Amplitude of Intracranial Induced Electric Fields Does Not Linearly Decrease with Age: A Computational Study of Anatomical Effects in Adults
Abstract
1. Introduction
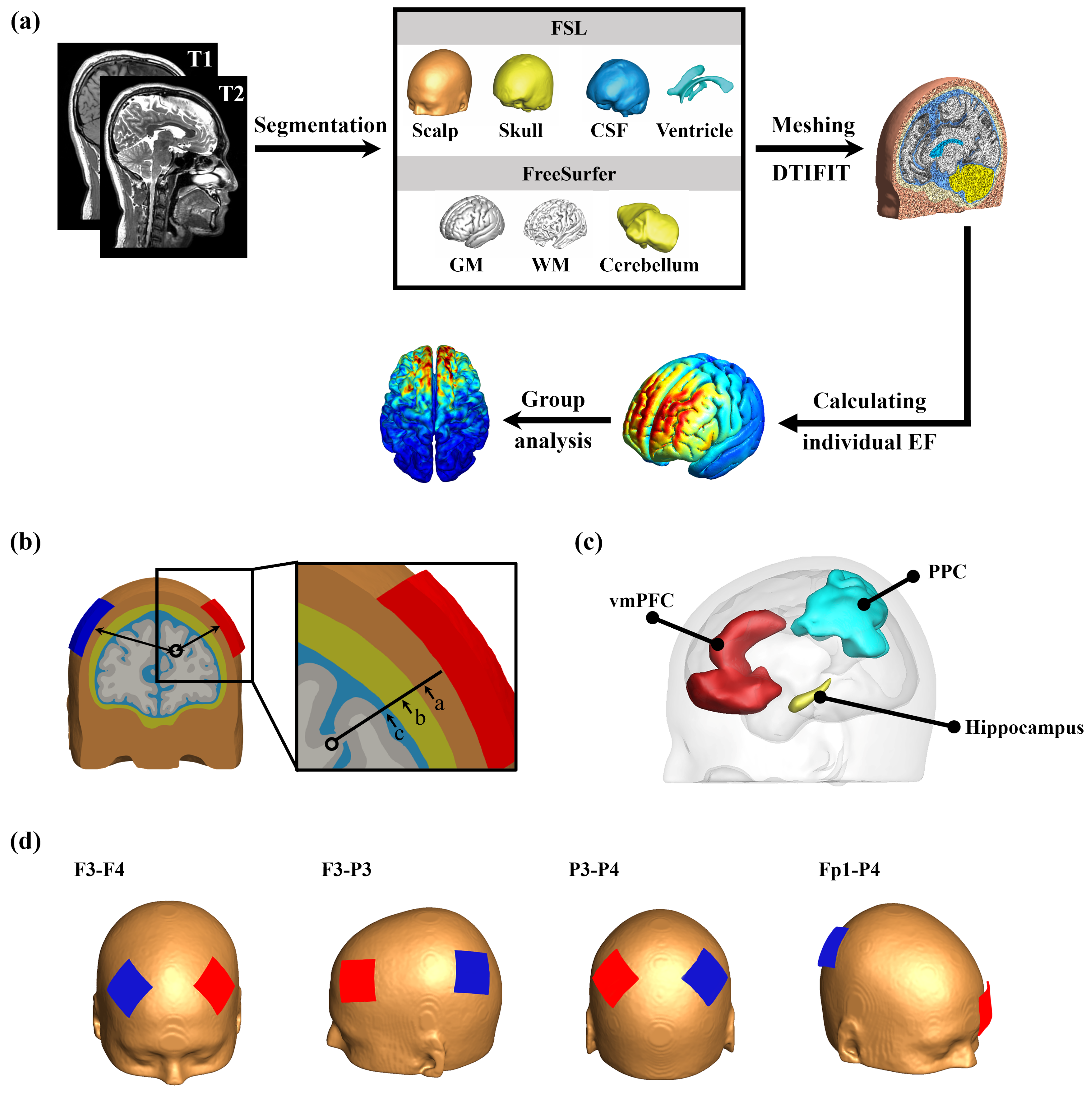
2. Materials and Methods
2.1. MRI Data Acquisition
2.2. Construction of Volume Conductor Models
2.3. Electrical Field (EF) Computations
2.4. Anatomical Parameters
2.5. ROI Definition
2.6. Statistical Analysis
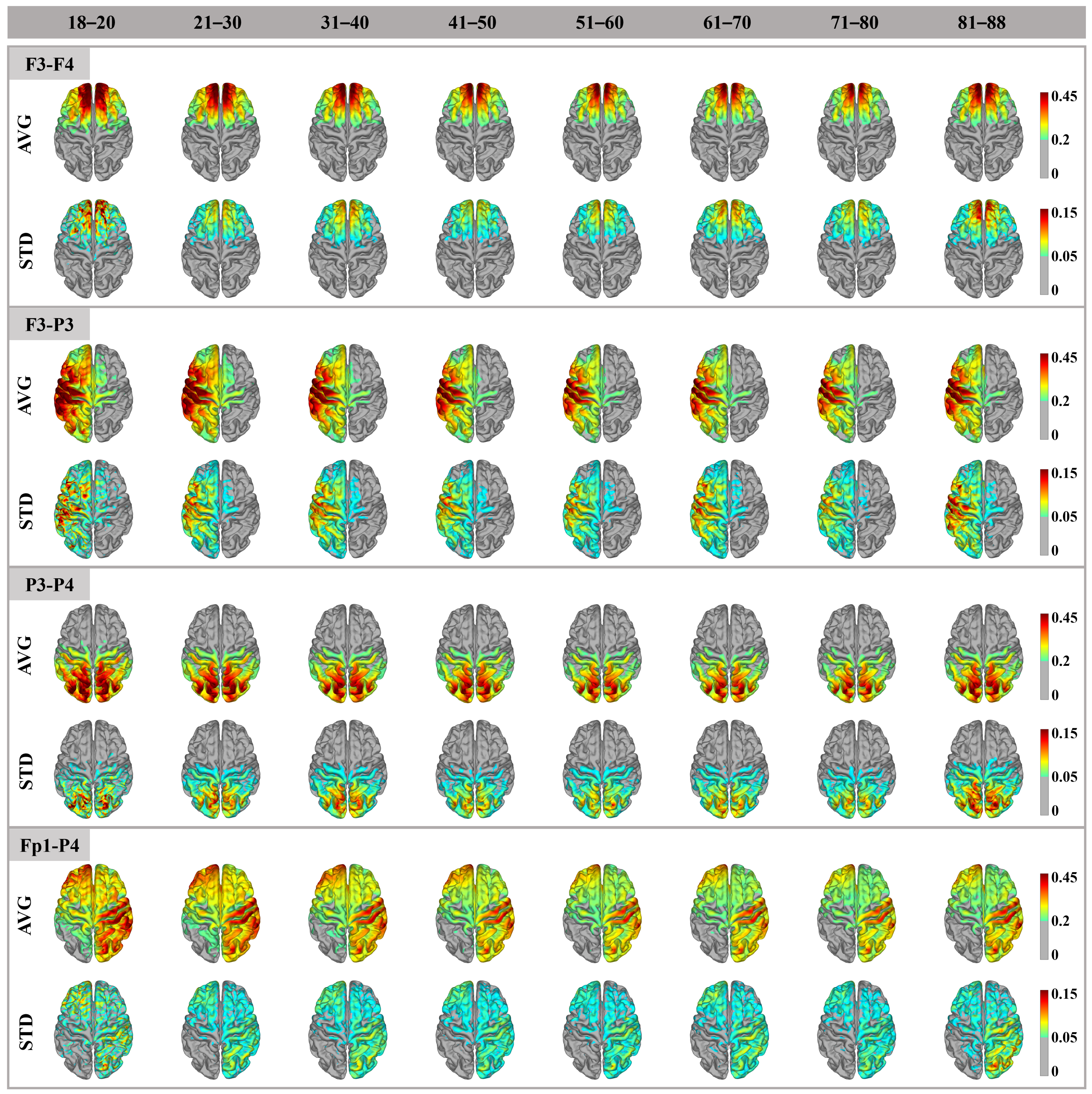
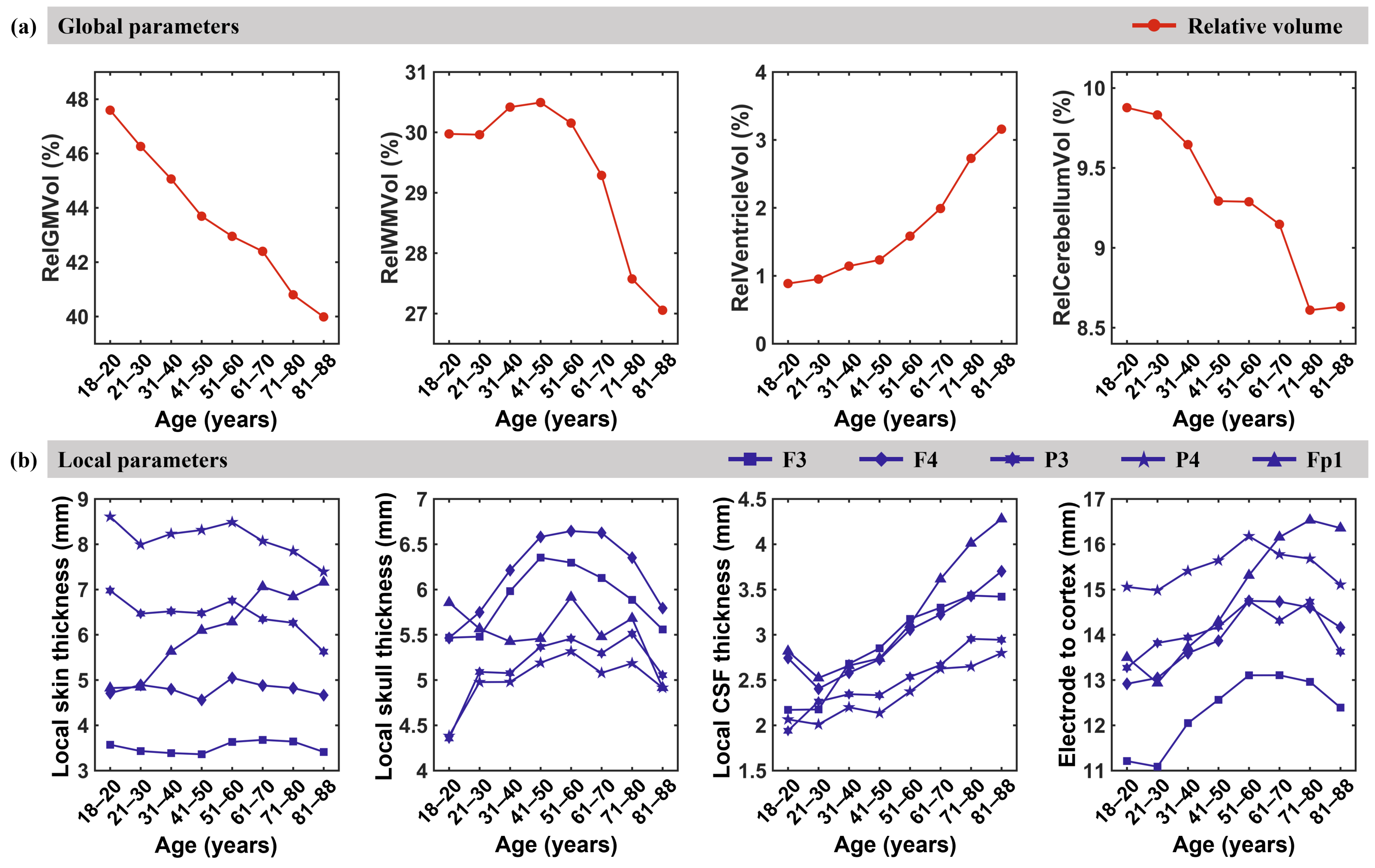
3. Results
3.1. Cortex Electric Field Distribution
3.2. ROI Electric Field Amplitude
3.3. Anatomical Parameters
3.4. Correlation Between Cortical E-Field Amplitude and Anatomical Parameters
4. Discussion
4.1. Distribution Pattern and Amplitude of the Electric Field
4.2. Global and Local Anatomical Parameters
4.3. Correlation Between Anatomical Parameters and Cortical Electric Field
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| tES | transcranial electrical stimulation |
| tDCS | transcranial direct current stimulation |
| tACS | transcranial alternating current stimulation |
| tRNS | transcranial random noise stimulation |
| TI | temporal interference |
| CSF | cerebrospinal fluid |
| GM | gray matter |
| WM | white matter |
| ROIs | regions of interest |
| DWI | diffusion-weighted imaging |
| TIV | total intracranial volume |
| vmPFC | ventromedial prefrontal cortex |
| PPC | posterior parietal cortex |
| RelGMVol | relative GM volume |
| RelWMVol | relative WM volume |
| RelVentVol | relative ventricle volume |
| RelCereVol | relative cerebellum volume |
Appendix A
| Age (Years) | F3-F4 | F3-P3 | P3-P4 | Fp1-P4 |
|---|---|---|---|---|
| 18–20 | 0.630 (0.639) | 0.710 (0.675) | 0.590 (0.588) | 0.653 (0.607) |
| 21–30 | 0.611 (0.596) | 0.675 (0.671) | 0.587 (0.582) | 0.621 (0.610) |
| 31–40 | 0.570 (0.571) | 0.599 (0.664) ** | 0.550 (0.577) | 0.565 (0.614) ** |
| 41–50 | 0.553 (0.553) | 0.575 (0.636) *** | 0.532 (0.550) | 0.542 (0.589) ** |
| 51–60 | 0.551 (0.520) | 0.580 (0.602) | 0.518 (0.535) | 0.538 (0.554) |
| 61–70 | 0.543 (0.529) | 0.574 (0.607) | 0.516 (0.544) | 0.533 (0.579) ** |
| 71–80 | 0.520 (0.516) | 0.555 (0.588) | 0.497 (0.534) ** | 0.518 (0.565) ** |
| 81–88 | 0.603 (0.543) | 0.625 (0.633) | 0.552 (0.560) | 0.589 (0.579) |
| Age (Years) | F3-F4 | F3-P3 | P3-P4 | Fp1-P4 |
|---|---|---|---|---|
| 18–20 | 7093.627 (7799.388) | 8112.669 (8994.119) | 15,074.122 (13,346.022) | 7432.177 (7078.626) |
| 21–30 | 6307.498 (6477.617) | 7905.436 (7981.395) | 12,606.733 (14,776.818) ** | 7428.422 (6767.201) |
| 31–40 | 6737.324 (6891.327) | 8531.659 (7292.458) ** | 12,406.133 (13,974.758) ** | 7012.688 (6430.987) ** |
