Noninvasive Continuous Glucose Monitoring Using Multimodal Near-Infrared, Temperature, and Pressure Signals on the Earlobe
Abstract
1. Introduction
- The MW-SE-NIRS Algorithm: This algorithm combines slope efficiency-based parameterization and post-warmup normalization to reduce hardware-induced variations and signal-to-noise variability.
- Multi-Channel Fusion: Multi-channel fusion integrates three wavelength channels optimized for earlobe tissue properties, reducing scattering artifacts through cross-channel compensation.
- The Low-Complexity Conv1D Model: The model achieves reliable glucose prediction with limited training data, leveraging streamlined architecture for biomedical applications.
- Clinical Feasibility: The clinical feasibility was validated on five healthy subjects (400–700 frames/subject over 4–6 h), highlighting the potential for optimization despite challenges in individual/environmental variability.
2. Methodology and Design
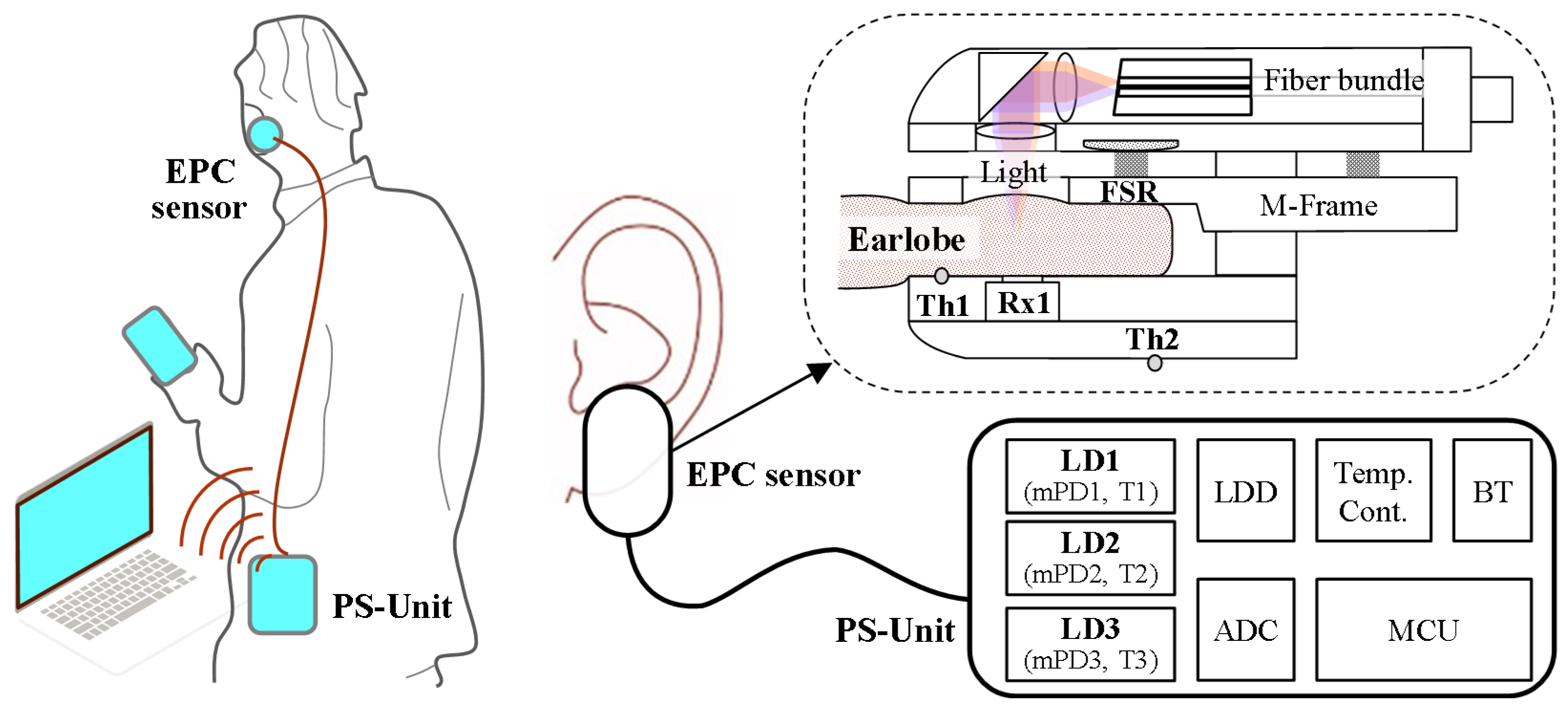
2.1. System Overview
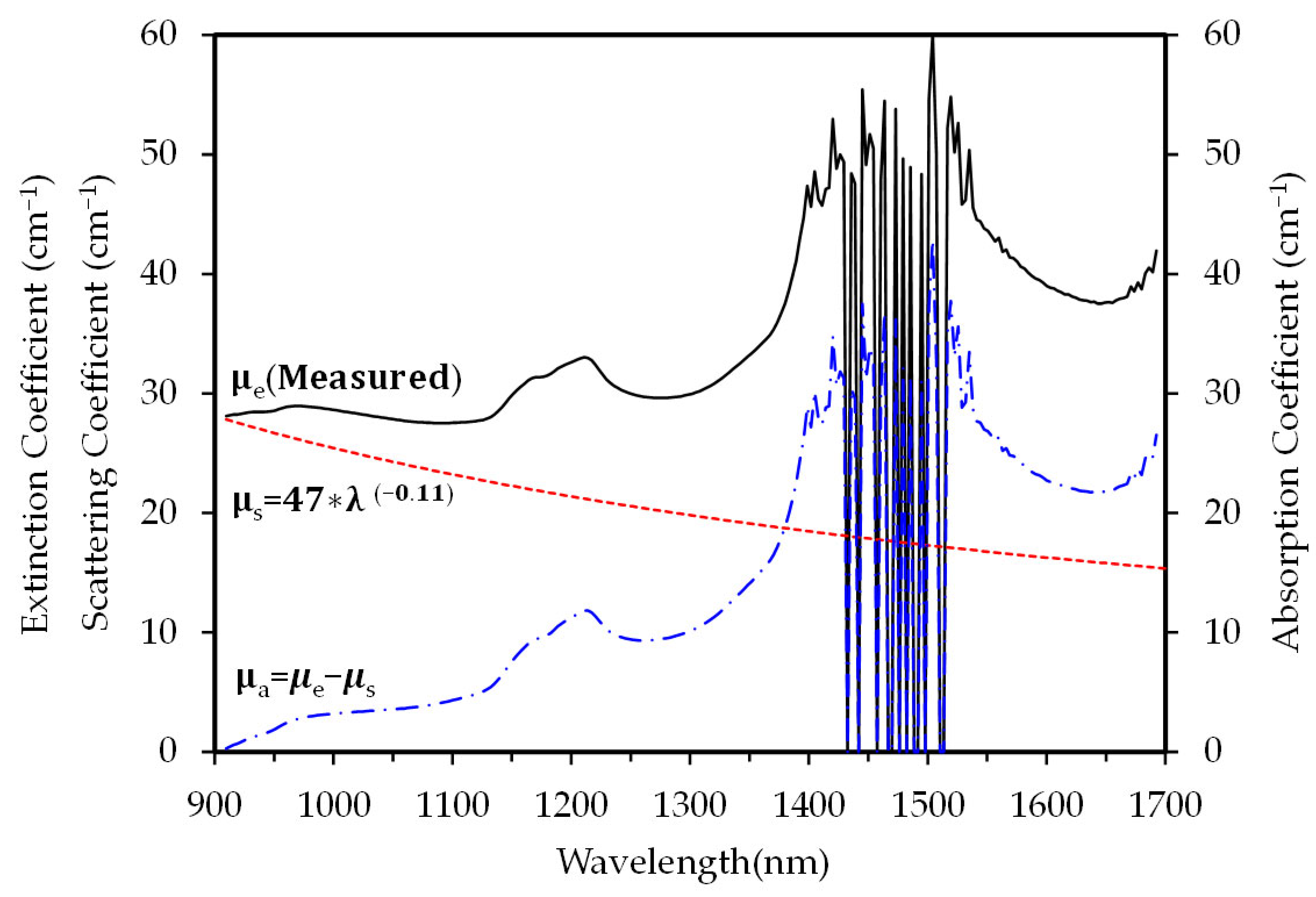
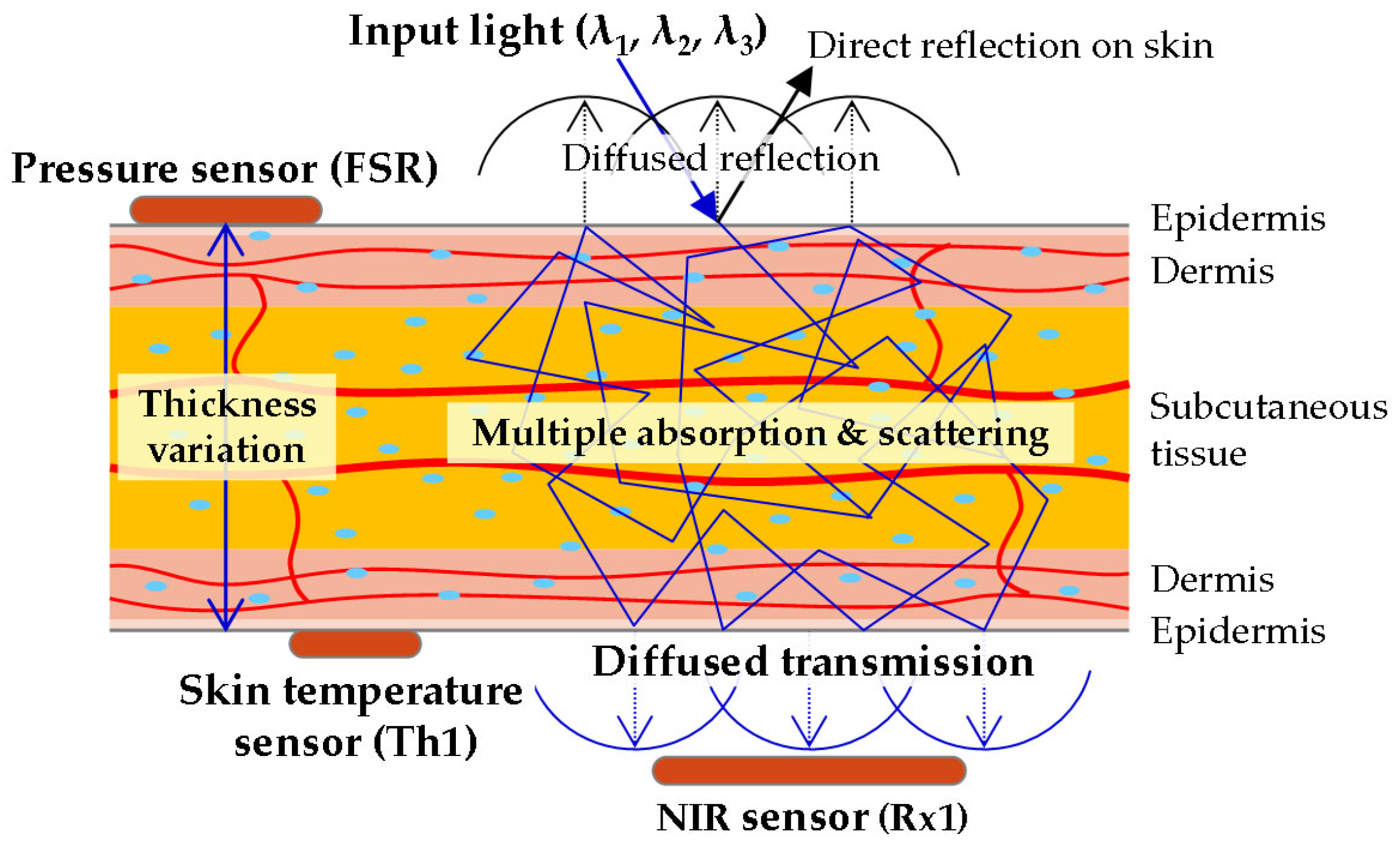
2.2. Optical Properties of Earlobe and Wavelengths
2.3. MW-SE-NIRS Algorithm
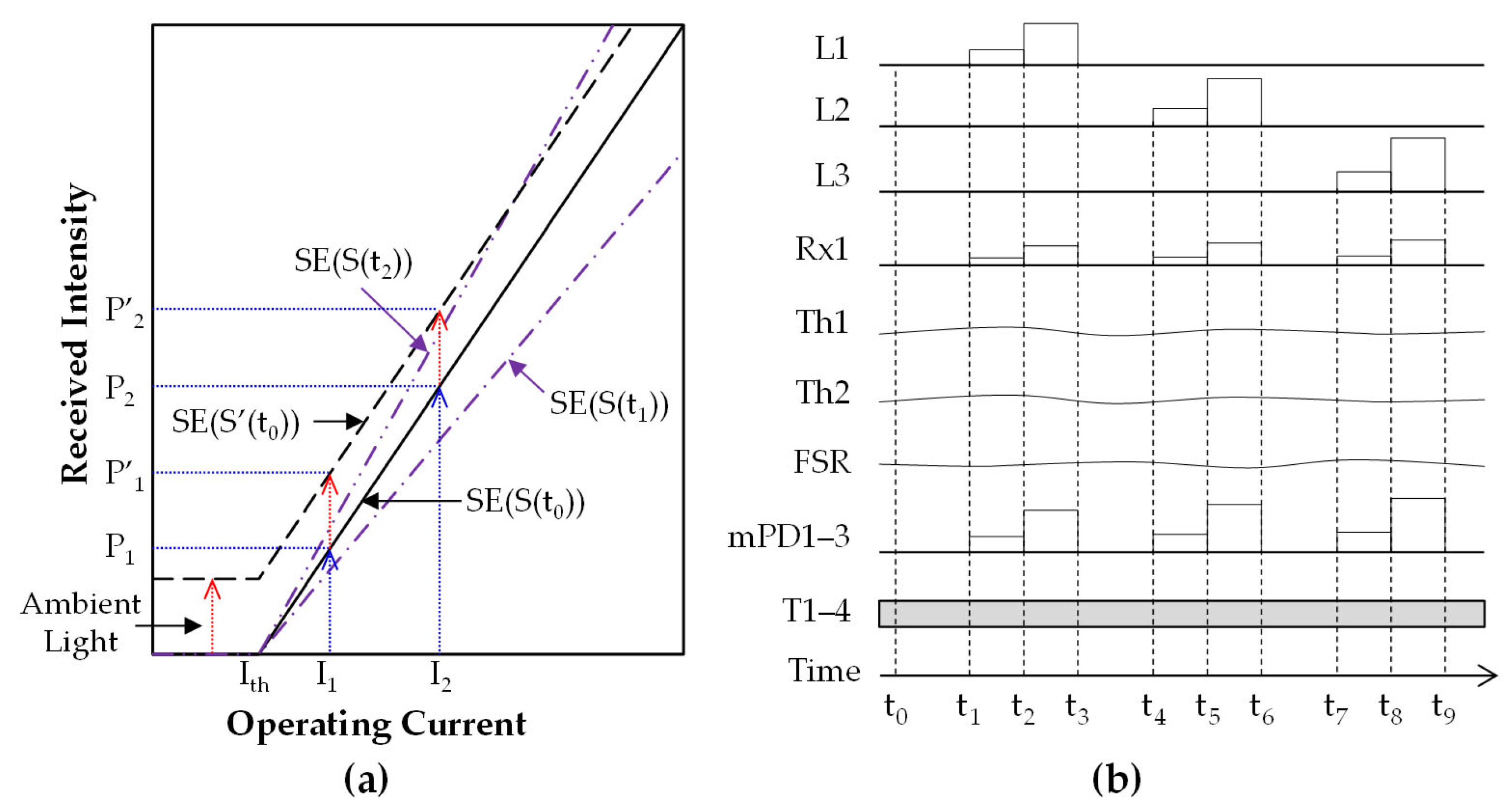
2.3.1. Signal Parameterization
