the doping induction of camphor sulfonic acid. J. Appl. Polym. Sci. 2002, 87, 1537–1540. [Google Scholar] [CrossRef]
- Fu, J.; Yun, J.; Wu, S.; Li, L.; Yu, L.; Kim, K.H. Architecturally Robust Graphene-Encapsulated MXene Ti2CTx@Polyaniline Composite for High-Performance Pouch-Type Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 34212–34221. [Google Scholar] [CrossRef]
- Liao, P.; Geng, Z.; Zhang, X.; Yan, W.; Qiu, Z.; Xu, H. High-performance Ti3C2Tx achieved by polyaniline intercalation and gelatinization as a high-energy cathode for zinc-ion capacitor. Nano Res. 2024, 17, 5305–5316. [Google Scholar] [CrossRef]
- Hou, Z.; Jiang, H.; Guo, Y.; Huang, K.; Zhao, F.; Xu, Y.; Peng, P.; Zou, S.; Yan, J.; Zhang, J. Enhancing acidic hydrogen evolution through pyrrolic nitrogen-doped reduced graphene oxide triggering two-electron oxygen reduction. Inorg. Chem. Front. 2024, 11, 4318–4328. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Rekertaitė, A.I.; Valiūnas, R.; Valiūnienė, A. Single-step procedure for the modification of graphite electrode by composite layer based on polypyrrole, Prussian blue and glucose oxidase. Sens. Actuators B Chem. 2017, 240, 220–223. [Google Scholar] [CrossRef]
- Li, J.; Koinkar, P.; Fuchiwaki, Y.; Yasuzawa, M. A fine pointed glucose oxidase immobilized electrode for low-invasive amperometric glucose monitoring. Biosens. Bioelectron. 2016, 86, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Shamili, C.; Pillai, A.S.; Saisree, S.; Chandran, A.; Varma, M.R.; Peethambharan, S.K. All-printed wearable biosensor based on MWCNT-iron oxide nanocomposite ink for physiological level detection of glucose in human sweat. Biosens. Bioelectron. 2024, 258, 116358. [Google Scholar] [CrossRef]
- Khumngern, S.; Nontipichet, N.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Smartphone-enabled flow injection amperometric glucose monitoring based on a screen-printed carbon electrode modified with PEDOT@PB and a GOx@PPtNPs@MWCNTs nanocomposite. Talanta 2024, 277, 126336. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef] [PubMed]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly(3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef]
- Pan, Y.; He, M.; Wu, J.; Qi, H.; Cheng, Y. One-step synthesis of MXene-functionalized PEDOT:PSS conductive polymer hydrogels for wearable and noninvasive monitoring of sweat glucose. Sens. Actuators B Chem. 2023, 401, 135055. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Wang, R.; Li, M.; Liu, W.; Li, C.; Chen, X.; Lu, Q.; Chang, Y. Wearable biosensors for human sweat glucose detection based on carbon black nanoparticles. Anal. Bioanal. Chem. 2024, 416, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef]
- Li, B.; Wu, X.; Shi, C.; Dai, Y.; Zhang, J.; Liu, W.; Wu, C.; Zhang, Y.; Huang, X.; Zeng, W. Flexible enzymatic biosensor based on graphene sponge for glucose detection in human sweat. Surf. Interfaces 2022, 36, 102525. [Google Scholar] [CrossRef]
- Müsse, A.; La Malfa, F.; Brunetti, V.; Rizzi, F.; De Vittorio, M. Flexible Enzymatic Glucose Electrochemical Sensor Based on Polystyrene-Gold Electrodes. Micromachines 2021, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, H.; Snyder, A.; Wang, M.-X.; Xie, J.; Porterfield, D.M.; Stanciu, L.A. An aqueous media based approach for the preparation of a biosensor platform composed of graphene oxide and Pt-black. Biosens. Bioelectron. 2012, 38, 314–320. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, M.; Ya, S.; Tian, H.; Li, K.; Zhang, Q.; Li, Y.; Wang, H.; Hou, C. Ti3C2TX MXene/Polyaniline-Modified Nylon Fabric Electrode for Wearable Non-Invasive Glucose Monitoring in Sweat. Biosensors 2025, 15, 531. https://doi.org/10.3390/bios15080531
Wang L, Li M, Ya S, Tian H, Li K, Zhang Q, Li Y, Wang H, Hou C. Ti3C2TX MXene/Polyaniline-Modified Nylon Fabric Electrode for Wearable Non-Invasive Glucose Monitoring in Sweat. Biosensors. 2025; 15(8):531. https://doi.org/10.3390/bios15080531
Chicago/Turabian StyleWang, Lichao, Meng Li, Shengnan Ya, Hang Tian, Kerui Li, Qinghong Zhang, Yaogang Li, Hongzhi Wang, and Chengyi Hou. 2025. "Ti3C2TX MXene/Polyaniline-Modified Nylon Fabric Electrode for Wearable Non-Invasive Glucose Monitoring in Sweat" Biosensors 15, no. 8: 531. https://doi.org/10.3390/bios15080531
APA StyleWang, L., Li, M., Ya, S., Tian, H., Li, K., Zhang, Q., Li, Y., Wang, H., & Hou, C. (2025). Ti3C2TX MXene/Polyaniline-Modified Nylon Fabric Electrode for Wearable Non-Invasive Glucose Monitoring in Sweat. Biosensors, 15(8), 531. https://doi.org/10.3390/bios15080531