| 41–50 | 6267.261 (6000.679) | 8220.783 (6717.638) *** | 12,371.470 (13,165.953) | 6823.989 (6217.212) ** |
| 51–60 | 6279.537 (5997.522) | 7812.807 (6797.435) ** | 11,852.961 (12,281.317) | 6530.629 (6282.867) |
| 61–70 | 5800.709 (5739.736) | 6987.422 (6641.700) | 10,638.890 (11,326.190) | 6223.979 (5433.416) ** |
| 71–80 | 5876.427 (5500.898) | 6338.130 (5683.273) ** | 10,270.464 (9783.348) | 6057.178 (5075.829) *** |
| 81–88 | 5103.779 (5294.637) | 6188.709 (5085.589) ** | 8566.855 (8907.962) | 5835.409 (5440.269) |
References
- Gu, X.; Ren, H. A Survey of Transoral Robotic Mechanisms: Distal Dexterity, Variable Stiffness, and Triangulation. Cyborg Bionic Syst. 2023, 4, 0007. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Ouyang, B.; Mo, H.; Ren, H.; Yang, S. Three-Dimensional Collision Avoidance Method for Robot-Assisted Minimally Invasive Surgery. Cyborg Bionic Syst. 2023, 4, 0042. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Kuo, P.H.; Su, M.I.; Huang, W.L. The efficacy of non-invasive, non-convulsive electrical neuromodulation on depression, anxiety and sleep disturbance: A systematic review and meta-analysis. Psychol. Med. 2022, 52, 801–812. [Google Scholar]
- Wang, H.X.; Wang, L.; Zhang, W.R.; Xue, Q.; Peng, M.; Sun, Z.C.; Li, L.P.; Wang, K.; Yang, X.T.; Jia, Y.; et al. Effect of transcranial alternating current stimulation for the treatment of chronic insomnia: A randomized, double-blind, parallel-group, placebo-controlled clinical trial. Psychother. Psychosom. 2020, 89, 38–47. [Google Scholar]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar]
- Wischnewski, M.; Alekseichuk, I.; Opitz, A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cogn. Sci. 2023, 27, 189–205. [Google Scholar]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Antal, A.; Boros, K.; Poreisz, C.; Chaieb, L.; Terney, D.; Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008, 1, 97–105. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Krause, B.; Cohen Kadosh, R. Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; López-Alonso, V.; Cheeran, B.; Suppa, A. Variability in non-invasive brain stimulation studies: Reasons and results. Neurosci. Lett. 2020, 719, 133330. [Google Scholar] [CrossRef] [PubMed]
- Chew, T.; Ho, K.A.; Loo, C.K. Inter- and Intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef]
- Wicherts, J.M.; Héroux, M.E.; Loo, C.K.; Taylor, J.L.; Gandevia, S.C. Questionable science and reproducibility in electrical brain stimulation research. PLoS ONE 2017, 12, e0175635. [Google Scholar] [CrossRef]
- Jones, K.T.; Arciniega, H.; Berryhill, M.E. Replacing tDCS with theta tACS provides selective, but not general WM benefits. Brain Res. 2019, 1720, 146324. [Google Scholar] [CrossRef]