2.3.2. Signal Processing for Multimodal Sensors in MW-SE-NIRS
2.4. Clinical Trial and Data Collection
2.5. Feature Engineering and Neural Network Design
2.5.1. Feature Selection and Exploratory Analysis
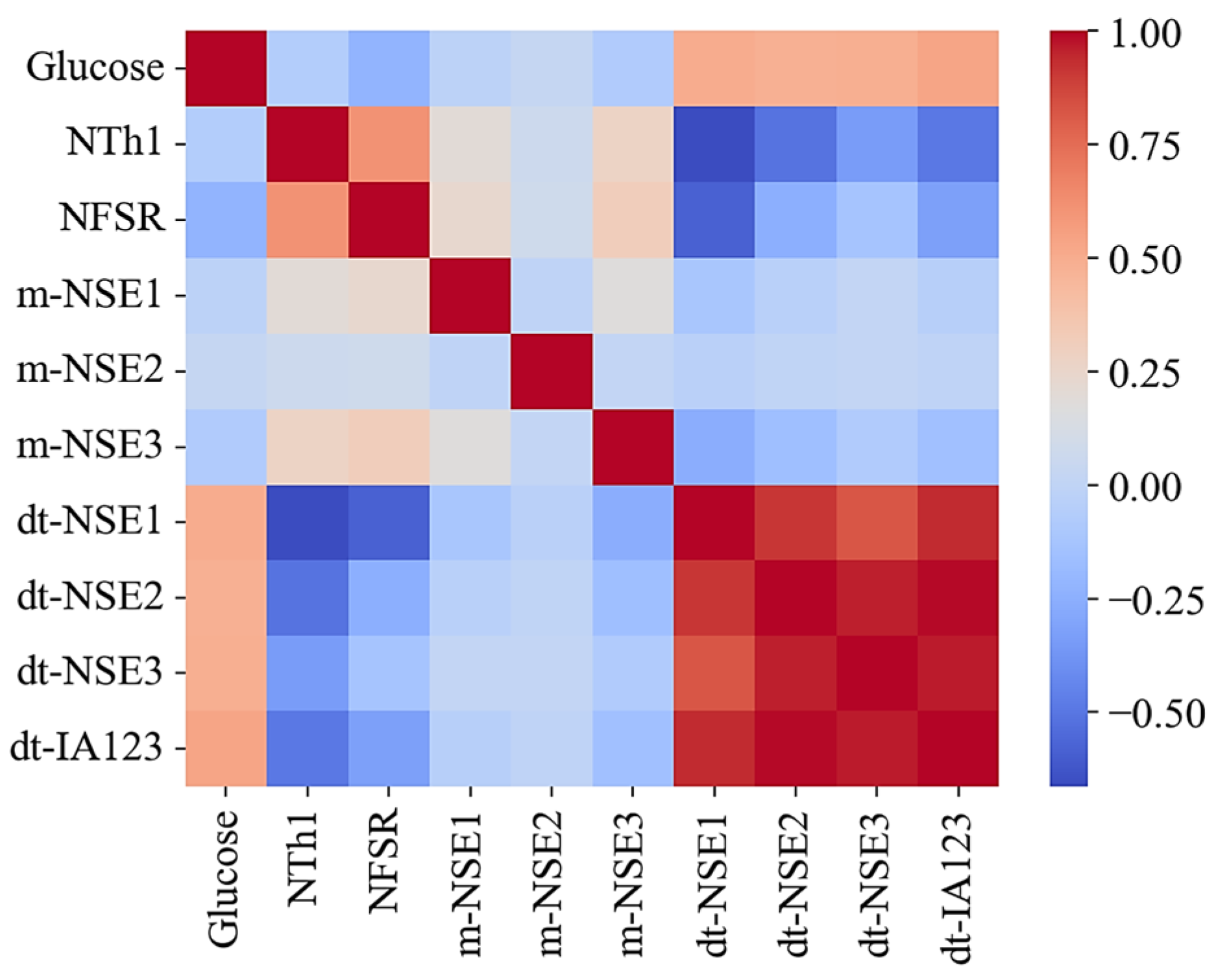
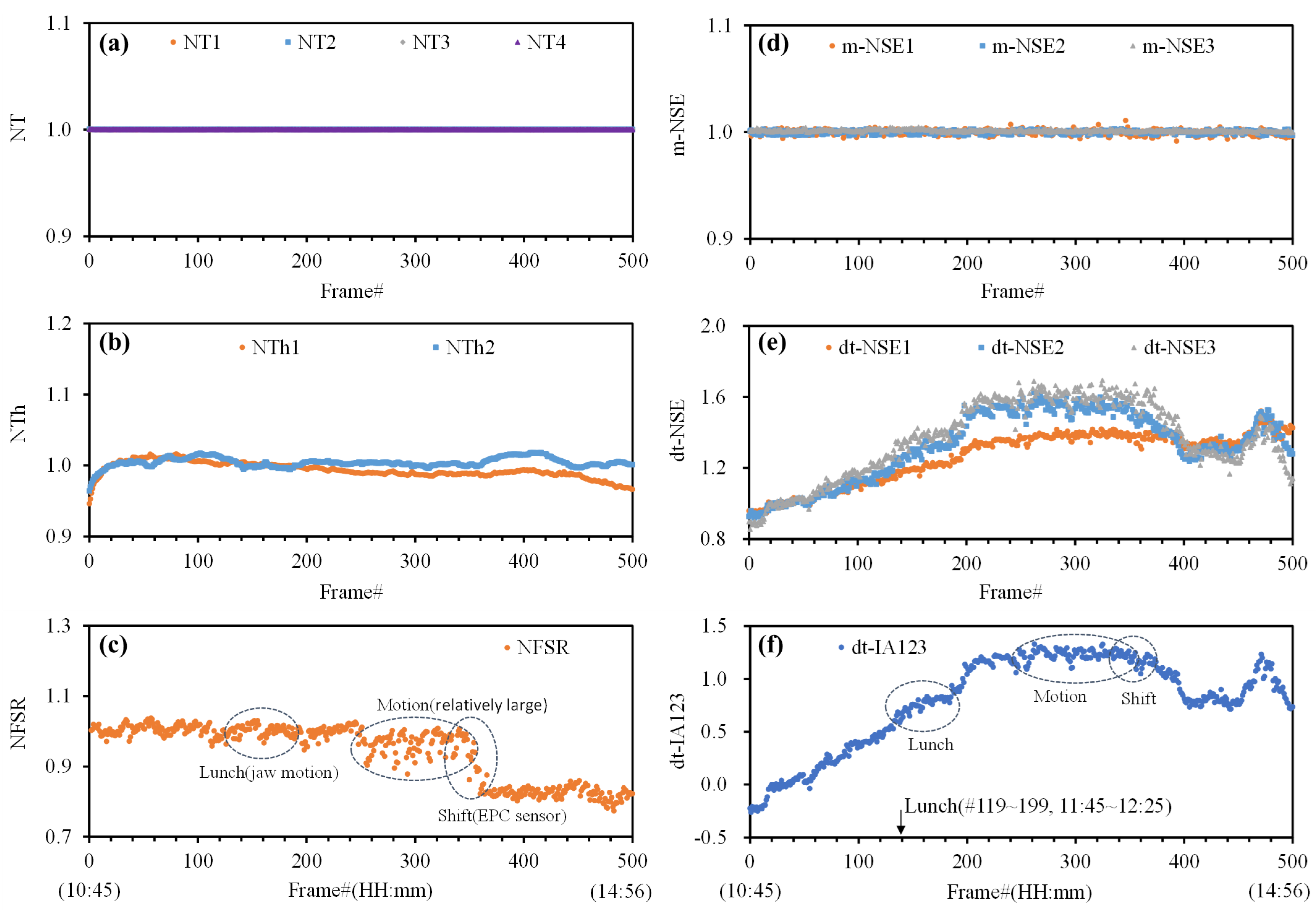
- The kernel group: This group includes dt-NSE1–3 and dt-IA123 data, which show strong correlations with glucose levels.
