Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases
Abstract
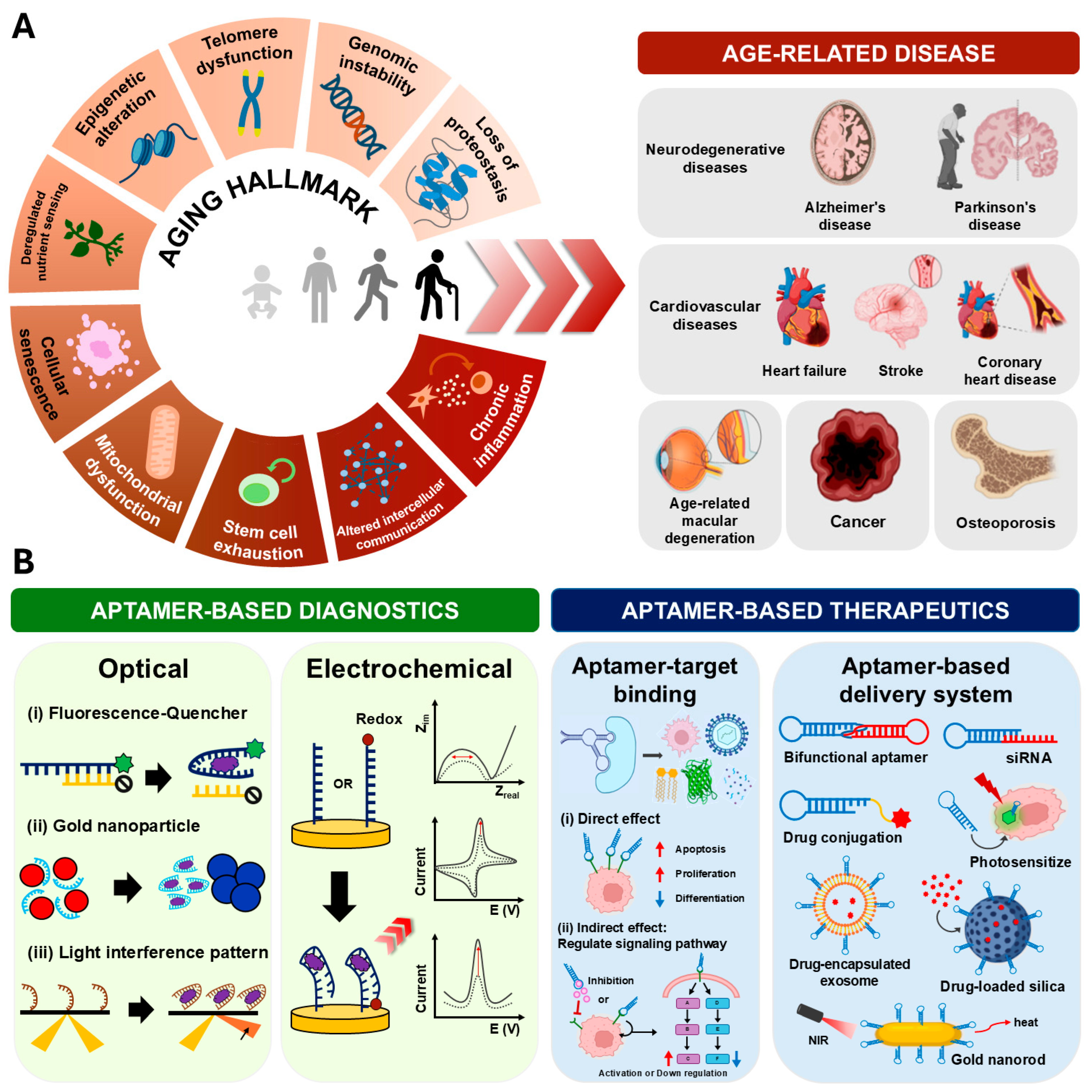
1. Introduction
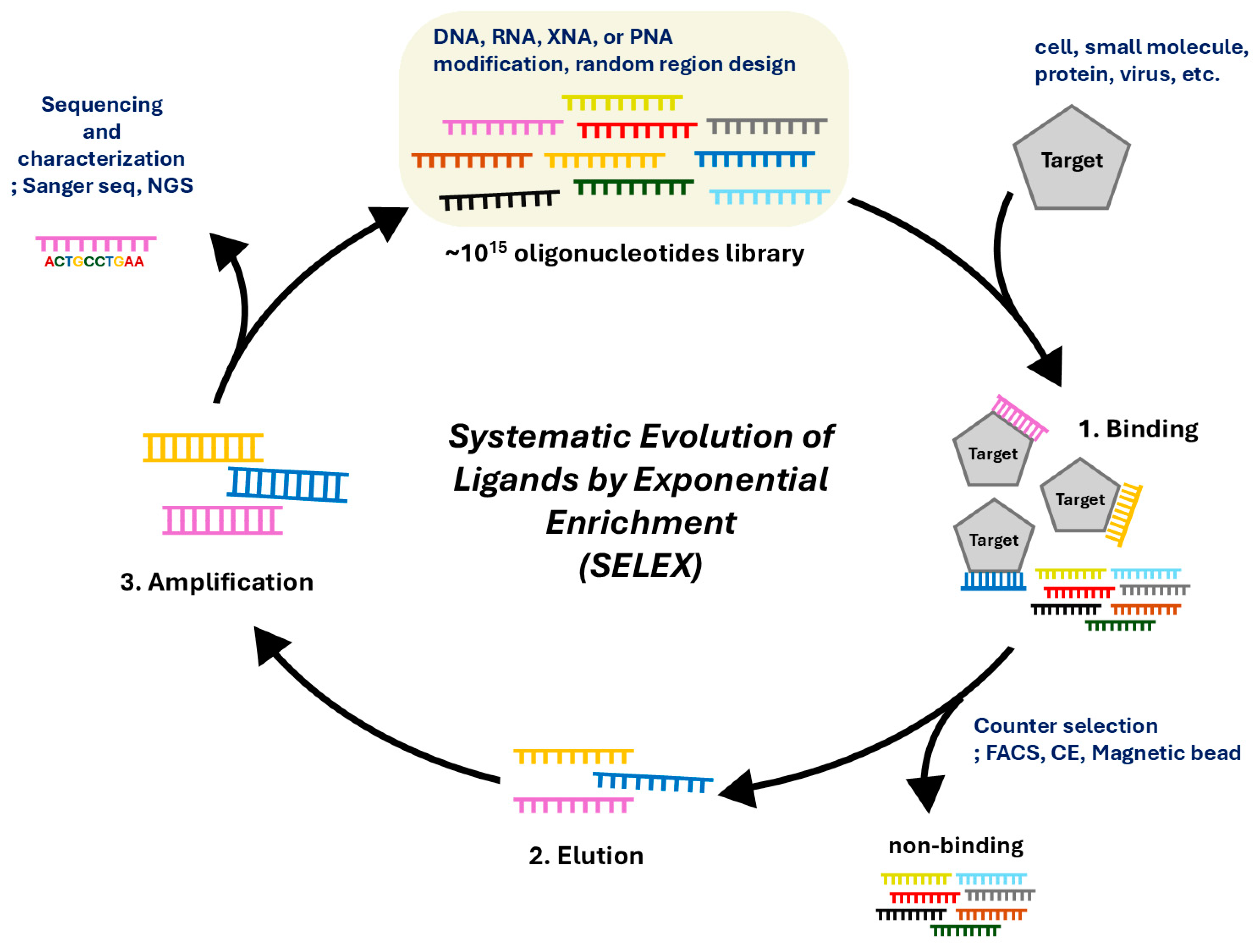
2. Systematic Evolution of Ligands by Exponential Enrichment (SELEX) for Isolation and Characterization of Aptamer
3. Biomarkers for Aging and Age-Related Diseases
| Diseases | Biomarkers | Function | Ref. |
|---|---|---|---|
| Neurodegenerative diseases | Amyloid beta (Aβ) | Formation of amyloid plaques; toxic to nerve cells | [33] |
| Tau protein | Neurofibrillary tangles; toxic to nerve cells | [77] | |
| Alpha-synuclein (α-syn) | Aggregates in Lewy bodies; decreases resistance to neuronal apoptosis | [98] | |
| Age-related macular degeneration (AMD) | Vascular endothelial growth factor (VEGF) | Induces abnormal blood vessel growth beneath the retina | [99] |
| Malondialdehyde (MDA) | Promote cytotoxicity and VEGF expression in retinal tissue | [100] | |
| Interleukin-6 (IL-6) | Pro-inflammatory cytokines; induces VEGF expression and choroidal neovascularization | [101] | |
| Cardiovascular disease (CVD) | C-reactive protein (CRP) | Inflammatory-associated biomarker; promotes endothelial dysfunction | [84] |
| Von Willebrand Factor (vWF) | Promotes platelet adhesion and clot formation; increases the risk of atherosclerosis | [88] | |
| Integrin avβ3 | Cell surface receptor; mediates angiogenesis, and endothelial cell adhesion | [102] | |
| Osteoporosis | Osteocalcin (OC) | Indicators of bone formation (osteogenesis) | [103] |
| Procollagen type 1 N-terminal propeptide (P1NP) | [104] | ||