- Veniero, D.; Benwell, C.S.Y.; Ahrens, M.M.; Thut, G. Inconsistent Effects of Parietal alpha-tACS on Pseudoneglect across Two Experiments: A Failed Internal Replication. Front. Psychol. 2017, 8, 952. [Google Scholar] [CrossRef]
- Koenigs, M.; Ukueberuwa, D.; Campion, P.; Grafman, J.; Wassermann, E. Bilateral frontal transcranial direct current stimulation: Failure to replicate classic findings in healthy subjects. Clin. Neurophysiol. 2009, 120, 80–84. [Google Scholar] [CrossRef]
- Lippold, O.C.J.; Redfearn, J.W.T. Mental Changes Resulting from the Passage of Small Direct Currents Through the Human Brain. Br. J. Psychiatry 2018, 110, 768–772. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Notturno, F.; Marzetti, L.; Pizzella, V.; Uncini, A.; Zappasodi, F. Local and remote effects of transcranial direct current stimulation on the electrical activity of the motor cortical network. Hum. Brain Mapp. 2014, 35, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject Variability in Electric Fields of Motor Cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Wang, H.; Sun, W.; Zhang, J.; Yan, Z.; Wang, C.; Wang, L.; Liu, T.; Li, C.; Chen, D.; Shintaro, F.; et al. Influence of layered skull modeling on the frequency sensitivity and target accuracy in simulations of transcranial current stimulation. Hum. Brain Mapp. 2021, 42, 5345–5356. [Google Scholar] [CrossRef]
- Antonenko, D.; Grittner, U.; Saturnino, G.; Nierhaus, T.; Thielscher, A.; Flöel, A. Inter-individual and age-dependent variability in simulated electric fields induced by conventional transcranial electrical stimulation. NeuroImage 2021, 224, 117413. [Google Scholar] [CrossRef]
- Indahlastari, A.; Albizu, A.; O’Shea, A.; Forbes, M.A.; Nissim, N.R.; Kraft, J.N.; Evangelista, N.D.; Hausman, H.K.; Woods, A.J.; Alzheimers Dis Neuroimaging, I. Modeling transcranial electrical stimulation in the aging brain. Brain Stimul. 2020, 13, 664–674. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Kashyap, R.; Goodwill, A.M.; O’Brien, B.A.; Rapp, B.; Oishi, K.; Desmond, J.E.; Chen, S.H.A. Sex difference in tDCS current mediated by changes in cortical anatomy: A study across young, middle and older adults. Brain Stimul. 2022, 15, 125–140. [Google Scholar] [CrossRef]
- Rezaee, Z.; Dutta, A. Lobule-Specific Dosage Considerations for Cerebellar Transcranial Direct Current Stimulation During Healthy Aging: A Computational Modeling Study Using Age-Specific Magnetic Resonance Imaging Templates. Neuromodulation Technol. Neural Interface 2020, 23, 341–365. [Google Scholar] [CrossRef]
