Electrochemical Aptamer-Based Biosensors for Sepsis Diagnosis: Recent Advances, Challenges, and Future Perspectives (2020–2025)
Abstract
1. Introduction
2. Clinical Overview of Sepsis and Diagnostic Methods
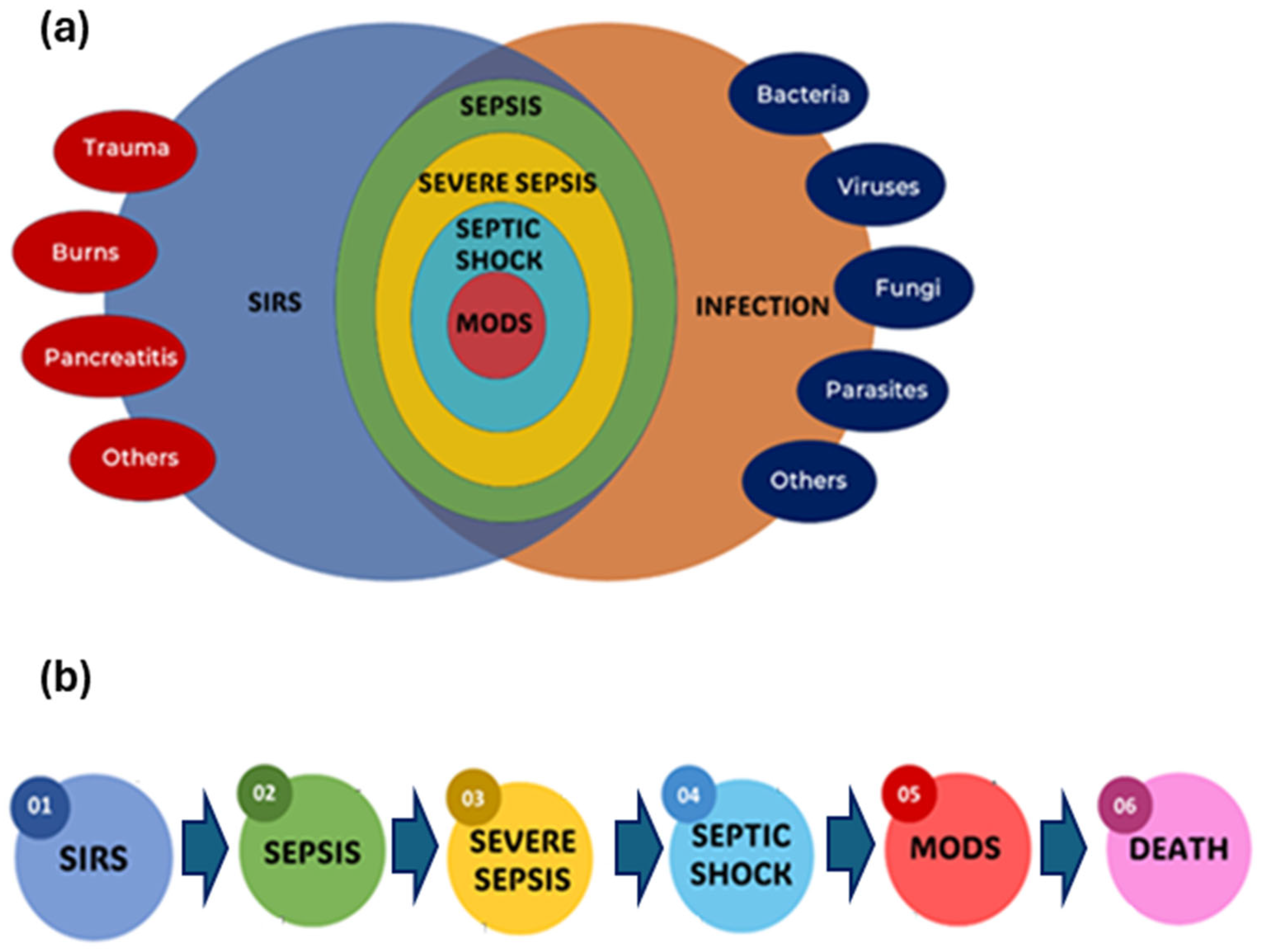
2.1. Definition and Progression of Sepsis Clinically
2.2. Diagnostic Techniques for Sepsis: Conventional and Advanced Analytical Approaches
2.2.1. Blood Cultures (BCs)
2.2.2. PCR-Based Techniques
2.2.3. Spectroscopy-Based Approaches
2.2.4. Nanotechnology-Based Sensors/Biosensors
2.2.5. Commercial Sepsis Diagnosis Kits
3. Electrochemical and Aptamer-Based Platforms: The Frontier of Sepsis Diagnostics
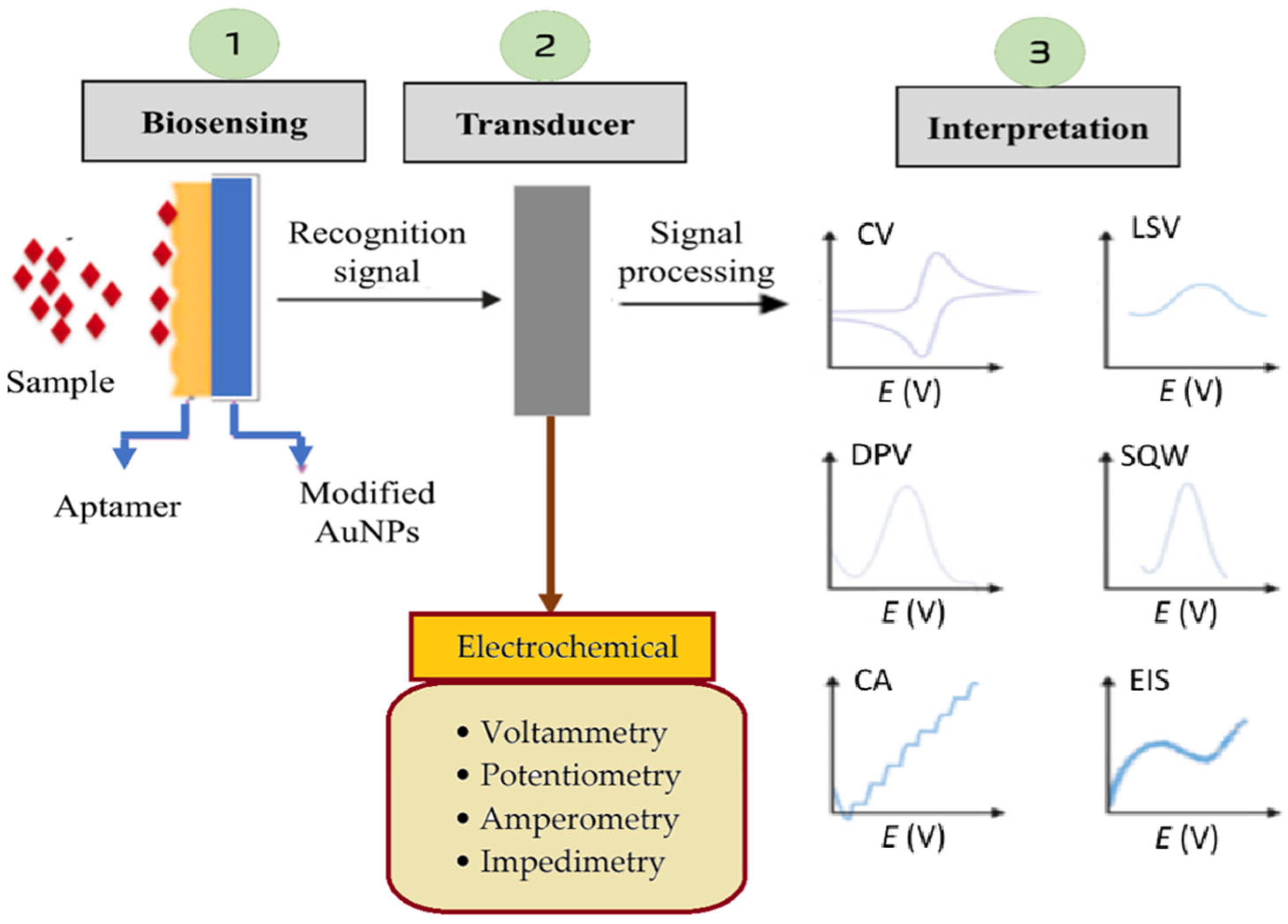
3.1. Principles of Electrochemical Biosensor Operation
3.2. Significance of Aptamers in Sepsis Biosensing as Superior Alternatives to Antibodies
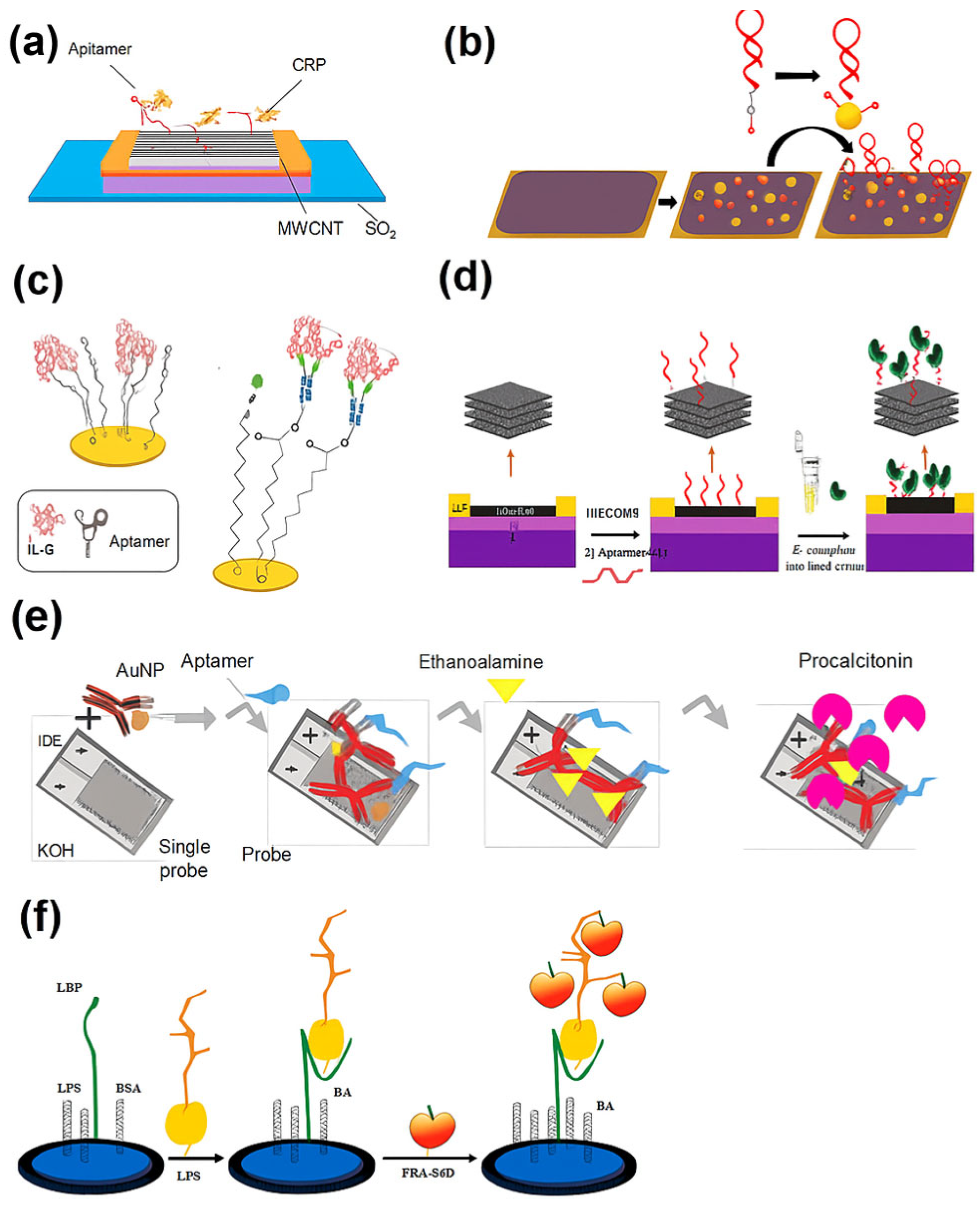
3.3. Recent Electrochemical Aptamer-Based Biosensors for Sepsis Biomarkers (January 2020–May 2025)
| Sensor Type | Target Biomarker | LOD | Linear Range | Response Time | Non- Clinical Matrix | Clinical Matrix | Electrochemical Transducer | Notable Features (Strengths) | Limitations | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| CNT-FET aptasensor | CRP | 150.000 pM | 0.05 – 5.00 mg L−1 | 8 min | Buffer | - | FET | Real-time label-free detection and high stability | Tested only in buffer; CRP overlaps with non-septic inflammation | [86] |
| Structure-switching EAB | CRP | 20.000 – 60.000 nM | 1.00–500.00 nM | 1 min | 50% human serum | Voltammetry | Single-step detection, reagentless, and reusable | Limited-to -moderate CRP levels; long-term stability untested | [87] | |
| OECT aptasensor | IL-6 | 60.000 pM | pM–nM | - | Buffer | Human serum | OECT (conductance modulation) | Low-voltage, aqueous compatibility, and miniaturizable | Selective for IL-6 but lacks multiplex capability | [88] |
| Capacitive EIS aptasensor | IL-6 | 5.000 pg mL−1 | 5.00 pg mL−1 – 1.00 ng mL−1 | - | - | 10% human serum | EIS | Capacitive mode, flexible sensor, and suitable for wearables | Capacitive EIS is less established in POC applications | [89] |
| Multiplex aptasensor with electrokinetic strip | IL-6, TNF-α, and miRNA-155 | 0.180 pg mL−1 (IL-6), 0.840 pg mL−1 (TNF-α), 0.001 pg mL−1 (miRNA-155) | - | 5 min | - | Murine sepsis serum | Amperometry/voltammetry (multiplex) | Multiplex detection, femtogram sensitivity, and fast hybridization | Needs validation in human serum; animal models only | [90] |
| IDE AuNP-aptamer– antibody | PCT | 10.000 ng mL−1 | 10.00 – 100.00 ng mL−1 | - | Spiked serum | - | Impedance/ amperometric (IDE) | Hybrid probe (aptamer + antibody) and good correlation in serum | Moderate sensitivity; aptamer/antibody ratio optimization needed | [91] |
| MXene-GO FET aptasensor | LPSs and E. coli | 1.000 pg mL−1 (LPS), 3.000 CFU mL−1 (E. coli) | - | 5 min | - | Human serum | FET | Rapid whole-cell and LPS detection, stable, and portable | Fabrication complexity; long-term reproducibility not shown | [92] |
| Nanopore aptasensor | LPSs | 10.000 ng mL−1 | - | Fast | Tap water | Human serum | Nanopore current blockade | Single-molecule detection and serotype resolution | Needs specialized nanopore equipment | [94] |
| Graphene-PBA aptasensor | LPSs | 3.900 fg mL−1 | - | - | - | - | Amperometry (graphene interface) | Signal amplification via PBA-cis-diol binding | Matrix interference not fully explored | [95] |
| Photo-ATRP amplified aptasensor | LPSs | 0.250 fg mL−1 | 1.00 fg mL−1 – 0.10 pg mL−1 | <4.5 h | - | Human serum | Photo-ATRP with ferrocene polymerization | Dual amplification (chemical + photocatalytic) and ultralow LOD | Longer detection time; red-light setup required | [96] |
