Metal Nanocluster-Based Biosensors for DNA Detection
Abstract
1. Introduction
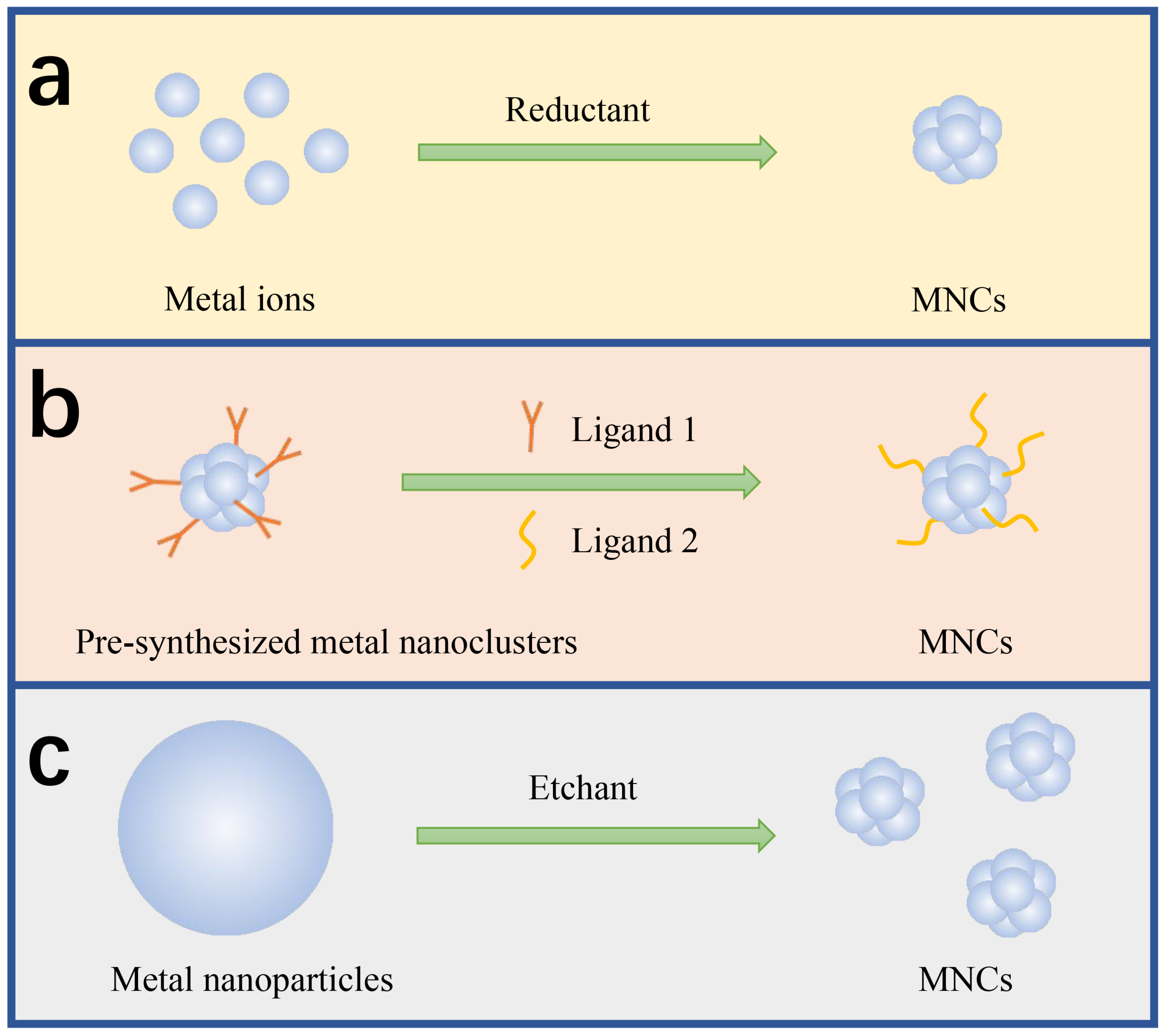
2. Synthesis of Metal Nanoclusters
2.1. Chemical Reduction
2.2. Ligand Exchange
2.3. Chemical Etching
2.4. Other Synthetic Methods
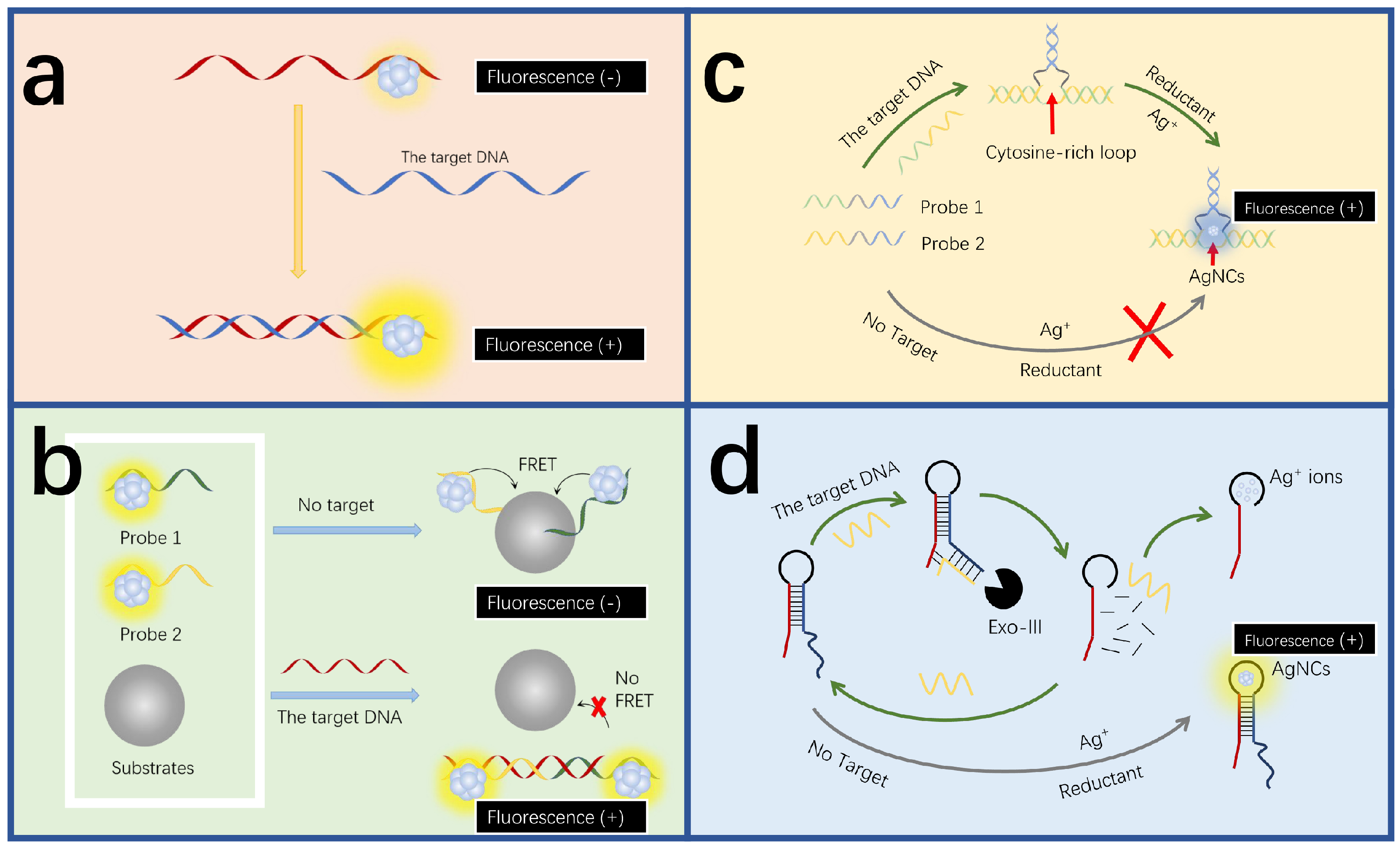
3. MNCs for DNA Detection
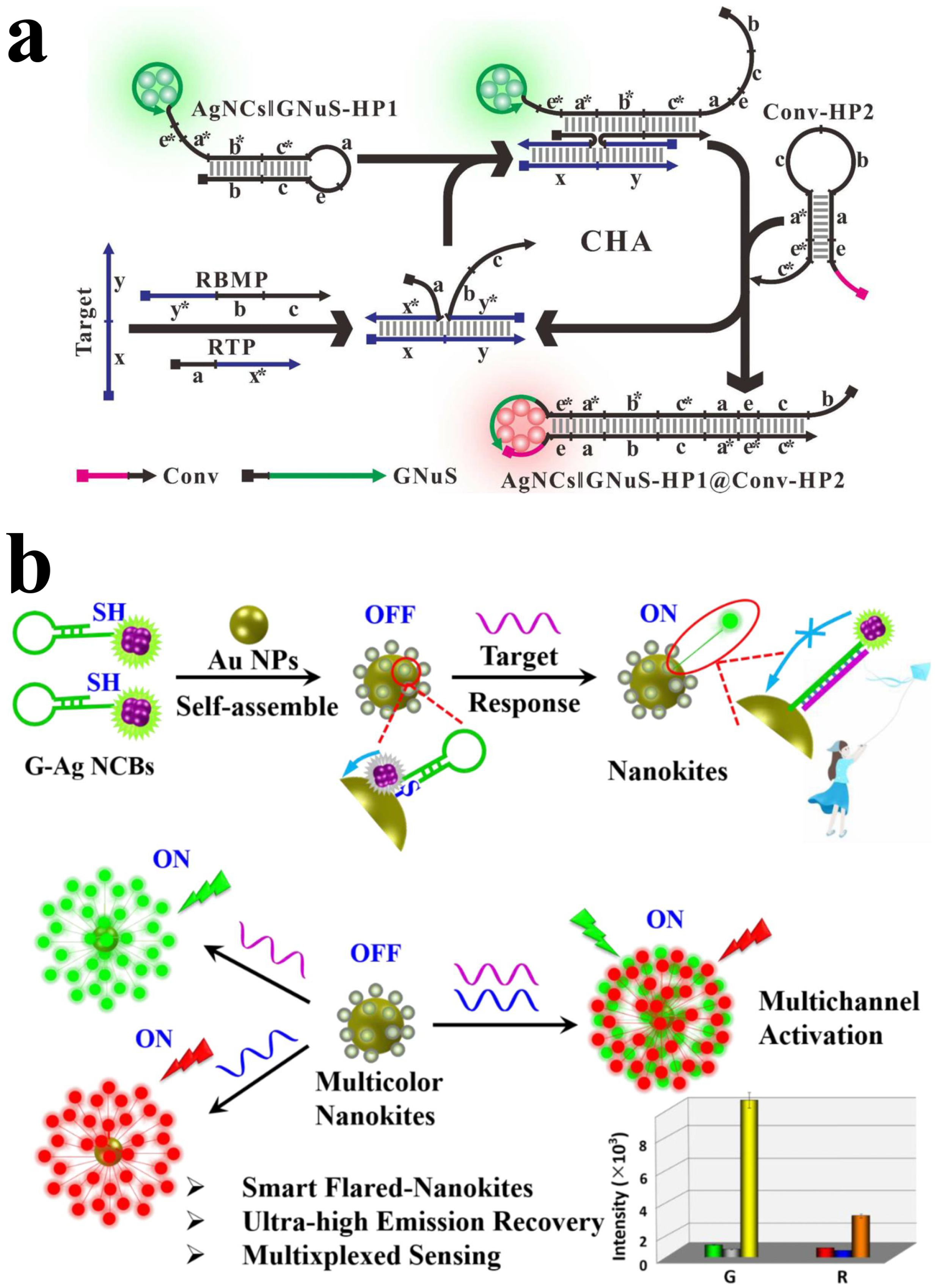
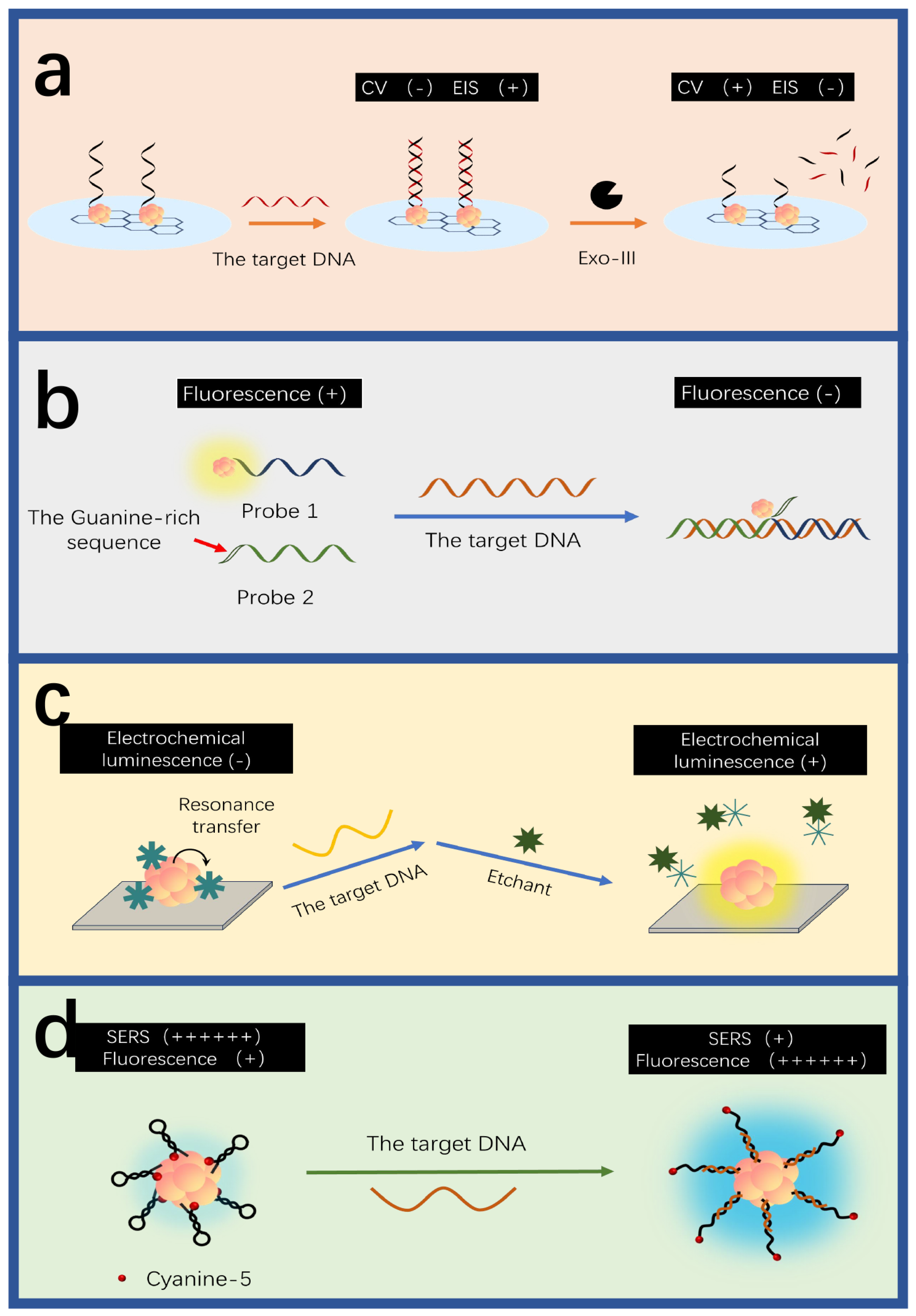
3.1. AgNCs for DNA Detection
3.2. AuNCs for DNA Detection
3.3. CuNCs for DNA Detection
3.4. Alloy MNCs for DNA Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xue, T.; Liu, Y.; Qi, J.; Jin, R.; Lin, Z. Luminescent metal nanoclusters for biomedical applications. Nano Res. 2019, 12, 1251–1265. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Luminescent metal nanoclusters for potential chemosensor applications. Chemosensors 2017, 5, 36. [Google Scholar] [CrossRef]
- Chen, Y.; Phipps, M.L.; Werner, J.H.; Chakraborty, S.; Martinez, J.S. DNA templated metal nanoclusters: From emergent properties to unique applications. Accounts Chem. Res. 2018, 51, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Li, R.D.; Wang, Q.; Yin, B.C.; Ye, B.C. Enzyme-free detection of sequence-specific microRNAs based on nanoparticle-assisted signal amplification strategy. Biosens. Bioelectron. 2016, 77, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xie, H.; Wu, G.; Xiao, F.X. Boosted solar water oxidation steered by atomically precise alloy nanocluster. Chin. Chem. Lett. 2025, 36, 110279. [Google Scholar] [CrossRef]
- Yang, J.; Peng, Y.; Li, S.; Mu, J.; Huang, Z.; Ma, J.; Shi, Z.; Jia, Q. Metal nanocluster-based hybrid Nanomaterials: Fabrication and application. Coordin. Chem. Rev. 2022, 456, 214391. [Google Scholar] [CrossRef]
- Kwak, K.; Lee, D. Electrochemistry of atomically precise metal nanoclusters. Accounts Chem. Res. 2018, 52, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xie, J. Engineering ultrasmall metal nanoclusters as promising theranostic agents. Trends Chem. 2020, 2, 665–679. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, Z.; Xu, J.J. Recent advances in near infrared (NIR) electrochemiluminescence luminophores. Chin. Chem. Lett. 2024, 35, 109622. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Mei, X. Diagnosis of cancer at early stages based on the multiplex detection of tumor markers using metal nanoclusters. Analyst 2020, 145, 7150–7161. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, Y.G.; Mozhi, A.; Goswami, N.; Yao, Q.; Xie, J. Gold nanocluster based nanocomposites for combinatorial antibacterial therapy for eradicating biofilm forming pathogens. Mater. Chem. Front. 2022, 6, 689–706. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Vikrant, K.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Nanomaterials as efficient platforms for sensing DNA. Biomaterials 2019, 214, 119215. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xu, Y.; Hu, P.; Yao, C.; Yang, D. Construction and applications of DNA-based Nanomaterials in cancer therapy. Chin. Chem. Lett. 2022, 33, 1131–1140. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Zeng, W.; Zhang, L.; He, N.; Lu, Z. High-throughput quantitative detection of triple-negative breast cancer-associated expressed miRNAs by rolling circle amplification on fluorescence-encoded microspheres. Chin. Chem. Lett. 2023, 34, 108141. [Google Scholar] [CrossRef]
- Zhou, X.; Pathak, P.; Jayawickramarajah, J. Design, synthesis, and applications of DNA–macrocyclic host conjugates. Chem. Commun. 2018, 54, 11668–11680. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Feng, C.; Liu, Z.; Ye, B.; Li, G.; Zou, L. A label-free electrochemical biosensor based on novel DNA nanotweezer coupled with G-quadruplex for sensitive DNA detection. Sens. Actuators B Chem. 2021, 331, 129437. [Google Scholar] [CrossRef]
- Wang, H.F.; Ma, R.N.; Sun, F.; Jia, L.P.; Zhang, W.; Shang, L.; Xue, Q.W.; Jia, W.L.; Wang, H.S. A versatile label-free electrochemical biosensor for circulating tumor DNA based on dual enzyme assisted multiple amplification strategy. Biosens. Bioelectron. 2018, 122, 224–230. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Ma, L.; Yang, H.; Si, F.; Liu, Y. Background-free and signal-amplified upconversion fluorescent biosensing platform for sensitive detection of CYFRA21-1. Talanta 2023, 262, 124659. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Zhao, S.; Chen, W.; Ma, W.; Yong, Q.; Xu, L.; Wang, L.; Xu, C. Rapid DNA detection by interface PCR on nanoparticles. Biosens. Bioelectron. 2011, 26, 2495–2499. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.; Zhu, L.; Liu, H.; Su, X.; Liu, Y.; Chen, Z.; Chen, H.; He, N. A novel cartridge for nucleic acid extraction, amplification and detection of infectious disease pathogens with the help of magnetic nanoparticles. Chin. Chem. Lett. 2023, 34, 108092. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Hubbert, N.L.; Wheeler, C.M.; Becker, T.M.; Lowy, D.R.; Schiller, J.T. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. JNCI J. Natl. Cancer Inst. 1994, 86, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Shani, H.; Goldwaser, T.; Keating, J.; Klugman, S. Chromosomal abnormalities not currently detected by cell-free fetal DNA: A retrospective analysis at a single center. Am. J. Obstet. Gynecol. 2016, 214, 729.e1–729.e11. [Google Scholar] [CrossRef]
- Wu, K.; Kong, F.; Zhang, J.; Tang, Y.; Chen, Y.; Chao, L.; Nie, L.; Huang, Z. Recent progress in single-nucleotide polymorphism biosensors. Biosensors 2023, 13, 864. [Google Scholar] [CrossRef]
