Innovative Applications of Nanopore Technology in Tumor Screening: An Exosome-Centric Approach
Abstract
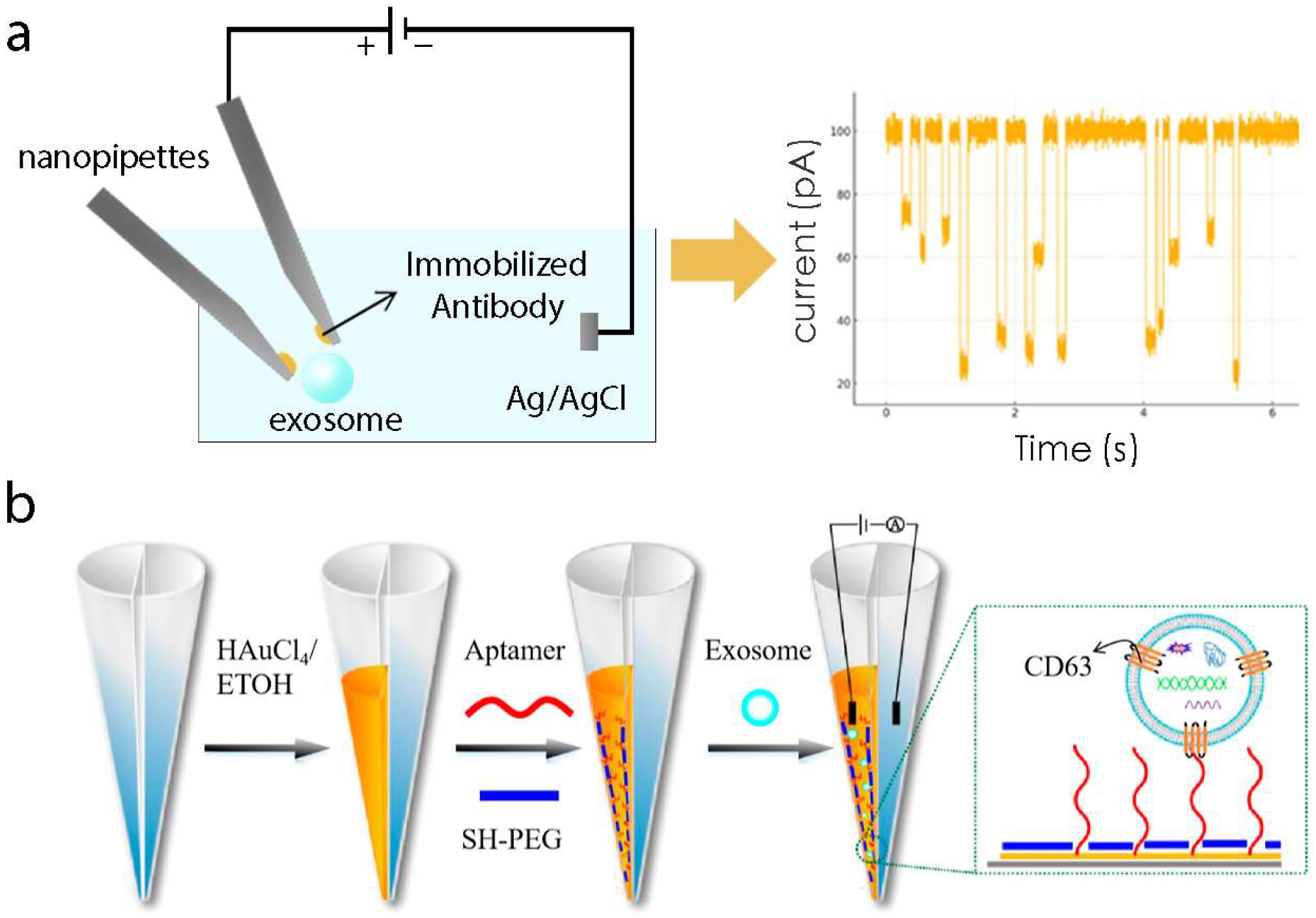
1. Introduction
2. Exosomes and Tumor Screening
2.1. The Importance of Exosomes in Tumor Screening
2.2. The Advantages of Exosomes in Tumor Screening
2.3. Applications of Multiple Biomarkers in Exosomes for Cancer Screening
2.4. The Limitations of Exosomes in Tumor Screening
3. Methodological Advances in Nanopore-Based Exosome Analysis
3.1. High-Throughput Exosome Separation Driven by Magnetic Nanopore Technology
3.2. Application of Electrochemical Signal Techniques in Precise Exosome Detection
3.3. Innovative Applications of Nanomaterial Assembly in Exosome Enrichment and Detection
3.4. Enhancing Exosome Detection Sensitivity Using Plasmon Resonance
4. Diverse Applications of Nanopore Technologies in Exosome Separation and Detection for Cancer Screening Across Tumor Types
5. Comparative Analysis of Nanopore-Based Approaches for Exosome Detection in Tumor Screening
6. Commercial Products for Cancer Diagnosis Based on Exosomes
7. Discussion and Future Perspectives
7.1. Improving Sensitivity and Specificity
7.2. Integration with Machine Learning and Data Analysis
7.3. Broadening Applications Beyond Oncology
7.4. Multiplexing and Multi-Biomarker Detection
7.5. Clinical Validation and Regulatory Challenges
Funding
Conflicts of Interest
Abbreviations
| AFP | Alpha-Fetoprotein |
| AuNC | Gold Nanocluster |
| AuR | Gold Nanorod |
| BPH | Benign Prostatic Hyperplasia |
| CA15-3 | Cancer Antigen 15-3 |
| CA19-9 | Carbohydrate Antigen 19-9 |
| CD63 | Cluster of Differentiation 63 (Exosome surface markers) |
| CNPs | Carbon Nanopipettes |
| CRPC | Castration-Resistant Prostate Cancer |
| ctDNA | Circulating Tumor DNA |
| CTCs | Circulating Tumor Cells |
| DCP | Des-Gamma-Carboxy Prothrombin |
| EGFR | Epidermal Growth Factor Receptor |
| ERP | Electrochemical Resistive Pulse |
| EVs | Extracellular Vesicles |
| GPC-1 | Glypican-1 |
| HCC | Hepatocellular Carcinoma |
| ILVs | Intraluminal Vesicles |
| MNNPs | Multifunctional Nanoelectrode-Nanopore Nanopipettes |
| MVBs | Multivesicular Bodies |
| nPLEX | Nanoplasmonic Exosome Technology |
| NSCLC | Non-Small Cell Lung Cancer |
| NCCN | National Comprehensive Cancer Network |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PMMA | Polymethyl Methacrylate |
| PSA | Prostate-Specific Antigen |
| ROS/RNS | Reactive Oxygen Species/Reactive Nitrogen Species |
| SEM | Scanning Electron Microscope |
| TSP1 | Thrombospondin-1 |
References
- Thill, M.; Kolberg-Liedtke, C.; Albert, U.S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.U.; Budach, W.; Dall, P.; Ditsch, N.; Fallenberg, E.M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2023. Breast Care 2023, 18, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Verma, V.; Simone, C.B., 2nd; Chetty, I.J.; Chun, S.G.; Donington, J.; Edelman, M.J.; Higgins, K.A.; Kestin, L.L.; Movsas, B.; et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in Oligometastatic or Oligoprogressive Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Munshi, V.N.; Perkins, R.B.; Sy, S.; Kim, J.J. Cost-effectiveness analysis of the 2019 American Society for Colposcopy and Cervical Pathology Risk-Based Management Consensus Guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Am. J. Obstet. Gynecol. 2022, 226, e1–e228. [Google Scholar] [CrossRef]
- Hayashi, K.; Kitago, M.; Abe, Y.; Yagi, H.; Hasegawa, Y.; Hori, S.; Tanaka, M.; Nakano, Y.; Asakura, K.; Masugi, Y.; et al. Long-term survival after surgical resection for bone metastasis from pancreatic cancer: A case report. Medicine 2023, 102, e35856. [Google Scholar] [CrossRef]
- Li, M.; Zheng, W. Metastasis of endometrial adenocarcinoma masquerading as a primary rectal cancer: A rare case report with literature review. Medicine 2023, 102, e36170. [Google Scholar] [CrossRef]
- Aleksandrowicz, K.; Hempel, D.; Politynska, B.; Wojtukiewicz, A.M.; Honn, K.V.; Tang, D.G.; Wojtukiewicz, M.Z. The Complex Role of Thrombin in Cancer and Metastasis: Focus on Interactions with the Immune System. Semin. Thromb. Hemost. 2024, 50, 462–473. [Google Scholar] [CrossRef]