| Magnetic Fe3O4@Au aptasensor | HSP70 | 0.525 pg mL−1 | 10.00 pg/mL – 200.00 ng/mL | - | - | Human serum | Impedance + magnetic enrichment | Magnetic recovery, reusability, and good performance in blood | Sensor regeneration steps may reduce throughput | [97] |
4. Critical Assessment and Future Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physicians |
| ASSURED | Affordable, Sensitive, Specific, User-friendly, Rapid, Equipment-free, and Deliverable |
| AuNPs | Gold nanoparticles |
| BCs | Blood cultures |
| CA | Chronoamperometry |
| CNTs | Carbon nanotubes |
| CRP | C-reactive protein |
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FET | Field-effect transistor |
| Fe3O4@Aus | Au-coated Fe3O4 nanoparticles |
| HSP70 | Heat shock protein 70 |
| IDE | Interdigitated electrode |
| ILs | Interleukins |
| IL-6 | Interleukin-6 |
| LMICs | Low- and middle-income countries |
| LOD | Limit of detection |
| LPSs | Lipopolysaccharides |
| LSPR | Localized surface plasmon resonance |
| LSV | Linear sweep voltammetry |
| MALDI-TOF | Matrix-Assisted Laser Desorption/Ionization Time of Flight |
| MIQEs | Minimum Information for Publication of Quantitative Real-Time PCR Experiments |
| MM | Micromotor |
| MODS | Multiple organ dysfunction syndrome |
| MXene-GO FET | Field-effect transistor aptasensor based on MXene and graphene oxide |
| NGS | Next-generation sequencing |
| OECT | Organic electrochemical transistor |
| PBA | Phenylboronic acid |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| POC | Point of care |
| SCCM | Society of Critical Care Medicine |
| SELEX | Systematic Evolution of Ligands by Exponential Enrichment |
| SERS | Surface-Enhanced Raman Spectroscopy |
| SIRS | Systemic inflammatory response syndrome |
| SPR | Surface plasmon resonance |
| SS-EABs | Structure-switching electrochemical aptamer-based sensors |
| SWV | Square wave voltammetry |
| TNF-α | Tumor necrosis factor-alpha |
References
- Goh, E.L.; See, K.C.; Chua, W.L. Call for a Singapore national action plan for sepsis (SNAPS): Stop sepsis, save lives. Ann. Acad. Med. Singap. 2024, 53, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-resistant sepsis: A critical healthcare challenge. Antibiotics 2024, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The global burden of sepsis and septic shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef]
- Samuel, L. Direct-from-blood detection of pathogens: A review of technology and challenges. J. Clin. Microbiol. 2023, 61, e0023121. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in chemical and biological methods to identify microorganisms—From past to present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Chen, S.; Wang, J. Advances in electrochemical detection of B-type natriuretic peptide as a heart failure biomarker. Int. J. Electrochem. Sci. 2024, 19, 100748. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-enabled biosensors: A review of fundamentals, design principles, materials, and applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- Kaushal, J.B.; Raut, P.; Kumar, S. Organic electronics in biosensing: A promising frontier for medical and environmental applications. Biosensors 2023, 13, 976. [Google Scholar] [CrossRef]
- da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; de Giarola, F.J.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef]