- Shi, N.; Jia, H.; Zhang, J.; Lu, P.; Cai, C.; Zhang, Y.; Zhang, L.; He, N.; Zhu, W.; Cai, Y.; et al. Accurate expression of neck motion signal by piezoelectric sensor data analysis. Chin. Chem. Lett. 2024, 35, 109302. [Google Scholar] [CrossRef]
- Han, S.; Zhao, Y.; Zhang, Z.; Xu, G. Recent advances in electrochemiluminescence and chemiluminescence of metal nanoclusters. Molecules 2020, 25, 5208. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Xie, T.J.; Luo, Y.J.; Mao, K.; Huang, C.Z.; Li, Y.F.; Zhen, S.J. Octopus-like DNA nanostructure coupled with graphene oxide enhanced fluorescence anisotropy for hepatitis B virus DNA detection. Chin. Chem. Lett. 2024, 35, 109137. [Google Scholar] [CrossRef]
- Xu, L.J.; Lei, Z.C.; Li, J.; Zong, C.; Yang, C.J.; Ren, B. Label-free surface-enhanced Raman spectroscopy detection of DNA with single-base sensitivity. J. Am. Chem. Soc. 2015, 137, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic carbon quantum dots as a fluorescent sensing platform for DNA detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- Chen, W.; Fang, X.; Li, H.; Cao, H.; Kong, J. DNA-mediated inhibition of peroxidase-like activities on platinum nanoparticles for simple and rapid colorimetric detection of nucleic acids. Biosens. Bioelectron. 2017, 94, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering luminescent metal nanoclusters for sensing applications. Coordin. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent noble metal nanoclusters as an emerging optical probe for sensor development. Chem.-Asian J. 2013, 8, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, Q.; Wu, S.; Zhang, L.; Feng, L.; Wang, Y.; Xie, Y.; Li, Y.; Zou, J.P.; Luo, S.L. Covalent coupling promoting charge transport of CdSeTe/UiO-66 for boosting photocatalytic CO2 reduction. Chin. Chem. Lett. 2023, 34, 107903. [Google Scholar] [CrossRef]
- Granger, J.H.; Schlotter, N.E.; Crawford, A.C.; Porter, M.D. Prospects for point-of-care pathogen diagnostics using surface-enhanced Raman scattering (SERS). Chem. Soc. Rev. 2016, 45, 3865–3882. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yao, H.; Song, W.; Jin, L.; Mosa, I.M.; Rusling, J.F.; Suib, S.L.; He, J. Ligand-free noble metal nanocluster catalysts on carbon supports via “soft” nitriding. J. Am. Chem. Soc. 2016, 138, 4718–4721. [Google Scholar] [CrossRef]
- Xue, R.; Geng, X.; Liang, F.; Liu, Y.; Yang, W.; Huang, Z. Natural plant compounds in synthesis and Luminescence modulation of metal nanoclusters: Toward sustainable nanoprobes for sensing and bioimaging. Mater. Today Adv. 2022, 16, 100279. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, W.; Chen, W. Copper nanoclusters: Synthesis, characterization and properties. Chin. Sci. Bull. 2012, 57, 41–47. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Bürgi, T. Copper nanoclusters: Designed synthesis, structural diversity, and multiplatform applications. Nanoscale 2021, 13, 6283–6340. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward total synthesis of thiolate-protected metal nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef]
- Karpushkin, E.; Ivanova, N.; Lopatina, L.; Sergeyev, V. Fluorescent gold nanoclusters prepared in the presence of DNA. Funct. Mater. Lett. 2020, 13, 2041002. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, Y.; Li, G.; Jin, R. Magic size Au64(S−c-C6H11)32 nanocluster protected by cyclohexanethiolate. Chem. Mater. 2014, 26, 2635–2641. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Zhu, X.; Xu, M.; Han, Z.; Wang, L.; Weng, L. NaBH4-mediated co-reduction synthesis of glutathione stabilized gold/silver nanoclusters for detection of magnesium Ions. Chemosensors 2023, 11, 435. [Google Scholar] [CrossRef]
- Nasirian, V.; Shamsipur, M.; Molaabasi, F.; Mansouri, K.; Sarparast, M.; Salim, V.; Barati, A.; Kashanian, S. miRNA-21 rapid diagnosis by one-pot synthesis of highly luminescent red emissive silver nanoclusters/DNA. Sens. Actuators B Chem. 2020, 308, 127673. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Zhuang, Q.; Ni, Y. One-pot aqueous synthesis of nucleoside-templated fluorescent copper nanoclusters and their application for discrimination of nucleosides. ACS Appl. Mater. Interfaces 2017, 9, 32135–32141. [Google Scholar] [CrossRef]
- Tiwari, N.; Mishra, R.K.; Gupta, S.; Srivastava, R.; Aggarwal, S.; Bandyopadhyay, P.; Munde, M. Synthetic tunability and biophysical basis for fabricating highly fluorescent and stable DNA copper nanoclusters. Langmuir 2021, 37, 9385–9395. [Google Scholar] [CrossRef]
- Katla, S.K.; Zhang, J.; Castro, E.; Bernal, R.A.; Li, X. Atomically precise Au25(SG)18 nanoclusters: Rapid single-step synthesis and application in photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, J.; Abbas, M.; Li, Y.; Hussain, S.Z.; Mumtaz, S.; Song, Z.; Hussain, I.; Tan, B. Facile synthesis of ultrastable fluorescent copper nanoclusters and their cellular imaging application. Nanomaterials 2020, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Chen, Y.; Das, A.; Jin, R. Transformation chemistry of gold nanoclusters: From one stable size to another. J. Phys. Chem. Lett. 2015, 6, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.; Shi, Y.; Li, G. Efficient synthesis of Au99(SR)42 nanoclusters. Nanoscale 2015, 7, 5987–5990. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, C.; Lin, J.; Yu, C.; Huang, J.; Li, G. One-pot synthesis of Au144(SCH2Ph)60 nanoclusters and their catalytic application. J. Mater. Chem. A 2015, 3, 20167–20173. [Google Scholar] [CrossRef]
