Kinematic Monitoring of the Thorax During the Respiratory Cycle Using a Biopolymer-Based Strain Sensor: A Chitosan–Glycerol–Graphite Composite
Abstract
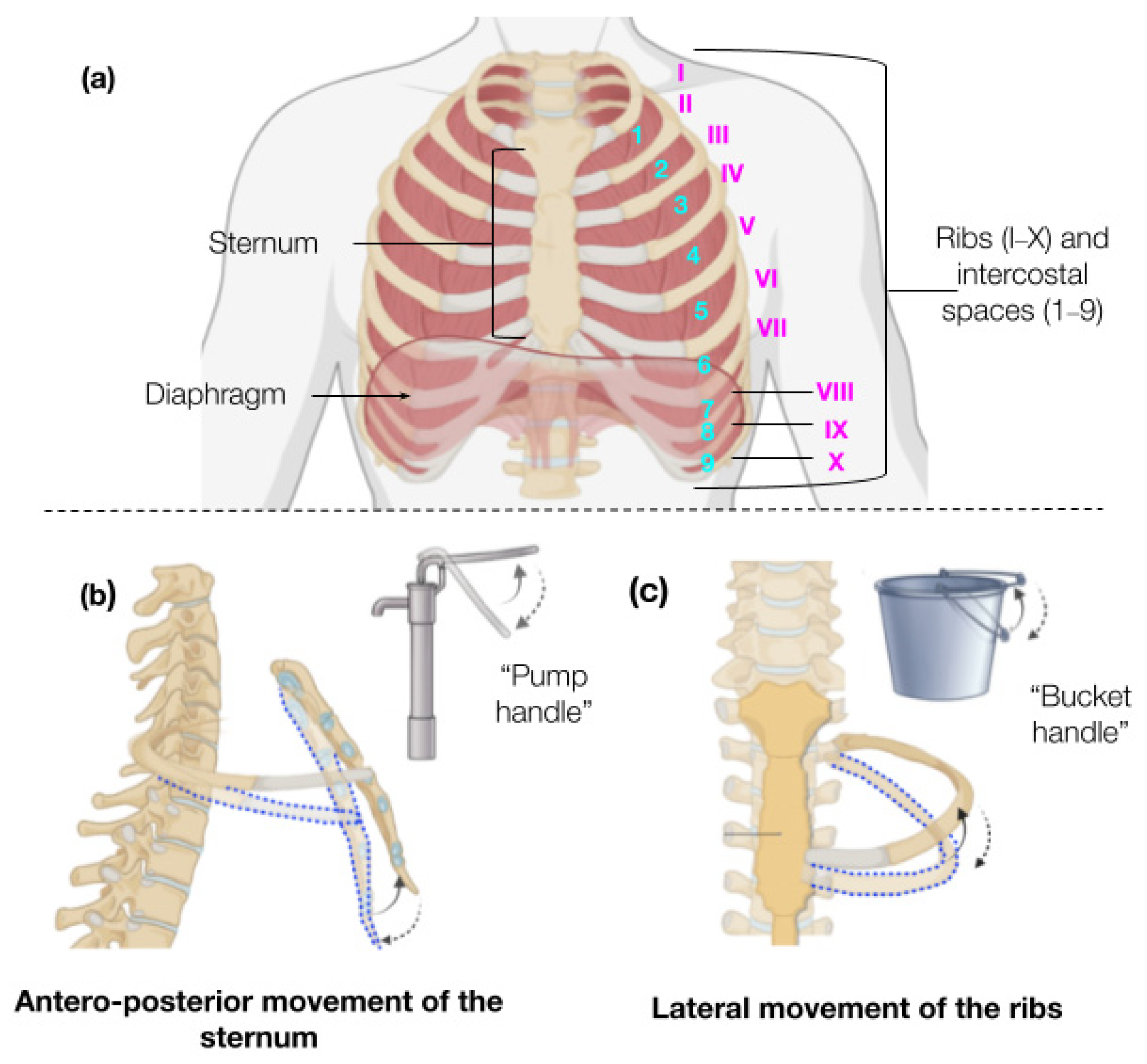
1. Introduction
2. Materials and Methods
2.1. Materials
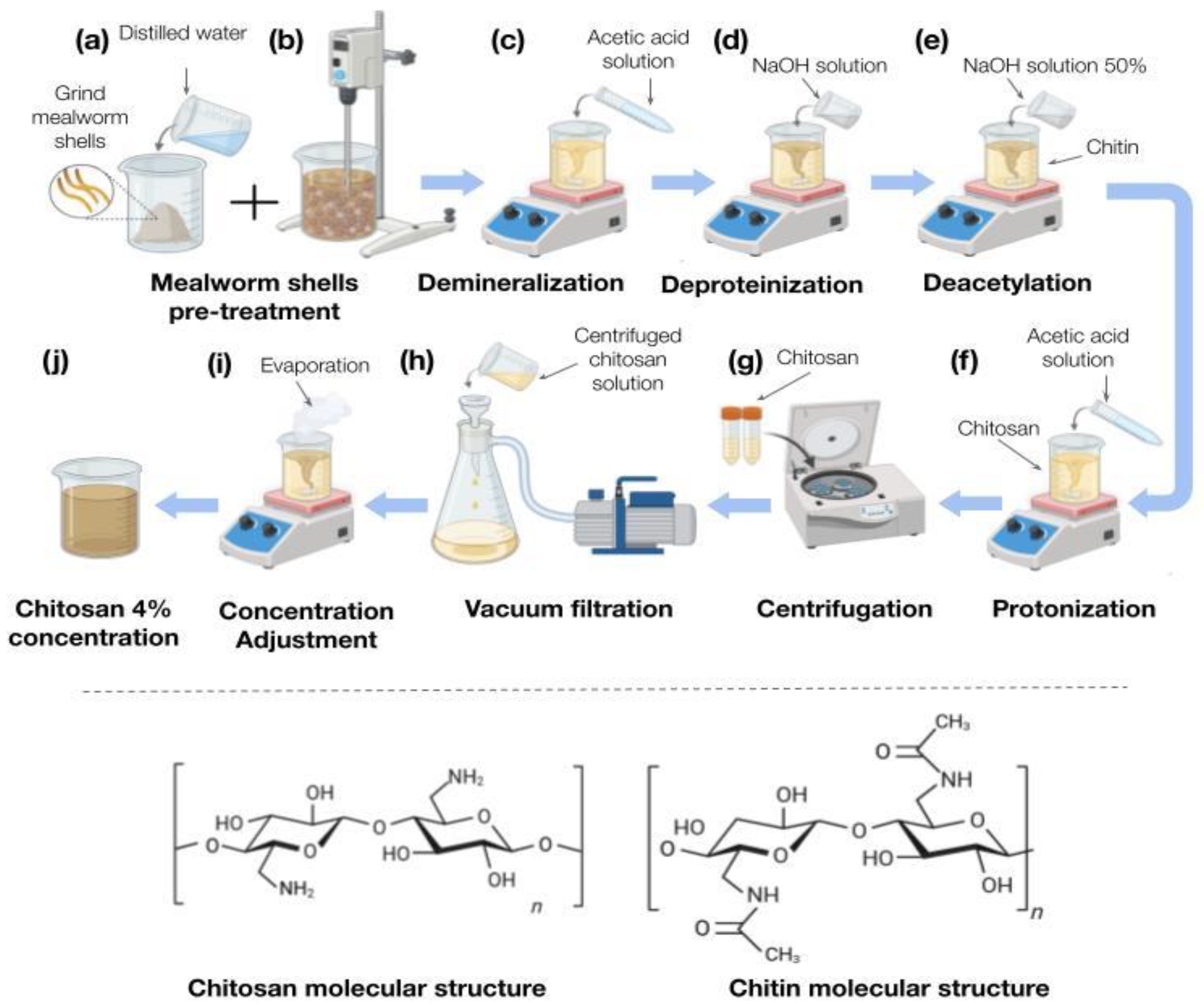
2.2. Extraction of Chitosan
2.2.1. New Sample Preparation
2.2.2. Demineralization
2.2.3. Deproteinization
2.2.4. Deacetylation
2.2.5. Protonation
2.2.6. Filtration
2.2.7. Concentration Adjustment
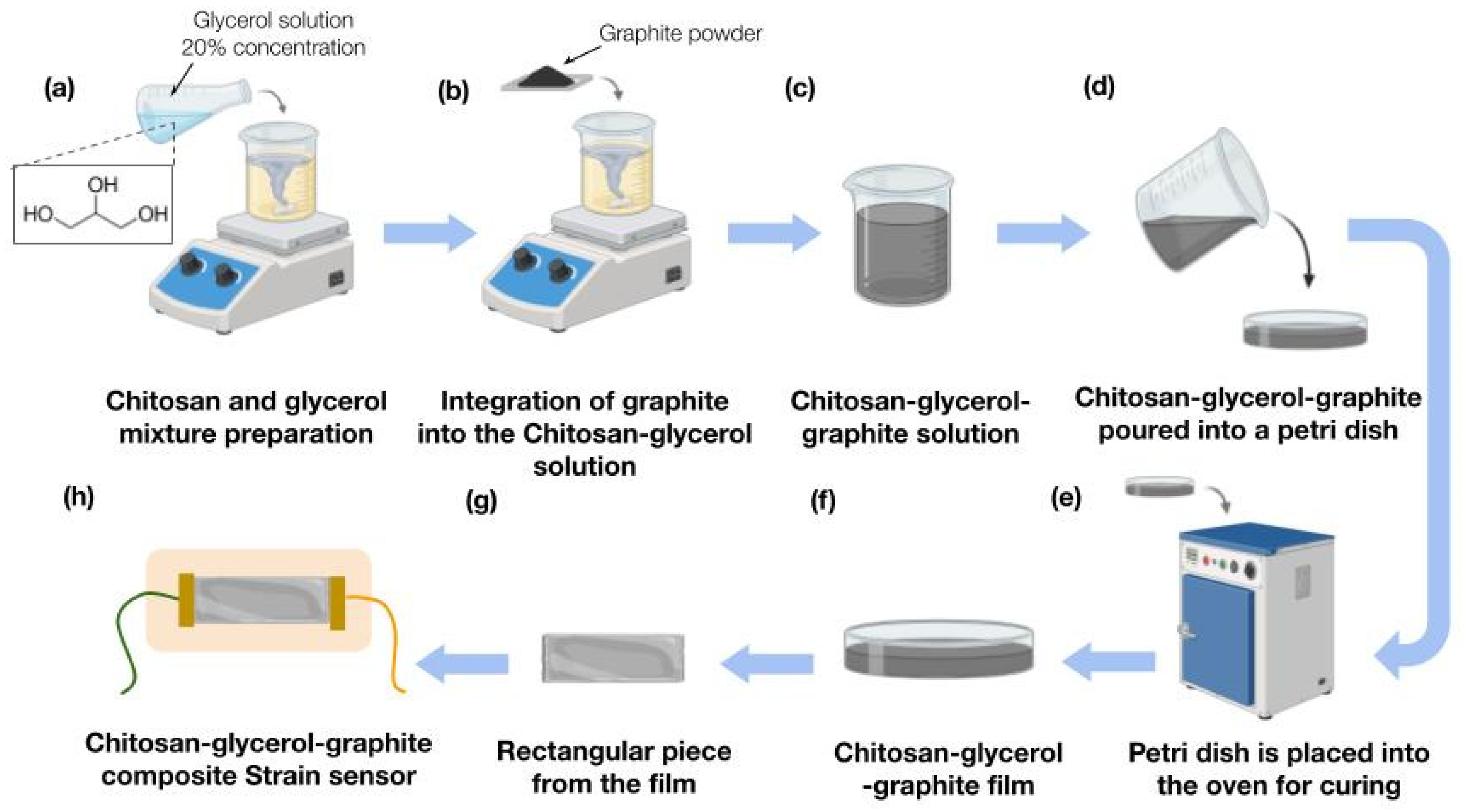
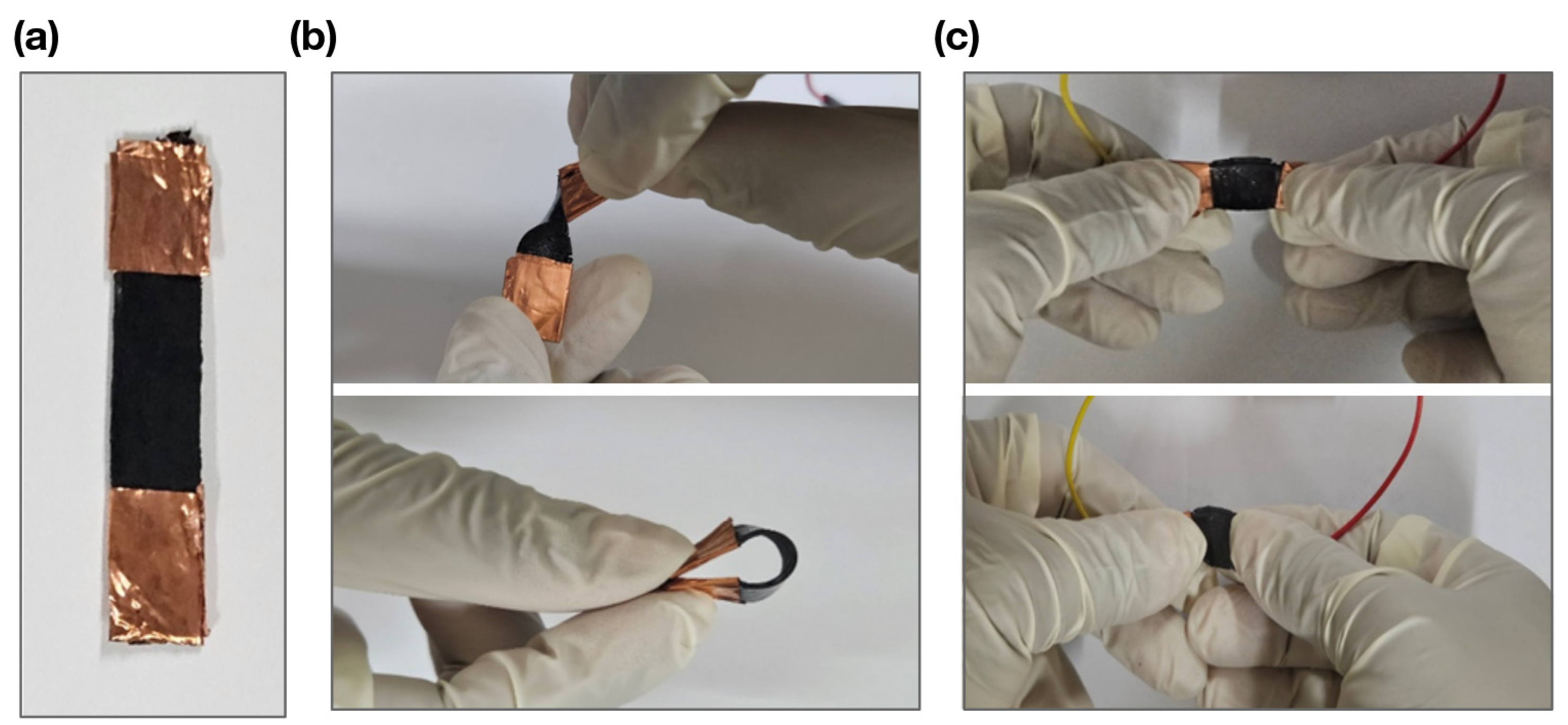
2.3. Strain Sensor Fabrication
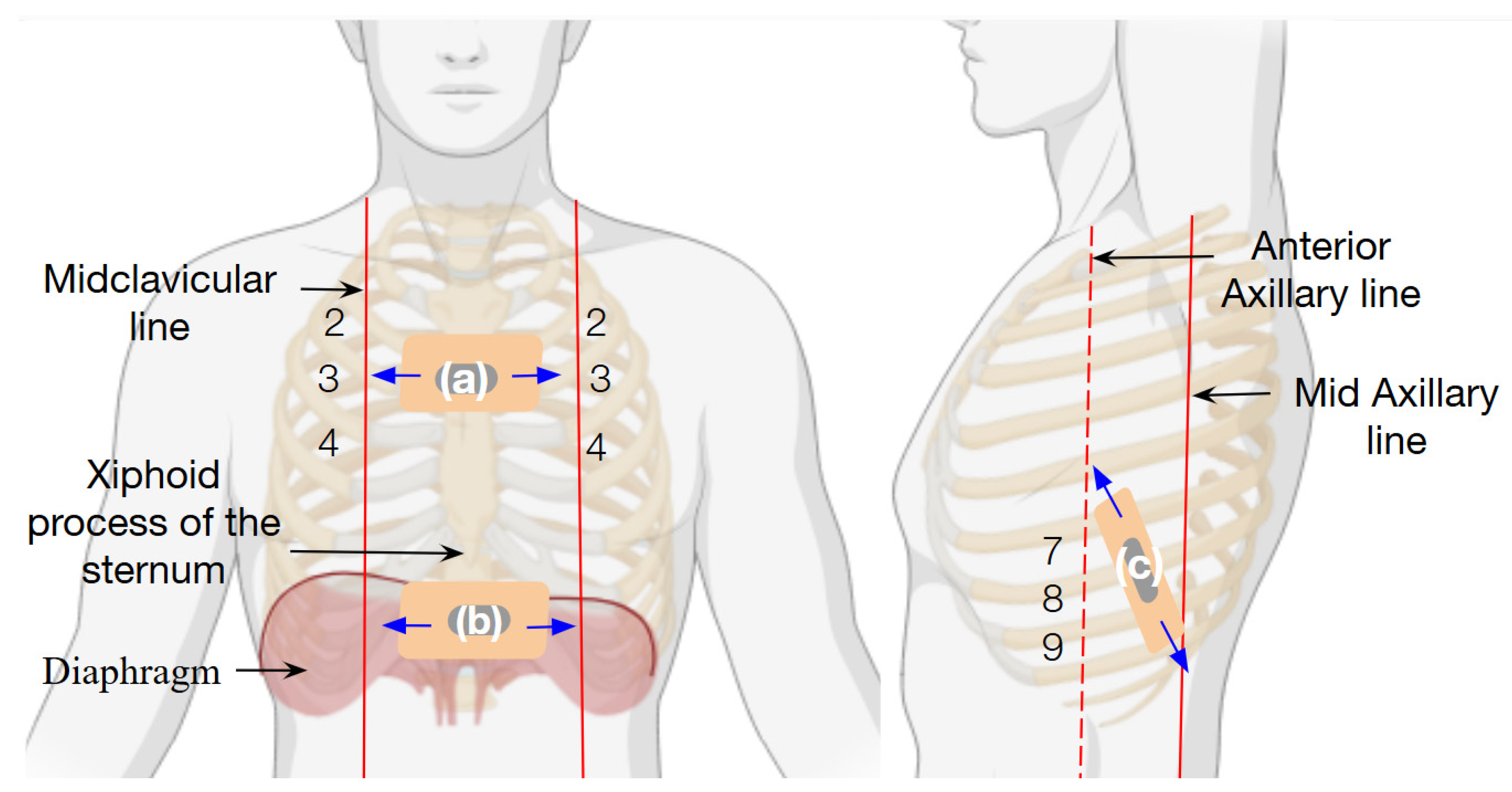
2.4. Strain Sensor Setup
2.5. Statistical Analysis
3. Results
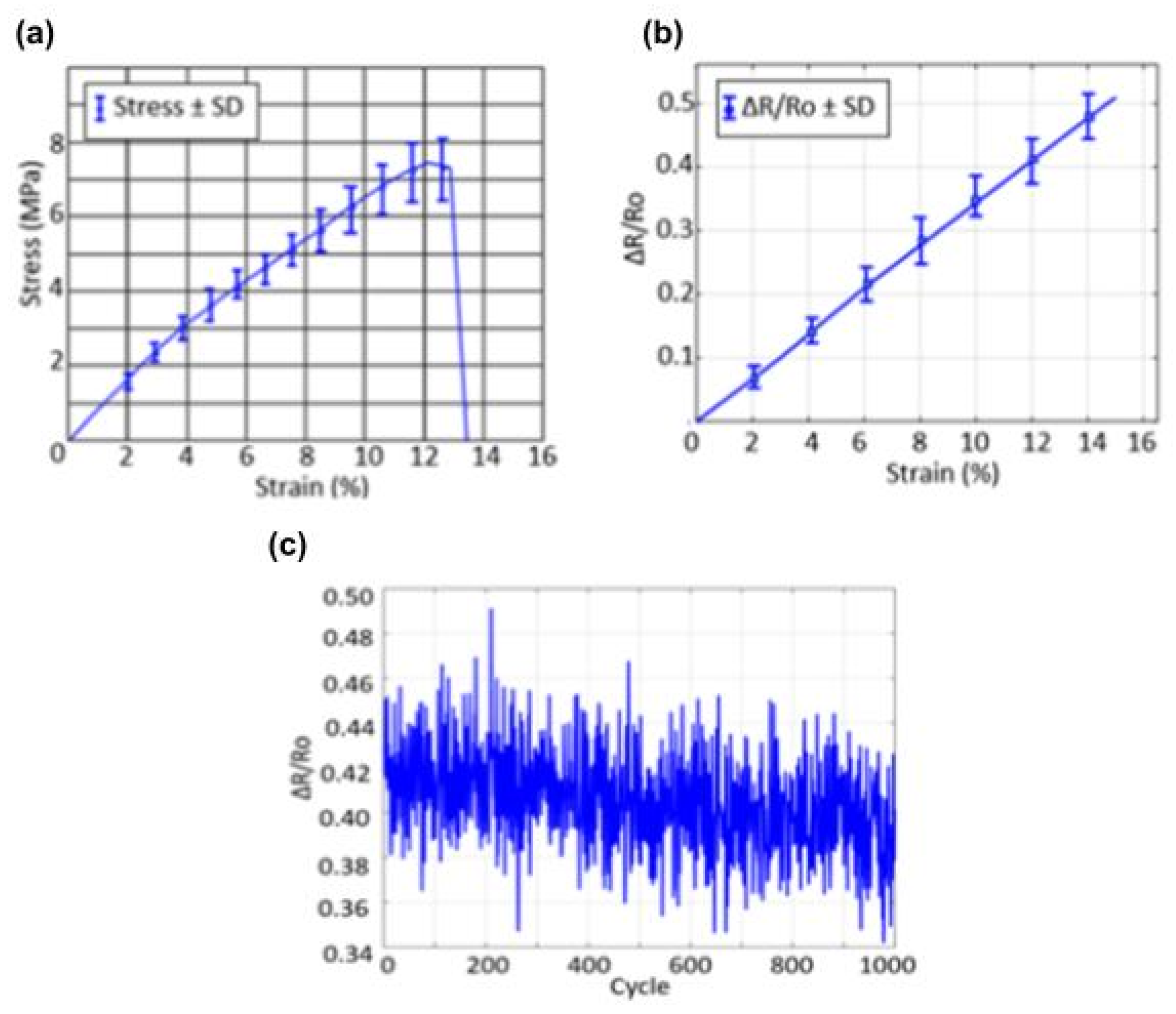
3.1. Mechanical Characterization of the Respiratory Sensor
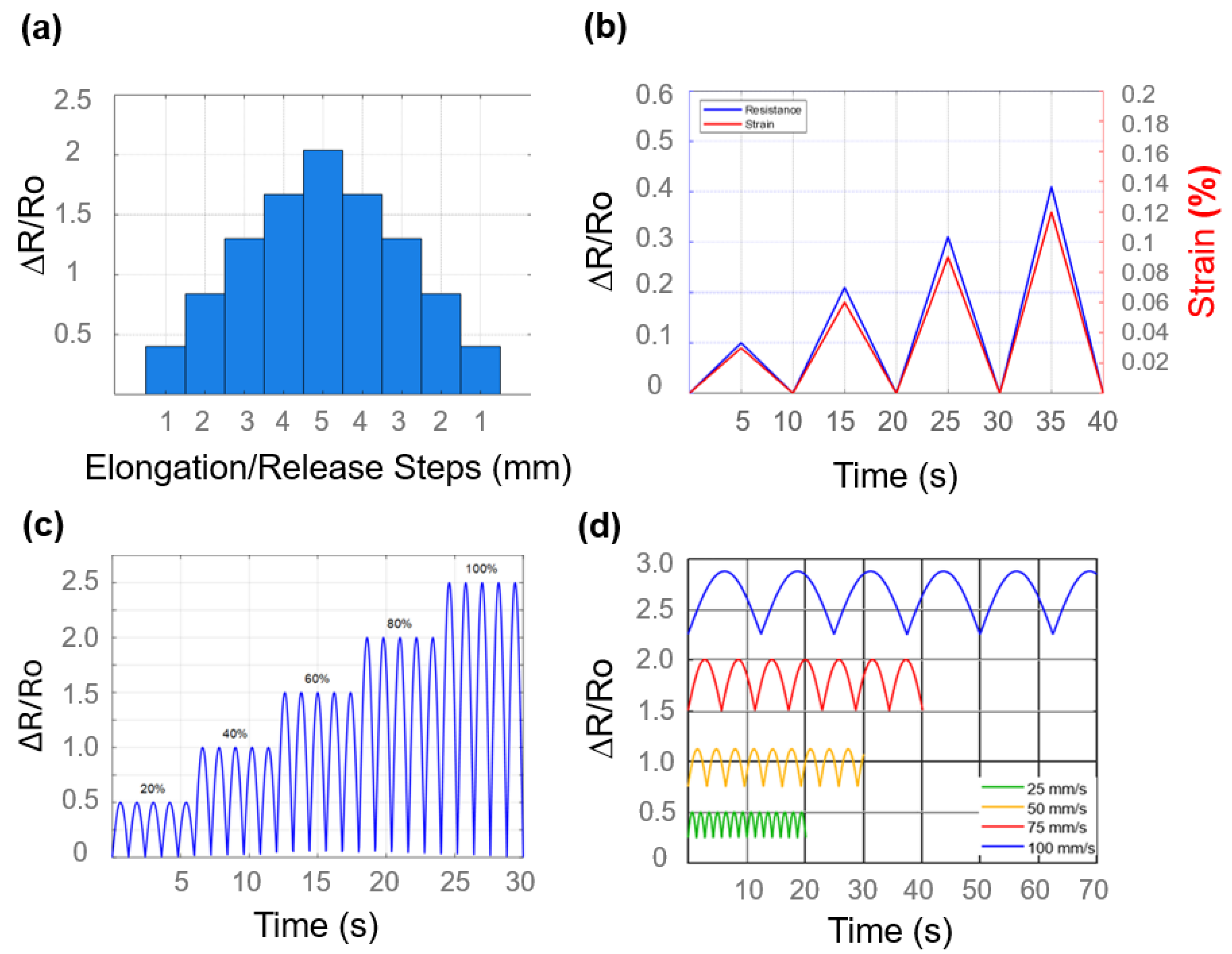
3.2. Strain Sensor Analysis
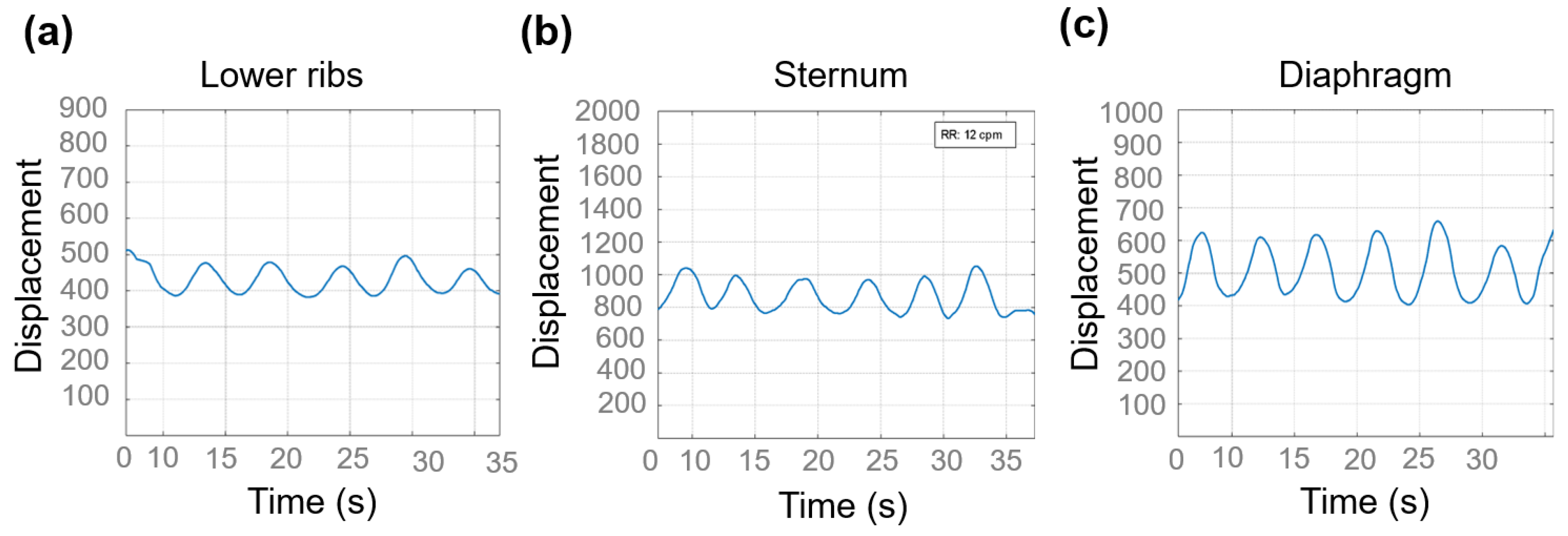
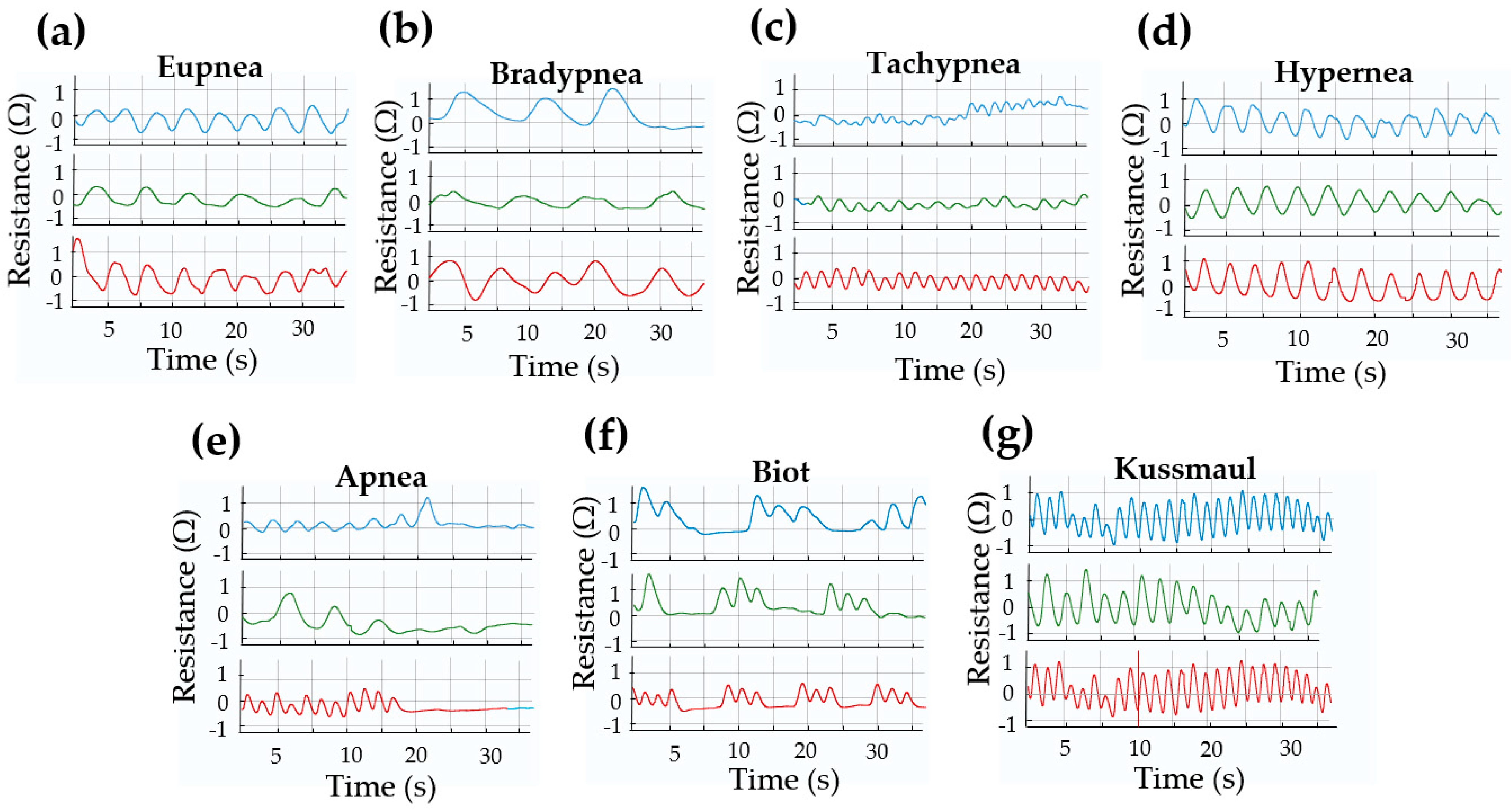
3.3. Respiratory Pattern Characterization
4. Discussion