- The assistant group: This group comprises NTh1 and NFSR data to monitor skin temperature and earlobe pressure, which may interfere with kernel group data.
- The additional group: This group contains NT1–4, m-NSE1–3 data to assess the sensor system stability, along with NTh2 to track ambient temperature changes during testing.
2.5.2. Design of Conv1D Models and Hyperparameter Optimization
2.5.3. Pearson Correlation and Multicollinearity
3. Results and Discussion
3.1. Characteristics of NI-CGM Frame Data
3.2. Test with Individual Dataset in Five Subjects
- Training Data Requirements: Effective temporal pattern learning requires training datasets exceeding 1.67× the minimum size threshold.
- Movement Artifacts: Subject movement during measurements reduced the accuracy, particularly in individuals with thinner earlobes, where fixed sensor pressure exacerbated the signal instability.
- Earlobe Biomechanics: Pre- and post-measurement thickness variations may stem from individual differences in skin elasticity or movement, though isolating biological versus behavioral factors requires further study.
- System Comparability: The two NI-CGM systems demonstrated comparable performances, but direct benchmarking was limited by hardware discrepancies (e.g., pressure calibration protocols, wavelength ranges).
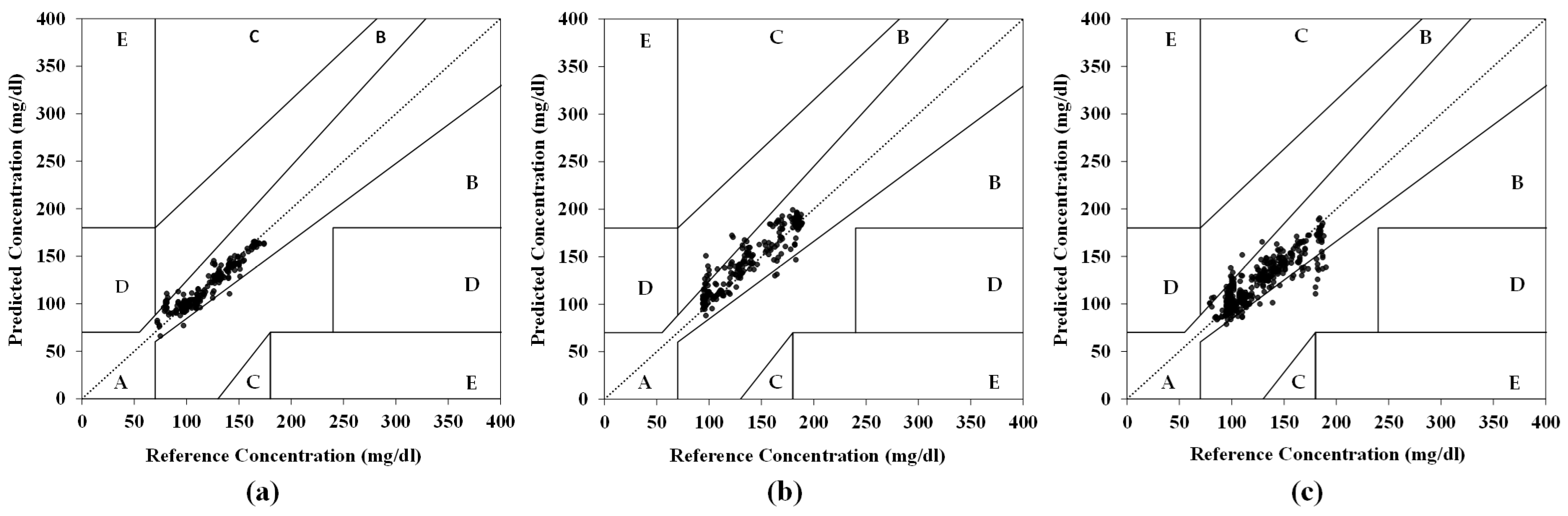
3.3. Test with Mixed Datasets in Five Subjects
- G1 (EPC sensor and PS-Unit #1): The model achieved 97.0% CEG Zone-A accuracy (Figure 8a), a 5.2% MARD, and a test RMSE of 7.95 mg/dL (vs. the training RMSE: 6.77 mg/dL), indicating no overfitting.