| Procollagen type 1 C-terminal propeptide (P1CP) | [105] | ||
| Carboxy-terminal cross-linking telopeptide of type I collagen (CTX-1) | Indicators of bone resorption (osteoclastogenesis) | [106] | |
| NF-κB ligand (RANKL) | Regulators of bone turnover | [90] | |
| Advanced glycation end products (AGEs) | [107] | ||
| Sclerostin | [108] | ||
| Cancer | Senescence-associated secretory phenotype (SASP) | Promotes proliferation and metastasis of cancer; induces inflammatory cytokine release | [51] |
| p16INK4a | Tumor suppressor; inhibits cyclin-dependent kinase CDK4 | [109] | |
| Programmed cell death ligand 1 (PD-L1) | Suppresses adaptive immune responses by binding to PD-1 | [110] | |
| Tyrosine-protein kinase-like 7 (PTK7) | Transduces extracellular signals across the cell membrane | [111] |
4. Aptamer-Based Diagnosis and Therapeutics for Aging and Age-Related Diseases
4.1. Aptamer-Based Diagnosis
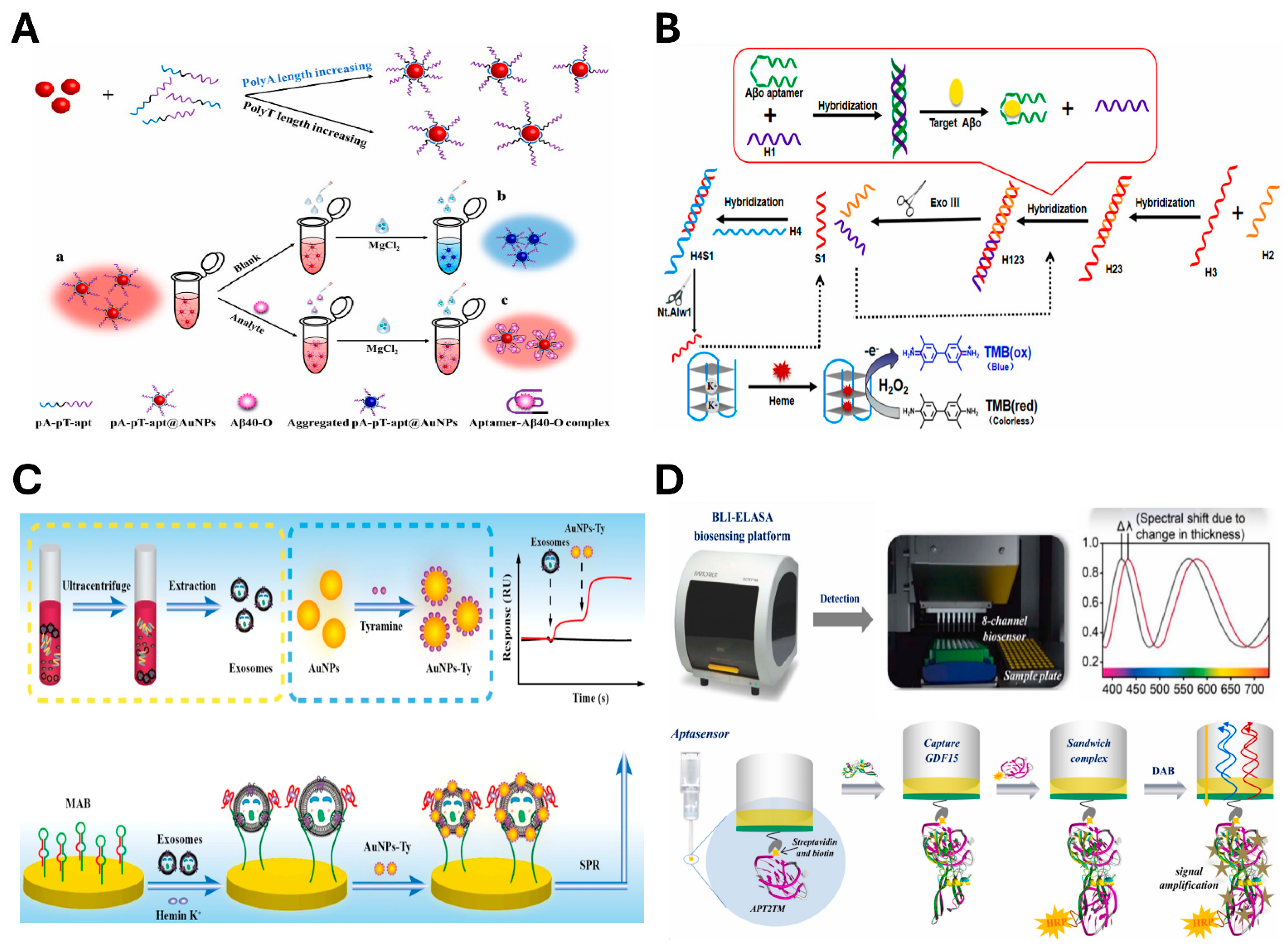
4.1.1. Optical Sensing
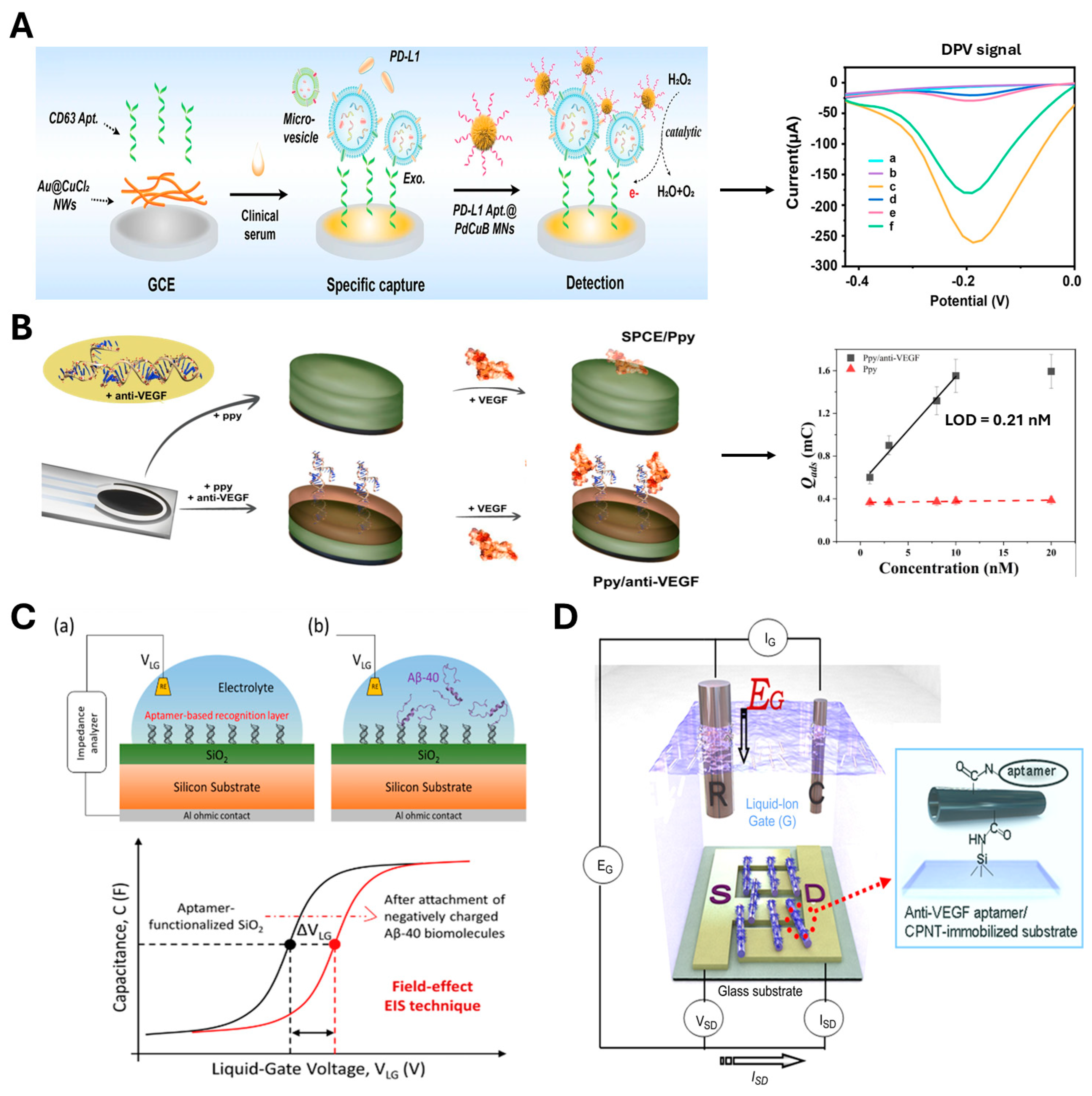
4.1.2. Electrochemical Sensing
4.2. Aptamer-Based Therapeutics
4.2.1. Anti-Aging Strategy
4.2.2. Treatment of Neurodegenerative Diseases
4.2.3. Treatment of Age-Related Macular Degeneration
4.2.4. Treatment of Cardiovascular Diseases
4.2.5. Treatment of Osteoporosis
4.2.6. Treatment of Cancer
5. Concluding Remarks and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Prospects 2024: Summary of Results. Available online: https://population.un.org/wpp/assets/Files/WPP2024_Summary-of-Results.pdf (accessed on 1 January 2025).