Futures Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Zhang, P.; Chen, Y.; Li, Y.; Zhang, Y.; Zhang, J.; Huang, L. A Flexible Strain Sensor Based on the Porous Structure of a Carbon Black/Carbon Nanotube Conducting Network for Human Motion Detection. Sensors 2020, 20, 1154. [Google Scholar] [CrossRef]
- Frenț, O.D.; Vicaș, L.; Jurca, T.; Ciocan, S.; Duteanu, N.; Pallag, A.; Muresan, M.; Marian, E.; Negrea, A.; Micle, O. A Review: Uses of Chitosan in Pharmaceutical Forms. In Reviews of Physiology, Biochemistry and Pharmacology; Pedersen, S.H.F., Ed.; Springer: Cham, Switzerland, 2021; Volume 184. [Google Scholar] [CrossRef]
- Yadav, H.; Malviya, R.; Kaushik, N. Chitosan in Biomedicine: A Comprehensive Review of Recent Advances. Mater. Adv. 2024, 5, 4222–4245. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Alkafaas, S.S.; Dladla, M.; Ghosh, S.; Elkafas, S.S.; Hafez, W.; Ezzat, S.M.; Khedr, S.A.; Hussien, A.M.; et al. Chitosan, Derivatives, and Its Nanoparticles: Preparation, Physicochemical Properties, Biological Activities, and Biomedical Applications—A Comprehensive Review. Int. J. Biol. Macromol. 2025, 313, 142832. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Bhattarai, P.; Sriprablom, J.; Zhang, R.; Nirmal, S.; Nirmal, N. Recent Progress of Functional Nano-Chitosan in Pharmaceutical and Biomedical Applications: An Updated Review. Int. J. Biol. Macromol. 