- Wang, T.W.; Chao, H.S.; Chiu, H.Y.; Lu, C.F.; Liao, C.Y.; Lee, Y.; Chen, J.R.; Shiao, T.H.; Chen, Y.M.; Wu, Y.T. Radiomics of metastatic brain tumor as a predictive image biomarker of progression-free survival in patients with non-small-cell lung cancer with brain metastasis receiving tyrosine kinase inhibitors. Transl. Oncol. 2024, 39, 101826. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, S.; Xu, Z.; Zhang, X.; Wang, H.; Fan, G.; Liao, X. Prevalence and risk factors of bone metastasis and the development of bone metastatic prognostic classification system: A pan-cancer population study. Aging 2023, 15, 13134–13149. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Z.Z.; Cai, Z.M.; Zhong, N.N.; Cao, L.M.; Huo, F.Y.; Liu, B.; Wu, Q.J.; Bu, L.L. Nanotheranostics in cancer lymph node metastasis: The long road ahead. Pharmacol. Res. 2023, 198, 106989. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xing, Z.; Dai, Q.; Cheng, H.; Wang, X. Role of aggressive locoregional surgery in treatment strategies for ipsilateral supraclavicular lymph node metastasis of breast cancer: A real-world cohort study. Front. Mol. Biosci. 2023, 10, 1248410. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, F.; Zhou, Q.; Xu, H.; Bian, J. Metastasis, characteristic, and treatment of breast cancer in young women and older women: A study from the Surveillance, Epidemiology, and End Results registration database. PLoS ONE 2023, 18, e0293830. [Google Scholar] [CrossRef] [PubMed]
- Gera, K.; Kahramangil, D.; Fenton, G.A.; Martir, D.; Rodriguez, D.N.; Ijaz, Z.; Lin, R.Y.; Rogers, S.C.; Ramnaraign, B.H.; George, T.J.; et al. Prognosis and Treatment Outcomes of Bone Metastasis in Gallbladder Adenocarcinoma: A SEER-Based Study. Cancers 2023, 15, 5055. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Childs, D.S.; Ahmed, M.E.; Tuba Kendi, A.; Johnson, G.B.; Orme, J.J.; Stish, B.J.; Phillips, R.M.; Park, S.S.; Davis, B.J.; et al. Treatment modalities and survival outcomes in prostate cancer parenchymal brain metastasis. Prostate 2024, 84, 237–244. [Google Scholar] [CrossRef]
- Bassani, S.; Lee, Y.K.; Campagnari, V.; Eccher, A.; Monzani, D.; Nocini, R.; Sacchetto, L.; Molteni, G. From Hype To Reality: A Narrative Review on the Promising Role of Artificial Intelligence in Larynx Cancer Detection and Transoral Microsurgery. Crit. Rev. Oncog. 2023, 28, 21–24. [Google Scholar] [CrossRef]
- Kawada, T.; Shim, S.R.; Quhal, F.; Rajwa, P.; Pradere, B.; Yanagisawa, T.; Bekku, K.; Laukhtina, E.; von Deimling, M.; Teoh, J.Y.; et al. Diagnostic Accuracy of Liquid Biomarkers for Clinically Significant Prostate Cancer Detection: A Systematic Review and Diagnostic Meta-analysis of Multiple Thresholds. Eur. Urol. Oncol. 2024, 7, 649–662. [Google Scholar] [CrossRef]
- Tan, H.; Hosein, P.J. Detection and therapeutic implications of homologous recombination repair deficiency in pancreatic cancer: A narrative review. J. Gastrointest. Oncol. 2023, 14, 2249–2259. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, A.; Xie, J. Detection of clinically important BRCA gene mutations in ovarian cancer patients using next generation sequencing analysis. Am. J. Cancer Res. 2023, 13, 5005–5020. [Google Scholar]
- Young, E.; Edwards, L.; Singh, R. The Role of Artificial Intelligence in Colorectal Cancer Screening: Lesion Detection and Lesion Characterization. Cancers 2023, 15, 5126. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 2020, 16, e1903916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Zhou, Q.; Wu, Y.L. The emerging roles of NGS-based liquid biopsy in non-small cell lung cancer. J. Hematol. Oncol. 2017, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Kawamura, S.; Iinuma, H.; Wada, K.; Takahashi, K.; Minezaki, S.; Kainuma, M.; Shibuya, M.; Miura, F.; Sano, K. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. J. Hepatobiliary Pancreat. Sci. 2019, 26, 63–72. [Google Scholar] [CrossRef]
- Nimir, M.; Ma, Y.; Jeffreys, S.A.; Opperman, T.; Young, F.; Khan, T.; Ding, P.; Chua, W.; Balakrishnar, B.; Cooper, A.; et al. Detection of AR-V7 in Liquid Biopsies of Castrate Resistant Prostate Cancer Patients: A Comparison of AR-V7 Analysis in Circulating Tumor Cells, Circulating Tumor RNA and Exosomes. Cells 2019, 8, 688. [Google Scholar] [CrossRef]
- Cai, X.; Janku, F.; Zhan, Q.; Fan, J.B. Accessing Genetic Information with Liquid Biopsies. Trends Genet. 2015, 31, 564–575. [Google Scholar] [CrossRef]
