Gaseous Ammonia Sensing from Liquids via a Portable Chemosensor with Signal Correction for Humidity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
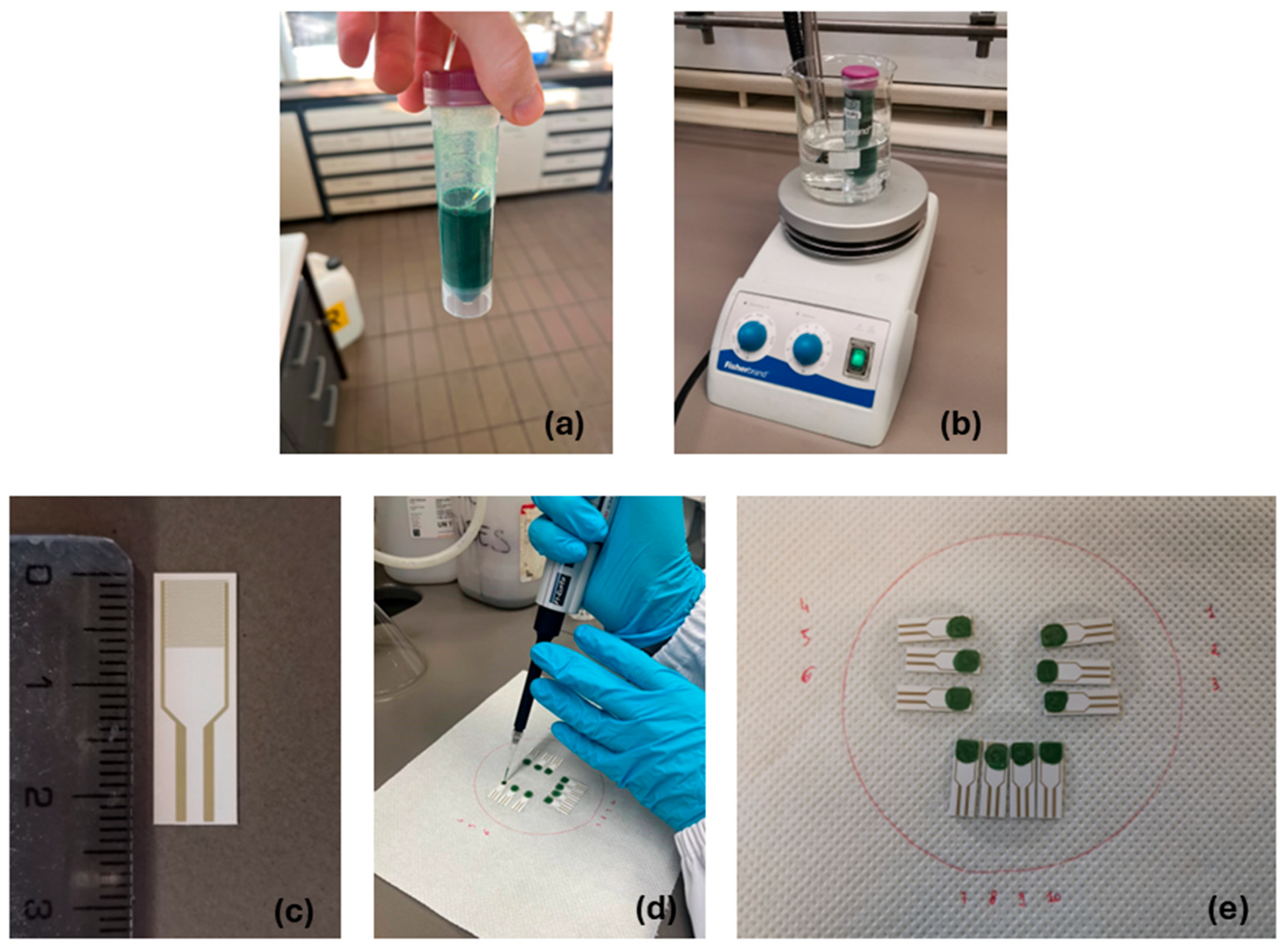
2.2. PANI Ink Formulation
2.3. Chemosensor Fabrication and Device Integration
2.3.1. Ink Deposition
2.3.2. Integration of Porous Membrane
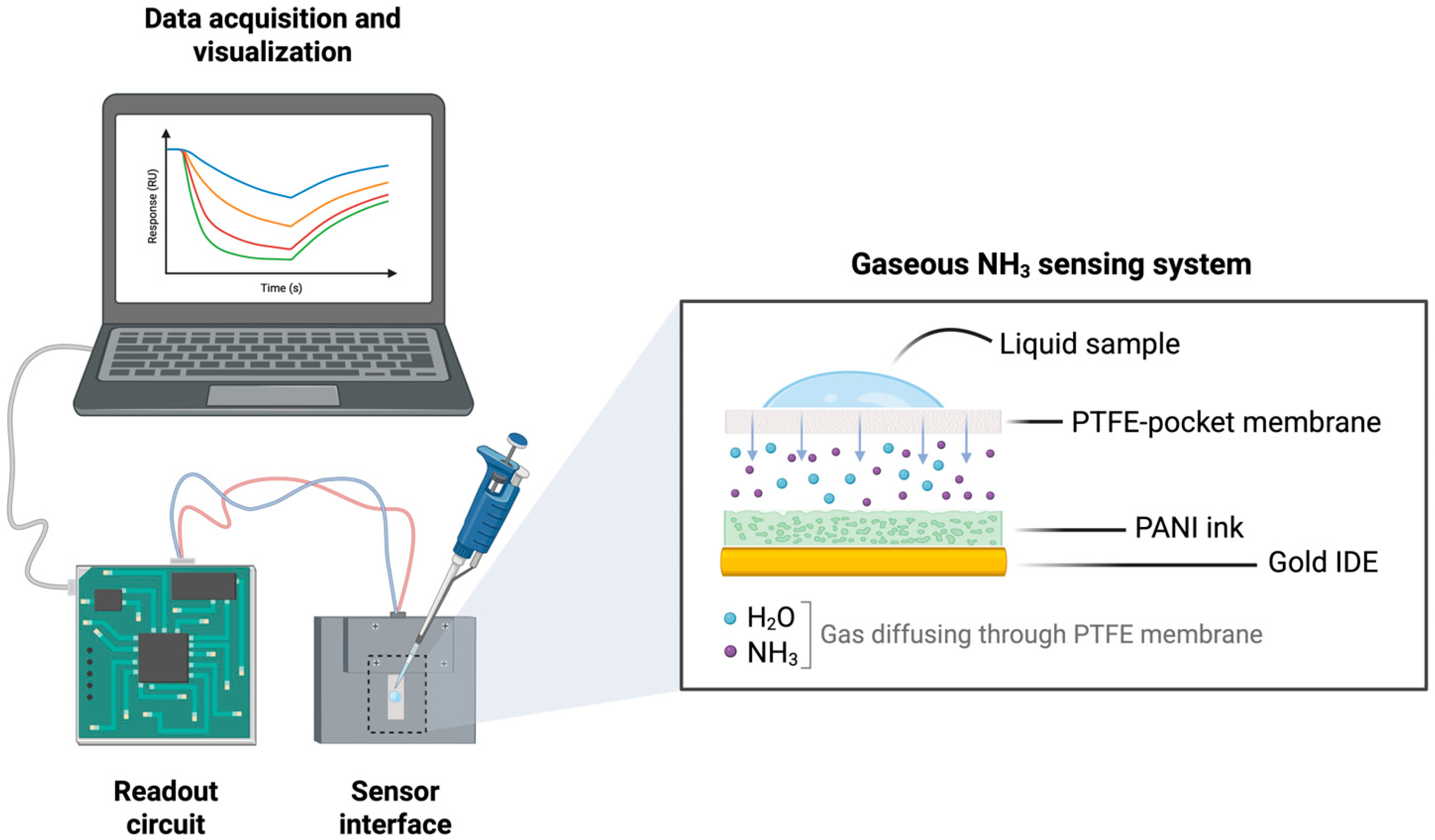
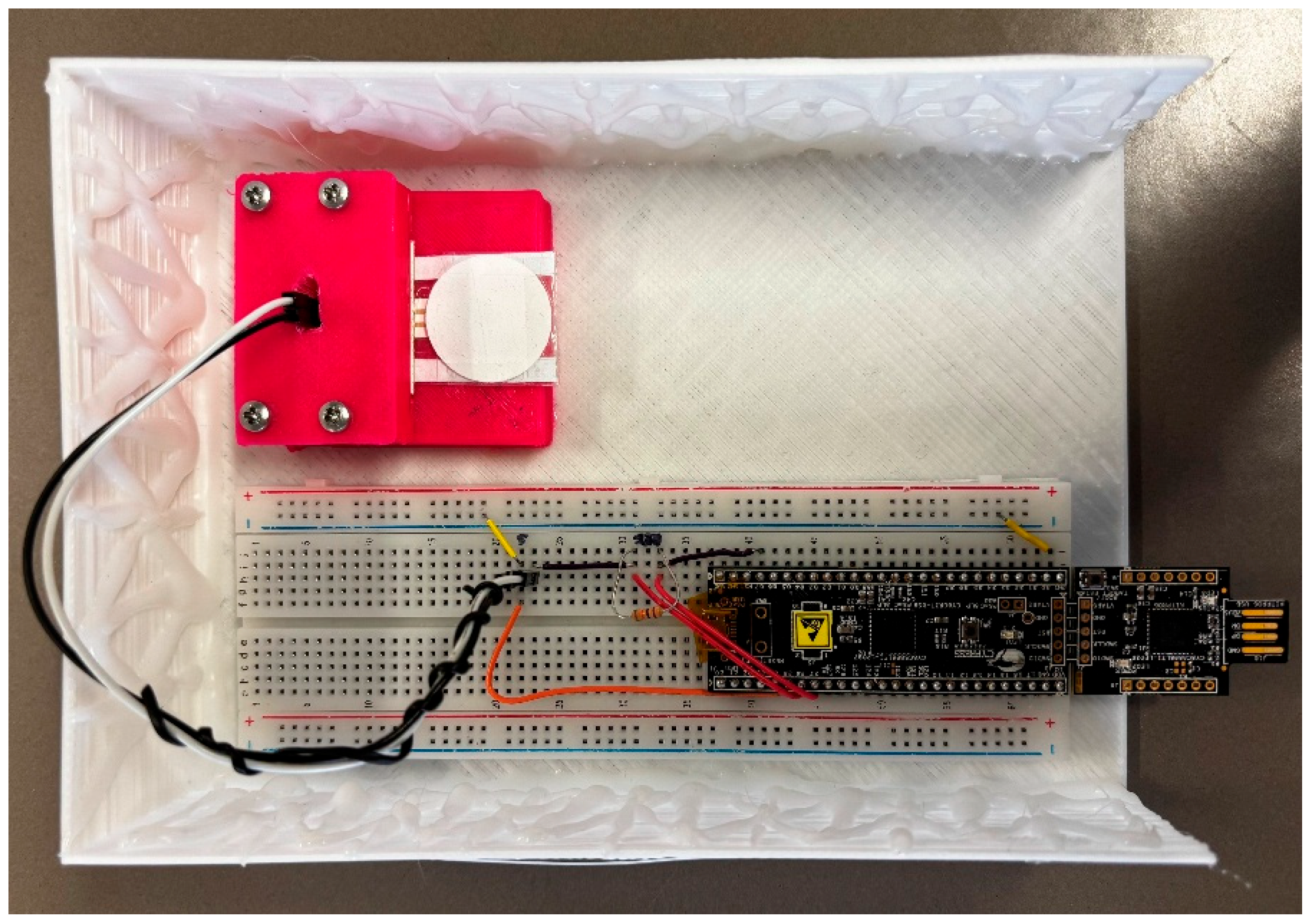
2.3.3. Portable Device
2.4. Experimental Protocol and Data Pre-Processing
- (a)
- The chemosensor was encapsulated within the pocket-membrane and connected to the measuring device. A 3 min baseline acquisition period was initiated to establish the sensor’s initial resistance. The mean value of the final 30 s, termed , served as the reference value for normalizing all subsequent resistance data;
- (b)
- A 50 μL liquid sample containing varying concentrations of ammonia was dropped in the center of the pocket-membrane. The chemosensor was subsequently exposed to the resulting gaseous ammonia for 10 min;
- (c)
- Following the exposure phase, the pocket-membrane was removed and the sensor re-exposed to ambient air for an additional 10 min to monitor its recovery characteristics.
3. Results and Discussion
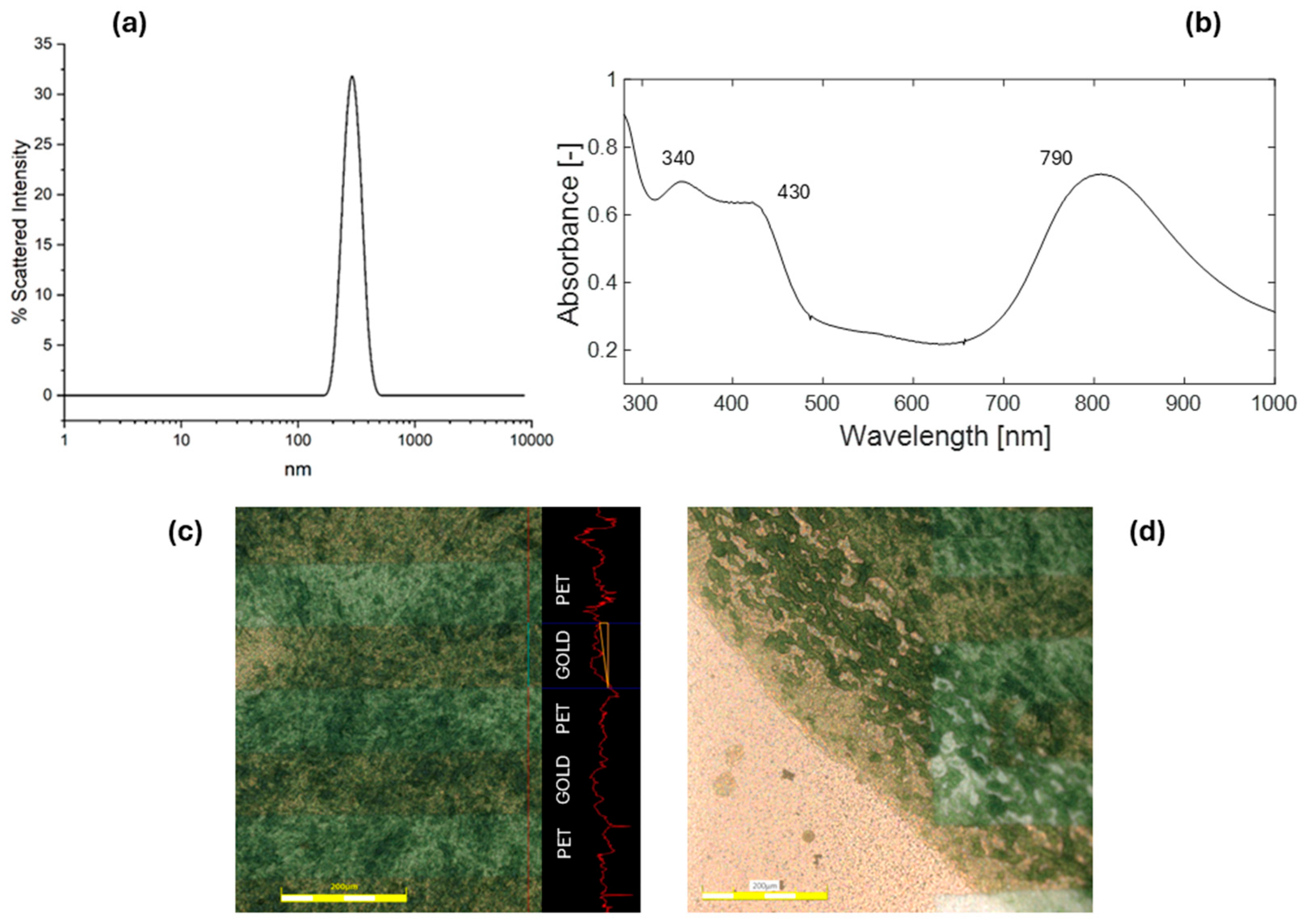
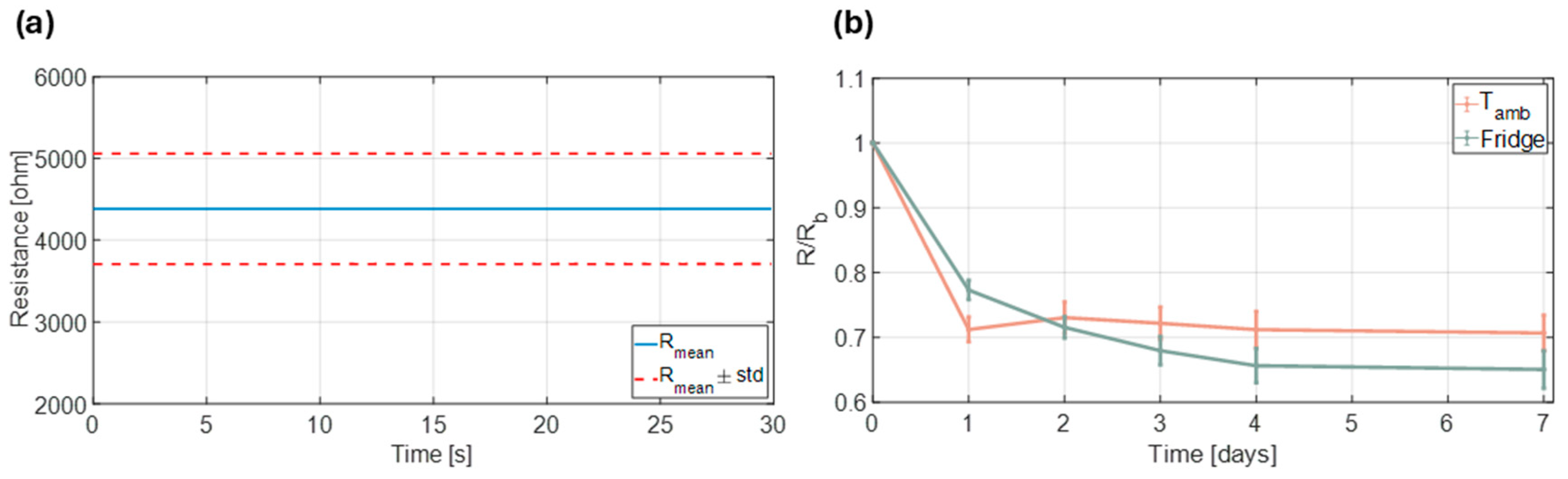
3.1. Chemosensor Assembly and Characterization
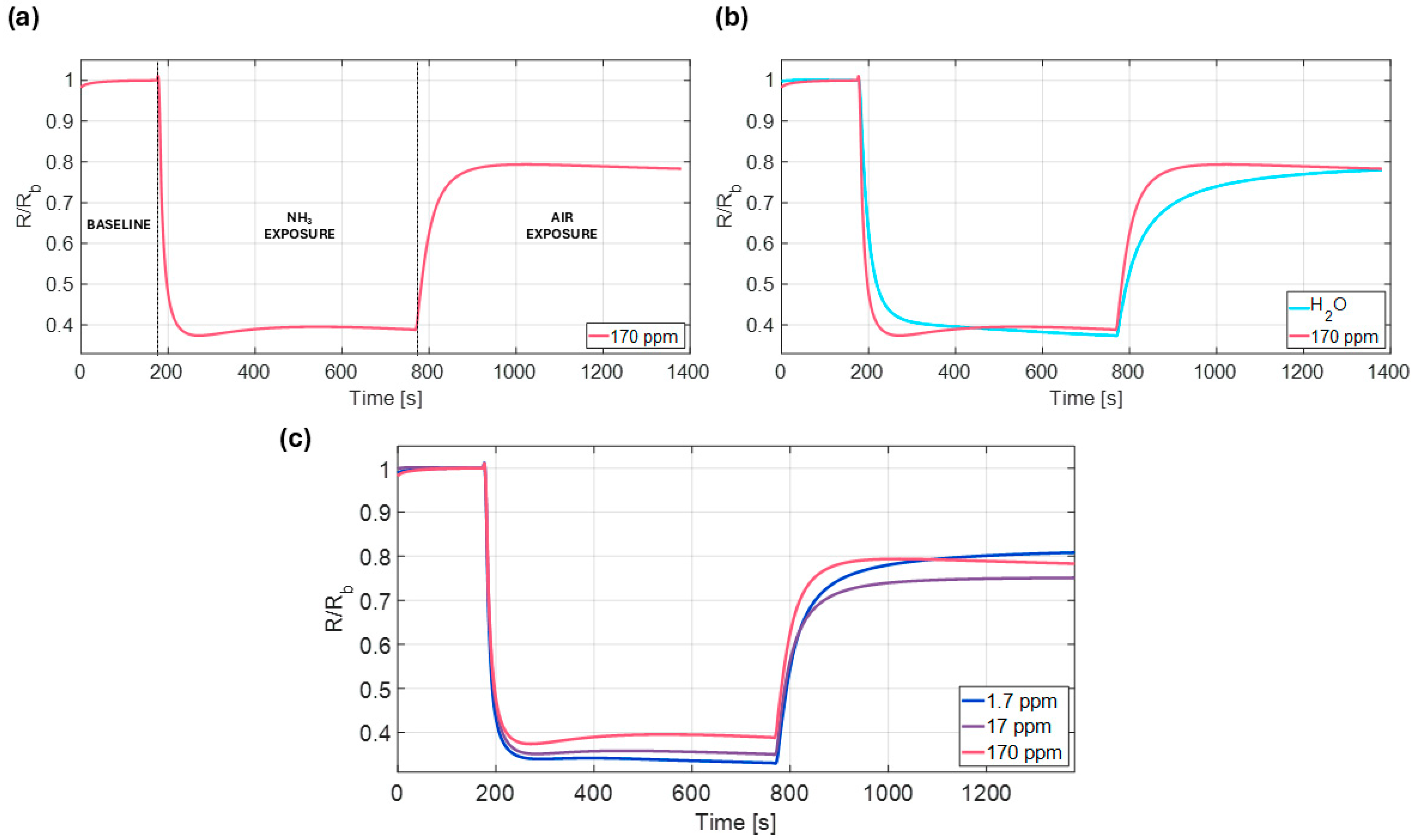