2025, 285, 138324. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. Glycine–Chitosan-Based Flexible Biodegradable Piezoelectric Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 9008–9016. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Rini, N.D.W.; Tsalsabila, A.; Birowosuto, M.D.; Wibowo, A. Chitosan-Based Smart Biomaterials for Biomedical Applications: Progress and Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 3084–3115. [Google Scholar] [CrossRef]
- Yang, C.; Bai, X.; Zhang, J. Glycerol-Crosslinked Chitosan/Polyvinyl Alcohol/Polyaniline Conductive Hydrogel for Wearable Strain Sensors with High Sensitivity and Environmental Durability. Int. Core J. Eng. 2025, 11, 18–26. [Google Scholar] [CrossRef]
- Blebea, N.-M.; Pușcașu, C.; Vlad, R.-A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene-Based Materials for Biomedical Applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Ikram, R.; Mohamed Jan, B.; Abdul Qadir, M.; Sidek, A.; Stylianakis, M.M.; Kenanakis, G. Recent Advances in Chitin and Chitosan/Graphene-Based Bio-Nanocomposites for Energetic Applications. Polymers 2021, 13, 3266. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A Review of Chitosan-Based Materials for Biomedical, Food, and Water Treatment Applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Glycerol Plasticisation of Chitosan/Carboxymethyl Cellulose Composites: Role of Interactions in Determining Structure and Properties. Int. J. Biol. Macromol. 2020, 163, 683–693. [Google Scholar] [CrossRef]
- Kusmono; Wildan, M.W.; Lubis, F.I. Fabrication and Characterization of Chitosan/Cellulose Nanocrystal/Glycerol Bio-Composite Films. Polymers 2021, 13, 1096. [Google Scholar] [CrossRef]
- West, J.B. Respiratory Physiology: The Essentials, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016. [Google Scholar]
- Levitzky, M.G. Pulmonary Physiology, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Bordoni, B.; Mahabadi, N.; Varacallo, M. Anatomy, Thorax, Respiratory Muscles. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- De Troyer, A.; Loring, S.H. Rib Cage Mechanics. Compr. Physiol. 2011, 1, 799–830. [Google Scholar] [CrossRef]