- G2 (EPC sensor and PS-Unit #2): The model achieved 93.2% CEG Zone-A accuracy (Figure 8b), with an RMSE of 14.37 mg/dL and a 7.56% MARD.
- Combined Group (G1 + G2): The model achieved 90.9% CEG Zone-A accuracy (Figure 8c), with an RMSE of 14.13 mg/dL and an 8.44% MARD, showing slightly reduced accuracy compared to the individual-group analyses.
3.4. Cross-Subject Generalization Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NI-CGM | Noninvasive Continuous Glucose Monitoring |
| MW-SE-NIRS | Multi-Wavelength Slope Efficiency Near-Infrared Spectroscopy |
| SE | Slope Efficiency |
| EPC | Earlobe-Parallel Clip |
| PS-Unit | Portable Sensor Unit |
| FSR | Force-Sensitive Resistor (pressure sensor signal) |
| LD | Laser Diode (light source) |
| L1–3 | Laser Output Signals From LD1–3 (wavelengths λ1, λ2, λ3) |
| mPD1-3 | Photodiodes, Monitoring Output Intensities From LD1–LD3. |
| Rx1 | Receiver 1 (optical receiving signal) |
| Th1 | Thermistor 1 (earlobe skin temperature) |
| Th2 | Thermistor 2 (sensor case/ambient temperature) |
| T1–T3, T4 | Temperatures of LD1-LD3 and Rx1 |
| NT1–4 | Normalized T1-4 |
| NTh1–2 | Normalized Th1-2 |
| NSE | Normalized Slope Efficiency |
| m-NSE1–3 | Generated Power Monitoring NSE From mPD1–3, as defined in Equation (2) |
| dt-NSE1–3 | Diffused Transmission NSE From Rx1, as defined in Equation (3) |
| dt-IA123 | Integrated Value of dt-NSE1–3, as defined in Equation (4) |
| Conv1D | 1D Convolutional Neural Network |
| CEG | Clarke Error Grid |
| MARD | Mean Absolute Relative Difference |
| RMSE | Root Mean Square Error |
References
- Lizzo, J.M.; Goyal, A.; Gupta, V. Adult Diabetic Ketoacidosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Calcutt, N.A.; Cooper, M.E.; Kern, T.S.; Schmidt, A.M. Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nat. Rev. Drug Discov. 2009, 8, 417–430. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring-a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Link, M.; Kamecke, U.; Waldenmaier, D.; Pleus, S.; Garcia, A.; Haug, C.; Freckmann, G. Comparative Accuracy Analysis of a Real-time and an Intermittent-Scanning Continuous Glucose Monitoring System. J. Diabetes Sci. Technol. 2021, 15, 287–293. [Google Scholar] [CrossRef]
- Lee, J.E.; Sridharan, B.; Kim, D.; Sung, Y.; Park, J.H.; Lim, H.G. Continuous glucose monitoring: Minimally and non-invasive technologies. Clin. Chim. Acta 2025, 575, 120358. [Google Scholar] [CrossRef]
- Pfützner, A.; Strobl, S.; Sachsenheimer, D.; Lier, A.; Ramljak, S.; Demircik, F. Evaluation of the non-invasive glucose monitoring device GlucoTrack® in patients with type 2 diabetes and subjects with prediabetes. J. Diabetes Treat. 2019, 1, 1070. [Google Scholar]
- Geng, Z.; Tang, F.; Ding, Y.; Li, S.; Wang, X. Noninvasive continuous glucose monitoring using a multisensor-based glucometer and time series analysis. Sci. Rep. 2017, 7, 12650. [Google Scholar] [CrossRef]
- Hina; Saadeh, W. Noninvasive blood glucose monitoring systems using near-infrared technology—A review. Sensors 2022, 22, 4855. [Google Scholar] [CrossRef]
- Todaro, F.; Begarani, F.; Sartori, F.; Luin, S. Is Raman the best strategy towards the development of non-invasive continuous glucose monitoring devices for diabetes management? Front. Chem. 2022, 10, 994272. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, J.; Zhu, J.; Liang, S. An accurate noninvasive blood glucose measurement system using portable near-infrared spectrometer and transfer learning framework. IEEE Sens. J. 2021, 21, 3506–3519. [Google Scholar] [CrossRef]
- Srichan, C.; Srichan, W.; Danvirutai, P.; Ritsongmuang, C.; Sharma, A.; Anutrakulchai, S. Non-invasively accuracy enhanced blood glucose sensor using shallow dense neural networks with NIR monitoring and medical features. Sci. Rep. 2022, 12, 1769. [Google Scholar] [CrossRef]
- Chan, P.Z.; Jin, E.; Jansson, M.; Chew, H.S.J. AI-based noninvasive blood glucose monitoring: Scoping review. J. Med. Internet Res. 2024, 26, e58892. [Google Scholar] [CrossRef]
- Gómez-Peralta, F.; Luque Romero, L.G.; Puppo-Moreno, A.; Riesgo, J. Performance of a non-invasive system for monitoring blood glucose levels based on near-infrared spectroscopy technology (Glucube®). Sensors 2024, 24, 7811. [Google Scholar] [CrossRef]
- Nakazawa, T.; Sekine, R.; Kitabayashi, M.; Hashimoto, Y.; Ienaka, A.; Morishita, K.; Fujii, T.; Ito, M.; Matsushita, F. Non-invasive blood glucose estimation method based on the phase delay between oxy- and deoxyhemoglobin using visible and near-infrared spectroscopy. J. Biomed. Opt. 2024, 29, 037001. [Google Scholar] [CrossRef]