- Trzeciak, A.; Pietropaoli, A.P.; Kim, M. Biomarkers and associated immune mechanisms for early detection and therapeutic management of sepsis. Immune Netw. 2020, 20, e23. [Google Scholar] [CrossRef] [PubMed]
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for point-of-care diagnosis of sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Tanak, A.S.; Sardesai, A.; Muthukumar, S.; Prasad, S. Simultaneous detection of sepsis host response biomarkers in whole blood using electrochemical biosensor. Bioeng. Transl. Med. 2022, 7, e10310. [Google Scholar] [CrossRef]
- Hosseinniay, S.; Rezayan, A.H.; Ghasemi, F.; Malekmohamadi, M.; Taheri, R.A.; Hosseini, M.; Alvandi, H. Fabrication and evaluation of optical nanobiosensor based on localized surface plasmon resonance (LSPR) of gold nanorod for detection of CRP. Anal. Chim. Acta 2023, 1237, 340580. [Google Scholar] [CrossRef] [PubMed]
- Gordón, J.; Arruza, L.; Ibáñez, M.D.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. On the move-sensitive fluorescent aptassay on board catalytic micromotors for the determination of interleukin-6 in Ultra-low serum volumes for neonatal sepsis diagnostics. ACS Sens. 2022, 7, 3144–3152. [Google Scholar] [CrossRef]
- Bonini, A.; Carota, A.G.; Poma, N.; Vivaldi, F.M.; Biagini, D.; Bottai, D.; Lenzi, A.; Tavanti, A.; Di Francesco, F.; Lomonaco, T. Emerging biosensing technologies towards early sepsis diagnosis and management. Biosensors 2022, 12, 894. [Google Scholar] [CrossRef]
- Liu, L.; Han, Z.; An, F.; Gong, X.; Zhao, C.; Zheng, W.; Mei, L.; Zhou, Q. Aptamer-based biosensors for the diagnosis of sepsis. J. Nanobiotechnol. 2021, 19, 216. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Ma, C.; Xu, G.-R.; Ma, J.; Xie, H.; Zhu, W.; Liu, H.; Wang, L.; Wang, Y. Utilizing electrochemical biosensors as an innovative platform for the rapid and on-site detection of animal viruses. Animals 2023, 13, 3141. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T.; Ramanathan, S.; Gopinath, S.C.B. Revolutionizing biosensing through cutting-edge nanomaterials: An in-depth exploration of recent technological advances. Nano-Struct. Nano-Objects 2024, 38, 101128. [Google Scholar] [CrossRef]
- Hanif, A.; Farooq, R.; Rehman, M.U.; Khan, R.; Majid, S.; Ganaie, M.A. Aptamer-based nanobiosensors: Promising healthcare devices. Saudi Pharm. J. 2019, 27, 312–319. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (bio)sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Balaji, S.; Iyengar, K.P.; Jain, V.K.; Rajendran, R.L.; Gangadaran, P. Nanomaterials in point-of-care diagnostics: Bridging the gap between laboratory and clinical practice. Pathol. Res. Pract. 2024, 263, 155685. [Google Scholar] [CrossRef] [PubMed]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Chen, X.; Zhang, Y.; Wang, X.; Zhou, N. Systematic bio-fabrication of aptamers and their applications in engineering biology. Syst. Microbiol. Biomanufacturing 2023, 3, 223–245. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in biosensor technologies for healthcare diagnostics and therapeutic drug monitoring: Applications, recent progress, and future research challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Napit, R.; Jaysawal, S.K.; Chowdhury, R.; Catague, J.; Melke, H.; Pham, C.V.; Xu, H.; Jia, L.; Lin, J.; Hou, Y.; et al. Aptasensors and advancement in molecular recognition technology. Adv. Mater. Technol. 2025, 10, 2400504. [Google Scholar] [CrossRef]
- Ling, L.; Mui, O.O.Y.; Laupland, K.B.; Lefrant, J.-Y.; Roberts, J.A.; Gopalan, P.D.; Lipman, J.; Joynt, G.M.; Stelfox, T.; Niven, D.; et al. Scoping review on diagnostic criteria and investigative approach in sepsis of unknown origin in critically Ill patients. J. Intensive Care 2022, 10, 44. [Google Scholar] [CrossRef]