- Ceccarelli, M.; D’Onofrio, M.; Ambrogi, V.; Russo, M. A Numerical Analysis of Ventilation Motion after Chest Surgery with a RESPIRholter Device. Respir. Med. Case Rep. 2024, 49, 102005. [Google Scholar] [CrossRef] [PubMed]
- Roussos, C.; Koutsoukou, A. Respiratory Failure. Eur. Respir. J. 2003, 22, 3s–14s. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Jafari, S.M. Bio-Nanocomposites of Graphene with Biopolymers: Fabrication, Properties, and Applications. Adv. Colloid Interface Sci. 2021, 292, 102416. [Google Scholar] [CrossRef]
- Tobin, M.J. Principles and Practice of Mechanical Ventilation, 3rd ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Shen, S.; Zhou, q.; Chen, G.; Fang, Y.; Kurilova, O.; Liu, Z.; Li, S.; Chen, J. Advances in wearable respiration sensors. Mater. Today 2024, 72, 140–162. [Google Scholar] [CrossRef]
- Zahri, N.N.A.H.; Nordin, A.N.; Azlan, N.Z.; Hassan, I.H.; Tung, L.H.; Lim, L.M.; Samsudin, Z. Wearable Strain Sensors: Design Shapes, Fabrication, Encapsulation and Performance Evaluation Methods. Sens. Diagn. 2024, 3, 1635–1650. [Google Scholar] [CrossRef]