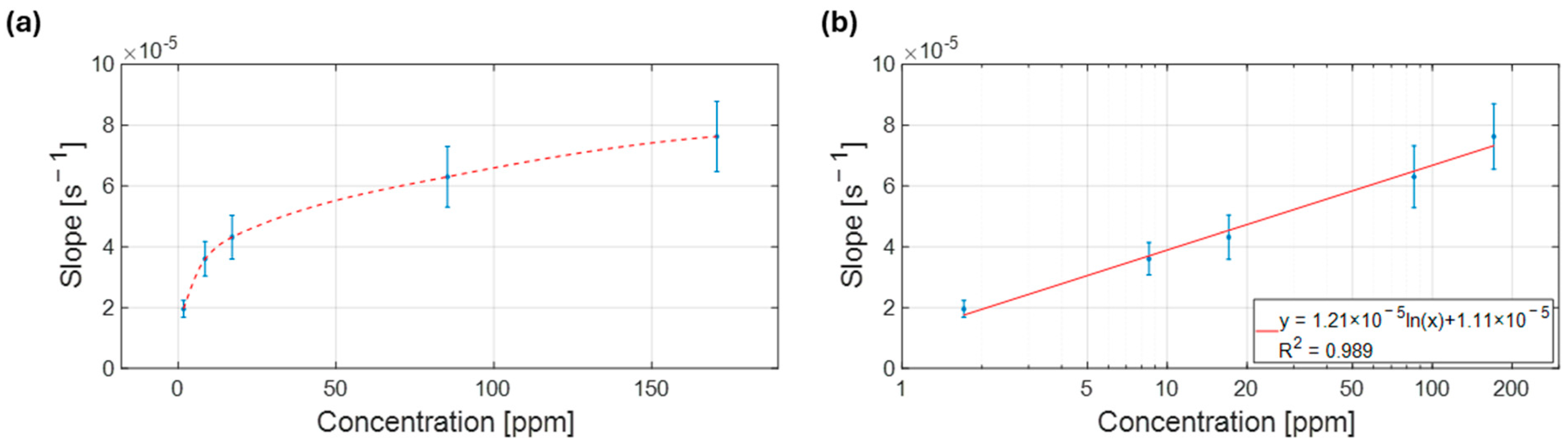
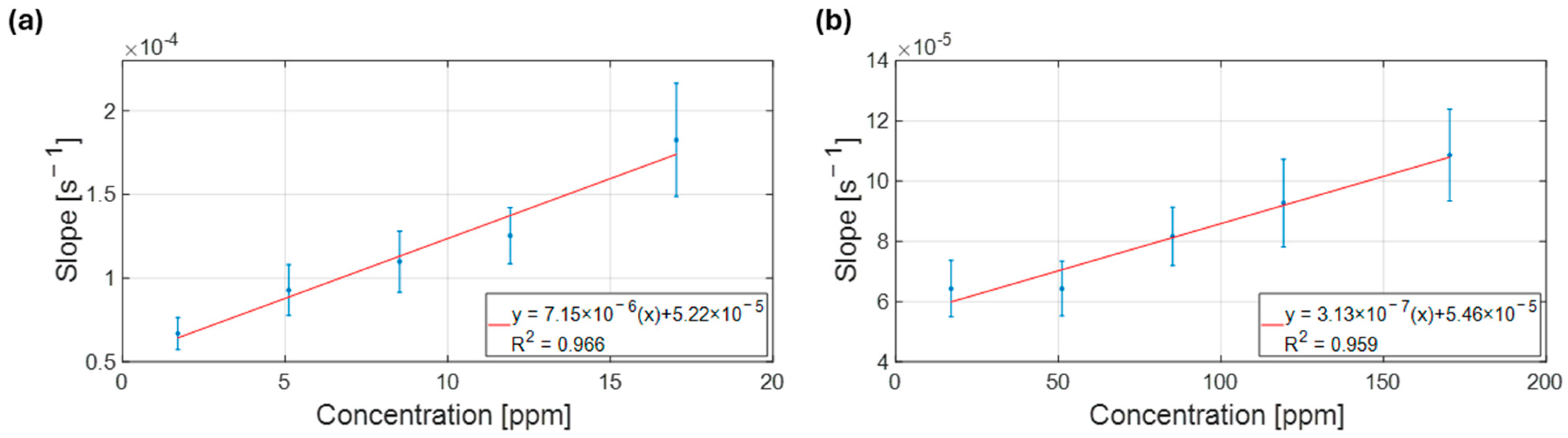
3.2. Exploring Operational Range—NH3 Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APS | Ammonium persulfate |
| DBSA | Dodecylbenzene sulfonic acid |
| DLS | Dynamic Light Scattering |
| IDE | Interdigitated electrode |
| NH3 | Ammonia |
| PANI | Polyaniline |
| PTFE | Polytetrafluoroethylene |
| POC | Point-of-care |
| SDS | Sodium dodecyl sulfate |
References
- Soler, P.; Faria, M.; Barata, C.; García-Galea, E.; Lorente, B.; Vinyoles, D. Improving water quality does not guarantee fish health: Effects of ammonia pollution on the behaviour of wild-caught pre-exposed fish. PLoS ONE 2021, 16, e0243404. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems–A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Xu, X.; Feng, J.; Ren, Y.; Bai, X.; Xia, X. Preparation of NH3- and H2S-sensitive intelligent pH indicator film from sodium alginate/black soybean seed coat anthocyanins and its use in monitoring meat freshness. Food Packag. Shelf Life 2023, 35, 100994. [Google Scholar] [CrossRef]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Rath, R.J.; Herrington, J.O.; Adeel, M.; Güder, F.; Dehghani, F.; Farajikhah, S. Ammonia detection: A pathway towards potential point-of-care diagnostics. Biosens. Bioelectron. 2024, 251, 116100. [Google Scholar] [CrossRef]