- Duncan, C.F.; Youngstein, T.; Kirrane, M.D.; Lonsdale, D.O. Diagnostic challenges in Sepsis. Curr. Infect. Dis. Rep. 2021, 23, 22. [Google Scholar] [CrossRef]
- Dünser, M.W.; Festic, E.; Dondorp, A.; Kissoon, N.; Ganbat, T.; Kwizera, A.; Haniffa, R.; Baker, T.; Schultz, M.J. Recommendations for sepsis management in resource-limited settings. Intensive Care Med. 2012, 38, 557–574. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the ‘golden age’ of bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef]
- Trung, N.T.; Thau, N.S.; Bang, M.H.; Song, L.H. PCR-based sepsis@quick test is superior in comparison with blood culture for identification of sepsis-causative pathogens. Sci. Rep. 2019, 9, 13663. [Google Scholar] [CrossRef]
- Rasooly, A.; Herold, K.E. Food microbial pathogen detection and analysis using DNA microarray technologies. Foodborne Pathog. Dis. 2008, 5, 531–550. [Google Scholar] [CrossRef]
- Liesenfeld, O.; Lehman, L.; Hunfeld, K.-P.; Kost, G. Molecular diagnosis of sepsis: New aspects and recent developments. Eur. J. Microbiol. Immunol. 2014, 4, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Valenzuela-Sánchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A review of advances in management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The surviving sepsis campaign bundle: 2018 update. Crit. Care Med. 2018, 46, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Narayana Iyengar, S.; Dietvorst, J.; Ferrer-Vilanova, A.; Guirado, G.; Muñoz-Berbel, X.; Russom, A. Toward rapid detection of viable bacteria in whole blood for early sepsis diagnostics and susceptibility testing. ACS Sens. 2021, 6, 3357–3366. [Google Scholar] [CrossRef]
- Mancini, N.; Carletti, S.; Ghidoli, N.; Cichero, P.; Burioni, R.; Clementi, M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin. Microbiol. Rev. 2010, 23, 235–251. [Google Scholar] [CrossRef]
- Peri, A.M.; Harris, P.N.A.; Paterson, D.L. Culture-independent detection systems for bloodstream infection. Clin. Microbiol. Infect. 2022, 28, 195–201. [Google Scholar] [CrossRef]
- Whelan, S.O.; Mulrooney, C.; Moriarty, F.; Cormican, M. Pediatric blood cultures—Turning up the volume: A before and after intervention study. Eur. J. Pediatr. 2024, 183, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Opota, O.; Croxatto, A.; Prod’Hom, G.; Greub, G. Blood culture-based diagnosis of bacteraemia: State of the art. Clin. Microbiol. Infect. 2015, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Book, M.; Lehmann, L.E.; Zhang, X.; Stüber, F. Monitoring infection: From blood culture to polymerase chain reaction (PCR). Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Wellinghausen, N.; Welte, T. Will polymerase chain reaction (PCR)-based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med. 2011, 37, 1069–1076. [Google Scholar] [CrossRef]
- Yamin, D.; Uskoković, V.; Wakil, A.M.; Goni, M.D.; Shamsuddin, S.H.; Mustafa, F.H.; Alfouzan, W.A.; Alissa, M.; Alshengeti, A.; Almaghrabi, R.H. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics 2023, 13, 3246. [Google Scholar] [CrossRef]
- Khodaparast, M.; Sharley, D.; Marshall, S.; Beddoe, T. Advances in point-of-care and molecular techniques to detect waterborne pathogens. NPJ Clean Water 2024, 7, 74. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A basic guide to real time PCR in microbial diagnostics: Definitions, parameters, and everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Ott, S.J.; Musfeldt, M.; Ullmann, U.; Hampe, J.; Schreiber, S. Quantification of intestinal bacterial populations by real-time PCR with a universal primer set and minor groove binder probes: A global approach to the enteric flora. J. Clin. Microbiol. 2004, 42, 2566–2572. [Google Scholar] [CrossRef]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef]