- Coupé, P.; Catheline, G.; Lanuza, E.; Manjón, J.V. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2017, 38, 5501–5518. [Google Scholar] [CrossRef]
- Shafto, M.A.; Tyler, L.K.; Dixon, M.; Taylor, J.R.; Rowe, J.B.; Cusack, R.; Calder, A.J.; Marslen-Wilson, W.D.; Duncan, J.; Dalgleish, T.; et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Williams, N.; Cusack, R.; Auer, T.; Shafto, M.A.; Dixon, M.; Tyler, L.K.; Cam, C.; Henson, R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage 2017, 144, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Puonti, O.; Nielsen, J.D.; Antonenko, D.; Madsen, K.H.H.; Thielscher, A. SimNIBS 2.1: A Comprehensive Pipeline for Individualized Electric Field Modelling for Transcranial Brain Stimulation. Brain Hum. Body Model. 2018, 2018, 3–25. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis—I. Segmentation and surface reconstruction. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis—II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 1999, 9, 195–207. [Google Scholar] [CrossRef]
- Windhoff, M.; Opitz, A.; Thielscher, A. Electric field calculations in brain stimulation based on finite elements: An optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp. 2013, 34, 923–935. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Nikolin, S.; Martin, D.; Loo, C.K.; Boonstra, T.W. Effects of TDCS dosage on working memory in healthy participants. Brain Stimul. 2018, 11, 518–527. [Google Scholar] [CrossRef]
- Borghini, G.; Candini, M.; Filannino, C.; Hussain, M.; Walsh, V.; Romei, V.; Zokaei, N.; Cappelletti, M. Alpha Oscillations Are Causally Linked to Inhibitory Abilities in Ageing. J. Neurosci. 2018, 38, 4418–4429. [Google Scholar] [CrossRef]
- Pahor, A.; Jaušovec, N. The Effects of Theta and Gamma tACS on Working Memory and Electrophysiology. Front. Hum. Neurosci. 2018, 11, 651. [Google Scholar] [CrossRef]
- He, Z.; Soullié, P.; Lefebvre, P.; Ambarki, K.; Felblinger, J.; Odille, F. Changes of in vivo electrical conductivity in the brain and torso related to age, fat fraction and sex using MRI. Sci. Rep. 2024, 14, 16109. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.A.; Zahn, M.; Grodzinsky, A.J.; Pascual-Leone, A. Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans. Biomed. Eng. 2004, 51, 1586–1594. [Google Scholar] [CrossRef]
- Treder, M.S.; Charest, I.; Michelmann, S.; Martín-Buro, M.C.; Roux, F.; Carceller-Benito, F.; Ugalde-Canitrot, A.; Rollings, D.T.; Sawlani, V.; Chelvarajah, R.; et al. The hippocampus as the switchboard between perception and memory. Proc. Natl. Acad. Sci. USA 2021, 118, e2114171118. [Google Scholar] [CrossRef]
