Signal Differentiation of Moving Magnetic Nanoparticles for Enhanced Biodetection and Diagnostics
Abstract
:1. Introduction
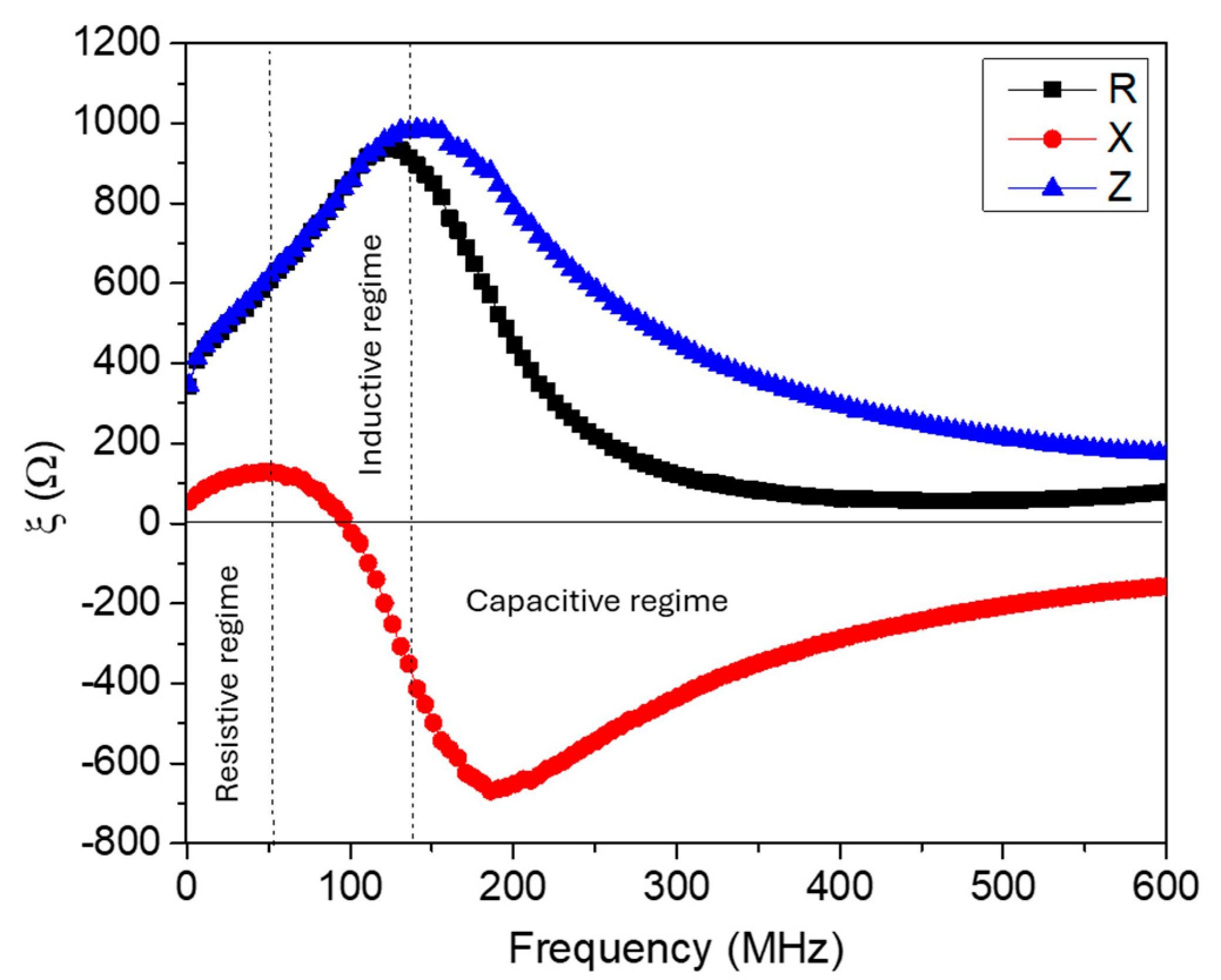