- de Magalhaes, J.P. Distinguishing between driver and passenger mechanisms of aging. Nat. Genet. 2024, 56, 204–211. [Google Scholar] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, J. Digital health for aging populations. Nat. Med. 2023, 29, 1623–1630. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Swaroop, K.N.; Chandu, K.; Gorrepotu, R.; Deb, S. A health monitoring system for vital signs using IoT. Internet Things 2019, 5, 116–129. [Google Scholar] [CrossRef]
- Mensa, E.; Latini, S.; Ramini, D.; Storci, G.; Bonafe, M.; Olivieri, F. The telomere world and aging: Analytical challenges and future perspectives. Ageing Res. Rev. 2019, 50, 27–42. [Google Scholar]
- Kim, W.; Shay, J.W. Long-range telomere regulation of gene expression: Telomere looping and telomere position effect over long distances (TPE-OLD). Differentiation 2018, 99, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Multani, A.S.; Cabrera, N.G.; Naylor, M.L.; Laud, P.; Lombard, D.; Pathak, S.; Guarente, L.; DePinho, R.A. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 2004, 36, 877–882. [Google Scholar]
- de Jesus, B.B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Safir Filho, M.; Dao, P.; Gesson, M.; Martin, A.R.; Benhida, R. Development of highly sensitive fluorescent probes for the detection of beta-galactosidase activity—Application to the real-time monitoring of senescence in live cells. Analyst 2018, 143, 2680–2688. [Google Scholar] [PubMed]
- Zhang, S.; Wang, X.; Wang, X.; Wang, T.; Liao, W.; Yuan, Y.; Chen, G.; Jia, X. A novel AIE fluorescent probe for beta-galactosidase detection and imaging in living cells. Anal. Chim. Acta 2022, 1198, 339554. [Google Scholar]
- Liu, J.; Ma, X.W.; Cui, C.; Chen, Z.X.; Wang, Y.; Deenik, P.R.; Cui, L.N. Noninvasive NIR Imaging of Senescence viaIn Situ Labeling. J. Med. Chem. 2021, 64, 17969–17978. [Google Scholar] [PubMed]
- Cai, Y.; Zhou, H.; Zhu, Y.; Sun, Q.; Ji, Y.; Xue, A.; Wang, Y.; Chen, W.; Yu, X.; Wang, L.; et al. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020, 30, 574–589. [Google Scholar]
- Munoz-Espin, D.; Rovira, M.; Galiana, I.; Gimenez, C.; Lozano-Torres, B.; Paez-Ribes, M.; Llanos, S.; Chaib, S.; Munoz-Martin, M.; Ucero, A.C.; et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018, 10, e9355. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Chen, Q.; Wan, Y.; Liu, Z.; Zhang, J.; Huang, C.; Xu, Z.; Li, S.; Lee, C.S.; et al. An Activatable NIR Probe for the Detection and Elimination of Senescent Cells. Anal. Chem. 2022, 94, 5425–5431. [Google Scholar]
- de Magalhaes, J.P.; Stevens, M.; Thornton, D. The Business of Anti-Aging Science. Trends Biotechnol. 2017, 35, 1062–1073. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar]
- Farooqui, T.; Farooqui, A.A. Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mech. Ageing Dev. 2009, 130, 203–215. [Google Scholar]
- Rodriguez, M.; Rodriguez-Sabate, C.; Morales, I.; Sanchez, A.; Sabate, M. Parkinson’s disease as a result of aging. Aging Cell 2015, 14, 293–308. [Google Scholar]
- Sengoku, R. Aging and Alzheimer’s disease pathology. Neuropathology 2020, 40, 22–29. [Google Scholar] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; da Cunha, L.C.; Saavedra-Rodriguez, R.; Rodrigues, K.L.; Naves, L.M.; Mourao, A.A.; da Silva, E.F.; Williams, N.E.E.; Martins, J.L.R.; Sousa, R.B.; et al. Aging-Induced Biological Changes and Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 7156435. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Ki, M.R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural Compounds for Preventing Age-Related Diseases and Cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, J. Age-related hearing loss or presbycusis. Eur. Arch. Otorhinolaryngol. 2010, 267, 1179–1191. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar]
- Han, Y.; Kim, D.H.; Pack, S.P. Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives. Mar. Drugs 2024, 22, 496. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Budde, B.; Schartner, J.; Tönges, L.; Kötting, C.; Nabers, A.; Gerwert, K. Reversible Immuno-Infrared Sensor for the Detection of Alzheimer’s Disease Related Biomarkers. ACS Sens. 2019, 4, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Gupta, A.K.; Purwidyantri, A.; Prabowo, B.A.; Chen, C.H.; Chuang, C.C.; Tian, Y.C.; Lu, Y.J.; Lai, C.S. Sensing Alzheimer’s Disease Utilizing Au Electrode by Controlling Nanorestructuring. Chemosensors 2022, 10, 94. [Google Scholar] [CrossRef]
- Adolfsson, O.; Pihlgren, M.; Toni, N.; Varisco, Y.; Buccarello, A.L.; Antoniello, K.; Lohmann, S.; Piorkowska, K.; Gafner, V.; Atwal, J.K.; et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J. Neurosci. 2012, 32, 9677–9689. [Google Scholar] [CrossRef]
- Guarente, L.; Sinclair, D.A.; Kroemer, G. Human trials exploring anti-aging medicines. Cell Metab. 2024, 36, 354–376. [Google Scholar]
- Dolton, M.J.; Chesterman, A.; Moein, A.; Sink, K.M.; Waitz, A.; Blondeau, K.; Kerchner, G.A.; Hu, N.; Brooks, L.; Roden, A.; et al. Safety, Tolerability, and Pharmacokinetics of High-Volume Subcutaneous Crenezumab, With and Without Recombinant Human Hyaluronidase in Healthy Volunteers. Clin. Pharmacol. Ther. 2021, 110, 1337–1348. [Google Scholar] [CrossRef]
- Yaku, K.; Okabe, K.; Nakagawa, T. NAD metabolism: Implications in aging and longevity. Ageing Res. Rev. 2018, 47, 1–17. [Google Scholar]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar]
- Herdewijn, P.; Marlière, P. Toward Safe Genetically Modified Organisms through the Chemical Diversification of Nucleic Acids. Chem. Biodivers. 2009, 6, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Cha, H.; Niu, J.; Soh, H.T.; Lee, J.H.; Pack, S.P. DNA-controlled protein fluorescence: Design of aptamer-split peptide hetero-modulator for GFP to respond to intracellular ATP levels. Nucleic Acids Res. 2024, 52, 8063–8071. [Google Scholar] [CrossRef]
- Park, K.S.; Park, T.I.; Lee, J.E.; Hwang, S.Y.; Choi, A.; Pack, S.P. Aptamers and Nanobodies as New Bioprobes for SARS-CoV-2 Diagnostic and Therapeutic System Applications. Biosensors 2024, 14, 146. [Google Scholar] [CrossRef]
- Fu, J.; Yao, F.; An, Y.; Li, X.; Wang, W.; Yang, X.-D. Novel bispecific aptamer targeting PD-1 and nucleolin for cancer immunotherapy. Cancer Nanotechnol. 2023, 14, 27. [Google Scholar] [CrossRef]
- Rubahamya, B.; Dong, S.; Thurber, G.M. Clinical translation of antibody drug conjugate dosing in solid tumors from preclinical mouse data. Sci. Adv. 2024, 10, eadk1894. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.K.; Son, R.G.; Pack, S.P. Novel enzymatic single-nucleotide modification of DNA oligomer: Prevention of incessant incorporation of nucleotidyl transferase by ribonucleotide-borate complex. Nucleic Acids Res. 2019, 47, e102. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, A.; Park, T.I.; Pack, S.P. Fluorometric and Colorimetric Method for SARS-CoV-2 Detection Using Designed Aptamer Display Particles. Biosensors 2024, 14, 113. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.S.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [PubMed]
- Mendonsa, S.D.; Bowser, M.T. In vitro evolution of functional DNA using capillary electrophoresis. J. Am. Chem. Soc. 2004, 126, 20–21. [Google Scholar] [PubMed]
- Bruno, J.G. In vitro selection of DNA to chloroaromatics using magnetic microbead-based affinity separation and fluorescence detection. Biochem. Biophys. Res. Commun. 1997, 234, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Ahmed, M.S.; Dolf, A.; Endl, E.; Knolle, P.A.; Famulok, M. Fluorescence-activated cell sorting for aptamer SELEX with cell mixtures. Nat. Protoc. 2010, 5, 1993–2004. [Google Scholar]
- Park, J.W.; Tatavarty, R.; Kim, D.W.; Jung, H.T.; Gu, M.B. Immobilization-free screening of aptamers assisted by graphene oxide. Chem. Commun. 2012, 48, 2071–2073. [Google Scholar]
- Sefah, K.; Shangguan, D.; Xiong, X.; O’Donoghue, M.B.; Tan, W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010, 5, 1169–1185. [Google Scholar]