- Shichibu, Y.; Negishi, Y.; Tsukuda, T.; Teranishi, T. Large-scale synthesis of thiolated Au25 clusters via ligand exchange reactions of phosphine-stabilized Au11 clusters. J. Am. Chem. Soc. 2005, 127, 13464–13465. [Google Scholar] [CrossRef]
- Shah, P.; Rørvig-Lund, A.; Chaabane, S.B.; Thulstrup, P.W.; Kjaergaard, H.G.; Fron, E.; Hofkens, J.; Yang, S.W.; Vosch, T. Design aspects of bright red emissive silver nanoclusters/DNA probes for microRNA detection. ACS Nano 2012, 6, 8803–8814. [Google Scholar] [CrossRef]
- Ganguly, M.; Pal, A.; Negishi, Y.; Pal, T. Synthesis of highly fluorescent silver clusters on gold (I) surface. Langmuir 2013, 29, 2033–2043. [Google Scholar] [CrossRef]
- Qu, F.; Li, N.B.; Luo, H.Q. Highly sensitive fluorescent and colorimetric pH sensor based on polyethylenimine-capped silver nanoclusters. Langmuir 2013, 29, 1199–1205. [Google Scholar] [CrossRef]
- Gregersen, S.; Vosch, T.; Jensen, K.J. Peptide-stabilized, fluorescent silver nanoclusters: Solid-hhase synthesis and screening. Chem.-Asian J. 2016, 22, 18492–18500. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Geng, J.; Ong, E.Y.X.; Chellappan, V.; Tan, Y.N. Bovine serum albulmin protein-templated silver nanocluster (BSA-Ag13): An effective singlet oxygen generator for photodynamic cancer therapy. Adv. Healthc. Mater. 2016, 5, 2528–2535. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Lee, P.Y.; Liao, J.H.; Chakrahari, K.K.; Kahlal, S.; Liu, Y.C.; Chiang, M.H.; Saillard, J.Y.; Liu, C. Eight-electron silver and mixed gold/silver nanoclusters stabilized by selenium donor ligands. Angew. Chem. Int. Ed. 2017, 56, 10178–10182. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Joshi, C.P.; Alhilaly, M.J.; Bakr, O.M. Switching a nanocluster core from hollow to nonhollow. Chem. Mater. 2016, 28, 3292–3297. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Jones, Z.R.; Leto, D.F.; Wu, G.; Scott, S.L.; Hayton, T.W. Ligand-exchange-induced growth of an atomically precise Cu29 nanocluster from a smaller cluster. Chem. Mater. 2016, 28, 8385–8390. [Google Scholar] [CrossRef]
- Cui, H.; Shao, Z.S.; Song, Z.; Wang, Y.B.; Wang, H.S. Development of gold nanoclusters: From preparation to applications in the field of biomedicine. J. Mater. Chem. C 2020, 8, 14312–14333. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Rogach, A.L. Synthesis, optical properties and applications of light-emitting copper nanoclusters. Nanoscale Horiz. 2017, 2, 135–146. [Google Scholar] [CrossRef]
- Le Guével, X.; Spies, C.; Daum, N.; Jung, G.; Schneider, M. Highly fluorescent silver nanoclusters stabilized by glutathione: A promising fluorescent label for bioimaging. Nano Res. 2012, 5, 379–387. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Zheng, Y.; Wang, Y.; Guo, Z.; Jiang, H.; Wang, X. A novel turn-on fluorescent sensor for the sensitive detection of glutathione via gold nanocluster preparation based on controllable ligand-induced etching. Analyst 2020, 145, 4265–4275. [Google Scholar] [CrossRef]
- Liu, C.; Ding, Y.; Li, Q.; Lin, Y. Photochemical synthesis of glutathione-stabilized silver nanoclusters for fluorometric determination of hydrogen peroxide. Microchim. Acta 2017, 184, 2497–2503. [Google Scholar] [CrossRef]
- Shen, Q.; Hossain, F.; Fang, C.; Shu, T.; Zhang, X.; Law, J.L.M.; Logan, M.; Houghton, M.; Tyrrell, D.L.; Joyce, M.A.; et al. Bovine serum albumin-protected gold nanoclusters for sensing of SARS-CoV-2 antibodies and virus. ACS Appl. Mater. Interfaces 2023, 15, 29914–29926. [Google Scholar] [CrossRef]
- Deng, H.H.; Li, K.L.; Zhuang, Q.Q.; Peng, H.P.; Zhuang, Q.Q.; Liu, A.L.; Xia, X.H.; Chen, W. An ammonia-based etchant for attaining copper nanoclusters with green fluorescence emission. Nanoscale 2018, 10, 6467–6473. [Google Scholar] [CrossRef]
- Saleh, S.M.; El-Sayed, W.A.; El-Manawaty, M.A.; Gassoumi, M.; Ali, R. An eco-friendly synthetic approach for copper nanoclusters and their potential in lead ions sensing and biological applications. Biosensors 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, V.A.; Jha, S.; Park, T.J.; Kailasa, S.K. Synthesis of copper nanoclusters from Bacopa monnieri leaves for fluorescence sensing of dichlorvos. Luminescence 2023, 38, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Bo, F.; Wang, Z.; Shao, H.; Xu, S.; Cui, Y. Microwave-assisted synthesis of fluorescent Ag nanoclusters in aqueous solution. ChemPhysChem 2012, 13, 2097–2101. [Google Scholar] [CrossRef] [PubMed]
- Naaz, S.; Chowdhury, P. Sunlight and ultrasound-assisted synthesis of photoluminescent silver nanoclusters: A unique ‘knock out’ sensor for thiophilic metal ions. Sens. Actuators B Chem. 2017, 241, 840–848. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, T.; Du, H.; Qiao, Y.; Guo, X.; Shi, L.; Zhang, Y.; Shuang, S.; Dong, C.; Ma, H. A reversible fluorescent pH-sensing system based on the one-pot synthesis of natural silk fibroin-capped copper nanoclusters. J. Mater. Chem. C 2016, 4, 3540–3545. [Google Scholar] [CrossRef]
- Xu, F.; Qing, T.; Qing, Z. DNA-coded metal nano-fluorophores: Preparation, properties and applications in biosensing and bioimaging. Nano Today 2021, 36, 101021. [Google Scholar] [CrossRef]