- Gomez-Tames, J.; Laakso, I.; Haba, Y.; Hirata, A.; Poljak, D.; Yamazaki, K. Computational Artifacts of the In Situ Electric Field in Anatomical Models Exposed to Low-Frequency Magnetic Field. IEEE Trans. Electromagn. Compat. 2018, 60, 589–597. [Google Scholar] [CrossRef]
- Gomez-Tames, J.; Asai, A.; Mikkonen, M.; Laakso, I.; Tanaka, S.; Uehara, S.; Otaka, Y.; Hirata, A. Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J. Neural Eng. 2019, 16, 036001. [Google Scholar] [CrossRef]
- Van Hoornweder, S.; Meesen, R.; Caulfield, K.A. On the importance of using both T1-weighted and T2-weighted structural magnetic resonance imaging scans to model electric fields induced by non-invasive brain stimulation in SimNIBS. Brain Stimul. 2022, 15, 641–644. [Google Scholar] [CrossRef]
- Thomas, C.; Datta, A.; Woods, A. Effect of Aging on Cortical Current Flow Due to Transcranial Direct Current Stimulation: Considerations for safety. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3084–3087. [Google Scholar] [CrossRef]
- von Conta, J.; Kasten, F.H.; Ćurčić Blake, B.; Aleman, A.; Thielscher, A.; Herrmann, C.S. Interindividual variability of electric fields during transcranial temporal interference stimulation (tTIS). Sci. Rep. 2021, 11, 20357. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Bos, M.G.N.; van de Kamp, F.C.; Peters, S.; Crone, E.A. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev. Cogn. Neurosci. 2018, 30, 212–222. [Google Scholar] [CrossRef]
- Terry, R.D.; Deteresa, R.; Hansen, L.A. Neocortical cell counts in normal human adult aging. Ann. Neurol. 1987, 21, 530–539. [Google Scholar] [CrossRef]
- Peters, A.; Morrison, J.H.; Rosene, D.L.; Hyman, B.T. Are neurons lost from the primate cerebral cortex during normal aging? Cereb. Cortex 1998, 8, 295–300. [Google Scholar] [CrossRef]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Westlye, L.T.; Walhovd, K.B.; Dale, A.M.; Bjørnerud, A.; Due-Tønnessen, P.; Engvig, A.; Grydeland, H.; Tamnes, C.K.; Østby, Y.; Fjell, A.M. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex 2010, 20, 2055–2068. [Google Scholar] [CrossRef]
- Yeatman, J.D.; Wandell, B.A.; Mezer, A.A. Lifespan maturation and degeneration of human brain white matter. Nat. Commun. 2014, 5, 4932. [Google Scholar] [CrossRef]
- Giorgio, A.; Santelli, L.; Tomassini, V.; Bosnell, R.; Smith, S.; De Stefano, N.; Johansen-Berg, H. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage 2010, 51, 943–951. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Fjell, A.M.; Reinvang, I.; Lundervold, A.; Dale, A.M.; Eilertsen, D.E.; Quinn, B.T.; Salat, D.; Makris, N.; Fischl, B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Bernard, J.A.; Seidler, R.D. Cerebellar contributions to visuomotor adaptation and motor sequence learning: An ALE meta-analysis. Front. Hum. Neurosci. 2013, 7, 27. [Google Scholar] [CrossRef]