- Liu, M.; Lake-Thompson, G.; Wescott, A.; Beeby, S.; Tudor, J.; Yang, K. Design and Development of a Stretchable Electronic Textile and Its Application in a Knee Sleeve Targeting Wearable Pain Management. Sens. Actuators A Phys. 2024, 369, 115102. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Liu, S.; Liu, C.; Ma, T.; Luo, Z.; Ge, G. Single-Line Multi-Channel Flexible Stress Sensor Arrays. Micromachines 2023, 14, 1554. [Google Scholar] [CrossRef]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Choi, S.; Kang, S.; Lee, J.; Park, J.; Kang, S. Recent advances in wearable iontronic sensors for healthcare applications. Front. Bioeng. Biotechnol. 2023, 11, 335188. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.-Z.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S.-T. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, 2207879. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tan, H.; Wen, W. Recent Advances in Wearable Healthcare Devices: From Material to Application. Bioengineering 2024, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, F.; Yan, Z.; Chen, P. Wearable bioelectronics based on emerging nanomaterials for telehealth applications. Device 2025, 3, 100676. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhao, Y.; Shi, W.; Li, H.; Cai, J.; Ding, F.; Qu, P. Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors. Chemosensors 2023, 11, 39. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A Stretchable Carbon Nanotube Strain Sensor for Human-Motion Detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Piuzzi, E.; Pisa, S.; Pittella, E.; Podestà, L.; Sangiovanni, S. Wearable Belt with Built-In Textile Electrodes for Cardio–Respiratory Monitoring. Sensors 2020, 20, 4500. [Google Scholar] [CrossRef]
- Moon, K.S.; Lee, S.Q. A Wearable Multimodal Wireless Sensing System for Respiratory Monitoring and Analysis. Sensors 2023, 23, 6790. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Das, P.S.; Chhetry, A.; Park, J.Y. A Flexible Capacitive Pressure Sensor for Wearable Respiration Monitoring System. IEEE Sens. J. 2017, 17, 6559–6567. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, J.; Weng, S.; Wu, X. Flexible Chitosan-Based Capacitive Humidity Sensors for Respiratory Monitoring. Sensors 2024, 24, 1352. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas Ebner, M.C.; Ackah, E.; Kim, S.