- Ning, L.; Li, Y.; Zhang, Z.; Zhou, Y.; Yang, L.; Yu, Q.; Yu, F.; Tong, Z. Primer exchange reaction coupled with DNA-templated silver nanoclusters for label-free and sensitive detection of microRNA. Appl. Biochem. Biotech. 2023, 195, 6334–6344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, Y.; Yin, X.; Zhang, Y.; Yang, Z.; Wang, H.; Yang, W.; Pang, P. Electrochemical determination of Pb2+ based on DNAzyme-triggered rolling circle amplification and DNA-templated silver nanoclusters amplification strategy. Microchem. J. 2023, 189, 108544. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Jin, L.; Li, Z.; He, N.; Miao, W. Recent advancements in DNA nanotechnology-enabled extracellular vesicles detection and diagnosis: A mini review. Chin. Chem. Lett. 2023, 34, 107926. [Google Scholar] [CrossRef]
- Tan, S.C.L.; He, Z.; Wang, G.; Yu, Y.; Yang, L. Protein-templated metal nanoclusters: Molecular-like hybrids for biosensing, diagnostics and pharmaceutics. Molecules 2023, 28, 5531. [Google Scholar] [CrossRef]
- Aires, A.; Lopez-Martinez, E.; Cortajarena, A.L. Sensors based on metal nanoclusters stabilized on designed proteins. Biosensors 2018, 8, 110. [Google Scholar] [CrossRef]
- Yang, C.; Shi, K.; Dou, B.; Xiang, Y.; Chai, Y.; Yuan, R. In situ DNA-templated synthesis of silver nanoclusters for ultrasensitive and label-free electrochemical detection of microRNA. ACS Appl. Mater. Interfaces 2015, 7, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xia, X.; Hu, S.; Yang, M.; Wang, J. Silver nanoclusters-based fluorescence assay of protein kinase activity and inhibition. Anal. Chem. 2015, 87, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, Y.C.; Li, H.W.; Chang, H.T. Fluorescent silver nanoclusters stabilized by DNA scaffolds. Chem. Commun. 2014, 50, 9800–9815. [Google Scholar] [CrossRef]
- Thyrhaug, E.; Bogh, S.A.; Carro-Temboury, M.R.; Madsen, C.S.; Vosch, T.; Zigmantas, D. Ultrafast coherence transfer in DNA-templated silver nanoclusters. Nat. Commun. 2017, 8, 15577. [Google Scholar] [CrossRef]
- Obliosca, J.M.; Liu, C.; Yeh, H.C. Fluorescent silver nanoclusters as DNA probes. Nanoscale 2013, 5, 8443–8461. [Google Scholar] [CrossRef]
- Yao, Y.; Li, N.; Zhang, X.; Ong’achwa Machuki, J.; Yang, D.; Yu, Y.; Li, J.; Tang, D.; Tian, J.; Gao, F. DNA-templated silver nanocluster/porphyrin/MnO2 platform for label-free intracellular Zn2+ imaging and fluorescence-/magnetic resonance imaging-guided photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 13991–14003. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Huang, M.; Li, P.; Xu, M.; Yao, C.; Yang, D. Hybridization chain reaction-based DNA Nanomaterials for biosensing, bioimaging and therapeutics. Chin. Chem. Lett. 2024, 35, 108601. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bahara, N.H.H.; Choong, Y.S.; Lim, T.S.; Tye, G.J. DNA fluorescence shift sensor: A rapid method for the detection of DNA hybridization using silver nanoclusters. J. Colloid Interface Sci. 2014, 433, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhu, J.; Fan, D.; Teng, Y.; Zhu, X.; Dong, S. A multicolor chameleon DNA-templated silver nanocluster and Its application for ratiometric fluorescence target detection with exponential signal response. Adv. Funct. Mater. 2017, 27, 1704092. [Google Scholar] [CrossRef]
- Ye, Y.D.; Xia, L.; Xu, D.D.; Xing, X.J.; Pang, D.W.; Tang, H.W. DNA-stabilized silver nanoclusters and carbon nanoparticles oxide: A sensitive platform for label-free fluorescence turn-on detection of HIV-DNA sequences. Biosens. Bioelectron. 2016, 85, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, F.; Duan, Y.; Li, Q.; Pan, Y.; Du, H.; He, P.; Shen, X.; Luo, Z.; Zhu, C.; et al. Label-free analysis of H5N1 virus based on three-segment branched DNA-templated fluorescent silver nanoclusters. ACS Appl. Mater. Interfaces 2020, 12, 48357–48362. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Qian, H.; Cheng, Y.; Xie, Y.; Yu, H.; Yao, W.; Pei, R.; Guo, Y.; Li, H.W. Three-way junction-promoted recycling amplification for sensitive DNA detection using highly bright DNA-silver nanocluster as label-free output. Talanta 2020, 206, 120216. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Liu, D.; Liu, S.; You, T. Ratiometric fluorescent sensing of mercury (II) ion based on the Pt nanozyme-triggered fluorescence resonance energy transfer between Si quantum dots and 2,3-diaminophenazine. Sens. Actuators B Chem. 2021, 331, 112976. [Google Scholar] [CrossRef]
- Liu, L.; Ga, L.; Ai, J. Ratiometric fluorescence sensing with logical operation: Theory, design and applications. Biosens. Bioelectron. 2022, 213, 114456. [Google Scholar] [CrossRef]
- Liu, X.; Hu, R.; Gao, Z.; Shao, N. Photoluminescence mechanism of DNA-templated silver nanoclusters: Coupling between surface plasmon and emitter and sensing of lysozyme. Langmuir 2015, 31, 5859–5867. [Google Scholar] [CrossRef]
- Ritchie, C.M.; Johnsen, K.R.; Kiser, J.R.; Antoku, Y.; Dickson, R.M.; Petty, J.T. Ag nanocluster formation using a cytosine oligonucleotide template. J. Phys. Chem. Lett. 2007, 111, 175–181. [Google Scholar] [CrossRef]
- Sengupta, B.; Ritchie, C.M.; Buckman, J.G.; Johnsen, K.R.; Goodwin, P.M.; Petty, J.T. Base-directed formation of fluorescent silver clusters. J. Phys. Chem. Lett. 2008, 112, 18776–18782. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H.C.; Sun, X.H.; Guo, F.N.; Feng, L.X.; Yang, T.; Wang, J.H. Detection of HIV/HCV virus DNA with homogeneous DNA machine-triggered in situ formation of silver nanoclusters. Sens. Actuators B Chem. 2022, 352, 131041. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, F.; Chen, C.; Cai, C. DNA-programming multicolor silver nanoclusters for sensitively simultaneous detection of two HIV DNAs. Sens. Actuators B Chem. 2019, 296, 126608. [Google Scholar] [CrossRef]