- Lefferts, M.J.; Castell, M.R. Ammonia breath analysis. Sens. Diagn. 2022, 1, 955–967. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Gifford, J.L.; Nguyen, W.N.T.; de Koning, L.; Seiden-Long, I. Stabilizing specimens for routine ammonia testing in the clinical laboratory. Clin. Chim. Acta 2018, 478, 37–43. [Google Scholar] [CrossRef]
- Barsotti, R.J. Measurement of ammonia in blood. J. Pediatr. 2001, 138, S11–S20. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G.; Blanco, N.; Donapetry, C. Ammonium metabolism in humans. Metabolism 2012, 61, 1495–1511. [Google Scholar] [CrossRef]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Fusamoto, H.; Kamada, T.; Sato, N. Mechanism of gastric mucosal damage induced by ammonia. Gastroenterology 1992, 102, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.C.; Spiller, R.C. The urea breath test for Helicobacter pylori. Gut 1994, 35, 723. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, L.R.; Goodman, W.; Patel, C.K.N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar] [CrossRef]
- Pandey, S.; Nanda, K.K. Au Nanocomposite Based Chemiresistive Ammonia Sensor for Health Monitoring. ACS Sens. 2016, 1, 55–62. [Google Scholar] [CrossRef]
- Cheng, A.; Hu, L.; Wang, Y.; Huang, L.; Zhao, L.; Zhang, C.; Liu, X.; Xu, R.; Liu, F.; Li, J.; et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106110. [Google Scholar] [CrossRef]