- Borst, A.; Box, A.T.A.; Fluit, A.C. False-positive results and contamination in nucleic acid amplification assays: Suggestions for a prevent and destroy strategy. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 289–299. [Google Scholar] [CrossRef]
- Wattrang, E.; Jäderblom, V.; Jinnerot, T.; Eriksson, H.; Bagge, E.; Persson, M.; Dalgaard, T.S.; Söderlund, R. Detection and quantification of erysipelothrix rhusiopathiae in blood from infected chickens–addressing challenges with detection of DNA from infectious agents in host species with nucleated red blood cells. J. Med. Microbiol. 2019, 68, 1003–1011. [Google Scholar] [CrossRef]
- Cason, C.; D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Comar, M.; Caselli, E. Next-generation sequencing and PCR technologies in monitoring the hospital microbiome and its drug resistance. Front. Microbiol. 2022, 13, 969863. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Kostrzewa, M. Rapid identification of pathogens in positive blood culture of patients with sepsis: Review and meta-analysis of the performance of the sepsityper kit. Int. J. Microbiol. 2015, 2015, 827416. [Google Scholar] [CrossRef]
- Papafilippou, L.; Claxton, A.; Dark, P.; Kostarelos, K.; Hadjidemetriou, M. Nanotools for sepsis diagnosis and treatment. Adv. Healthc. Mater. 2021, 10, 2001378. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Y.; Li, Y.; Shi, L. Recent advances and prospects in nanomaterials for bacterial sepsis management. J. Mater. Chem. B 2023, 11, 10778–10792. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Mackraj, I.; Govender, T. Advances in sepsis diagnosis and management: A paradigm shift towards nanotechnology. J. Biomed. Sci. 2021, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; Santos, T. Nanotechnology applications in sepsis: Essential knowledge for clinicians. Pharmaceutics 2023, 15, 1682. [Google Scholar] [CrossRef]
- Digital PCR Assays. Available online: https://www.rainsureglobal.com/Digital-PCR-Assays-pl3726068.html (accessed on 4 April 2025).
- Loop Diagnostic Product. Available online: https://loopdx.com/product/ (accessed on 4 April 2025).
- PCT test kit. Available online: https://www.medicalexpo.com/prod/guangzhou-kofa-biotechnology-co-ltd/product-121805-849117.html (accessed on 4 April 2025).
- Sepsis Test Kit Optical Q™. Available online: https://www.medicalexpo.com/prod/optibio-co-ltd/product-299840-991270.html (accessed on 4 April 2025).
- Sepsis Test Kit. Available online: https://www.medicalexpo.com/prod/boditech-med-inc/product-67878-874396.html (accessed on 4 April 2025).
- Sepsis Test Kit BCID. Available online: https://www.medicalexpo.com/prod/genmark-diagnostics/product-128668-948599.html (accessed on 4 April 2025).
- Sepsis Assay Kit PATHFAST™ B・R・A・H・M・S. Available online: https://www.medicalexpo.com/prod/phc-europe-bv-phcbi/product-70908-1023517.html (accessed on 4 April 2025).
- PCT Assay Kit Lumipulse® G B・R・A・H・M・S. Available online: https://www.medicalexpo.com/prod/fujirebio/product-95077-832090.html (accessed on 4 April 2025).
- Sepsis Test Kit OTK002. Available online: https://www.medicalexpo.com/prod/hangzhou-singclean-medical-products/product-92787-1104781.html (accessed on 4 April 2025).