- Huyan, T.; Li, H.; Peng, H.; Chen, J.; Yang, R.; Zhang, W.; Li, Q. Extracellular Vesicles—Advanced Nanocarriers in Cancer Therapy: Progress and Achievements. Int. J. Nanomedicine 2020, 15, 6485–6502. [Google Scholar] [CrossRef]
- Ailuno, G.; Baldassari, S.; Lai, F.; Florio, T.; Caviglioli, G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells 2020, 9, 2569. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, P.; Shen, K. Role of Exosome Shuttle RNA in Cell-to-Cell Communication. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016, 38, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Issadore, D. Diagnostic technologies for circulating tumour cells and exosomes. Biosci. Rep. 2015, 36, e00292. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Sun, J.; Xianyu, Y.; Jiang, X. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef]
- Quesada-Gonzalez, D.; Merkoci, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent Advances in Electrochemical Immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef]
- Turner, A.P. Biosensors: Fundamentals and applications—Historic book now open access. Biosens. Bioelectron. 2015, 65, A1. [Google Scholar] [CrossRef]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Ortiz, N.; Sahmoud, A.; Black, T.; Aiello, N.M.; McKenzie, L.; O’Hara, M.; Redlinger, C.; et al. Combining Machine Learning and Nanofluidic Technology To Diagnose Pancreatic Cancer Using Exosomes. ACS Nano 2017, 11, 11182–11193. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef]
- Min, J.; Son, T.; Hong, J.S.; Cheah, P.S.; Wegemann, A.; Murlidharan, K.; Weissleder, R.; Lee, H.; Im, H. Plasmon-Enhanced Biosensing for Multiplexed Profiling of Extracellular Vesicles. Adv. Biosyst. 2020, 4, e2000003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cheng, L.; Hu, L.; Lou, D.; Zhang, T.; Li, J.; Zhu, Q.; Liu, F. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens. Bioelectron. 2020, 163, 112290. [Google Scholar] [CrossRef]
- Yi, K.; Wang, Y.; Rong, Y.; Bao, Y.; Liang, Y.; Chen, Y.; Liu, F.; Zhang, S.; He, Y.; Liu, W.; et al. Transcriptomic Signature of 3D Hierarchical Porous Chip Enriched Exosomes for Early Detection and Progression Monitoring of Hepatocellular Carcinoma. Adv. Sci. 2024, 11, e2305204. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Rotenberg, S.A.; Mirkin, M.V. Electrochemical Resistive-Pulse Sensing of Extracellular Vesicles. Anal. Chem. 2022, 94, 12614–12620. [Google Scholar] [CrossRef] [PubMed]
- Wen-Nan, F.; He, J.; Li, S.-Y.; Yang, D.; Li, H.-N.; Yang, G.-C.; Fu, Q.; Shan, Y.-P. Detection of Secretion of Exosomes from Individual Cell in Real-Time by Multifunctional Nanoelectrode. Chin. J. Anal. Chem. 2020, 48, e20061–e20068. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Zhao, T.; Chen, Y.; Luo, Y.; Dong, Y.; Tang, H.; Jiang, J. Real-Time Monitoring of Exosomes Secretion from Single Cell Using Dual-Nanopore Biosensors. ACS Sens. 2023, 8, 2583–2590. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Gusachenko, O.N.; Zenkova, M.A.; Vlassov, V.V. Nucleic acids in exosomes: Disease markers and intercellular communication molecules. Biochemistry 2013, 78, 1–7. [Google Scholar] [CrossRef]
- Bullock, M.D.; Silva, A.M.; Kanlikilicer-Unaldi, P.; Filant, J.; Rashed, M.H.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. Exosomal Non-Coding RNAs: Diagnostic, Prognostic and Therapeutic Applications in Cancer. Noncoding RNA 2015, 1, 53–68. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Melo, S.A.; Sugimoto, H.; O’Connell, J.T.; Kato, N.; Villanueva, A.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Caradec, J.; Kharmate, G.; Hosseini-Beheshti, E.; Adomat, H.; Gleave, M.; Guns, E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin. Biochem. 2014, 47, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Mallik, S.; Devi, A. Exosomal microRNAs (exoMIRs): Micromolecules with macro impact in oral cancer. 3 Biotech 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef] [PubMed]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Lasser, C. Identification and analysis of circulating exosomal microRNA in human body fluids. Methods Mol. Biol. 2013, 1024, 109–128. [Google Scholar] [CrossRef]