- Deféver, T.; Druet, M.; Evrard, D.; Marchal, D.; Limoges, B. Real-time electrochemical PCR with a DNA intercalating redox probe. Anal. Chem. 2011, 83, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Su, J.; Xiang, Y.; Yuan, R.; Chai, Y. In situ hybridization chain reaction amplification for universal and highly sensitive electrochemiluminescent detection of DNA. Anal. Chem. 2012, 84, 7750–7755. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, X.; Liu, Q.; Wang, L.; Lin, Z.; Chen, G. Ultrasensitive electrochemical biosensor for detection of DNA from Bacillus subtilis by coupling target-induced strand displacement and nicking endonuclease signal amplification. Anal. Chem. 2014, 86, 8785–8790. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, M.; Chen, G.; Mao, H.; Chen, W.; Wang, W.; Chen, J. An electrochemical biosensor based on DNA “nano-bridge” for amplified detection of exosomal microRNAs. Chin. Chem. Lett. 2021, 32, 3474–3478. [Google Scholar] [CrossRef]
- Ma, J.; Gong, L.; Cen, Y.; Feng, L.; Su, Y.; Liu, X.; Chao, J.; Wan, Y.; Su, S.; Wang, L. Electrochemical analysis of microRNAs with hybridization chain reaction-based triple signal amplification. Chin. Chem. Lett. 2023, 34, 108012. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wei, S.; Zhao, C.; Song, X.; Xu, K.; Li, J.; Pang, B.; Wang, J. Applications of hybridization chain reaction optical detection incorporating Nanomaterials: A review. Anal. Chim. Acta 2022, 1190, 338930. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, J.; Zheng, X.; Wu, Z.S. Hybridization chain reaction and its applications in biosensing. Talanta 2021, 234, 122637. [Google Scholar] [CrossRef] [PubMed]
- Wong, Z.W.; New, S.Y. An enzyme-free turn-on fluorescent strategy for nucleic acid detection based on hybridization chain reaction and transferable silver nanoclusters. Microchim. Acta 2023, 190, 16. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Xin, C.; Zhao, J.; Liu, S. A cascade autocatalytic strand displacement amplification and hybridization chain reaction event for label-free and ultrasensitive electrochemical nucleic acid biosensing. Biosens. Bioelectron. 2018, 113, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; You, Y.; Zhu, Y.; Zheng, H. Recent advances in the exonuclease III-assisted target signal amplification strategy for nucleic acid detection. Anal. Methods 2021, 13, 5103–5119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, L.; Lu, F.; Wu, X.; Zhu, J.J. A universal upconversion sensing platform for the sensitive detection of tumour-related ncRNA through an Exo III-assisted cycling amplification strategy. Small 2018, 14, 1703858. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jin, T.; Zhang, J.; Huang, X.; Tan, C.; Jiang, Y.; Tan, Y. A novel aptasensor strategy for protein detection based on G-quadruplex and exonuclease III-aided recycling amplification. Chin. Chem. Lett. 2020, 31, 155–158. [Google Scholar] [CrossRef]
- Li, Z.M.; Zhong, Z.H.; Liang, R.P.; Qiu, J.D. The colorimetric assay of DNA methyltransferase activity based on strand displacement amplification. Sens. Actuators B Chem. 2017, 238, 626–632. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Kang, Q.; Qi, Y.; Du, Y.; Xiang, H. A novel DNA detection using spherical identification probe and strand displacement reaction-initiated silver nanocluster switch. Anal. Sci. 2023, 39, 275–284. [Google Scholar] [CrossRef]
- Qian, J.; Yang, Y.; Gong, F.; Shan, X.; Ji, X.; He, Z. Ratiometric fluorescence biosensing of silver NanoCluster Beacons for ATP detection based on ligation-triggered rolling cycle amplification. Microchem. J. 2023, 190, 108663. [Google Scholar] [CrossRef]
- Kuo, Y.A.; Jung, C.; Chen, Y.A.; Kuo, H.C.; Zhao, O.S.; Nguyen, T.D.; Rybarski, J.R.; Hong, S.; Chen, Y.I.; Wylie, D.C.; et al. Massively parallel selection of nanocluster beacons. Adv. Mater. 2022, 34, 2204957. [Google Scholar] [CrossRef]
- Feng, D.Q.; Zhang, W.; Wang, W.; Liu, G. Smart flared-nanokites with ultra-high fluorescence enhancement for multiplexing virus DNA biosensing. Sens. Actuators B Chem. 2023, 387, 133813. [Google Scholar] [CrossRef]
- Ge, L.; Sun, X.; Hong, Q.; Li, F. Ratiometric catalyzed-assembly of nanocluster beacons: A nonenzymatic approach for amplified DNA detection. ACS Appl. Mater. Interfaces 2017, 9, 32089–32096. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Sun, X.; Hong, Q.; Li, F. Ratiometric NanoCluster Beacon: A label-free and sensitive fluorescent DNA detection platform. ACS Appl. Mater. Interfaces 2017, 9, 13102–13110. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, S.; Sun, J.; Xia, M.; Xu, G. Ratiometric fluorescence detection of bleomycin based on proximity-dependent fluorescence conversion of DNA-templated silver nanoclusters. Chin. Chem. Lett. 2021, 32, 906–909. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, D.; Ma, Y.; Bie, Z.; Liu, Z. Multimodal plasmonic assay of copper (II) ion via stimuli-responsive state transformation of silver molecular nanoparticles. Anal. Chem. 2016, 88, 8123–8128. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Feng, D.Q.; Zhu, J.J.; Wang, W. Silver nanoclusters beacon as stimuli-responsive versatile platform for multiplex DNAs detection and aptamer–substrate complexes sensing. Anal. Chem. 2017, 89, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Samandari, L.; Farzin, L.; Molaabasi, F.; Mousazadeh, M.H. Dual-modal label-free genosensor based on hemoglobin@ gold nanocluster stabilized graphene nanosheets for the electrochemical detection of BCR/ABL fusion gene. Talanta 2020, 217, 121093. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, T.; Zeng, X.; Xiong, H.; Wen, W.; Zhang, X.; Wang, S. Ultrasensitive electrochemical DNA biosensor based on functionalized gold clusters/graphene nanohybrids coupling with exonuclease III-aided cascade target recycling. Biosens. Bioelectron. 2017, 91, 183–189. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X.; Wen, W.; Zhang, X.; Wang, S. Ultrasensitive electrochemical biosensor for HIV gene detection based on graphene stabilized gold nanoclusters with exonuclease amplification. ACS Appl. Mater. Interfaces 2015, 7, 18872–18879. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, F.; Wang, D.; Xiong, D.; Yan, S.; Liu, S.; Tang, H. Fabrication of avidin-stabilized gold nanoclusters with dual emissions and their application in biosensing. J. Nanobiotechnol. 2022, 20, 306. [Google Scholar] [CrossRef]
- Wang, H.B.; Bai, H.Y.; Dong, G.L.; Liu, Y.M. DNA-templated Au nanoclusters coupled with proximity-dependent hybridization and guanine-rich DNA induced quenching: A sensitive fluorescent biosensing platform for DNA detection. Nanoscale Adv. 2019, 1, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Zou, Z.; Huang, Z.; Deng, H.; Chen, W.; Peng, H. Split-type electrochemiluminescent gene assay platform based on gold nanocluster probe for human papillomavirus diagnosis. Biosens. Bioelectron. 2021, 178, 113044. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Zhao, K.R.; Liu, Z.J.; Wang, L.; Ye, S.Y.; Liang, G.X. Cas12a-based electrochemiluminescence biosensor for target amplification-free DNA detection. Biosens. Bioelectron. 2021, 176, 112954. [Google Scholar] [CrossRef]
- Guo, T.; Li, W.; Qian, L.; Yan, X.; Cui, D.; Zhao, J.; Ni, H.; Zhao, X.; Zhang, Z.; Li, X.; et al. Highly-selective detection of EGFR mutation gene in lung cancer based on surface enhanced Raman spectroscopy and asymmetric PCR. J. Pharmaceut. Biomed. 2020, 190, 113522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, M.; Bao, Y.; Xie, X.; Nie, Y.; Lv, Y.; Su, X. Construction of a sensing platform based on DNA-encoded magnetic beads and copper nanoclusters for viral gene analysis with target recycling amplification. ACS Appl. Bio Mater. 2021, 4, 5669–5677. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, M.; Zhou, X.; Nie, Y.; Su, X. Highly sensitive label-free fluorescence determination of lymphotropic virus DNA based on exonuclease assisted target recycling amplification and in-situ generation of fluorescent copper nanoclusters. Sens. Actuators B Chem. 2021, 326, 128847. [Google Scholar] [CrossRef]
- Tao, Y.; Yi, K.; Wang, H.; Li, K.; Li, M. Metal nanoclusters combined with CRISPR-Cas12a for hepatitis B virus DNA detection. Sens. Actuators B Chem. 2022, 361, 131711. [Google Scholar] [CrossRef]
- Liu, L.; Bai, Q.; Zhang, X.; Lu, C.; Li, Z.; Liang, H.; Chen, L. Fluorescent biosensor based on hairpin DNA stabilized copper nanoclusters for chlamydia trachomatis detection. J. Fluoresc. 2022, 32, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; Xiao, B.; Deng, M.; Yu, P.; Qing, T. Fluorometric determination of the breast cancer 1 gene based on the target-induced conformational change of a DNA template for copper nanoclusters. Anal. Methods 2021, 13, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J. A fluorescence nanobiosensor for detection of Campylobacter jejuni DNA in milk based on Au/Ag bimetallic nanoclusters. J. Food Meas. Character. 2019, 13, 1797–1804. [Google Scholar] [CrossRef]
- Wu, F.; Lin, Q.; Wang, L.; Zou, Y.; Chen, M.; Xia, Y.; Lan, J.; Chen, J. A DNA electrochemical biosensor based on triplex DNA-templated Ag/Pt nanoclusters for the detection of single-nucleotide variant. Talanta 2020, 207, 120257. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Qamhieh, N.; Iratni, R.; Ayesh, A.I. Real-time nucleic acid detection via field-effect transistor sensors based on graphite oxide decorated with trimetallic nanocluster of gold, silver, and platinum. New J. Phys. 2021, 23, 103041. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.; Lai, J.; Xu, G. Gold nanoclusters: Synthetic strategies and recent advances in fluorescent sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Yuan, X.; Fung, V.; Yu, Y.; Leong, D.T.; Jiang, D.e.; Xie, J. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017, 8, 927. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.; Shen, Y.; Zhang, J.; Zhu, J.J. Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Anal. Chem. 2011, 83, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Farzin, L.; Tabrizi, M.A.; Molaabasi, F. Highly sensitive label free electrochemical detection of VGEF165 tumor marker based on “signal off” and “signal on” strategies using an anti-VEGF165 aptamer immobilized BSA-gold nanoclusters/ionic liquid/glassy carbon electrode. Biosens. Bioelectron. 2015, 74, 369–375. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Shang, L. Ratiometric fluorescence and visual sensing of ATP based on gold nanocluster-encapsulated metal-organic framework with a smartphone. Chin. Chem. Lett. 2023, 34, 108093. [Google Scholar] [CrossRef]