- Ok, F.; Erdogan, O.; Durmus, E.; Carkci, S.; Canik, A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID-19 patients. J. Med. Virol. 2021, 93, 786–793. [Google Scholar] [CrossRef]
- Ricci, P.P.; Gregory, O.J. Sensors for the detection of ammonia as a potential biomarker for health screening. Sci. Rep. 2021, 11, 7185. [Google Scholar] [CrossRef]
- Veltman, T.R.; Tsai, C.J.; Gomez-Ospina, N.; Kanan, M.W.; Chu, G. Point-of-Care Analysis of Blood Ammonia with a Gas-Phase Sensor. ACS Sens. 2020, 5, 2415–2421. [Google Scholar] [CrossRef]
- Veeralingam, S.; Sahatiya, P.; Badhulika, S. Low cost, flexible and disposable SnSe2 based photoresponsive ammonia sensor for detection of ammonia in urine samples. Sens. Actuators B Chem. 2019, 297, 126725. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Hong, Y. Portable Sensing Probe for Real-Time Quantification of Ammonia in Blood Samples. ACS Appl. Mater. Interfaces 2024, 16, 47242–47256. [Google Scholar] [CrossRef]
- Deepak, K.; Balapure, A.; Priya, P.; Kumar, P.; Dubey, S.K.; Javed, A.; Chattopadhyay, S.; Goel, S. Development of a microfluidic device for the dual detection and quantification of ammonia and urea from the blood serum. Sens. Actuators A Phys. 2024, 369, 115174. [Google Scholar] [CrossRef]