- Vijg, J.; Dong, X. Pathogenic Mechanisms of Somatic Mutation and Genome Mosaicism in Aging. Cell 2020, 182, 12–23. [Google Scholar]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar]
- Tedone, E.; Huang, E.; O’Hara, R.; Batten, K.; Ludlow, A.T.; Lai, T.P.; Arosio, B.; Mari, D.; Wright, W.E.; Shay, J.W. Telomere length and telomerase activity in T cells are biomarkers of high-performing centenarians. Aging Cell 2019, 18, e12859. [Google Scholar]
- Oh, E.S.; Petronis, A. Origins of human disease: The chrono-epigenetic perspective. Nat. Rev. Genet. 2021, 22, 533–546. [Google Scholar]
- Seale, K.; Horvath, S.; Teschendorff, A.; Eynon, N.; Voisin, S. Making sense of the ageing methylome. Nat. Rev. Genet. 2022, 23, 585–605. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Lipinski, M.M.; Zheng, B.; Lu, T.; Yan, Z.; Py, B.F.; Ng, A.; Xavier, R.J.; Li, C.; Yankner, B.A.; Scherzer, C.R.; et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2010, 107, 14164–14169. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Ruzankina, Y.; Brown, E.J. Relationships between stem cell exhaustion, tumour suppression and ageing. Br. J. Cancer 2007, 97, 1189–1193. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Ellison-Hughes, G.M. Editorial: Stem cell exhaustion in aging. Front. Aging 2024, 5, 1433702. [Google Scholar] [CrossRef]
- Fafian-Labora, J.A.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [PubMed]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome-How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar]
- Hou, Y.J.; Dan, X.L.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar]
- Beharry, C.; Cohen, L.S.; Di, J.; Ibrahim, K.; Briffa-Mirabella, S.; Alonso, A.D. Tau-induced neurodegeneration: Mechanisms and targets. Neurosci. Bull. 2014, 30, 346–358. [Google Scholar]
- Ingelsson, M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016, 10, 408. [Google Scholar]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic Interactions between Aβ, Tau, and α-Synuclein: Acceleration of Neuropathology and Cognitive Decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid beta, Tau, and alpha-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; de Jong, P.T. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Kommineni, V.K.; William, A.; Detrick, B.; Hooks, J.J. Regulation of VEGF expression in human retinal cells by cytokines: Implications for the role of inflammation in age-related macular degeneration. J. Cell. Physiol. 2012, 227, 116–126. [Google Scholar]
- Droho, S.; Cuda, C.M.; Perlman, H.; Lavine, J.A. Macrophage-derived interleukin-6 is necessary and sufficient for choroidal angiogenesis. Sci. Rep. 2021, 11, 18084. [Google Scholar] [CrossRef]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. S1), 9–14. [Google Scholar] [CrossRef]
- Zhuang, Q.; Shen, C.; Chen, Y.; Zhao, X.; Wei, P.; Sun, J.; Ji, Y.; Chen, X.; Yang, S. Association of high sensitive C-reactive protein with coronary heart disease: A Mendelian randomization study. BMC Med. Genet. 2019, 20, 170. [Google Scholar] [CrossRef]
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ. 2005, 173, 1191–1202. [Google Scholar] [CrossRef]
- Jenkins, W.S.; Vesey, A.T.; Stirrat, C.; Connell, M.; Lucatelli, C.; Neale, A.; Moles, C.; Vickers, A.; Fletcher, A.; Pawade, T.; et al. Cardiac alpha(V)beta(3) integrin expression following acute myocardial infarction in humans. Heart 2017, 103, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Eastell, R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: Clinical utility in metabolic bone disease assessment. J. Clin. Endocrinol. Metab. 2005, 90, 6323–6331. [Google Scholar]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 2006, 8, 1291–1297. [Google Scholar]
- Bent, E.H.; Gilbert, L.A.; Hemann, M.T. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 2016, 30, 1811–1821. [Google Scholar]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar]
- Ferrara, N.; Mass, R.D.; Campa, C.; Kim, R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu. Rev. Med. 2007, 58, 491–504. [Google Scholar]
- Ye, F.; Kaneko, H.; Hayashi, Y.; Takayama, K.; Hwang, S.J.; Nishizawa, Y.; Kimoto, R.; Nagasaka, Y.; Tsunekawa, T.; Matsuura, T.; et al. Malondialdehyde induces autophagy dysfunction and VEGF secretion in the retinal pigment epithelium in age-related macular degeneration. Free Radic. Biol. Med. 2016, 94, 121–134. [Google Scholar] [PubMed]
- Xiao, R.; Lei, C.; Zhang, Y.; Zhang, M. Interleukin-6 in retinal diseases: From pathogenesis to therapy. Exp. Eye Res. 2023, 233, 109556. [Google Scholar] [PubMed]
- Zhang, S.; Zhang, Q.; Lu, Y.; Chen, J.; Liu, J.; Li, Z.; Xie, Z. Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 4096. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, D.; Lal, A.K. Serum osteocalcin as a diagnostic biomarker for primary osteoporosis in women. J. Clin. Diagn. Res. JCDR 2015, 9, RC04–RC07. [Google Scholar]
- Krege, J.H.; Lane, N.E.; Harris, J.M.; Miller, P.D. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos. Int. 2014, 25, 2159–2171. [Google Scholar] [PubMed]
- Hassager, C.; Jensen, L.T.; Johansen, J.S.; Riis, B.J.; Melkko, J.; Podenphant, J.; Risteli, L.; Christiansen, C.; Risteli, J. The Carboxy-Terminal Propeptide of Type-I Procollagen in Serum as a Marker of Bone-Formation—The Effect of Nandrolone Decanoate and Female Sex-Hormones. Metab.-Clin. Exp. 1991, 40, 205–208. [Google Scholar]
- Baim, S.; Miller, P.D. Assessing the Clinical Utility of Serum CTX in Postmenopausal Osteoporosis and Its Use in Predicting Risk of Osteonecrosis of the Jaw. J. Bone Miner. Res. 2009, 24, 561–574. [Google Scholar]
- Yamagishi, S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets 2011, 12, 2096–2102. [Google Scholar]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.A.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C.E. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar]
- Krishnamurthty, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.S.; Sharpless, N.E. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv324. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, M.; Yue, B.; Yang, M.; Carter, C.; Al-Quran, S.Z.; Li, B.; Li, Y. PTK7: A new biomarker for immunophenotypic characterization of maturing T cells and T cell acute lymphoblastic leukemia. Leuk. Res. 2012, 36, 1347–1353. [Google Scholar]
- Song, S.P.; Wang, L.H.; Li, J.; Zhao, J.L.; Fan, C.H. Aptamer-based biosensors. TrAC-Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug. Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.C.; Wang, X.B. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Matsui, T. Therapeutic Potential of DNA-aptamers Raised Against AGE-RAGE Axis in Diabetes-related Complications. Curr. Pharm. Des. 2018, 24, 2802–2809. [Google Scholar]
- Phan, L.M.T.; Cho, S. Fluorescent Aptasensor and Colorimetric Aptablot for p-tau231 Detection: Toward Early Diagnosis of Alzheimer’s Disease. Biomedicines 2022, 10, 93. [Google Scholar] [CrossRef]
- Shan, S.; He, Z.; Mao, S.; Jie, M.; Yi, L.; Lin, J.M. Quantitative determination of VEGF165 in cell culture medium by aptamer sandwich based chemiluminescence assay. Talanta 2017, 171, 197–203. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Chai, K.; Hu, X.; Hu, Y.; Shi, S.; Yao, T. A turn-on unlabeled colorimetric biosensor based on aptamer-AuNPs conjugates for amyloid-beta oligomer detection. Talanta 2023, 260, 124649. [Google Scholar]
- Chen, M.; Man, Y.; Xu, S.; Wu, H.; Ling, P.; Gao, F. A label-free dually-amplified aptamer sensor for the specific detection of amyloid-beta peptide oligomers in cerebrospinal fluids. Anal. Chim. Acta 2023, 1266, 341298. [Google Scholar] [CrossRef]
- Yang, X.H.; Wang, Y.N.; Wang, K.M.; Wang, Q.; Wang, P.; Lin, M.; Chen, N.; Tan, Y.Y. DNA aptamer-based surface plasmon resonance sensing of human C-reactive protein. RSC Adv. 2014, 4, 30934–30937. [Google Scholar]