- Lin, B.; Lei, Y.; Wang, J.; Zhu, L.; Wu, Y.; Zhang, H.; Wu, L.; Zhang, P.; Yang, C. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods 2021, 5, e2001131. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Sharma, S.; Salomon, C. Techniques Associated with Exosome Isolation for Biomarker Development: Liquid Biopsies for Ovarian Cancer Detection. Methods Mol. Biol. 2020, 2055, 181–199. [Google Scholar] [CrossRef]
- Ghosh, S.; Rajendran, R.L.; Mahajan, A.A.; Chowdhury, A.; Bera, A.; Guha, S.; Chakraborty, K.; Chowdhury, R.; Paul, A.; Jha, S.; et al. Harnessing exosomes as cancer biomarkers in clinical oncology. Cancer Cell Int. 2024, 24, 278. [Google Scholar] [CrossRef]
- Xiao, X. Screening, dignosis and follow-up in lung cancer in early stage. Zhonghua Yi Xue Za Zhi 2015, 95, 3481–3483. [Google Scholar] [PubMed]
- Zhang, X.C.; Lu, S.; Zhang, L.; Wang, C.L.; Cheng, Y.; Li, G.D.; Mok, T.; Huang, C.; Liu, X.Q.; Wang, J.; et al. Consensus on dignosis for ALK positive non-small cell lung cancer in China, the 2013 version. Zhonghua Bing Li Xue Za Zhi 2013, 42, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Soon, R.H.; Zhang, P.; Jiang, K.; Lim, C.T. Cancer diagnosis: From tumor to liquid biopsy and beyond. Lab Chip 2018, 19, 11–34. [Google Scholar] [CrossRef]
- Tschuschke, M.; Kocherova, I.; Bryja, A.; Mozdziak, P.; Angelova Volponi, A.; Janowicz, K.; Sibiak, R.; Piotrowska-Kempisty, H.; Izycki, D.; Bukowska, D.; et al. Inclusion Biogenesis, Methods of Isolation and Clinical Application of Human Cellular Exosomes. J. Clin. Med. 2020, 9, 436. [Google Scholar] [CrossRef]
- Garcia-Romero, N.; Esteban-Rubio, S.; Rackov, G.; Carrion-Navarro, J.; Belda-Iniesta, C.; Ayuso-Sacido, A. Extracellular vesicles compartment in liquid biopsies: Clinical application. Mol. Aspects Med. 2018, 60, 27–37. [Google Scholar] [CrossRef]
- Salciccia, S.; Frisenda, M.; Bevilacqua, G.; Gobbi, L.; Bucca, B.; Moriconi, M.; Viscuso, P.; Gentilucci, A.; Mariotti, G.; Cattarino, S.; et al. Exosome Analysis in Prostate Cancer: How They Can Improve Biomarkers’ Performance. Curr. Issues Mol. Biol. 2023, 45, 6085–6096. [Google Scholar] [CrossRef]
- Saad, M.G.; Beyenal, H.; Dong, W.J. Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques. Biosensors 2021, 11, 518. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, W.; Lv, M.; Fan, S.; Lu, Y.; Wu, Q.; Pi, J. Potentiality of Exosomal Proteins as Novel Cancer Biomarkers for Liquid Biopsy. Front. Immunol. 2022, 13, 792046. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.M.; Xu, Y.M.; Huang, L.F.; Wang, X.Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2021, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.; Wu, Y. Recent Advances in Exosomal Protein Detection Via Liquid Biopsy Biosensors for Cancer Screening, Diagnosis, and Prognosis. AAPS J. 2018, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Di, H.; Mi, Z.; Sun, Y.; Liu, X.; Liu, X.; Li, A.; Jiang, Y.; Gao, H.; Rong, P.; Liu, D. Nanozyme-assisted sensitive profiling of exosomal proteins for rapid cancer diagnosis. Theranostics 2020, 10, 9303–9314. [Google Scholar] [CrossRef]
- Nakano, T.; Chen, I.H.; Wang, C.C.; Chen, P.J.; Tseng, H.P.; Huang, K.T.; Hu, T.H.; Li, L.C.; Goto, S.; Cheng, Y.F.; et al. Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am. J. Transplant. 2019, 19, 3250–3262. [Google Scholar] [CrossRef]
- Preis, M.; Gardner, T.B.; Gordon, S.R.; Pipas, J.M.; Mackenzie, T.A.; Klein, E.E.; Longnecker, D.S.; Gutmann, E.J.; Sempere, L.F.; Korc, M. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2011, 17, 5812–5821. [Google Scholar] [CrossRef]
- Joshi, G.K.; Deitz-McElyea, S.; Liyanage, T.; Lawrence, K.; Mali, S.; Sardar, R.; Korc, M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano 2015, 9, 11075–11089. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, C.; Lu, L.; Wang, C.; Sun, Z.; Xiao, R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens. Bioelectron. 2019, 130, 204–213. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, e1804968. [Google Scholar] [CrossRef]
- Wu, W.; Yu, X.; Wu, J.; Wu, T.; Fan, Y.; Chen, W.; Zhao, M.; Wu, H.; Li, X.; Ding, S. Surface plasmon resonance imaging-based biosensor for multiplex and ultrasensitive detection of NSCLC-associated exosomal miRNAs using DNA programmed heterostructure of Au-on-Ag. Biosens. Bioelectron. 2021, 175, 112835. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef] [PubMed]