- Bao, Y.; Yeh, H.C.; Zhong, C.; Ivanov, S.A.; Sharma, J.K.; Neidig, M.L.; Vu, D.M.; Shreve, A.P.; Dyer, R.B.; Werner, J.H.; et al. Formation and stabilization of fluorescent gold nanoclusters using Small Molecules. J. Phys. Chem. C 2010, 114, 15879–15882. [Google Scholar] [CrossRef]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, T.; Wang, S.; Ma, J.; Zhou, T.; Wang, F.; Wang, X.; Zhang, G. DNA-templated gold nanocluster as a novel fluorometric sensor for glutathione determination. J. Photochem. Photobiol. A 2019, 370, 89–93. [Google Scholar] [CrossRef]
- Yang, X.; Shi, M.; Zhou, R.; Chen, X.; Chen, H. Blending of HAuCl4 and histidine in aqueous solution: A simple approach to the Au10 cluster. Nanoscale 2011, 3, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, Y.; Wei, L.; Wang, J.; Zhang, M.; Yang, X.; Wang, W.; Yang, G. The assembly of protein-templated gold nanoclusters for enhanced fluorescence emission and multifunctional applications. Acta Biomater. 2020, 101, 436–443. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Imanpour, A.; Rezavand, M.; Arjmandi Tash, A.M.; Antonini, S.; Rezvani, S.J.; Abdi, Y. Electrochemoresistance sensor: A borophene-based sensor with simultaneous electrochemical and chemoresistance sensing capability. ACS Mater. Lett. 2024, 6, 933–942. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Zhu, Y.; Jia, X.; Zhang, S.; Wang, H.; Li, Y.; Hu, G. Fe2O3-decorated boron/nitrogen-co-doped carbon nanosheets as an electrochemical sensing platform for ultrasensitive determination of paraquat in natural water. Chin. Chem. Lett. 2023, 34, 107299. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.; Wang, S.; Cai, G.; Xue, L.; Li, Y.; Liao, M.; Lin, J. A microfluidic biosensor for rapid detection of Salmonella typhimurium based on magnetic separation, enzymatic catalysis and electrochemical impedance analysis. Chin. Chem. Lett. 2022, 33, 3156–3160. [Google Scholar] [CrossRef]
- Danis, A.S.; Potts, K.P.; Perry, S.C.; Mauzeroll, J. Combined spectroelectrochemical and simulated insights into the electrogenerated chemiluminescence coreactant mechanism. Anal. Chem. 2018, 90, 7377–7382. [Google Scholar] [CrossRef]
- Tao, X.L.; Pan, M.C.; Yang, X.; Yuan, R.; Zhuo, Y. CDs assembled metal-organic framework: Exogenous coreactant-free biosensing platform with pore confinement-enhanced electrochemiluminescence. Chin. Chem. Lett. 2022, 33, 4803–4807. [Google Scholar] [CrossRef]
- Xia, S.; Pan, J.; Dai, D.; Dai, Z.; Yang, M.; Yi, C. Design of portable electrochemiluminescence sensing systems for point-of-care-testing applications. Chin. Chem. Lett. 2023, 34, 107799. [Google Scholar] [CrossRef]
- Yin, F.; Yang, E.; Ge, X.; Sun, Q.; Mo, F.; Wu, G.; Shen, Y. Coupling WO3-x dots-encapsulated metal-organic frameworks and template-free branched polymerization for dual signal-amplified electrochemiluminescence biosensing. Chin. Chem. Lett. 2024, 35, 108753. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Liu, R.; Wang, Y.; Li, A.; Tian, S.; Cheng, W.; Ding, S.; Li, W.; Zhao, M.; et al. A novel CRISPR/Cas14a-based electrochemical biosensor for ultrasensitive detection of Burkholderia pseudomallei with PtPd@ PCN-224 nanoenzymes for signal amplification. Biosens. Bioelectron. 2023, 225, 115098. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Peng, S.; Tan, Z.; Chen, S.; Lei, C.; Nie, Z. Integrating CRISPR-Cas12a with a DNA circuit as a generic sensing platform for amplified detection of microRNA. Chem. Sci. 2020, 11, 7362–7368. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, J.; Luan, X.; Liu, X.; Li, X.; Yang, J.; Huang, T.; Sun, L.; Wang, Y.; Lin, Y.; et al. Near-infrared upconversion–activated CRISPR-Cas9 system: A remote-controlled gene editing platform. Sci. Adv. 2019, 5, eaav7199. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Li, S.; Lv, Z.; Ding, X.; Li, F.; Yang, D. Engineering CRISPR/Cas-based nanosystems for therapeutics, diagnosis and bioimaging. Chin. Chem. Lett. 2023, 34, 108134. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath analysis based on surface-enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, Z.; Li, J.; Bai, H.; Xi, G. Plasmonic rhenium trioxide self-assembled microtubes for highly sensitive, stable and reproducible surface-enhanced Raman spectroscopy detection. Chin. Chem. Lett. 2023, 34, 107572. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Wang, S.; Wu, L.; Wang, Y.; Dong, J. A distinction of gliomas at cellular and tissue level by surface-enhanced Raman scattering spectroscopy. Chin. Chem. Lett. 2024, 35, 109383. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Hao, Q.; Wang, S.; Yang, Y.; Xu, J.; Yin, Z.; Zhang, L.; Chen, Z. Ultrastable graphene isolated AuAg nanoalloy for SERS biosensing and photothermal therapy of bacterial infection. Chin. Chem. Lett. 2024, 35, 108636. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, H.; Tang, Y.; Zhang, H.; Yang, L.; Wang, X.; Zhao, B. g-C3N4/TiO2−X heterojunction with high-efficiency carrier separation and multiple charge transfer paths for ultrasensitive SERS sensing. Chin. Chem. Lett. 2024, 35, 109415. [Google Scholar] [CrossRef]
- Tian, S.; Huang, W.; Hu, J.; Wang, H.; Zhang, Z.; Xu, L.; Li, J.; Sun, Y. Exploring the frontiers of plant health: Harnessing NIR fluorescence and surface-enhanced Raman scattering modalities for innovative detection. Chin. Chem. Lett. 2024, 36, 110336. [Google Scholar] [CrossRef]
- Cao, Q.; Li, J.; Wang, E. Recent advances in the synthesis and application of copper Nanomaterials based on various DNA scaffolds. Biosens. Bioelectron. 2019, 132, 333–342. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q.; Jin, Y.; Li, B. Fluorescent enzyme-linked immunoassay strategy based on enzyme-triggered in-situ synthesis of fluorescent copper nanoclusters. Sens. Actuators B Chem. 2019, 281, 28–33. [Google Scholar] [CrossRef]