- Romero, J.E.; Coupe, P.; Lanuza, E.; Catheline, G.; Manjón, J.V. Toward a unified analysis of cerebellum maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2021, 42, 1287–1303. [Google Scholar] [CrossRef]
- Urban, J.E.; Weaver, A.A.; Lillie, E.M.; Maldjian, J.A.; Whitlow, C.T.; Stitzel, J.D. Evaluation of morphological changes in the adult skull with age and sex. J. Anat. 2014, 229, 838–846. [Google Scholar] [CrossRef]
- McCann, H.; Beltrachini, L. Does participant’s age impact on tDCS induced fields? Insights from computational simulations. Biomed. Phys. Eng. Express 2021, 7, 045018. [Google Scholar] [CrossRef]
- Rice, J.K.; Rorden, C.; Little, J.S.; Parra, L.C. Subject position affects EEG magnitudes. NeuroImage 2013, 64, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Hernández, P.A.; Von Ellenrieder, N.; Ojeda-Gonzalez, A.; Kochen, S.; Alemán-Gómez, Y.; Muravchik, C.; Valdés-Sosa, P.A. Approximate average head models for EEG source imaging. J. Neurosci. Methods 2009, 185, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Muffel, T.; Kirsch, F.; Shih, P.C.; Kalloch, B.; Schaumberg, S.; Villringer, A.; Sehm, B. Anodal Transcranial Direct Current Stimulation Over S1 Differentially Modulates Proprioceptive Accuracy in Young and Old Adults. Front. Aging Neurosci. 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Syeda, F.; Magsood, H.; Lee, E.G.; El-Gendy, A.A.; Jiles, D.C.; Hadimani, R.L. Effect of anatomical variability in brain on transcranial magnetic stimulation treatment. AIP Adv. 2017, 7, 056711. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef]
- McCann, H.; Pisano, G.; Beltrachini, L. Variation in Reported Human Head Tissue Electrical Conductivity Values. Brain Topogr. 2019, 32, 825–858. [Google Scholar] [CrossRef]



| Age Range | Number of Subjects | Sex (M/F) | Mean Age (SD) |
|---|---|---|---|
| 18–20 | 7 | 5/2 | 19.38 (0.69) |
| 21–30 | 49 | 17/32 | 26.66 (2.55) |
| 31–40 | 76 | 41/35 | 35.96 (2.87) |
| 41–50 | 79 | 39/40 | 46.21 (2.83) |
| 51–60 | 73 | 40/33 | 55.67 (2.95) |
| 61–70 | 72 | 36/36 | 65.78 (2.95) |
| 71–80 | 84 | 38/46 | 76.43 (3.13) |
| 81–88 | 36 | 19/17 | 84.11 (1.99) |
| Total | 476 | 235/241 | 54.78 (18.53) |
| Age (Years) | E-Field Amplitude | E-Field Focality | ||||||
|---|---|---|---|---|---|---|---|---|
| F3-F4 | F3-P3 | P3-P4 | Fp1-P4 | F3-F4 | F3-P3 | P3-P4 | Fp1-P4 | |
| 18–20 | 0.633 (0.043) | 0.700 (0.039) | 0.640 (0.031) | 0.589 (0.026) | 7.295 (0.522) | 8.365 (0.358) | 7.331 (0.228) | 14.580 (0.851) |