- Wu, B.; Jiang, R.; Wang, Q.; Huang, J.; Yang, X.; Wang, K.; Li, W.; Chen, N.; Li, Q. Detection of C-reactive protein using nanoparticle-enhanced surface plasmon resonance using an aptamer-antibody sandwich assay. Chem. Commun. 2016, 52, 3568–3571. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Cheng, W.; Wu, T.; Li, J.; Li, X.; Liu, L.; Bai, H.; Ding, S.; Li, X.; et al. Surface plasmon resonance biosensor for exosome detection based on reformative tyramine signal amplification activated by molecular aptamer beacon. J. Nanobiotechnology 2021, 19, 450. [Google Scholar] [CrossRef]
- Ziu, I.; Laryea, E.T.; Alashkar, F.; Wu, C.G.; Martic, S. A dip-and-read optical aptasensor for detection of tau protein. Anal. Bioanal. Chem. 2020, 412, 1193–1201. [Google Scholar] [CrossRef]
- Gao, S.; Li, Q.; Zhang, S.; Sun, X.; Zhou, H.; Wang, Z.; Wu, J. A novel biosensing platform for detection of glaucoma biomarker GDF15 via an integrated BLI-ELASA strategy. Biomaterials 2023, 294, 121997. [Google Scholar] [CrossRef]
- Torrini, F.; Palladino, P.; Brittoli, A.; Baldoneschi, V.; Minunni, M.; Scarano, S. Characterization of troponin T binding aptamers for an innovative enzyme-linked oligonucleotide assay (ELONA). Anal. Bioanal. Chem. 2019, 411, 7709–7716. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Wang, Z.; Wu, F.; He, Y.; Liu, Y.; Deng, Y.; Bing, T.; Qiu, L.; Tan, W. DNA aptamer-based sensitive electrochemical biosensor for NAD(H) detection. Biosens. Bioelectron. 2025, 271, 116996. [Google Scholar] [CrossRef]
- Meirinho, S.G.; Dias, L.G.; Peres, A.M.; Rodrigues, L.R. Electrochemical aptasensor for human osteopontin detection using a DNA aptamer selected by SELEX. Anal. Chim. Acta 2017, 987, 25–37. [Google Scholar] [CrossRef]
- Fu, X.M.; Liu, Z.J.; Cai, S.X.; Zhao, Y.P.; Wu, D.Z.; Li, C.Y.; Chen, J.H. Electrochemical aptasensor for the detection of vascular endothelial growth factor (VEGF) based on DNA-templated Ag/Pt bimetallic nanoclusters. Chin. Chem. Lett. 2016, 27, 920–926. [Google Scholar] [CrossRef]
- Kutovyi, Y.; Hlukhova, H.; Boichuk, N.; Menger, M.; Offenhausser, A.; Vitusevich, S. Amyloid-beta peptide detection via aptamer-functionalized nanowire sensors exploiting single-trap phenomena. Biosens. Bioelectron. 2020, 154, 112053. [Google Scholar] [CrossRef]
- Jiang, L.; Li, D.; Su, M.; Qiu, Y.; Chen, F.; Qin, X.; Wang, L.; Gui, Y.; Zhao, J.; Guo, H.; et al. A Label-Free Electrochemical Aptamer Sensor for Sensitive Detection of Cardiac Troponin I Based on AuNPs/PB/PS/GCE. Nanomaterials 2024, 14, 1579. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; Chen, R.; Sun, X.; Yang, Y.; Huang, C.; Ding, S.; Liu, C.; Cheng, W. Microporous PdCuB nanotag-based electrochemical aptasensor with Au@CuCl2 nanowires interface for ultrasensitive detection of PD-L1-positive exosomes in the serum of lung cancer patients. J. Nanobiotechnol. 2023, 21, 86. [Google Scholar] [CrossRef]
- Liustrovaite, V.; Ratautaite, V.; Ramanaviciene, A.; Plikusiene, I.; Malinovskis, U.; Erts, D.; Sarvutiene, J.; Ramanavicius, A. Electrochemical sensor for vascular endothelial growth factor based on self-assembling DNA aptamer structure. Sci. Total Environ. 2024, 955, 177151. [Google Scholar]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar] [CrossRef]
- Peltomaa, R.; Glahn-Martinez, B.; Benito-Pena, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef]
- Napit, R.; Jaysawal, S.K.; Chowdhury, R.; Catague, J.; Melke, H.; Pham, C.V.; Xu, H.; Jia, L.; Lin, J.; Hou, Y.C.; et al. Aptasensors and Advancement in Molecular Recognition Technology. Adv. Mater. Technol. 2025, 10, 2400504. [Google Scholar]
- Downs, A.M.; Gerson, J.; Leung, K.K.; Honeywell, K.M.; Kippin, T.; Plaxco, K.W. Improved calibration of electrochemical aptamer-based sensors. Sci. Rep. 2022, 12, 5535. [Google Scholar]
- Mei, C.; Zhang, Y.; Pan, L.; Dong, B.; Chen, X.; Gao, Q.; Xu, H.; Xu, W.; Fang, H.; Liu, S.; et al. A One-Step Electrochemical Aptasensor Based on Signal Amplification of Metallo Nanoenzyme Particles for Vascular Endothelial Growth Factor. Front. Bioeng. Biotechnol. 2022, 10, 850412. [Google Scholar]
- Mikula, E.; Malecka-Baturo, K. An Overview of the Latest Developments in the Electrochemical Aptasensing of Neurodegenerative Diseases. Coatings 2023, 13, 235. [Google Scholar] [CrossRef]
- Liu, Y.X.; Dykstra, G. Recent progress on electrochemical (bio)sensors based on aptamer-molecularly imprinted polymer dual recognition. Sens. Actuator Rep. 2022, 4, 100112. [Google Scholar]
- Lang, M.; Luo, D.; Yang, G.; Mei, Q.; Feng, G.; Yang, Y.; Liu, Z.; Chen, Q.; Wu, L. An ultrasensitive electrochemical sensing platform for the detection of cTnI based on aptamer recognition and signal amplification assisted by TdT. RSC Adv. 2020, 10, 36396–36403. [Google Scholar]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R.; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Kang, C. Avacincaptad Pegol: First Approval. Drugs 2023, 83, 1447–1453. [Google Scholar]
- Choi, S.; Han, J.; Kim, J.H.; Kim, A.R.; Kim, S.H.; Lee, W.; Yoon, M.Y.; Kim, G.; Kim, Y.S. Advances in dermatology using DNA aptamer “Aptamin C” innovation: Oxidative stress prevention and effect maximization of vitamin C through antioxidation. J. Cosmet. Dermatol. 2020, 19, 970–976. [Google Scholar]
- Salman, R.F.; Al-Sudani, B.T.; Mshimesh, B.A.R. Protective Action of SIRT1 Activator Aptamer in Human Skin Cell Line. J. Popul. Ther. Clin. Pharmacol. 2023, 30, E342–E359. [Google Scholar]
- Xia, Y.; Li, J.; Wang, L.; Xie, Y.; Zhang, L.; Han, X.; Tan, W.; Liu, Y. Engineering Hierarchical Recognition-Mediated Senolytics for Reliable Regulation of Cellular Senescence and Anti-Atherosclerosis Therapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214169. [Google Scholar]
- Xie, Y.; Li, J.; Wu, P.; Wang, L.; Hong, D.; Wang, J.; Liu, Y. Targeted regulation of senescence-associated secretory phenotype with an aptamer-conjugated activatable senomorphic. Aging Pathobiol. Ther. 2023, 5, 59–65. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Li, S.Y.; Geng, X.H.; Zou, L.Y.; Jin, M.M.; Zou, Q.Q.; Wang, Q.; Yang, X.H.; Wang, K.M. Development of DNA Aptamer as a β-Amyloid Aggregation Inhibitor. ACS Appl. Bio Mater. 2020, 3, 8611–8618. [Google Scholar]
- Kim, J.H.; Kim, E.; Choi, W.H.; Lee, J.; Lee, J.H.; Lee, H.; Kim, D.E.; Suh, Y.H.; Lee, M.J. Inhibitory RNA Aptamers of Tau Oligomerization and Their Neuroprotective Roles against Proteotoxic Stress. Mol. Pharm. 2016, 13, 2039–2048. [Google Scholar]
- Zheng, Y.; Qu, J.; Xue, F.Q.; Zheng, Y.; Yang, B.; Chang, Y.C.; Yang, H.; Zhang, J.L. Novel DNA Aptamers for Parkinson’s Disease Treatment Inhibit α-Synuclein Aggregation and Facilitate its Degradation. Mol. Ther.-Nucl. Acids 2018, 11, 228–242. [Google Scholar]
- Ren, X.X.; Zhao, Y.; Xue, F.Q.; Zheng, Y.; Huang, H.X.; Wang, W.; Chang, Y.C.; Yang, H.; Zhang, J.L. Exosomal DNA Aptamer Targeting α-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther.-Nucl. Acids 2019, 17, 726–740. [Google Scholar]
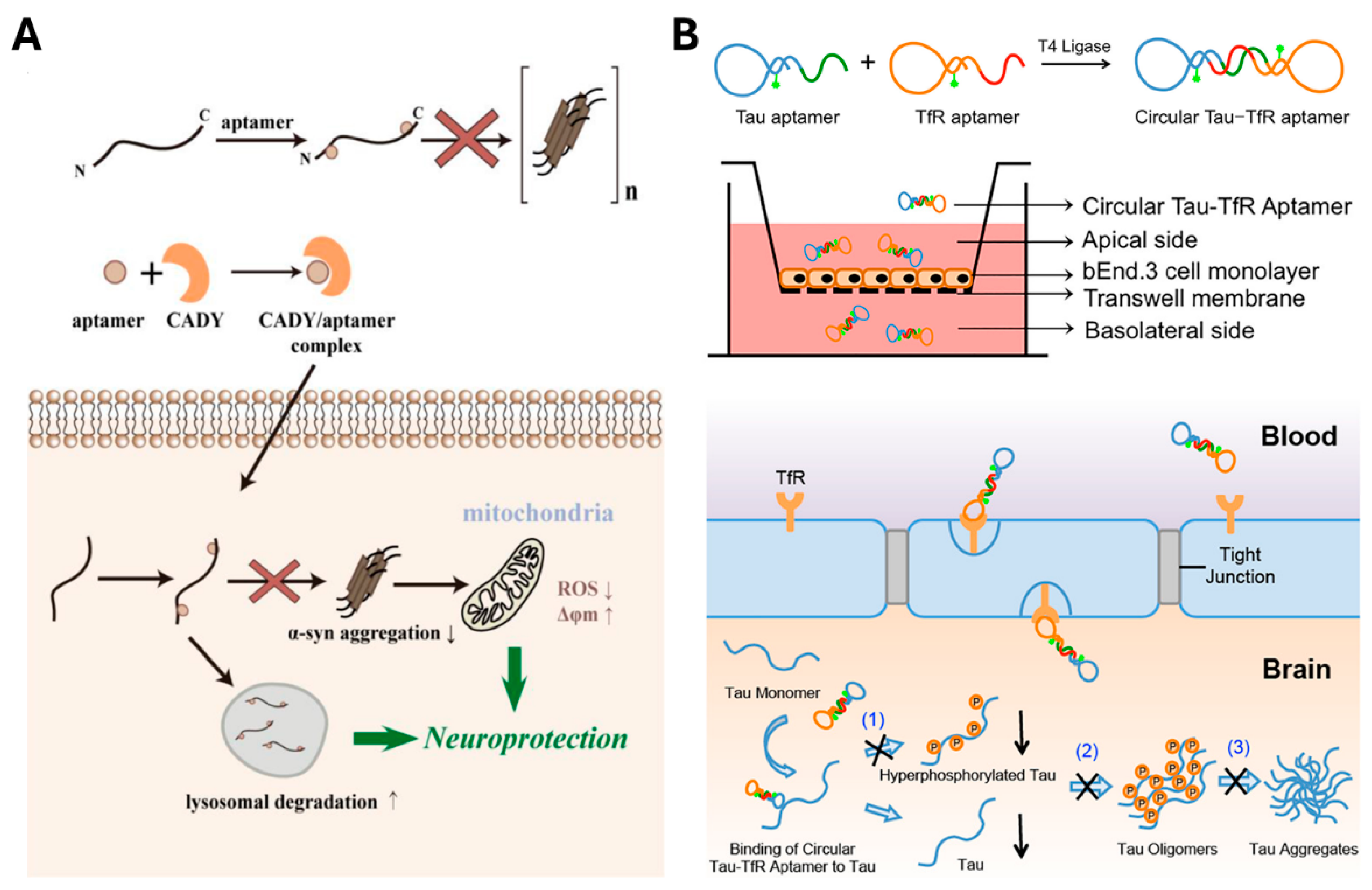
- Li, X.W.; Yang, Y.; Zhao, H.Z.; Zhu, T.; Yang, Z.H.; Xu, H.Y.; Fu, Y.Q.; Lin, F.; Pan, X.S.; Li, L.; et al. Enhanced in Vivo Blood-Brain Barrier Penetration by Circular Tau-Transferrin Receptor Bifunctional Aptamer for Tauopathy Therapy. J. Am. Chem. Soc. 2020, 142, 3862–3872. [Google Scholar]
- Martin, D.F.; Klein, M.; Haller, J.; Adamis, A.; Gragoudas, E.; Miller, J.; Blumenkrantz, M.; Goldberg, M.; Yannuzzi, L.; Henninger, D.; et al. Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina 2002, 22, 143–152. [Google Scholar]
- Akiyama, H.; Kachi, S.; Silva, R.L.E.; Umeda, N.; Hackett, S.F.; McCauley, D.; McCauley, T.; Zoltoski, A.; Epstein, D.M.; Campochiaro, P.A. Intraocular injection of an aptamer that binds PDGF-B: A potential treatment for proliferative retinopathies. J. Cell. Physiol. 2006, 207, 407–412. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Eliott, D.; Wells, J.A.; Prenner, J.L.; Papp, A.; Patel, S. A Phase 1 Study of Intravitreous E10030 in Combination with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology 2016, 123, 78–85. [Google Scholar] [CrossRef]