- Qing, T.; Qing, Z.; Mao, Z.; He, X.; Xu, F.; Wen, L.; He, D.; Shi, H.; Wang, K. dsDNA-templated fluorescent copper nanoparticles: Poly (AT-TA)-dependent formation. RSC Adv. 2014, 4, 61092–61095. [Google Scholar] [CrossRef]
- Fan, K.; Kang, W.; Qu, S.; Li, L.; Qu, B.; Lu, L. A label-free and enzyme-free fluorescent aptasensor for sensitive detection of acetamiprid based on AT-rich dsDNA-templated copper nanoparticles. Talanta 2019, 197, 645–652. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Cao, Z.; Lau, C.; Lu, J. Additive and enhanced fluorescence effects of hairpin DNA template-based copper nanoparticles and their application for the detection of NAD+. Talanta 2016, 154, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, M.K.; Das, P. Biosensing of solitary and clustered abasic site DNA damage lesions with copper nanoclusters and carbon dots. Sens. Actuators B Chem. 2018, 255, 763–774. [Google Scholar] [CrossRef]
- Carrillo-Torres, R.; García-Soto, M.; Morales-Chávez, S.; Garibay-Escobar, A.; Hernández-Paredes, J.; Guzmán, R.; Barboza-Flores, M.; Álvarez-Ramos, M. Hollow Au–Ag bimetallic nanoparticles with high photothermal stability. RSC Adv. 2016, 6, 41304–41312. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Yang, L.; Zhao, J.; Han, G.; Yu, X.; ShenTu, X.; Ye, Z. Surface engineered bimetallic gold/silver nanoclusters for in situ imaging of mercury ions in living organisms. Anal. Bioanal. Chem. 2022, 414, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mu, X.; Liu, H.; Wang, Q.; Bai, X.; Wang, J.; Liu, H.; Xu, F.; Jing, Y.; Dai, H.; et al. Structure, Luminescence, and bioimaging of bimetallic CuAu nanoclusters. Opt. Mater. 2018, 86, 291–297. [Google Scholar] [CrossRef]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
| Synthesis Method of MNCs | Yield | Scalability | Reproducibility |
|---|---|---|---|
| Chemical reduction | Regulated by reactant concentration, reducing agent, temperature, and time | Higher | Higher |
| Ligand exchange | Governed by equilibrium constant, ligand concentration, and reaction activity | Low | Low |
| Chemical etching | Depends on material surface area, etchant concentration, and etching time | Medium | Medium |
| Microwave-assisted and green synthesis techniques | Influenced by microwave power, radiation time, reactant concentration, green solvents, and biocatalysts | High | High |
| Material | Synthesis Template | Detecting Technology | Target | Detection Limit (M) | Linear Range (M) | Ref. |
|---|---|---|---|---|---|---|
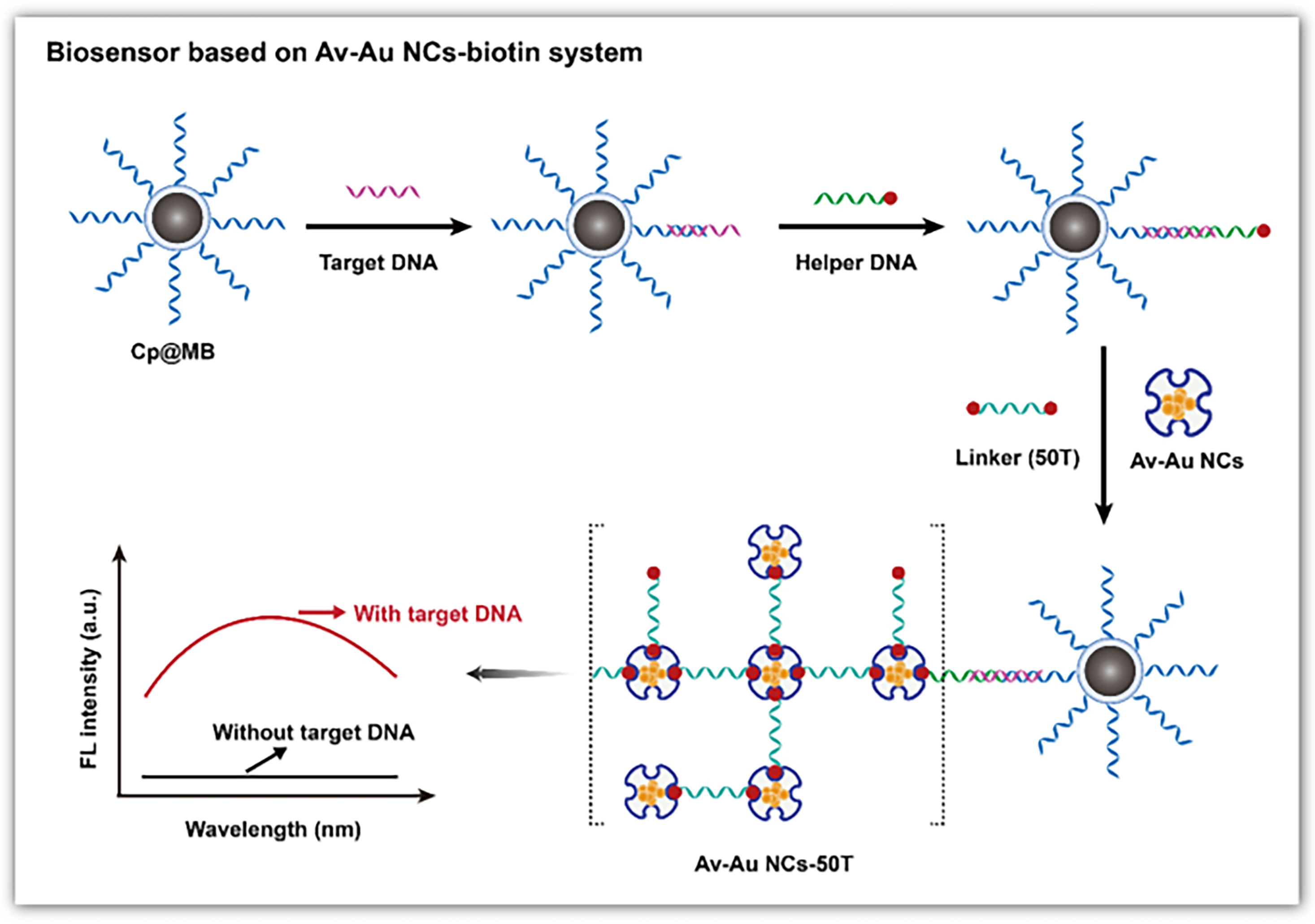
| AgNCs | DNA | Fluorescence | FOXP3-DNA | - | [89] | |
| AgNCs | DNA | Ratiometric fluorescence | HIV-DNA | – | [90] | |
| HBV-DNA | ||||||
| AgNCs | - | Fluorescence | HIV-DNA | – | [91] | |
| AgNCs | - | Fluorescence | H5N1-DNA | – | [92] | |
| AgNCs | DNA | Fluorescence | two HIV DNA | – | [100] | |
| AgNCs | DNA | Fluorescence | DNA-214 | 0– | [108] | |
| AgNCs | DNA | Electrochemistry | - | – | [109] | |
| AgNCs | DNA | Fluorescence | Alzheimer disease DNA | – | [114] | |
| AgNCs | DNA | Fluorescence | - | – | [93] | |
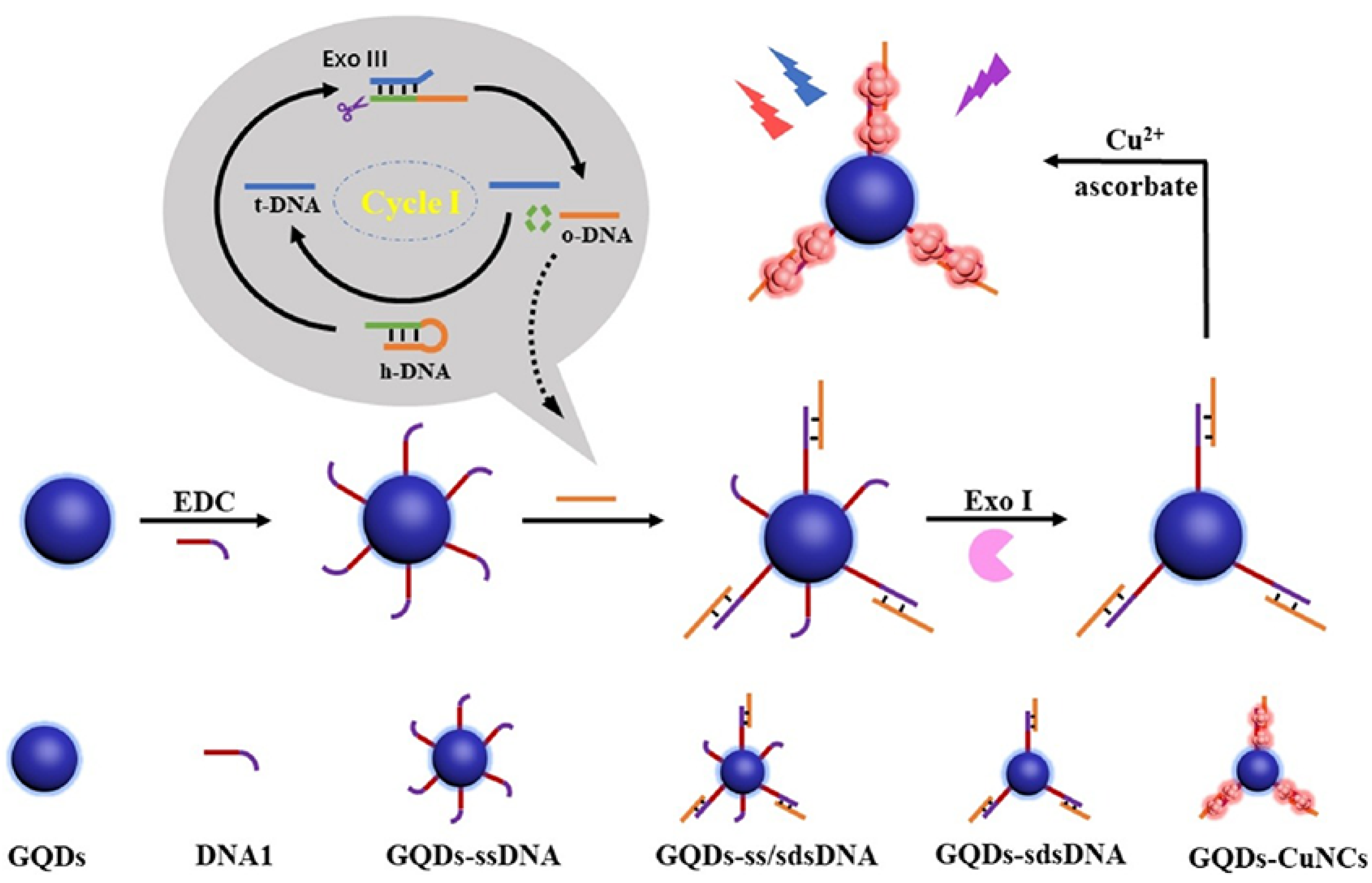
| AgNCBs | DNA | Fluorescence | H1N1-DNA | – | [117] | |
| H5N1-DNA | ||||||
| AgNCBs | DNA | Ratiometric fluorescence | WS-DNA | 0– | [118] | |
| AgNCBs | DNA | Ratiometric fluorescence | HAV-DNA | – | [119] | |
| AuNCs | hemoglobin | Electrochemistry | BCR/ABL-DNA | – | [123] | |
| AuNCs | graphene | Electrochemistry | - | – | [124] | |
| AuNCs | graphene | Electrochemistry | HIV-DNA | – | [125] | |
| AuNCs | avidin | Fluorescence | - | – | [126] | |
| AuNCs | DNA | Fluorescence | H1N1-DNA | – | [127] | |
| AuNCs | - | Electrochemiluminescence | HPV16 E7-DNA | – | [128] | |
| AuNCs | - | Electrochemiluminescence | HPV-16DNA | – | [129] | |
| AuNCs | L-methionine | Surface-enhanced Raman | ctDNA | 0– | [130] | |
| spectroscopy | ||||||
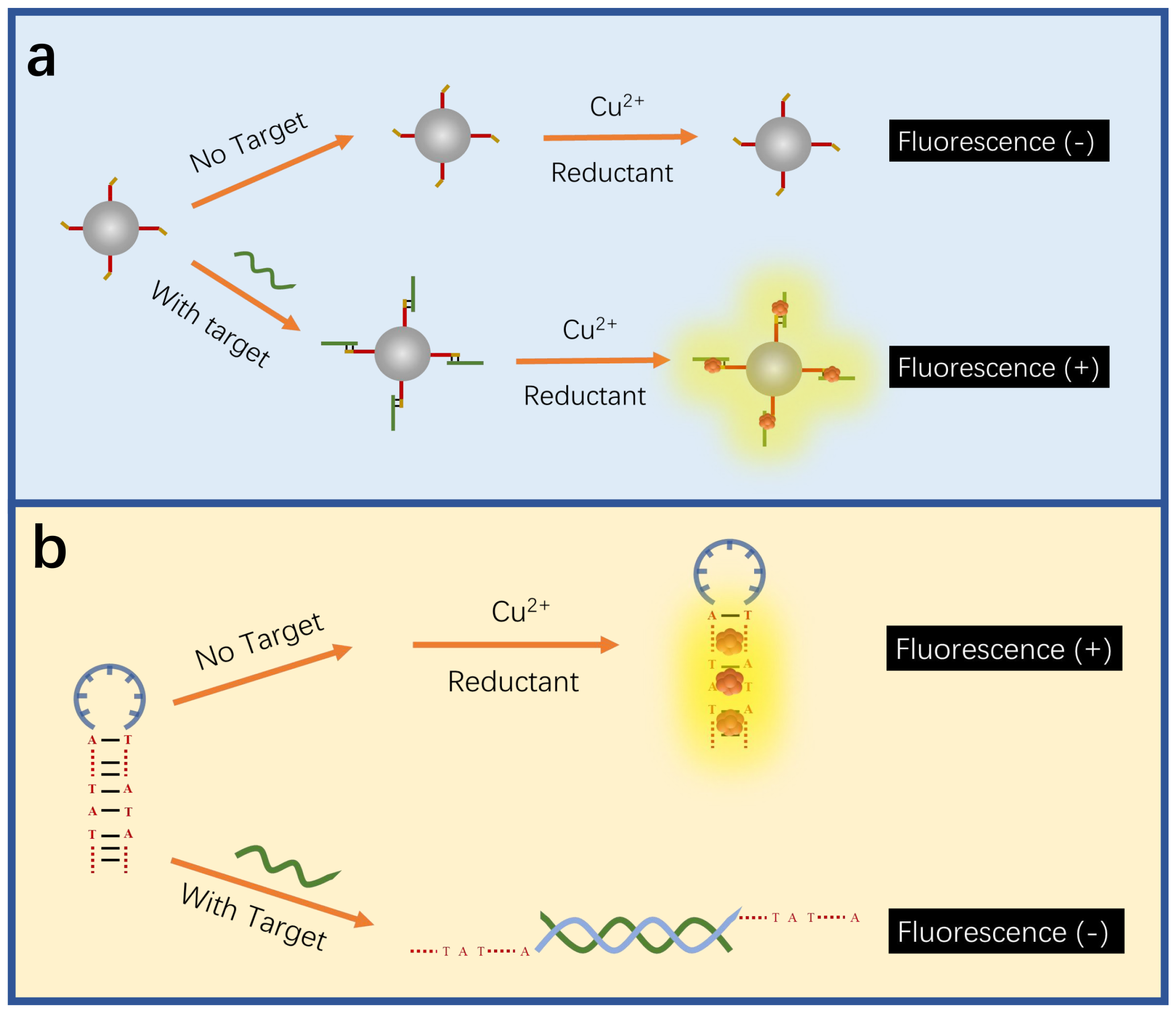
| CuNCs | DNA | Fluorescence | - | – | [131] | |
| CuNCs | DNA | Ratiometric fluorescence | HTLV-1 | – | [132] | |
| CuNCs | DNA | Fluorescence | HBV-DNA | – | [133] | |
| CuNCs | DNA | Fluorescence | C.trachomatis DNA | – | [134] | |
| CuNCs | DNA | Fluorescence | BRCA1-DNA | – | [135] | |
| Au/AgNCs | DNA | Fluorescence | campylobacter jejuni-DNA | – | [136] | |
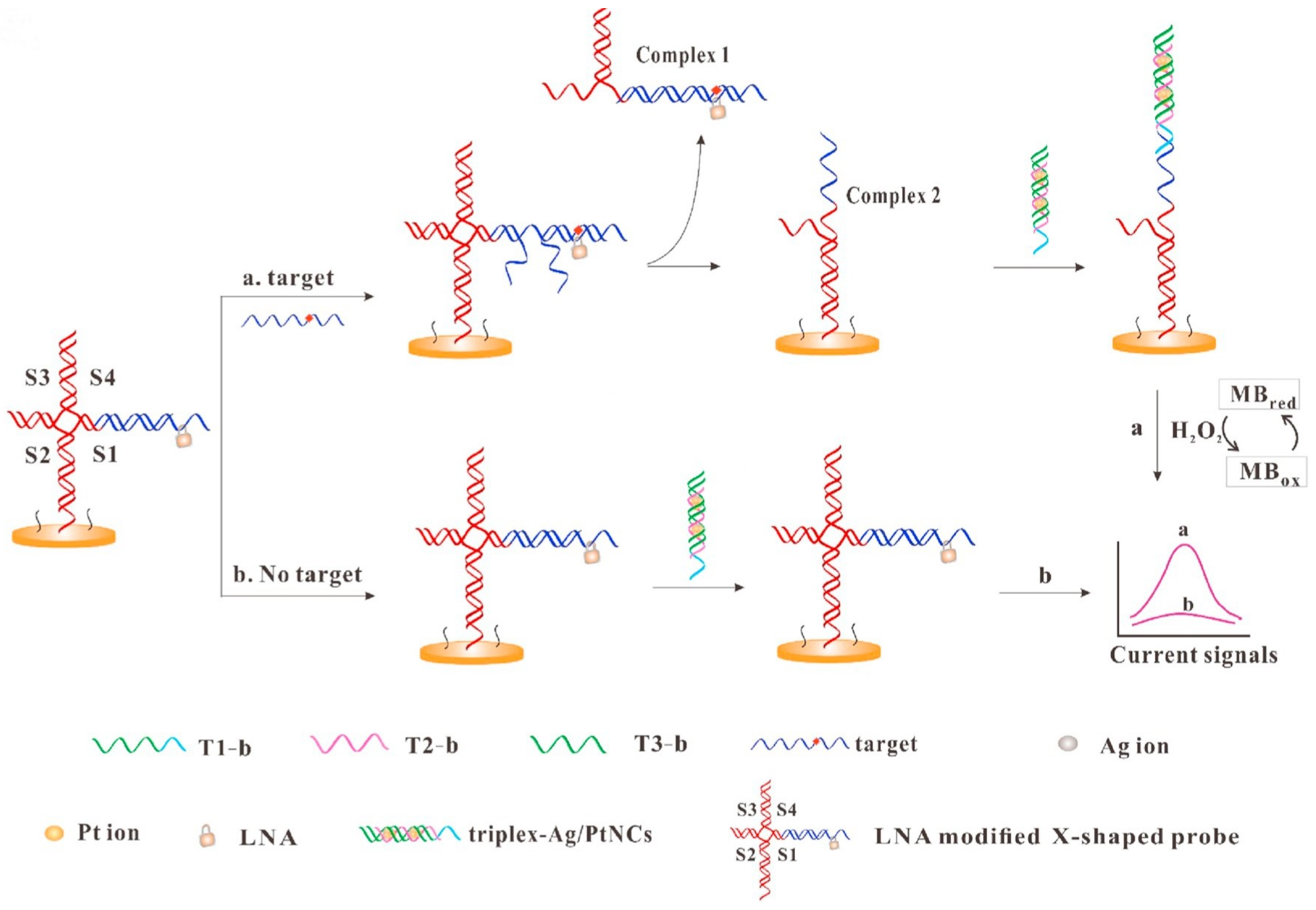
| Au/Ag/PtNCs | DNA | Electrochemistry | -thalassemia-DNA | – | [137] | |
| Ag/PtNCs | - | Field-effect transistor | - | - | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Wang, S.; Ju, F.; Huang, Z.; Gao, Y.; Zhang, J.; He, N.; Nie, L. Metal Nanocluster-Based Biosensors for DNA Detection. Biosensors 2025, 15, 72. https://doi.org/10.3390/bios15020072
He R, Wang S, Ju F, Huang Z, Gao Y, Zhang J, He N, Nie L. Metal Nanocluster-Based Biosensors for DNA Detection. Biosensors. 2025; 15(2):72. https://doi.org/10.3390/bios15020072
Chicago/Turabian StyleHe, Ran, Sheng Wang, Feiye Ju, Zhao Huang, Yuan Gao, Jing Zhang, Nongyue He, and Libo Nie. 2025. "Metal Nanocluster-Based Biosensors for DNA Detection" Biosensors 15, no. 2: 72. https://doi.org/10.3390/bios15020072
APA StyleHe, R., Wang, S., Ju, F., Huang, Z., Gao, Y., Zhang, J., He, N., & Nie, L. (2025). Metal Nanocluster-Based Biosensors for DNA Detection. Biosensors, 15(2), 72. https://doi.org/10.3390/bios15020072