- Dorayappan, K.D.P.; Wallbillich, J.J.; Cohn, D.E.; Selvendiran, K. The biological significance and clinical applications of exosomes in ovarian cancer. Gynecol. Oncol. 2016, 142, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Drummer, I.V.C.; Shao, Y.; Sun, Y.; Lu, Y.; Xu, K.; Ni, D.; Jiang, X.; Wang, H.; Yang, X. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharmacol. Ther. 2021, 220, 107715. [Google Scholar] [CrossRef]
- Grimaldi, A.; Zarone, M.R.; Irace, C.; Zappavigna, S.; Lombardi, A.; Kawasaki, H.; Caraglia, M.; Misso, G. Non-coding RNAs as a new dawn in tumor diagnosis. Semin. Cell Dev. Biol. 2018, 78, 37–50. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Chen, C.; Zhou, Q.; Yang, S.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Xu, J.; et al. The Biological Effect and Clinical Application of Long Noncoding RNAs in Colorectal Cancer. Cell Physiol. Biochem. 2018, 46, 431–441. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, Y.; Sun, G.; Wang, Z.; Chen, J.; Zhang, X.; Zhao, J.; Zhu, X.; Dai, J.; Liu, Z.; et al. Exosomal TUBB3 mRNA expression of metastatic castration-resistant prostate cancer patients: Association with patient outcome under abiraterone. Cancer Med. 2021, 10, 6282–6290. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.; Dai, Y.; Zhu, Q.; Chen, L.A. Update on liquid biopsy in clinical management of non-small cell lung cancer. Onco Targets Ther. 2019, 12, 5097–5109. [Google Scholar] [CrossRef]
- Aidoo-Brown, J.; Moschou, D.; Estrela, P. Multiplexed Prostate Cancer Companion Diagnostic Devices. Sensors 2021, 21, 5023. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Chen, W.; Yin, L.; Zhu, J.; Zhang, H.; Cai, C.; Li, P.; Huang, L.; Ma, P. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J. Cancer 2018, 9, 2659–2665. [Google Scholar] [CrossRef]
- Dobhal, G.; Datta, A.; Ayupova, D.; Teesdale-Spittle, P.; Goreham, R.V. Isolation, characterisation and detection of breath-derived extracellular vesicles. Sci. Rep. 2020, 10, 17381. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- An, M.; Wu, J.; Zhu, J.; Lubman, D.M. Comparison of an Optimized Ultracentrifugation Method versus Size-Exclusion Chromatography for Isolation of Exosomes from Human Serum. J. Proteome Res. 2018, 17, 3599–3605. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, J.; Wang, S. Recent Progress in Isolation and Detection of Extracellular Vesicles for Cancer Diagnostics. Adv. Healthc. Mater. 2018, 7, e1800484. [Google Scholar] [CrossRef]
- Hu, T.; Wolfram, J.; Srivastava, S. Extracellular Vesicles in Cancer Detection: Hopes and Hypes. Trends Cancer 2021, 7, 122–133. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzas, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef]
- Consortium, E.-T.; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, O.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Gong, H.; Luo, S.; Cui, Y. The Role of Exosomes and Their Applications in Cancer. Int. J. Mol. Sci. 2021, 22, 12204. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Li, J.; Wu, H.C.; Liang, X.J.; Guo, P. Solid-State and Biological Nanopore for Real-Time Sensing of Single Chemical and Sequencing of DNA. Nano Today 2013, 8, 56–74. [Google Scholar] [CrossRef]
- Ying, Y.L.; Cao, C.; Long, Y.T. Single molecule analysis by biological nanopore sensors. Analyst 2014, 139, 3826–3835. [Google Scholar] [CrossRef]
- Lenhart, B.; Wei, X.; Zhang, Z.; Wang, X.; Wang, Q.; Liu, C. Nanopore Fabrication and Application as Biosensors in Neurodegenerative Diseases. Crit. Rev. Biomed. Eng. 2020, 48, 29–62. [Google Scholar] [CrossRef]
- Kasianowicz, J.J.; Brandin, E.; Branton, D.; Deamer, D.W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. USA 1996, 93, 13770–13773. [Google Scholar] [CrossRef]
- Ito, T.; Sun, L.; Crooks, R.M. Simultaneous determination of the size and surface charge of individual nanoparticles using a carbon nanotube-based Coulter counter. Anal. Chem. 2003, 75, 2399–2406. [Google Scholar] [CrossRef]
- Choi, Y.; Baker, L.A.; Hillebrenner, H.; Martin, C.R. Biosensing with conically shaped nanopores and nanotubes. Phys. Chem. Chem. Phys. 2006, 8, 4976–4988. [Google Scholar] [CrossRef]
- Ito, T.; Sun, L.; Henriquez, R.R.; Crooks, R.M. A carbon nanotube-based coulter nanoparticle counter. Acc. Chem. Res. 2004, 37, 937–945. [Google Scholar] [CrossRef]