- Sepsis Test Kit OTK004. Available online: https://www.medicalexpo.com/prod/hangzhou-singclean-medical-products/product-92787-1104786.html (accessed on 4 April 2025).
- PCT Test Kit. Available online: https://www.medicalexpo.com/prod/nanjing-vazyme-medical-technology-co-ltd/product-303509-1109705.html (accessed on 4 April 2025).
- Sepsis Test Kit IF 1088. Available online: https://www.medicalexpo.com/prod/getein-biotech-inc/product-92691-1017693.html (accessed on 4 April 2025).
- Nurlely; Musa, A.; Lee, Y.H.; Tan, L.L. Potentiometric enzyme biosensor for rapid determination of formaldehyde based on succinimide-functionalized polyacrylate ion-selective membrane. Measurement 2011, 175, 109112. [Google Scholar] [CrossRef]
- Demkiv, O.; Nogala, W.; Stasyuk, N.; Klepach, H.; Danysh, T.; Gonchar, M. Highly sensitive amperometric sensors based on laccase-mimetic nanozymes for the detection of dopamine. RSC Adv. 2024, 14, 5472–5478. [Google Scholar] [CrossRef]
- Nadiah, I.; Gan, K.B.; Nurul Yuziana, M.Y.; Goh, C.T.; Niranjana, K.B.; Tan, L.L. Electrochemical genosensor based on RNA-responsive human telomeric G-quadruplex DNA: A proof-of-concept with SARS-CoV-2 RNA. Talanta 2024, 274, 125916. [Google Scholar] [CrossRef]
- Chin, J.J.C.C.; Muhamad Afiq, A.; Nurul Yuziana, M.Y.; Pike, A.; Goh, C.T.; Shuhadah, M.; Tan, L.L. Enhancing early warning: A DNA biosensor with polyaniline/graphene nanocomposite for label-free voltammetric detection of saxitoxin-producing harmful algae. Chemosphere 2024, 364, 143114. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Dedi, F.; Lee, Y.H.; Alizar, U.; Adlin Azlina, A.K.; Zamri, I. Voltammetric genosensor from silica nanocomposites for transgenic soybean analysis. J. Food Compos. Anal. 2025, 140, 107277. [Google Scholar] [CrossRef]
- Ong, J.Y.; Phang, S.; Goh, C.T.; Pike, A.; Tan, L.L. Impedimetric polyaniline-based aptasensor for aflatoxin B1 determination in agricultural products. Foods 2023, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Mahirah, T.; Tan, L.L.; Nurul Huda, A.K.; Goh, C.T.; Lee, Y.H.; Bahariah, K. Reflectance aptasensor based on metal salphen label for rapid and facile determination of insulin. Talanta 2020, 207, 120321. [Google Scholar] [CrossRef]
- Ning, Y.; Hu, J.; Lu, F. Aptamers used for biosensors and targeted therapy. Biomed. Pharmacother. 2020, 132, 110902. [Google Scholar] [CrossRef]
- Sequeira-Antunes, B.; Ferreira, H.A. Nucleic acid aptamer-based biosensors: A review. Biomedicines 2023, 11, 3201. [Google Scholar] [CrossRef]
- Li, H.-Y.; Jia, W.-N.; Li, X.-Y.; Zhang, L.; Liu, C.; Wu, J. Advances in detection of infectious agents by aptamer-based technologies. Emerg. Microbes Infect. 2020, 9, 1671–1681. [Google Scholar] [CrossRef]
- Graziani, A.C.; Stets, M.I.; Lopes, A.L.K.; Schluga, P.H.C.; Marton, S.; Ferreira, I.M.; De Andrade, A.S.R.; Krieger, M.A.; Cardoso, J. High efficiency binding aptamers for a wide range of bacterial sepsis agents. J. Microbiol. Biotechnol. 2017, 27, 838–843. [Google Scholar] [CrossRef]
- Saad, M.; Faucher, S.P. Aptamers and aptamer-coupled biosensors to detect water-borne pathogens. Front. Microbiol. 2021, 12, 643797. [Google Scholar] [CrossRef]
- Trunzo, N.E.; Hong, K.L. Recent progress in the identification of aptamers against bacterial origins and their diagnostic applications. Int. J. Mol. Sci. 2020, 21, 5074. [Google Scholar] [CrossRef]
- Nur Diyana, J.; Nur-Fadhilah, M.; Tan, L.L.; Nurul Yuziana, M.Y.; Bahariah, K. G-quadruplex microspheres-based optical RNA biosensor for arthropod-borne virus pathogen detection: A proof-of-concept with dengue serotype 2. Int. J. Biol. Macromol. 2022, 199, 1–9. [Google Scholar] [CrossRef]