- Matsuda, Y.; Nonaka, Y.; Futakawa, S.; Imai, H.; Akita, K.; Nishihata, T.; Fujiwara, M.; Ali, Y.; Bhisitkul, R.B.; Nakamura, Y. Anti-Angiogenic and Anti-Scarring Dual Action of an Anti-Fibroblast Growth Factor 2 Aptamer in Animal Models of Retinal Disease. Mol. Ther.-Nucl. Acids 2019, 17, 819–828. [Google Scholar] [CrossRef]
- Wu, H.B.; Wang, Z.W.; Shi, F.; Ren, Z.L.; Li, L.C.; Hu, X.P.; Hu, R.; Li, B.W. Avbeta3 Single-Stranded DNA Aptamer Attenuates Vascular Smooth Muscle Cell Proliferation and Migration via Ras-PI3K/MAPK Pathway. Cardiovasc. Ther. 2020, 2020, 6869856. [Google Scholar] [CrossRef]
- Thiel, W.H.; Esposito, C.L.; Dickey, D.D.; Dassie, J.P.; Long, M.E.; Adam, J.; Streeter, J.; Schickling, B.; Takapoo, M.; Flenker, K.S.; et al. Smooth Muscle Cell-targeted RNA Aptamer Inhibits Neointimal Formation. Mol. Ther. 2016, 24, 779–787. [Google Scholar] [CrossRef]
- Gilbert, J.C.; DeFeo-Fraulini, T.; Hutabarat, R.M.; Horvath, C.J.; Merlino, P.G.; Marsh, H.N.; Healy, J.M.; BouFakhreddine, S.; Holohan, T.V.; Schaub, R.G. First-in-human evaluation of anti-von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 2007, 116, 2678–2686. [Google Scholar] [CrossRef]
- Huang, R.H.; Fremont, D.H.; Diener, J.L.; Schaub, R.G.; Sadler, J.E. A Structural Explanation for the Antithrombotic Activity of ARC1172, a DNA Aptamer that Binds von Willebrand Factor Domain A1. Structure 2009, 17, 1476–1484. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Dornbos, D.; Pitoc, G.A.; Wheeler, D.G.; Layzer, J.M.; Venetos, N.; Huttinger, A.; Talentino, S.E.; Musgrave, N.J.; Moody, H.; et al. Preclinical Development of a vWF Aptamer to Limit Thrombosis and Engender Arterial Recanalization of Occluded Vessels. Mol. Ther. 2019, 27, 1228–1241. [Google Scholar] [CrossRef]
- Zhu, S.H.; Gilbert, J.C.; Hatala, P.; Harvey, W.; Liang, Z.C.; Gao, S.; Kang, D.W.; Jilma, B. The development and characterization of a long acting anti-thrombotic von Willebrand factor (VWF) aptamer. J. Thromb. Haemost. 2020, 18, 1113–1123. [Google Scholar]
- Wallukat, G.; Müller, J.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.J.; Vetter, R.; Salzsieder, E.; Kreutz, R.; Schimke, I. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 2016, 244, 44–47. [Google Scholar]
- Liang, C.; Guo, B.S.; Wu, H.; Shao, N.S.; Li, D.F.; Liu, J.; Dang, L.; Wang, C.; Li, H.; Li, S.H.; et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015, 21, 288–294. [Google Scholar]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar]
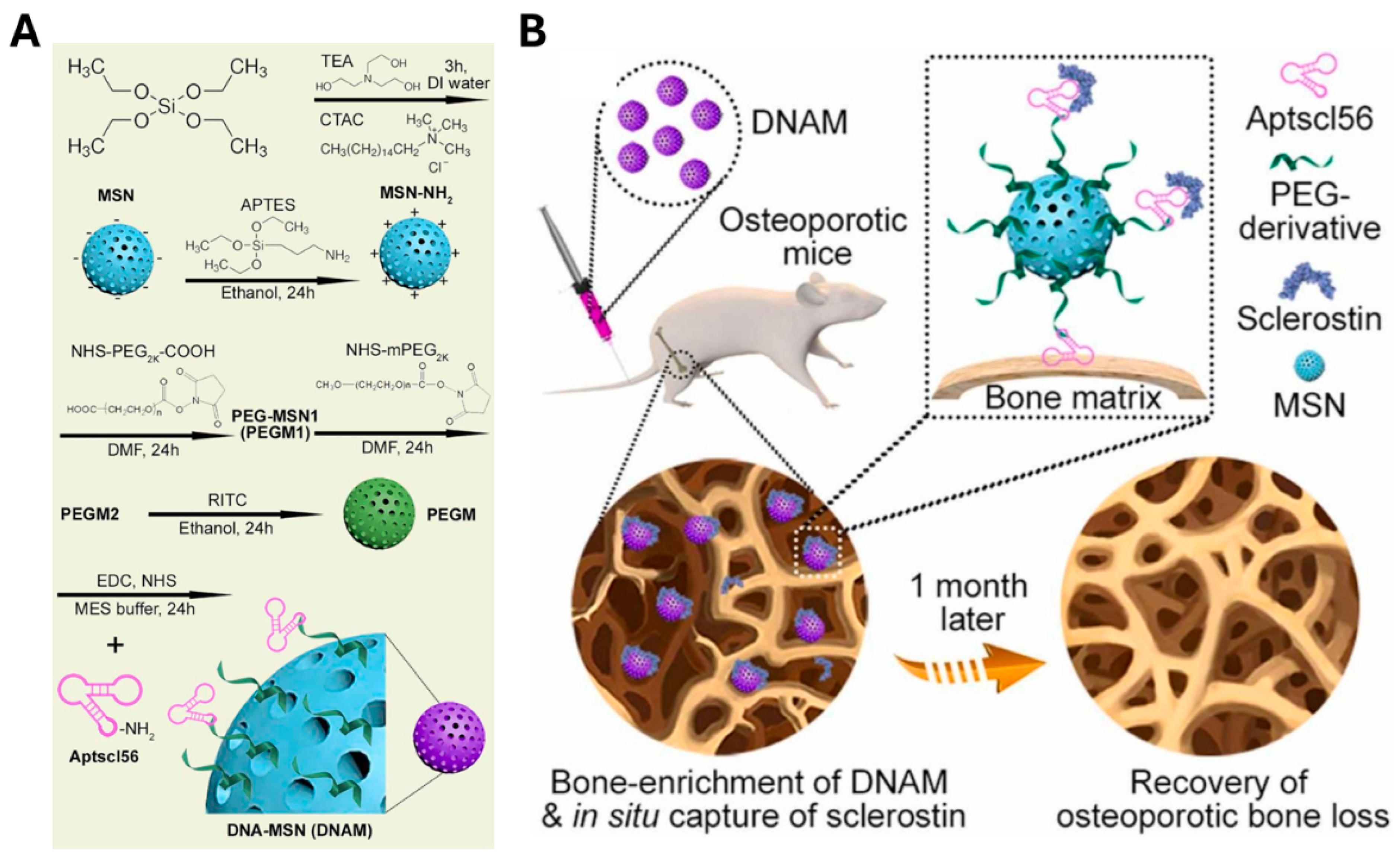
- Niu, Y.T.; Yang, Y.; Yang, Z.; Wang, X.; Zhang, P.; Lv, L.W.; Liu, Y.; Liu, Y.S.; Zhou, Y.S. Aptamer-immobilized bone-targeting nanoparticles in situ reduce sclerostin for osteoporosis treatment. Nano Today 2022, 45, 101529. [Google Scholar]
- Lai, W.Y.; Huang, B.T.; Wang, J.W.; Lin, P.Y.; Yang, P.C. A Novel PD-L1-targeting Antagonistic DNA Aptamer With Antitumor Effects. Mol. Ther. Nucleic Acids 2016, 5, e397. [Google Scholar]
- Kruspe, S.; Hahn, U. An Aptamer Intrinsically Comprising 5-Fluoro-2′-deoxyuridine for Targeted Chemotherapy. Angew. Chem. Int. Ed. 2014, 53, 10541–10544. [Google Scholar]
- Soundararajan, S.; Chen, W.W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl.-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, L.W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective Delivery of an Anticancer Drug with Aptamer-Functionalized Liposomes to Breast Cancer Cells in Vitro and in Vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar]
- Chen, H.C.; Tian, J.W.; Liu, D.Y.; He, W.J.; Guo, Z.J. Dual aptamer modified dendrigraft poly-L-lysine nanoparticles for overcoming multi-drug resistance through mitochondrial targeting. J. Mater. Chem. B 2017, 5, 972–979. [Google Scholar]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar]
- Mallikaratchy, P.; Tang, Z.W.; Tan, W.H. Cell specific aptamer-photosensitizer conjugates as a molecular tool in photodynamic therapy. Chemmedchem 2008, 3, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, J.; Wang, K.; Li, W.; Cui, L.; Li, X. Angiogenin-mediated photosensitizer-aptamer conjugate for photodynamic therapy. ChemMedChem 2011, 6, 1778–1780. [Google Scholar] [CrossRef]
- Huang, Y.F.; Sefah, K.; Bamrungsap, S.; Chang, H.T.; Tan, W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 2008, 24, 11860–11865. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, J.H.; Kim, S.H.; Sim, T.H.; Kim, Y.J. NXP032 ameliorates cognitive impairment by alleviating the neurovascular aging process in aged mouse brain. Sci. Rep. 2023, 13, 8594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar]
- Lai, W.Y.; Wang, J.W.; Huang, B.T.; Lin, E.P.Y.; Yang, P.C. A Novel TNF-α-Targeting Aptamer for TNF-α-Mediated Acute Lung Injury and Acute Liver Failure. Theranostics 2019, 9, 1741–1751. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, Y.T.; Huang, Z.H.; Qin, X.; Zhou, M.; Xiao, D.X.; Cui, W.T.; Liu, Z.Q.; Lin, Y.F. Targeted therapy for autoimmune diseases based on multifunctional frame nucleic acid system: Blocking TNF-α-NF-ΚB signaling and mediating macrophage polarization. Chem. Eng. J. 2023, 454, 140399. [Google Scholar] [CrossRef]
- Cheng, C.S.; Chen, Y.H.; Lennox, K.A.; Behlke, M.A.; Davidson, B.L. SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther.-Nucl. Acids 2013, 2, e67. [Google Scholar] [CrossRef]
- Choi, J.W.; Seo, M.; Kim, K.; Kim, A.R.; Lee, H.; Kim, H.S.; Park, C.G.; Cho, S.W.; Kang, J.H.; Joo, J.; et al. Aptamer Nanoconstructs Crossing Human Blood-Brain Barrier Discovered via Microphysiological System-Based SELEX Technology. ACS Nano 2023, 17, 8153–8166. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.Q.; Yin, H.F.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [PubMed]
- Jellinek, D.; Green, L.S.; Bell, C.; Janjic, N. Inhibition of Receptor-Binding by High-Affinity Rna Ligands to Vascular Endothelial Growth-Factor. Biochemistry 1994, 33, 10450–10456. [Google Scholar] [CrossRef]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2′-fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165): Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; Grp, A.S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [PubMed]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L.; Deg, C.A.-r.M. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration. Ophthalmology 2020, 127, S135–S145. [Google Scholar]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group, S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar]
- Bhisitkul, R.B.; Desai, S.J.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Fellow Eye Comparisons for 7-Year Outcomes in Ranibizumab-Treated AMD Subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP Study). Ophthalmology 2016, 123, 1269–1277. [Google Scholar]
- Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.; Fine, S.L.; Macular, C.A.-r. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar]
- Chen, X.Y.; Ma, Y.; Xie, Y.Q.; Pu, J. Aptamer-based applications for cardiovascular disease. Front. Bioeng. Biotechnol. 2022, 10, 1002285. [Google Scholar] [CrossRef] [PubMed]
- Thiel, W.H.; Bair, T.; Peek, A.S.; Liu, X.; Dassie, J.; Stockdale, K.R.; Behlke, M.A.; Miller, F.J., Jr.; Giangrande, P.H. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS ONE 2012, 7, e43836. [Google Scholar] [CrossRef]
- Udofot, O.; Lin, L.H.; Thiel, W.H.; Erwin, M.; Turner, E.; Miller, F.J., Jr.; Giangrande, P.H.; Yazdani, S.K. Delivery of Cell-Specific Aptamers to the Arterial Wall with an Occlusion Perfusion Catheter. Mol. Ther. Nucleic Acids 2019, 16, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, K.D.; Greisenegger, S.; Langer, A.; Gelbenegger, G.; Buchtele, N.; Pabinger, I.; Petroczi, K.; Zhu, S.H.; Gilbert, J.C.; Jilma, B. The aptamer BT200 blocks von Willebrand factor and platelet function in blood of stroke patients. Sci. Rep. 2021, 11, 3092. [Google Scholar] [CrossRef]