- An, R.; Uram, J.D.; Yusko, E.C.; Ke, K.; Mayer, M.; Hunt, A.J. Ultrafast laser fabrication of submicrometer pores in borosilicate glass. Opt. Lett. 2008, 33, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Bhagwat, N.; Black, T.; Yee, S.S.; Na, Y.J.; Fisher, S.; Kim, J.; Carpenter, E.L.; Stanger, B.Z.; Issadore, D. miRNA Profiling of Magnetic Nanopore-Isolated Extracellular Vesicles for the Diagnosis of Pancreatic Cancer. Cancer Res. 2018, 78, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Bhagwat, N.; Yee, S.S.; Black, T.; Redlinger, C.; Romeo, J.; O’Hara, M.; Raj, A.; Carpenter, E.L.; Stanger, B.Z.; et al. A magnetic micropore chip for rapid (<1 hour) unbiased circulating tumor cell isolation and in situ RNA analysis. Lab Chip 2017, 17, 3086–3096. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.; Shang, W.; Issadore, D. Track-etched magnetic micropores for immunomagnetic isolation of pathogens. Adv. Healthc. Mater. 2014, 3, 1078–1085. [Google Scholar] [CrossRef]
- Lin, A.A.; Shen, H.; Spychalski, G.; Carpenter, E.L.; Issadore, D. Modeling and optimization of parallelized immunomagnetic nanopore sorting for surface marker specific isolation of extracellular vesicles from complex media. Sci. Rep. 2023, 13, 13292. [Google Scholar] [CrossRef]
- Yu, R.J.; Hu, Y.X.; Chen, K.L.; Gu, Z.; Ying, Y.L.; Long, Y.T. Confined Nanopipet as a Versatile Tool for Precise Single Cell Manipulation. Anal. Chem. 2022, 94, 12948–12953. [Google Scholar] [CrossRef]
- Lu, S.M.; Peng, Y.Y.; Ying, Y.L.; Long, Y.T. Electrochemical Sensing at a Confined Space. Anal. Chem. 2020, 92, 5621–5644. [Google Scholar] [CrossRef]
- Hu, K.; Jia, R.; Hatamie, A.; Le Vo, K.L.; Mirkin, M.V.; Ewing, A.G. Correlating Molecule Count and Release Kinetics with Vesicular Size Using Open Carbon Nanopipettes. J. Am. Chem. Soc. 2020, 142, 16910–16914. [Google Scholar] [CrossRef]
- Morris, C.A.; Friedman, A.K.; Baker, L.A. Applications of nanopipettes in the analytical sciences. Analyst 2010, 135, 2190–2202. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Mirkin, M.V. Resistive-pulse and rectification sensing with glass and carbon nanopipettes. Proc. Math. Phys. Eng. Sci. 2017, 473, 20160931. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Su, B.; Shao, Y. Fabrication and Use of Nanopipettes in Chemical Analysis. Annu. Rev. Anal. Chem. 2018, 11, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Bayley, H.; Martin, C.R. Resistive-Pulse Sensing-From Microbes to Molecules. Chem. Rev. 2000, 100, 2575–2594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Zhang, S.H.; Wang, L.; Xiao, R.R.; Liu, W.; Zhang, X.W.; Zhou, Z.; Amatore, C.; Huang, W.H. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew. Chem. Int. Ed. Engl. 2014, 53, 12456–12460. [Google Scholar] [CrossRef]
- Li, X.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A.G. Quantitative measurement of transmitters in individual vesicles in the cytoplasm of single cells with nanotip electrodes. Angew. Chem. Int. Ed. Engl. 2015, 54, 11978–11982. [Google Scholar] [CrossRef] [PubMed]
- Cadinu, P.; Campolo, G.; Pud, S.; Yang, W.; Edel, J.B.; Dekker, C.; Ivanov, A.P. Double Barrel Nanopores as a New Tool for Controlling Single-Molecule Transport. Nano Lett. 2018, 18, 2738–2745. [Google Scholar] [CrossRef]
- Choi, Y.; Park, Y.; Kang, T.; Lee, L.P. Selective and sensitive detection of metal ions by plasmonic resonance energy transfer-based nanospectroscopy. Nat. Nanotechnol. 2009, 4, 742–746. [Google Scholar] [CrossRef]
- Shi, L.; Jing, C.; Ma, W.; Li, D.W.; Halls, J.E.; Marken, F.; Long, Y.T. Plasmon resonance scattering spectroscopy at the single-nanoparticle level: Real-time monitoring of a click reaction. Angew. Chem. Int. Ed. Engl. 2013, 52, 6011–6014. [Google Scholar] [CrossRef]
- Nam, S.; Choi, I.; Fu, C.C.; Kim, K.; Hong, S.; Choi, Y.; Zettl, A.; Lee, L.P. Graphene nanopore with a self-integrated optical antenna. Nano Lett. 2014, 14, 5584–5589. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; de la Rica, R.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604–607. [Google Scholar] [CrossRef]
- Lee, K.; Fraser, K.; Ghaddar, B.; Yang, K.; Kim, E.; Balaj, L.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Multiplexed Profiling of Single Extracellular Vesicles. ACS Nano 2018, 12, 494–503. [Google Scholar] [CrossRef]