- Sun, Y.; Cano-Garcia, H.; Kallos, E.; O’Brien, F.; Akintonde, A.; Motei, D.; Ancu, O.; Mackenzie, R.W.A.; Kosmas, P. Random forest analysis of combined millimeter-wave and near-infrared sensing for noninvasive glucose detection. IEEE Sens. J. 2023, 23, 20294–20309. [Google Scholar] [CrossRef]
- Oñate, W.; Ramos-Zurita, E.; Pallo, J.-P.; Manzano, S.; Ayala, P.; Garcia, M.V. NIR-based electronic platform for glucose monitoring for the prevention and control of diabetes mellitus. Sensors 2024, 24, 4190. [Google Scholar] [CrossRef]
- Vanaja, S.; Ravi Babu, T.; Malathi, M.; Saxena, K.K.; Sheela, J.J.J.; Suruthi, S.; Stalin, B.; Nagaprasad, M.; Krishnaraj, R.; Bandhu, D.; et al. Non-invasive glucometer monitoring system through optical based near-infrared sensor method. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2024, 12, 2327423. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wei, J.; Zheng, X.; Huang, X.; Jiang, J.; Guo, X.; Liang, Y. Noninvasive blood glucose detection system with infrared pulse sensor and hybrid feature neural network. IEEE Sens. J. 2024, 24, 13385–13394. [Google Scholar] [CrossRef]
- Hina; Saadeh, W. A 186μW photoplethysmography-based noninvasive glucose sensing SoC. IEEE Sens. J. 2022, 22, 14185–14195. [Google Scholar] [CrossRef]
- Valero, M.; Pola, P.; Falaiye, O.; Ingram, K.H.; Zhao, L.; Shahriar, H.; Ahamed, S.I. Development of a noninvasive blood glucose monitoring system prototype: Pilot study. JMIR Form. Res. 2022, 6, e38664. [Google Scholar] [CrossRef]
- Heise, H.M.; Delbeck, S.; Marbach, R. Noninvasive monitoring of glucose using near-infrared reflection spectroscopy of skin-constraints and effective novel strategy in multivariate calibration. Biosensors 2021, 11, 64. [Google Scholar] [CrossRef]
- Uwadaira, Y.; Ikehata, A.; Momose, A.; Miura, M. Identification of informative bands in the short-wavelength NIR region for non-invasive blood glucose measurement. Biomed. Opt. Express 2016, 7, 2729–2737. [Google Scholar] [CrossRef]
- Maruo, K.; Yamada, Y. Near-infrared noninvasive blood glucose prediction without using multivariate analyses: Introduction of imaginary spectra due to scattering change in the skin. J. Biomed. Opt. 2015, 20, 047003. [Google Scholar] [CrossRef]
- Olesberg, J.T.; Arnold, M.A.; Mermelstein, C.; Schmit, J.; Wagner, J. Comparison of Combination and First Overtone Spectral Regions for Near-Infrared Calibration Models for Glucose and Other Biomolecules in Aqueous Solutions. Anal. Chem. 2004, 76, 5405–5413. [Google Scholar]
- Ghazaryan, A.; Ovsepian, S.V.; Ntziachristos, V. Extended Near-Infrared Optoacoustic Spectrometry for Sensing Physiological Concentrations of Glucose. Front. Endocrinol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Fathimal, S.M.; Kumar, J.S.; Prabha, J.A.; Selvaraj, J.; Shilparani, F.J.F.; Kirubha, A.S.P. Potential of Near-Infrared Optical Techniques for Non-Invasive Blood Glucose Measurement: A Pilot Study. IRBM 2025, 46, 100870. [Google Scholar] [CrossRef]
- Zilinsky, I.; Erdmann, D.; Weissman, O.; Hammer, N.; Sora, M.C.; Schenck, T.L.; Cotofana, S. Reevaluation of the arterial blood supply of the auricle. J. Anat. 2017, 230, 315–324. [Google Scholar] [CrossRef]
- Uluç, N.; Glasl, S.; Gasparin, F.; Yuan, T.; He, H.; Jüstel, D.; Pleitez, M.A.; Ntziachristos, V. Non-invasive measurements of blood glucose levels by time-gating mid-infrared optoacoustic signals. Nat. Metab. 2024, 6, 678–686. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.K.; Huh, C. Noninvasive glucose monitoring evaluation with diffused transmitted NIRS in soft silicone-based tissue phantoms. In Proceedings of the IEEE SENSORS, Vienna, Austria, 29 October–1 November 2023; pp. 1–4. [Google Scholar]
- Filatova, S.A.; Shcherbakov, I.A.; Tsvetkov, V.B. Optical properties of animal tissues in the wavelength range from 350 to 2600 nm. J. Biomed. Opt. 2017, 22, 035009. [Google Scholar] [CrossRef]
- Tena, F.; Garnica, O.; Lanchares, J.; Hidalgo, J.I. Ensemble Models of Cutting-Edge Deep Neural Networks for Blood Glucose Prediction in Patients with Diabetes. Sensors 2021, 21, 7090. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Avci, O.; Abdeljaber, O.; Ince, T.; Gabbouj, M.; Inman, D.J. 1D convolutional neural networks and applications: A survey. Mech. Syst. Signal Process. 2021, 151, 107398. [Google Scholar] [CrossRef]