- Brannelly, N.T.; Killard, A.J. An electrochemical sensor device for measuring blood ammonia at the point of care. Talanta 2017, 167, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, L.; Zhang, D.; Fan, X.; Jin, Y.; Guo, L. Room temperature ammonia gas sensor based on polyaniline/copper ferrite binary nanocomposites. Sens. Actuators B Chem. 2020, 322, 128615. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Xiao, Y.; Chen, T.; Xiao, X.; Wang, Y. Pt/MoS2/Polyaniline Nanocomposite as a Highly Effective Room Temperature Flexible Gas Sensor for Ammonia Detection. ACS Appl. Mater. Interfaces 2023, 15, 9604–9617. [Google Scholar] [CrossRef]
- Wu, G.; Du, H.; Cha, Y.L.; Lee, D.; Kim, W.; Feyzbar-Khalkhali-Nejad, F.; Oh, T.; Zhang, X.; Kim, D. A wearable mask sensor based on polyaniline/CNT nanocomposites for monitoring ammonia gas and human breathing. Sens. Actuators B Chem. 2023, 375, 132858. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Okamoto, A.; Sakai, Y. Effect of humidity on NH3 gas sensitivity of polyaniline blend films. Sens. Actuators B Chem. 2003, 94, 46–52. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Duan, Z.; Zhao, Q.; Zhang, Y.; Xie, G.; Jiang, Y.; Li, S.; Tai, H. PANI nanofibers-supported Nb2CTx nanosheets-enabled selective NH3 detection driven by TENG at room temperature. Sens. Actuators B Chem. 2021, 327, 128923. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Liu, B.; Duan, Z.; Pan, H.; Yuan, Z.; Xie, G.; Wang, J.; Fang, Z.; Tai, H. Ultrathin Nb2CTx nanosheets-supported polyaniline nanocomposite: Enabling ultrasensitive NH3 detection. Sens. Actuators B Chem. 2021, 343, 130069. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, P.; Yu, Z.; Tao, M.; Zhou, D.; Yang, B.; Zhang, T. Light-driven, ultra-sensitive and multifunctional ammonia wireless sensing system by plasmonic-functionalized Nb2CTx MXenes towards smart agriculture. Nano Energy 2023, 108, 108216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319, 128293. [Google Scholar] [CrossRef]
- Duan, X.; Duan, Z.; Zhang, Y.; Liu, B.; Li, X.; Zhao, Q.; Yuan, Z.; Jiang, Y.; Tai, H. Enhanced NH3 sensing performance of polyaniline via a facile morphology modification strategy. Sens. Actuators B Chem. 2022, 369, 132302. [Google Scholar] [CrossRef]
- Ngamna, O.; Morrin, A.; Killard, A.J.; Moulton, S.E.; Smyth, M.R.; Wallace, G.G. Inkjet Printable Polyaniline Nanoformulations. Langmuir 2007, 23, 8569–8574. [Google Scholar] [CrossRef]
- Passador, F.; de Faria, P.; Backes, E.; Montanheiro, T.; Montagna, L.; de Souza Pinto, S.; Pessan, L.; Rezende, M. Thermal, mechanical and electromagnetic properties of LLDPE/PANI composites. Polym. Bull. 2017, 74, 2701–2717. [Google Scholar] [CrossRef]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. A review of composite conducting polymer-based sensors for detection of industrial waste gases. Sens. Actuators Rep. 2023, 5, 100143. [Google Scholar] [CrossRef]