- Fraser, K.; Jo, A.; Giedt, J.; Vinegoni, C.; Yang, K.S.; Peruzzi, P.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Characterization of single microvesicles in plasma from glioblastoma patients. Neuro Oncol. 2019, 21, 606–615. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, G.; Wang, H.; Li, H.; Zhang, T.; Tao, N.; Ding, X.; Yu, H. Interferometric plasmonic imaging and detection of single exosomes. Proc. Natl. Acad. Sci. USA 2018, 115, 10275–10280. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Guo, K.; Adkins, G.B.; Jiang, Q.; Liu, Y.; Sedano, S.; Duan, Y.; Yan, W.; Wang, S.E.; Bergersen, K.; et al. A Single Extracellular Vesicle (EV) Flow Cytometry Approach to Reveal EV Heterogeneity. Angew. Chem. Int. Ed. Engl. 2018, 57, 15675–15680. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett. 2018, 18, 4226–4232. [Google Scholar] [CrossRef]
- Bang, D.; Jo, E.J.; Hong, S.; Byun, J.Y.; Lee, J.Y.; Kim, M.G.; Lee, L.P. Asymmetric Nanocrescent Antenna on Upconversion Nanocrystal. Nano Lett. 2017, 17, 6583–6590. [Google Scholar] [CrossRef]
- Lee, K.; Cui, Y.; Lee, L.P.; Irudayaraj, J. Quantitative imaging of single mRNA splice variants in living cells. Nat. Nanotechnol. 2014, 9, 474–480. [Google Scholar] [CrossRef]
- Waldeisen, J.R.; Wang, T.; Ross, B.M.; Lee, L.P. Disassembly of a core-satellite nanoassembled substrate for colorimetric biomolecular detection. ACS Nano 2011, 5, 5383–5389. [Google Scholar] [CrossRef]
- Li, Y.; Hu, K.; Yu, Y.; Rotenberg, S.A.; Amatore, C.; Mirkin, M.V. Direct Electrochemical Measurements of Reactive Oxygen and Nitrogen Species in Nontransformed and Metastatic Human Breast Cells. J. Am. Chem. Soc. 2017, 139, 13055–13062. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; Margolis, E.; Partin, A.; Carter, B.; Brown, G.; Torkler, P.; Noerholm, M.; Skog, J.; Shore, N.; et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10ng/ml at Initial Biopsy. Eur. Urol. 2018, 74, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Loo, J.F.; Yu, S.; Kong, S.K.; Chan, T.F. Monitoring bacterial growth using tunable resistive pulse sensing with a pore-based technique. Appl. Microbiol. Biotechnol. 2014, 98, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.; Kozak, D.; Coleman, V.A.; Jamting, A.K.; Trau, M. A comparative study of submicron particle sizing platforms: Accuracy, precision and resolution analysis of polydisperse particle size distributions. J. Colloid. Interface Sci. 2013, 405, 322–330. [Google Scholar] [CrossRef] [PubMed]
- de Vrij, J.; Maas, S.L.; van Nispen, M.; Sena-Esteves, M.; Limpens, R.W.; Koster, A.J.; Leenstra, S.; Lamfers, M.L.; Broekman, M.L. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine 2013, 8, 1443–1458. [Google Scholar] [CrossRef]
- Maas, S.L.; De Vrij, J.; Broekman, M.L. Quantification and size-profiling of extracellular vesicles using tunable resistive pulse sensing. J. Vis. Exp. 2014, 92, e51623. [Google Scholar] [CrossRef]
- Blundell, E.L.; Vogel, R.; Platt, M. Particle-by-Particle Charge Analysis of DNA-Modified Nanoparticles Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 1082–1090. [Google Scholar] [CrossRef]
- Chung, M.C.; Dean, S.; Marakasova, E.S.; Nwabueze, A.O.; van Hoek, M.L. Chitinases are negative regulators of Francisella novicida biofilms. PLoS ONE 2014, 9, e93119. [Google Scholar] [CrossRef]
- Boing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Chen, J.; Dong, H.; Fu, R.; Liu, X.; Xue, F.; Liu, W.; Chen, Y.; Sun, T.; Ju, M.; Dai, X.; et al. Machine learning analyses constructed a novel model to predict recurrent thrombosis in adults with essential thrombocythemia. J. Thromb. Thrombolysis 2023, 56, 291–300. [Google Scholar] [CrossRef]
- Li, J.; Liang, Y.; Zhao, X.; Wu, C. Integrating machine learning algorithms to systematically assess reactive oxygen species levels to aid prognosis and novel treatments for triple -negative breast cancer patients. Front. Immunol. 2023, 14, 1196054. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, X.Y.; Xia, J.; Li, X.; Yang, T.; Wang, J.H. Ratiometric 3D DNA Machine Combined with Machine Learning Algorithm for Ultrasensitive and High-Precision Screening of Early Urinary Diseases. ACS Nano 2021, 15, 19522–19534. [Google Scholar] [CrossRef] [PubMed]