- Zeynali, M.; Alipour, K.; Tarvirdizadeh, B.; Ghamari, M. Non-invasive blood glucose monitoring using PPG signals with various deep learning models and implementation using TinyML. Sci. Rep. 2025, 15, 581. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Reiterer, F.; Polterauer, P.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Freckmann, G.; Heinemann, L.; del Re, L. Significance and reliability of MARD for the accuracy of CGM systems. J. Diabetes Sci. Technol. 2017, 11, 59–67. [Google Scholar] [CrossRef]
- Munoz-Organero, M. Deep Physiological Model for Blood Glucose Prediction in T1DM Patients. Sensors 2020, 20, 3896. [Google Scholar] [CrossRef]
- Hossain, D.; Ghosh, T.; Haider Imtiaz, M.; and Sazonov, E. Ear canal pressure sensor for food intake detection. Front. Electron. 2023, 4, 1173607. [Google Scholar] [CrossRef]
- Langarica, S.; de la Vega, D.; Cariman, N.; Miranda, M.; Andrade, D.C.; Nunez, F.; Rodriguez-Fernandez, M. Deep Learning-Based Glucose Prediction Models: A Guide for Practitioners and a Curated Dataset for Improved Diabetes Management. IEEE Open J. Eng. Med. Biol. 2024, 5, 467–475. [Google Scholar] [CrossRef]
- Ghimire, S.; Celik, T.; Gerdes, M.; Omlin, C.W. Deep learning for blood glucose level prediction: How well do models generalize across different data sets? PLoS ONE 2024, 19, e0310801. [Google Scholar] [CrossRef]
- Anand, P.K.; Shin, D.R.; Memon, M.L. Adaptive Boosting Based Personalized Glucose Monitoring System (PGMS) for Non-Invasive Blood Glucose Prediction with Improved Accuracy. Diagnostics 2020, 10, 285. [Google Scholar] [CrossRef]
- Bian, Q.; As’arry, A.; Cong, X.; Rezali, K.A.B.M.; Raja Ahmad, R.M.K.B. A hybrid Transformer-LSTM model apply to glucose prediction. PLoS ONE 2024, 19, e0310084. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.; Liang, Z.; Liu, R.; Chen, P.; Liu, Y.; Li, J.; Feng, Z.; Li, L.M.; Sheng, B.; et al. A pretrained transformer model for decoding individual glucose dynamics from continuous glucose monitoring data. Nat. Sci. Rev. 2025, 12, 5. [Google Scholar] [CrossRef]

| Volunteer | Earlobe Thickness (mm) | NI-CGM Sensor | |||
|---|---|---|---|---|---|
| Subject | HbA1c | Before | After | Variation | Prototype |
| V1 | Normal | 4.06 | 4.09 | +0.74% | EPC and PS-Unit #1 |
| V2 | Normal | 4.80 | 4.72 | −1.67% | |
| V3 | Pre-diabetic | 4.47 | 4.08 | −8.72% | |
| V4 | Pre-diabetic | 3.68 | 3.67 | −0.27% | EPC and PS-Unit #2 |
| V5 | Normal | 3.77 | 3.50 | −7.16% | |
| Volunteer | Earlobe Thickness | NI-CGM Data | Performances | ||||
|---|---|---|---|---|---|---|---|
| Group | Subject | Activity | Variation | System# | Frame# | RMSE | R2 |
| G1 | V1 | Low | 0.74% | EPC and PS-Unit #1 | 501 | 4.52 | 0.95 |
| V2 | Middle | –1.67% | 469 | 5.13 | 0.92 | ||
| V3 | High | –8.72% | 385 | 7.91 | 0.84 | ||
| G2 | V4 | Low | –0.27% | EPC and PS-Unit #2 | 701 | 7.85 | 0.95 |
| V5 | High | –7.16% | 466 | 14.90 | 0.75 | ||
| Volunteers | Frame# | Test Results | ||||
|---|---|---|---|---|---|---|
| Group | Subjects | Mixed | Test | CEG Zone-A | RMSE | MARD |
| G1 | V1~V3 | 1355 | 271 | 97.0% | 7.95 | 5.20% |
| G2 | V4~V5 | 1167 | 234 | 93.2% | 14.37 | 7.56% |
| G1 and G2 | V1~V5 | 2522 | 505 | 90.9% | 14.13 | 8.44% |
| Classification | Cross-Subjects | Test Results | ||
|---|---|---|---|---|
| Training | Testing | RMSE | MARD | |
| Intra-Group (G1) | V2 + V3 | V1 | 25.06 | 16.54 |
| V1 + V3 | V2 | 39.73 | 24.95 | |
| V1 + V2 | V3 | 58.66 | 62.57 | |
| Cross-Group (G1, G2) | V1 + V2 + V3 | V4 | 69.07 | 30.21 |
| V1 + V2 + V3 + V5 | V4 | 50.50 | 24.98 | |
| V1 + V2 + V3 | V5 | 25.08 | 17.33 | |
| V1 + V2 + V3 + V4 | V5 | 28.76 | 20.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, B.K.; Park, M.-R.; Cho, H.; Huh, C. Noninvasive Continuous Glucose Monitoring Using Multimodal Near-Infrared, Temperature, and Pressure Signals on the Earlobe. Biosensors 2025, 15, 406. https://doi.org/10.3390/bios15070406
Kim J, Kim BK, Park M-R, Cho H, Huh C. Noninvasive Continuous Glucose Monitoring Using Multimodal Near-Infrared, Temperature, and Pressure Signals on the Earlobe. Biosensors. 2025; 15(7):406. https://doi.org/10.3390/bios15070406
Chicago/Turabian StyleKim, Jongdeog, Bong Kyu Kim, Mi-Ryong Park, Hyoyoung Cho, and Chul Huh. 2025. "Noninvasive Continuous Glucose Monitoring Using Multimodal Near-Infrared, Temperature, and Pressure Signals on the Earlobe" Biosensors 15, no. 7: 406. https://doi.org/10.3390/bios15070406
APA StyleKim, J., Kim, B. K., Park, M.-R., Cho, H., & Huh, C. (2025). Noninvasive Continuous Glucose Monitoring Using Multimodal Near-Infrared, Temperature, and Pressure Signals on the Earlobe. Biosensors, 15(7), 406. https://doi.org/10.3390/bios15070406