- Nur-Fadhilah, M.; Edison, E.S.; Nur Syamimi, M.; Mukram, M.M.; Tan, L.L. On-site sensing for aflatoxicosis poisoning via ultraviolet excitable aptasensor based on fluorinated ethylene propylene strip: A promising forensic tool. Sci. Rep. 2024, 14, 17357. [Google Scholar] [CrossRef]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in point-of-care diagnosis: An emerging futuristic approach for biosensing. Nano-Micro Lett. 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Firoozbakhtian, A.; Rezayan, A.H.; Hajghassem, H.; Rahimi, F.; Ghazani, M.F.; Kalantar, M.; Mohamadsharifi, A. Buried-gate MWCNT FET-based nanobiosensing device for real-time detection of CRP. ACS Omega 2022, 7, 7341–7349. [Google Scholar] [CrossRef]
- Whitehouse, W.L.; Lo, L.H.Y.; Kinghorn, A.B.; Shiu, S.C.C.; Tanner, J.A. Structure-switching electrochemical aptasensor for rapid, reagentless, and single-step nanomolar detection of C-reactive protein. ACS Appl. Bio Mater. 2024, 7, 3721–3730. [Google Scholar] [CrossRef]
- Diacci, C.; Burtscher, B.; Berto, M.; Ruoko, T.-P.; Lienemann, S.; Greco, P.; Berggren, M.; Borsari, M.; Simon, D.T.; Bortolotti, C.A. Organic electrochemical transistor aptasensor for interleukin-6 detection. ACS Appl. Mater. Interfaces 2024, 16, 61467–61474. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-Los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Corrigan, D.K. Comparing nanobody and aptamer-based capacitive sensing for detection of interleukin-6 (IL-6) at physiologically relevant levels. Anal. Bioanal. Chem. 2023, 415, 7035–7045. [Google Scholar] [CrossRef]
- Ondevilla, N.A.P.; Liu, P.-W.; Huang, W.-T.; Weng, T.-P.; Lee, N.-Y.; Ma, S.-C.; Huang, J.-J.; Wong, T.-W.; Chang, H.-C. A point-of-care electrochemical biosensor for the rapid and sensitive detection of biomarkers in murine models with LPS-induced sepsis. Biosens. Bioelectron. 2024, 254, 116202. [Google Scholar] [CrossRef]
- Yang, B.; Tian, F.; Yu, H. Gold-antibody-aptamer complexed electrochemical sensing surface for septic arthritis biomarker determination. Heliyon 2024, 10, e34677. [Google Scholar] [CrossRef]
- Alvandi, H.; Asadi, F.; Rezayan, A.H.; Hajghassem, H.; Rahimi, F. Ultrasensitive biosensor based on MXene-GO field-effect transistor for the rapid detection of endotoxin and whole-cell E. coli in human blood serum. Anal. Chim. Acta 2025, 1348, 343816. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M. Environmental protection based on the nanobiosensing of bacterial lipopolysaccharides (LPSs): Material and method overview. RSC Adv. 2022, 12, 9704–9724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Qin, F.; Zheng, X.; Fang, S.; Ding, J.; Wang, D.; Liang, L. Single-molecule lipopolysaccharides identification and the interplay with biomolecules via nanopore readout. Biosens. Bioelectron. 2023, 240, 115641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, F.; Li, G.; Yang, Q.; Huang, X.; Ding, X.; Hu, Q. Sensitive detection of lipopolysaccharides using phenylboronic acid-functionalized graphene as a signal enhancement strategy. Microchem. J. 2025, 209, 112884. [Google Scholar] [CrossRef]
- Yu, W.; Yu, S.; Zhang, F.; Xu, Q.; Zhang, X.; Kong, J. Ultrasensitive electrochemical sensor for lipopolysaccharide detection catalyzed by 3, 4, 9, 10-perylenetetracarboxylic diimide. Anal. Chim. Acta 2025, 1352, 343926. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Liu, Y.; Wang, Y.; Si, Q.; Agyapong, D.A.Y.; Feng, J.; Fang, R.; Tang, L.; Cao, F. A highly sensitive and reusable magnetic nano-electrochemical biosensor for the detection of the liver cancer biomarker heat shock protein 70. Chem. Eng. J. 2025, 505, 159860. [Google Scholar] [CrossRef]