- Gorgzadeh, A.; Nazari, A.; Ali Ehsan Ismaeel, A.; Safarzadeh, D.; Hassan, J.A.K.; Mohammadzadehsaliani, S.; Kheradjoo, H.; Yasamineh, P.; Yasamineh, S. A state-of-the-art review of the recent advances in exosome isolation and detection methods in viral infection. Virol. J. 2024, 21, 34. [Google Scholar] [CrossRef]
- Deng, F.; Miller, J. A review on protein markers of exosome from different bio-resources and the antibodies used for characterization. J. Histotechnol. 2019, 42, 226–239. [Google Scholar] [CrossRef]
- Bei, Y.; Yu, P.; Cretoiu, D.; Cretoiu, S.M.; Xiao, J. Exosomes-Based Biomarkers for the Prognosis of Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 998, 71–88. [Google Scholar] [CrossRef]
- Yousif, G.; Qadri, S.; Haik, M.; Haik, Y.; Parray, A.S.; Shuaib, A. Circulating Exosomes of Neuronal Origin as Potential Early Biomarkers for Development of Stroke. Mol. Diagn. Ther. 2021, 25, 163–180. [Google Scholar] [CrossRef]
- Nistico, N.; Maisano, D.; Iaccino, E.; Vecchio, E.; Fiume, G.; Rotundo, S.; Quinto, I.; Mimmi, S. Role of Chronic Lymphocytic Leukemia (CLL)-Derived Exosomes in Tumor Progression and Survival. Pharmaceuticals 2020, 13, 244. [Google Scholar] [CrossRef]
- Ko, J.; Oh, J.; Ahmed, M.S.; Carlson, J.C.T.; Weissleder, R. Ultra-fast cycling for multiplexed cellular fluorescence imaging. Angew. Chem. Weinheim Bergstr. Ger. 2020, 132, 6906–6913. [Google Scholar] [CrossRef]
- Giedt, R.J.; Pathania, D.; Carlson, J.C.T.; McFarland, P.J.; Del Castillo, A.F.; Juric, D.; Weissleder, R. Single-cell barcode analysis provides a rapid readout of cellular signaling pathways in clinical specimens. Nat. Commun. 2018, 9, 4550. [Google Scholar] [CrossRef]



| Nanopore Approach | Magnetic Nanopores | Electrochemical Sensing | Nanomaterial Assembly | Plasmonic Nanopores |
|---|---|---|---|---|
| Core Function | High-throughput isolation | Single-particle detection | Ultrasensitive detection | Multiplex biomarker profiling |
| Sensitivity/ Detection Limit (LOD) | Moderate (Sensitivity improves with magnetic nanoparticles and functionalization) | High (Single-molecule detection) | High /~1000 particles/mL (Signal amplification with nanomaterials improves detection limits) | High /~670 aM (Detects low abundance biomarkers) |
| Specificity | High (Capable of effectively distinguishing target exosomes from other particles) | High (Selective recognition of target molecules after functionalization) | High (Functionalized nanomaterials enhance the recognition of target exosomes) | Very high (Utilizes resonance to detect molecular interactions) |
| Throughput | High | Moderate | Moderate | Low |
| Operational Complexity | Low (Automated systems) | High (Requires skilled handling) | Moderate (Nanomaterial synthesis) | High (Optical alignment) |
| Clinical Application | Used in exosome isolation and tumor biomarker detection, potential for liquid biopsy applications | Not widely adopted yet in clinical trials, but showing potential for real-time cancer detection | Limited clinical application; mainly in research or early stage commercial use | Not widely used in clinical settings yet, mostly in research and specialized diagnostics |
| Cost | Low to moderate (Magnetic nanoparticles and separation equipment) | Moderate (Depends on sensor integration and detection setup) | Moderate to high (High cost of nanomaterials and assembly processes) | High (Requires specialized equipment and maintenance) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, H.; Shi, L.; Gan, S.; Fan, G.; Dong, Y. Innovative Applications of Nanopore Technology in Tumor Screening: An Exosome-Centric Approach. Biosensors 2025, 15, 199. https://doi.org/10.3390/bios15040199
Chi H, Shi L, Gan S, Fan G, Dong Y. Innovative Applications of Nanopore Technology in Tumor Screening: An Exosome-Centric Approach. Biosensors. 2025; 15(4):199. https://doi.org/10.3390/bios15040199
Chicago/Turabian StyleChi, Heng, Liuxin Shi, Songlin Gan, Guangyi Fan, and Yuliang Dong. 2025. "Innovative Applications of Nanopore Technology in Tumor Screening: An Exosome-Centric Approach" Biosensors 15, no. 4: 199. https://doi.org/10.3390/bios15040199
APA StyleChi, H., Shi, L., Gan, S., Fan, G., & Dong, Y. (2025). Innovative Applications of Nanopore Technology in Tumor Screening: An Exosome-Centric Approach. Biosensors, 15(4), 199. https://doi.org/10.3390/bios15040199