- Stamenov, P.; Madathil, R.; Coey, J.M.D. Dynamic response of ammonia sensors constructed from polyaniline nanofibre films with varying morphology. Sens. Actuators B Chem. 2012, 161, 989–999. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Xie, L.; Buckley, L.J.; Josefowicz, J.Y. Observations of polyaniline surface morphology modification during doping and de-doping using atomic force microscopy. J. Mater. Sci. 1994, 29, 4200–4204. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Kumar, D.; Ram, M.K.; Chandra, S.; Trivedi, D.C. Application of conducting polyaniline as sensor material for ammonia. Sens. Actuators B Chem. 1997, 40, 99–103. [Google Scholar] [CrossRef]
- Sharma, A.L.; Kumar, K.; Deep, A. Nanostructured polyaniline films on silicon for sensitive sensing of ammonia. Sens. Actuators A Phys. 2013, 198, 107–112. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, T. Gas sensing properties of a Cu-doped PANI nanocomposite towards ammonia. Mater. Adv. 2024, 5, 7387–7400. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Hernandez, A.; O’Malley, E.; Whitten, P.; Wallace, G.; Smyth, M.; Killard, A. Fabrication of an ammonia gas sensor using inkjet-printed polyaniline nanoparticles. Talanta 2008, 77, 710–717. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Horio, K.; Uchida, A.; Kakunaka, R.; Shiba, S. A Flexible Ammonia Gas Sensor Based on a Grafted Polyaniline Grown on a Polyethylene Terephthalate Film. Sensors 2024, 24, 3695. [Google Scholar] [CrossRef] [PubMed]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef]