| [0.53, 0.74] | [0.60, 0.80] | [0.53, 0.65] | [0.56, 0.72] | [6.02, 8.57] | [7.49, 9.24] | [12.50, 16.66] | [6.77, 7.89] | |
| 21–30 | 0.601 (0.010) | 0.672 (0.012) | 0.614 (0.013) | 0.584 (0.007) | 6.419 (0.163) | 7.955 (0.240) | 6.997 (0.216) | 14.024 (0.434) |
| [0.58, 0.62] | [0.65, 0.70] | [0.57, 0.60] | [0.59, 0.64] | [6.09, 6.75] | [7.47, 8.44] | [13.15, 14.90] | [6.56, 7.43] | |
| 31–40 | 0.571 (0.011) | 0.629 (0.010) | 0.587 (0.012) | 0.562 (0.007) | 6.808 (0.152) | 7.961 (0.185) | 6.745 (0.137) | 13.129 (0.332) |
| [0.55, 0.59] | [0.61, 0.65] | [0.55, 0.58] | [0.56, 0.61] | [6.50, 7.11] | [7.59, 8.33] | [12.47, 13.79] | [6.47, 7.02] | |
| 41–50 | 0.553 (0.009) | 0.606 (0.009) | 0.566 (0.009) | 0.541 (0.006) | 6.132 (0.155) | 7.460 (0.193) | 6.517 (0.132) | 12.774 (0.306) |
| [0.54, 0.57] | [0.59, 0.62] | [0.53, 0.55] | [0.55, 0.58] | [5.82, 6.44] | [7.08, 7.84] | [12.16, 13.38] | [6.25, 6.78] | |
| 51–60 | 0.537 (0.010) | 0.590 (0.010) | 0.545 (0.009) | 0.526 (0.007) | 6.152 (0.154) | 7.354 (0.216) | 6.419 (0.165) | 12.047 (0.321) |
| [0.52, 0.56] | [0.57, 0.61] | [0.51, 0.54] | [0.53, 0.56] | [5.85, 6.46] | [6.92, 7.78] | [11.41, 12.69] | [6.09, 6.75] | |
| 61–70 | 0.536 (0.012) | 0.590 (0.012) | 0.556 (0.011) | 0.530 (0.008) | 5.770 (0.128) | 6.815 (0.174) | 5.829 (0.121) | 10.983 (0.288) |
| [0.51, 0.56] | [0.57, 0.61] | [0.51, 0.55] | [0.54, 0.58] | [5.52, 6.02] | [6.47, 7.16] | [10.41, 11.56] | [5.59, 6.07] | |
| 71–80 | 0.518 (0.009) | 0.573 (0.009) | 0.544 (0.009) | 0.518 (0.006) | 5.671 (0.117) | 5.980 (0.166) | 5.520 (0.121) | 10.004 (0.248) |
| [0.50, 0.54] | [0.56, 0.59] | [0.51, 0.53] | [0.53, 0.56] | [5.44, 5.90] | [5.65, 6.31] | [9.51, 10.50] | [5.28, 5.76] | |
| 81–88 | 0.575 (0.018) | 0.629 (0.019) | 0.584 (0.020) | 0.556 (0.012) | 5.194 (0.170) | 5.668 (0.218) | 5.649 (0.221) | 8.728 (0.326) |
| [0.54, 0.61] | [0.59, 0.67] | [0.53, 0.58] | [0.54, 0.62] | [4.85, 5.54] | [5.23, 6.11] | [8.07, 9.39] | [5.20, 6.10] | |
| ROI | Age (Years) | F3-F4 | F3-P3 | P3-P4 | Fp1-P4 |
|---|---|---|---|---|---|
| vmPFC | 18–20 | 0.348 (0.014) | 0.355 (0.016) | 0.102 (0.006) | 0.523 (0.028) |
| 21–30 | 0.337 (0.006) | 0.357 (0.007) | 0.092 (0.002) | 0.523 (0.009) | |
| 31–40 | 0.327 (0.005) | 0.343 (0.005) | 0.088 (0.001) | 0.505 (0.007) | |
| 41–50 | 0.319 (0.004) | 0.326 (0.005) | 0.091 (0.001) | 0.482 (0.008) | |
| 51–60 | 0.307 (0.004) | 0.327 (0.005) | 0.088 (0.001) | 0.473 (0.008) | |
| 61–70 | 0.310 (0.006) | 0.338 (0.006) | 0.087 (0.001) | 0.477 (0.009) | |
| 71–80 | 0.299 (0.005) | 0.324 (0.005) | 0.086 (0.001) | 0.461 (0.007) | |