-W.; Seok, Y.-S.; Choi, S.H. Kinematic Monitoring of the Thorax During the Respiratory Cycle Using a Biopolymer-Based Strain Sensor: A Chitosan–Glycerol–Graphite Composite. Biosensors 2025, 15, 523. https://doi.org/10.3390/bios15080523
Rivas Ebner MC, Ackah E, Kim S-W, Seok Y-S, Choi SH. Kinematic Monitoring of the Thorax During the Respiratory Cycle Using a Biopolymer-Based Strain Sensor: A Chitosan–Glycerol–Graphite Composite. Biosensors. 2025; 15(8):523. https://doi.org/10.3390/bios15080523
Chicago/Turabian StyleRivas Ebner, María Claudia, Emmanuel Ackah, Seong-Wan Kim, Young-Seek Seok, and Seung Ho Choi. 2025. "Kinematic Monitoring of the Thorax During the Respiratory Cycle Using a Biopolymer-Based Strain Sensor: A Chitosan–Glycerol–Graphite Composite" Biosensors 15, no. 8: 523. https://doi.org/10.3390/bios15080523
APA StyleRivas Ebner, M. C., Ackah, E., Kim, S.-W., Seok, Y.-S., & Choi, S. H. (2025). Kinematic Monitoring of the Thorax During the Respiratory Cycle Using a Biopolymer-Based Strain Sensor: A Chitosan–Glycerol–Graphite Composite. Biosensors, 15(8), 523. https://doi.org/10.3390/bios15080523