- Wallukat, G.; Schimke, I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin. Immunopathol. 2014, 36, 351–363. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T. Adverse Effects of Bisphosphonates: Implications for Osteoporosis Management. Mayo Clin. Proc. 2009, 84, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Quint, P.; Ruan, M.; Pederson, L.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Sclerostin is expressed in osteoclasts from aged mice and reduces osteoclast-mediated stimulation of mineralization. J. Cell. Biochem. 2013, 114, 1901–1907. [Google Scholar] [CrossRef]
- Rysanek, D.; Vasicova, P.; Kolla, J.N.; Sedlak, D.; Andera, L.; Bartek, J.; Hodny, Z. Synergism of BCL-2 family inhibitors facilitates selective elimination of senescent cells. Aging 2022, 14, 6381–6414. [Google Scholar] [CrossRef]
- Morales-Valencia, J.; Lau, L.; Marti-Nin, T.; Ozerdem, U.; David, G. Therapy-induced senescence promotes breast cancer cells plasticity by inducing Lipocalin-2 expression. Oncogene 2022, 41, 4361–4370. [Google Scholar] [CrossRef]
- Jo, H.; Shim, K.; Jeoung, D. The Potential of Senescence as a Target for Developing Anticancer Therapy. Int. J. Mol. Sci. 2023, 24, 3436. [Google Scholar] [CrossRef]
- Palazzo, A.; Hernandez-Vargas, H.; Goehrig, D.; Médard, J.J.; Vindrieux, D.; Flaman, J.M.; Bernard, D. Transformed cells after senescence give rise to more severe tumor phenotypes than transformed non-senescent cells. Cancer Lett. 2022, 546, 215850. [Google Scholar]
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics 2015, 5, 322–344. [Google Scholar] [PubMed]
- Intlekofer, A.M.; Thompson, C.B. At the Bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [PubMed]
- Meyer, C.; Eydeler, K.; Magbanua, E.; Zivkovic, T.; Piganeau, N.; Lorenzen, I.; Grötzinger, J.; Mayer, G.; Rose-John, S.; Hahn, U. Interleukin-6 receptor specific RNA aptamers for cargo delivery into target cells. RNA Biol. 2012, 9, 67–80. [Google Scholar] [PubMed]
- Mittelberger, F.; Meyer, C.; Waetzig, G.H.; Zacharias, M.; Valentini, E.; Svergun, D.I.; Berg, K.; Lorenzen, I.; Grötzinger, J.; Rose-John, S.; et al. RAID3-An interleukin-6 receptor-binding aptamer with post-selective modification-resistant affinity. RNA Biol. 2015, 12, 1043–1053. [Google Scholar]
- Hahn, U. Charomers-Interleukin-6 Receptor Specific Aptamers for Cellular Internalization and Targeted Drug Delivery. Int. J. Mol. Sci. 2017, 18, 2641. [Google Scholar] [CrossRef]
- Thongchot, S.; Aksonnam, K.; Thuwajit, P.; Yenchitsomanus, P.T.; Thuwajit, C. Nucleolin-based targeting strategies in cancer treatment: Focus on cancer immunotherapy (Review). Int. J. Mol. Med. 2023, 52, 81. [Google Scholar] [CrossRef]
- Ireson, C.R.; Kelland, L.R. Discovery and development of anticancer aptamers. Mol. Cancer Ther. 2006, 5, 2957–2962. [Google Scholar] [CrossRef]
- Soundararajan, S.; Wang, L.; Sridharan, V.; Chen, W.W.; Courtenay-Luck, N.; Jones, D.; Spicer, E.K.; Fernandes, D.J. Plasma Membrane Nucleolin Is a Receptor for the Anticancer Aptamer AS1411 in MV4-11 Leukemia Cells. Mol. Pharmacol. 2009, 76, 984–991. [Google Scholar] [CrossRef]
- Aravind, A.; Veeranarayanan, S.; Poulose, A.C.; Nair, R.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Aptamer-functionalized silica nanoparticles for targeted cancer therapy. BioNanoScience 2012, 2, 1–8. [Google Scholar]
- Kesharwani, P.; Ma, R.Y.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Kazemi, Y.; Dehghani, S.; Soltani, F.; Abnous, K.; Alibolandi, M.; Taghdisi, S.M.; Ramezani, M. PNA-ATP aptamer-capped doxorubicin-loaded silica nanoparticles for targeted cancer therapy. Nanomedicine 2022, 45, 102588. [Google Scholar] [CrossRef]
- Roy, K.; Kanwar, R.K.; Cheung, C.H.A.; Fleming, C.L.; Veedu, R.N.; Krishnakumar, S.; Kanwar, J.R. Locked nucleic acid modified bi-specific aptamer-targeted nanoparticles carrying survivin antagonist towards effective colon cancer therapy. RSC Adv. 2015, 5, 29008–29016. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, H.; Gubu, A.; Yu, S.; Zhang, H.; Dai, H.; Zhang, Y.; Zhang, B.; Ma, Y.; Lu, A.; et al. Chemically modified aptamers for improving binding affinity to the target proteins via enhanced non-covalent bonding. Front. Cell Dev. Biol. 2023, 11, 1091809. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.P.; Wang, L.; Lo, Y.; Shiu, S.C.C.; Kinghorn, A.B.; Tanner, J.A. Aptamer-Enabled Nanomaterials for Therapeutics, Drug Targeting and Imaging. Cells 2022, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.H.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Ma, X.; Fang, X.; Xiang, L.; Yi, Y.; Li, J.; Luo, Z.; Li, G. Aptamer-Functionalized Nanochannels for One-Step Detection of SARS-CoV-2 in Samples from COVID-19 Patients. Anal. Chem. 2021, 93, 16646–16654. [Google Scholar] [CrossRef]
- Jung, H.H.; Lee, H.; Yea, J.; Jang, K.-I. Wearable electrochemical sensors for real-time monitoring in diabetes mellitus and associated complications. Soft Sci. 2024, 4, 15. [Google Scholar] [CrossRef]
- Li, J.; Xiong, M.; Fu, X.H.; Fan, Y.; Dong, C.; Sun, X.; Zheng, F.; Wang, S.W.; Liu, L.; Xu, M.; et al. Determining a multimodal aging clock in a cohort of Chinese women. Med 2023, 4, 825–848.e13. [Google Scholar] [CrossRef]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Mamoshina, P. Deep Aging Clocks: The Emergence of AI-Based Biomarkers of Aging and Longevity. Trends Pharmacol. Sci. 2019, 40, 546–549. [Google Scholar] [PubMed]
- Froy, O. Circadian rhythms, aging, and life span in mammals. Physiology 2011, 26, 225–235. [Google Scholar] [PubMed]
- Cao, Y.W.; Wang, R.H. Associations among Metabolism, Circadian Rhythm and Age-Associated Diseases. Aging Dis. 2017, 8, 314–333. [Google Scholar]
- Lee, C.; Etchegaray, J.P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [PubMed]
- Zvonic, S.; Ptitsyn, A.A.; Conrad, S.A.; Scott, L.K.; Floyd, Z.E.; Kilroy, G.; Wu, X.; Goh, B.C.; Mynatt, R.L.; Gimble, J.M. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006, 55, 962–970. [Google Scholar]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Yeom, J.W.; Kim, H.; Pack, S.P.; Lee, H.J.; Cheong, T.; Cho, C.H. Exploring the Psychological and Physiological Insights Through Digital Phenotyping by Analyzing the Discrepancies Between Subjective Insomnia Severity and Activity-Based Objective Sleep Measures: Observational Cohort Study. JMIR. Ment. Health 2025, 12, e67478. [Google Scholar] [CrossRef]
- Nakazawa, K.; Matsuo, M.; Kikuchi, Y.; Nakajima, Y.; Numano, R. Melanopsin DNA aptamers can regulate input signals of mammalian circadian rhythms by altering the phase of the molecular clock. Front. Neurosci. 2024, 18, 1186677. [Google Scholar]

| Aptamer | Antibody | Small Molecule | Peptide | |
|---|---|---|---|---|
| Size | ~30 kDa | 150~180 kDa | less than 1 kDa | ~5 kDa |
| Affinity | nM-pM | nM-pM | μM-nM | μM-nM |
| Isolation process | In vitro process using SELEX (2–8 weeks) | In vivo biological production (weeks to months) | High-performance liquid chromatography (HPLC), Solid-phase extraction (SPE), Affinity purification (hours to days) | Ion-exchange chromatography, HPLC, lyophilization (hours to days) |
| Production cost | Low | High | Low | Low |
| Stability (pH, temperature) | Stable | Unstable | Stable | Unstable |
| Specificity | High | High | Low | Moderate |
| Chemical modification | Easy | Limited | Easy | Easy |
| Target potential | Proteins, peptides, cells, tissues, virus, bacteria, ions, small molecules | Proteins, peptides, cells, tissues, virus, bacteria, ions, small molecules | Proteins, enzymes, receptors, ion channels, DNA, RNA | Mainly proteins, peptides |
| In vivo half-life | Very short (minutes) | Long (days to weeks) | Variable (hours to days) | Short (minutes to hours) |
| Immunogenicity | Low | High | Low | Moderate |