- Russek-Cohen, E.; Feldblyum, T.; Whitaker, K.B.; Hojvat, S. FDA Perspectives on Diagnostic Device Clinical Studies for Respiratory Infections. Clin. Infect. Dis. 2011, 52, S305–S311. [Google Scholar] [CrossRef]

| Method | Product | Target Biomarker | LOD | Response Time | Matrix | Quantification Range | Qualitative/Quantitative | Manufacturer | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Digital PCR | Sepsis Pathogenic Microorganism Detection Kit | 21 bacteria and fungi | 3–5 copies µL−1 | <5 h | Whole blood | - | Both | RainSure SCIENTIFIC | [58] |
| Lateral flow | Rapid sepsis test SeptiLoop | Bacteria | - | 3 h | Whole blood | - | Quantitative | Loop Diagnostics | [59] |
| PCT test kit | PCT | - | 15 min | Serum and plasma | - | Quantitative | Guangzhou KOFA Biotechnology Co., Ltd. | [60] | |
| Sepsis test kit Optical Q™ | PCT | - | 15 min | Blood, serum, and tissue | 0.10–100.00 ng mL−1 | Quantitative | OptiBio Co., Ltd. | [61] | |
| Sepsis test kit | PCT | - | 12 min | Serum, plasma, and whole blood | 0.1–100 ng mL−1 | Quantitative | Boditech Med Inc. | [62] | |
| Blood cultures | Sepsis test kit BCID | Virus, bacteria, fungi | - | 1.5 h | Whole blood | - | Quantitative | GenMark Diagnostics | [63] |
| ELISA | Sepsis assay kit PATHFAST™ | PCT | - | 17 min | Serum, plasma, and whole blood | - | Quantitative | PHC Europe B.V./PHCbi | [64] |
| PCT assay kit Lumipulse® G | CRP, PCT | - | 6 h | Serum and plasma | - | Quantitative | Fujirebio | [65] | |
| Fluorescence immunochromatography | Sepsis test kit OTK002 | PCT | - | - | Serum, plasma, and whole blood | - | Quantitative | Hangzhou Singclean Medical Products | [66] |
| Sepsis test kit OTK004 | IL-6 | - | - | Serum, plasma, whole blood, and bone marrow | - | Quantitative | Hangzhou Singclean Medical Products | [67] | |
| PCT test kit | PCT | - | 15 min | Serum and plasma | 0.02–400 ng mL−1 | Quantitative | Nanjing Vazyme Medical Technology Co., Ltd | [68] | |
| Fluorescence immune assay | Sepsis test kit FiCA™ | PCT | 0.1–100 ng mL−1 | - | Serum, plasma, and whole blood | - | Quantitative | Medlere Limited | [69] |
| Sepsis test kit IF 1088 | IL-6 | - | 15 min | Serum, plasma, and whole blood | - | Quantitative | Getein Biotech Inc. | [69] |
| Sepsis Detection Technologies | Advantages | Disadvantages |
|---|---|---|
| BCs |
|
|
| PCR-based techniques |
|
|
| ELISA |
|
|
| Spectroscopy-based approaches |
|
|
| Nanotechnology-based sensors/biosensors |
|
|
| Electrochemical aptasensors |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.L.; Mohamad, N.S. Electrochemical Aptamer-Based Biosensors for Sepsis Diagnosis: Recent Advances, Challenges, and Future Perspectives (2020–2025). Biosensors 2025, 15, 402. https://doi.org/10.3390/bios15070402
Tan LL, Mohamad NS. Electrochemical Aptamer-Based Biosensors for Sepsis Diagnosis: Recent Advances, Challenges, and Future Perspectives (2020–2025). Biosensors. 2025; 15(7):402. https://doi.org/10.3390/bios15070402
Chicago/Turabian StyleTan, Ling Ling, and Nur Syamimi Mohamad. 2025. "Electrochemical Aptamer-Based Biosensors for Sepsis Diagnosis: Recent Advances, Challenges, and Future Perspectives (2020–2025)" Biosensors 15, no. 7: 402. https://doi.org/10.3390/bios15070402
APA StyleTan, L. L., & Mohamad, N. S. (2025). Electrochemical Aptamer-Based Biosensors for Sepsis Diagnosis: Recent Advances, Challenges, and Future Perspectives (2020–2025). Biosensors, 15(7), 402. https://doi.org/10.3390/bios15070402