| 81–88 | 0.322 (0.011) | 0.344 (0.009) | 0.090 (0.002) | 0.483 (0.010) | |
| PPC | 18–20 | 0.199 (0.012) | 0.477 (0.033) | 0.507 (0.030) | 0.327 (0.016) |
| 21–30 | 0.178 (0.004) | 0.469 (0.011) | 0.484 (0.013) | 0.313 (0.005) | |
| 31–40 | 0.167 (0.003) | 0.451 (0.010) | 0.463 (0.012) | 0.295 (0.005) | |
| 41–50 | 0.167 (0.003) | 0.428 (0.009) | 0.432 (0.008) | 0.286 (0.005) | |
| 51–60 | 0.168 (0.003) | 0.411 (0.010) | 0.424 (0.010) | 0.287 (0.006) | |
| 61–70 | 0.163 (0.003) | 0.415 (0.009) | 0.424 (0.010) | 0.283 (0.006) | |
| 71–80 | 0.166 (0.003) | 0.402 (0.008) | 0.426 (0.008) | 0.278 (0.005) | |
| 81–88 | 0.161 (0.005) | 0.431 (0.014) | 0.461 (0.018) | 0.277 (0.010) | |
| Hippocampus | 18–20 | 0.177 (0.006) | 0.269 (0.013) | 0.142 (0.008) | 0.335 (0.022) |
| 21–30 | 0.169 (0.004) | 0.301 (0.006) | 0.140 (0.003) | 0.382 (0.008) | |
| 31–40 | 0.168 (0.003) | 0.284 (0.005) | 0.133 (0.002) | 0.356 (0.007) | |
| 41–50 | 0.164 (0.003) | 0.272 (0.004) | 0.133 (0.002) | 0.338 (0.006) | |
| 51–60 | 0.163 (0.003) | 0.255 (0.004) | 0.130 (0.003) | 0.310 (0.005) | |
| 61–70 | 0.163 (0.003) | 0.256 (0.006) | 0.129 (0.002) | 0.307 (0.007) | |
| 71–80 | 0.159 (0.003) | 0.231 (0.005) | 0.124 (0.002) | 0.275 (0.006) | |
| 81–88 | 0.177 (0.005) | 0.252 (0.006) | 0.135 (0.004) | 0.278 (0.008) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yan, Z.; Kang, A.; Ouyang, J.; Ma, L.; Wang, X.; Wu, J.; Suo, D.; Funahashi, S.; Meng, W.; et al. Amplitude of Intracranial Induced Electric Fields Does Not Linearly Decrease with Age: A Computational Study of Anatomical Effects in Adults. Biosensors 2025, 15, 185. https://doi.org/10.3390/bios15030185
Zhang J, Yan Z, Kang A, Ouyang J, Ma L, Wang X, Wu J, Suo D, Funahashi S, Meng W, et al. Amplitude of Intracranial Induced Electric Fields Does Not Linearly Decrease with Age: A Computational Study of Anatomical Effects in Adults. Biosensors. 2025; 15(3):185. https://doi.org/10.3390/bios15030185
Chicago/Turabian StyleZhang, Jianxu, Zilong Yan, Anshun Kang, Jian Ouyang, Lihua Ma, Xinyue Wang, Jinglong Wu, Dingjie Suo, Shintaro Funahashi, Wei Meng, and et al. 2025. "Amplitude of Intracranial Induced Electric Fields Does Not Linearly Decrease with Age: A Computational Study of Anatomical Effects in Adults" Biosensors 15, no. 3: 185. https://doi.org/10.3390/bios15030185
APA StyleZhang, J., Yan, Z., Kang, A., Ouyang, J., Ma, L., Wang, X., Wu, J., Suo, D., Funahashi, S., Meng, W., Wang, L., & Zhang, J. (2025). Amplitude of Intracranial Induced Electric Fields Does Not Linearly Decrease with Age: A Computational Study of Anatomical Effects in Adults. Biosensors, 15(3), 185. https://doi.org/10.3390/bios15030185