| Cytotoxicity | Low | Variable | Variable | Variable |
| In vivo dosage | Low (μg-mg/mL) | High (mg/mL) | High (μg-mg/mL) | High (μg-mg/mL) |
| Transducer | Mechanism | Sensitivity | Applicability | |
|---|---|---|---|---|
| Optical | Fluorescence | Recognition of fluorescence signal change through aptamer conformational change | fM-pM | - |
| Luminescence | Recognition of emission light from chemical reactions | fM-pM | in vitro | |
| Colorimetric | Recognition of color alterations | pM-nM | - | |
| Surface plasmon resonance (SPR) | Recognition of alterations in the refractive index | fM-pM | in vitro | |
| Biolayer interferometry (BLI) | Recognition of alterations in the interference pattern of light | pM-nM | - | |
| BLI-enzyme-linked aptamer sorbent assay (ELASA) | Highly sensitive detection, real-time monitoring by combining BLI and ELISA | fM | in vitro | |
| Enzyme-Linked Oligonucleotide Assay (ELONA) | Recognition of enzyme-substrate reactions | pM-nM | - | |
| Electrochemical | Voltametric sensor | Recognition of current response of reaction between aptamers and molecules at electrode surface | fM-pM | in vitro |
| Amperometric sensor | Recognition of current alterations by electrochemical reduction or oxidation of molecules at an electrode | pM-nM | - | |
| Impedance, Potentiometric sensor | Recognition of alterations in electrical potential or in impedance of the sensor surface | fM | - | |
| Aptamer | Target | Therapeutic Principles | Ref. | |
|---|---|---|---|---|
| Aging | Aptamin C | Vitamin C | Reduction of ROS levels by improving vitamin C half-life | [143] |
| SIRT1 aptamer | Sirtuin1 | Reduction of ROS levels by Sirtuin 1 activation | [144] | |
| yly12 | Cell adhesion molecule L1 | Induction of senescent cell apoptosis via aptamer-prodrug conjugation and SA-β-gal selective activity | [145,146] | |
| Neurodegenerative diseases | Aβ7-92-1H1 (Aβ-Apt) | Aβ42 monomer | Inhibition of Aβ42 aggregation | [147] |
| Tau-1 aptamer | Tau protein | Inhibition of tau protein aggregation | [148] | |
| F5R1, F5R2 | α-syn | Inhibition of α-syn aggregation | [149] | |
| Inhibition of α-syn aggregation of in vivo neuronal cells via exosome-based F5R2 delivery | [150] | |||
| Circular Tau–TfR bispecific aptamer | Tau protein +TfR | Blood-brain barrier permeation and tau aggregation inhibition via tau-transferrin receptor bifunctional aptamer | [151] | |
| AMD | EYE001 | VEGF | Blocking the binding of VEGF to the human VEGF receptor | [141,152] |
| Pegaptanib | ||||
| E10030 | Platelet-derived growth factor (PDGF) | Inhibition of VEGF co-activation via PDGF receptor binding blockade | [153,154] | |
| RBM-007 | Fibroblast growth factor 2 (FGF2) | Inhibition of FGF2 function | [155] | |
| CVD | avβ3 aptamer | Integrin avβ3 | Prevention of vascular smooth muscle cell (VSMC) proliferation and migration via suppression of Ras-phosphatidylinositol-4,5-bisphosphate 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) signaling activity | [156] |
| Apt14 | VSMC | Inhibition of VSMC migration through activation of platelet-derived growth factor receptor (PDGFR)-β and inhibition of phosphatidylinositol 3-kinase/protein kinase-B (PI3K/Akt) signaling | [157] | |
| ARC1779 | vWF | Inhibition of platelet aggregation and promotion of revascularization in platelet-rich thrombotic occlusions | [158] | |
| ARC1172 | [159] | |||
| DTRI-031 | [160] | |||
| BT200 | [161] | |||
| BC007 | Autoantibodies against G-protein coupled receptors (GPCR-AABs) | Treatment of autoimmune-induced cardiomyopathy through neutralization of autoantibodies against G-protein coupled receptors (GPCR-AABs) | [162] | |
| Osteoporosis | CH6 | Rat and human osteoblasts | Promotion of bone formation and enhancing mechanical performance by delivery of pleckstrin homology domain-containing family O member 1 (Plekho1) siRNA-encapsulated nanoparticles | [163] |
| BMSC-targeting aptamer | Bone marrow mesenchymal stem cells (BMSCs) | Increasing bone formation and decreasing fat accumulation through antagomiR-188 delivery to BMSCs | [164] | |
| Aptscl56 | Sclerostin | Direct inhibition of sclerostin activity around hydroxyapatite via binding to bone calcium | [165] | |
| Cancer | AptPD-L1 | PD-L1 | Restoration of T-cell function and inhibition of tumor growth by blockade of PD-1 and PD-L1 interactions | [166] |
| AIR-3A | human IL-6 receptor (hIL-6R) | Inhibiting DNA biosynthesis and killing cancer cells via 5-fluoro-2′-deoxyuridine (5-FUdR) delivery | [167] | |
| AS1411 | Nucleolin | Induction of cancer cell death by suppressing DNA replication and inhibition of cancer cell proliferation by disrupting stabilization of BCL-2 mRNA | [168] | |
| Elimination of cancer cells via delivery of DOX-loaded liposomes | [169] | |||
| ATP aptamer +Cyt c aptamer +AS1411 | ATP/cytochrome c/nucleolin | Elimination of cancer cells by releasing doxorubicin (DOX) under high ATP conditions in cancer cell mitochondria | [170] | |
| A10 RNA aptamer | Prostate-specific membrane antigen (PSMA) | Cancer treatment through delivery of a cytotoxic platinum-encapsulated nanoparticle | [171] | |
| TD05 | Ramos cells | Radiation-induced photodynamic cancer therapy via delivery of the photosensitizer chlorine e6 (Ce6) | [172] | |
| Angiogenin aptamer | Human angiogenin | [173] | ||
| sgc8 | PTK7 | Photothermal cancer therapy using gold nanorods | [174] |
| Transducer | Aptamer Name | Target | Limit of Detection (LOD) | Ref. | |
|---|---|---|---|---|---|
| Optical | Fluorescence | IT3 | p-tau231 | 3.64 ng/mL | [116] |
| Luminescence | VEGF Apt1, VEGF Apt2 | Vascular endothelial growth factor (VEGF) | 1 ng/mL | [117] | |
| Colorimetric | pA-pT-apt | Amyloid-β1-40 oligomer (Aβ40-O) | 3.03 nM | [118] | |
| Aβo, H1 aptamer | Amyloid-β oligomer (Aβo) | 0.23 pM | [119] | ||
| Surface plasmon resonance (SPR) | CRP-40-17, CRP-80-17 | Human C-reactive protein (CRP) | 0.35 nmol/L | [120] | |
| 3′-thiol-modified 6th-62-40 | CRP | 10 pM | [121] | ||
| HER2 aptamer | Human epidermal growth factor receptor 2 (HER2)-positive exosomes | 104–107 particles/mL | [122] | ||
| Biolayer interferometry (BLI) | Biotinylated DNA aptamer | Tau441 protein | 6.7 nM | [123] | |
| BLI-ELASA | APT2TM | Growth differentiation factor-15 (GDF15) | 5–6 pg/mL | [124] | |
| ELONA | Apt.1, Apt.2 | Recombinant human cardiac troponin T isoform 6 (cTnT3) | 3.42 nM, 3.13 nM | [125] | |
| Electro-chemical | Square wave voltammetry (SWV) | cTnI aptamer | Cardiac troponin I (cTnI) | 40 pg/mL | [125] |
| NAD3-1a | NAD(H) | 0.601 pM | [126] | ||
| C10K2 | Recombinant human osteopontin (rhOPN) | 1.3 ± 0.1 nM | [127] | ||
| Cyclic voltammetry (CV) | Amino-Apt13, Template-Apt12 | VEGF | 4.6 pmol/L | [128] | |
| Aβ-40-specific aptamer | Aß-40 peptides | 20 fM | [129] | ||
| Differential pulse voltammetry (DPV) | Thiol-functionalized aptamer Tro4 | cTnI | 2.03 fg/mL | [130] | |
| PD-L1 Apt., CD63 Apt. | Programmed cell death ligand 1 protein-positive (PD-L1+) exosomes | 36 particles/mL | [131] | ||
| Pulsed amperometric detection (PAD) | combSl2B, stalkGTG, r_stalkGTG | VEGF | 0.21 nM | [132] | |
| Field-effect transistor (FET) | Anti-VEGF RNA aptamer (CPNT2-aptamer) | VEGF | 400 fM | [133] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.-I.; Yang, A.H.; Kanth, B.K.; Pack, S.P. Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases. Biosensors 2025, 15, 232. https://doi.org/10.3390/bios15040232
Park T-I, Yang AH, Kanth BK, Pack SP. Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases. Biosensors. 2025; 15(4):232. https://doi.org/10.3390/bios15040232
Chicago/Turabian StylePark, Tae-In, Ah Hyun Yang, Bashistha Kumar Kanth, and Seung Pil Pack. 2025. "Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases" Biosensors 15, no. 4: 232. https://doi.org/10.3390/bios15040232
APA StylePark, T.-I., Yang, A. H., Kanth, B. K., & Pack, S. P. (2025). Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases. Biosensors, 15(4), 232. https://doi.org/10.3390/bios15040232