- Wen, J.; Wang, S.; Feng, J.; Ma, J.; Zhang, H.; Wu, P.; Li, G.; Wu, Z.; Meng, F.; Li, L.; et al. Recent progress in polyaniline-based chemiresistive flexible gas sensors: Design, nanostructures, and composite materials. J. Mater. Chem. A Mater. 2024, 12, 6190–6210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rescalli, A.; Porello, I.; Cerveri, P.; Cellesi, F. Gaseous Ammonia Sensing from Liquids via a Portable Chemosensor with Signal Correction for Humidity. Biosensors 2025, 15, 407. https://doi.org/10.3390/bios15070407
Rescalli A, Porello I, Cerveri P, Cellesi F. Gaseous Ammonia Sensing from Liquids via a Portable Chemosensor with Signal Correction for Humidity. Biosensors. 2025; 15(7):407. https://doi.org/10.3390/bios15070407
Chicago/Turabian StyleRescalli, Andrea, Ilaria Porello, Pietro Cerveri, and Francesco Cellesi. 2025. "Gaseous Ammonia Sensing from Liquids via a Portable Chemosensor with Signal Correction for Humidity" Biosensors 15, no. 7: 407. https://doi.org/10.3390/bios15070407
APA StyleRescalli, A., Porello, I., Cerveri, P., & Cellesi, F. (2025). Gaseous Ammonia Sensing from Liquids via a Portable Chemosensor with Signal Correction for Humidity. Biosensors, 15(7), 407. https://doi.org/10.3390/bios15070407