2. Magneto-LC-Resonance (MLCR)-Based Biosensing: Theoretical Considerations
3. Experimental Section
3.1. Sensor Design, Fabrication, and Testing
3.2. Synthesis and Characterization of Magnetic Nanoparticles
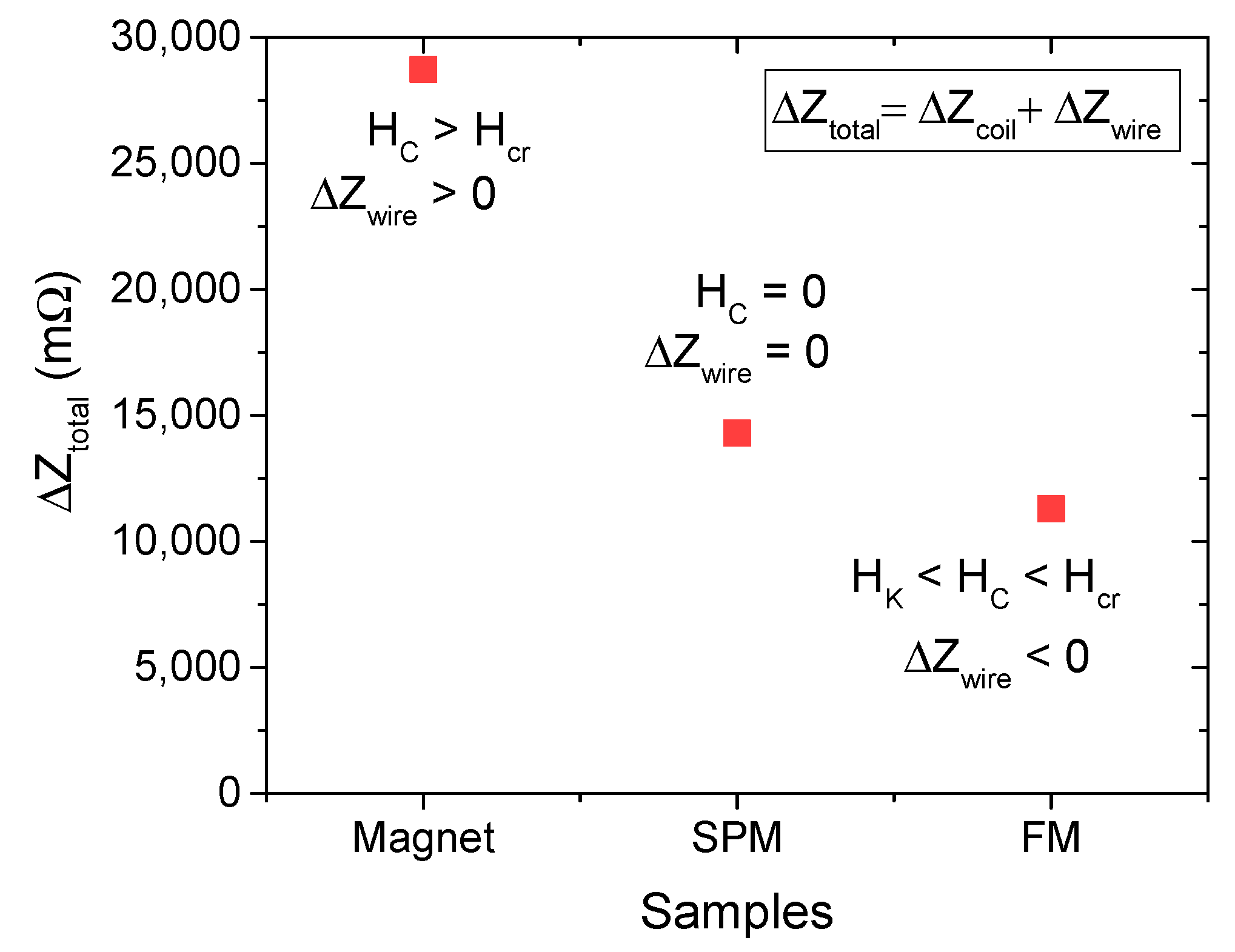
4. Results and Discussion
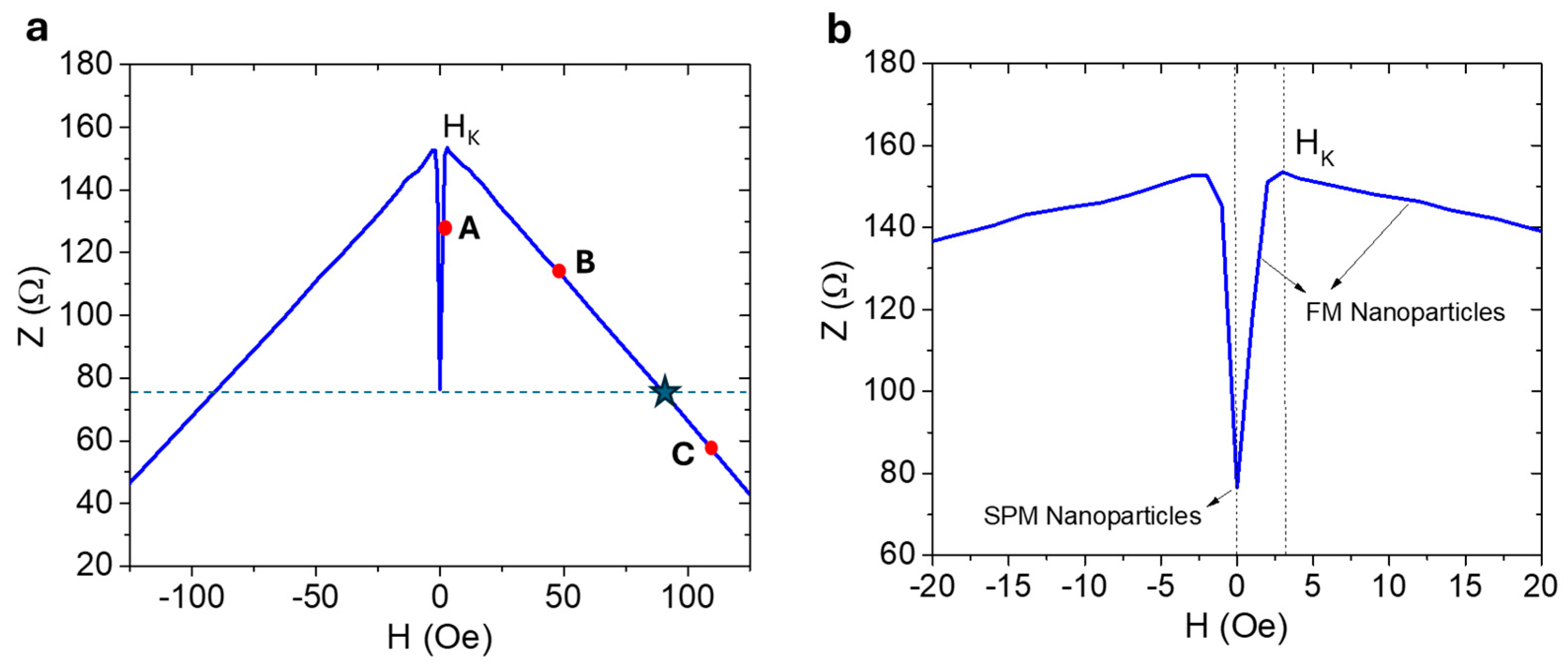
4.1. Iron Oxide Superparticles: Superparamagnetism Versus Ferrimagnetism
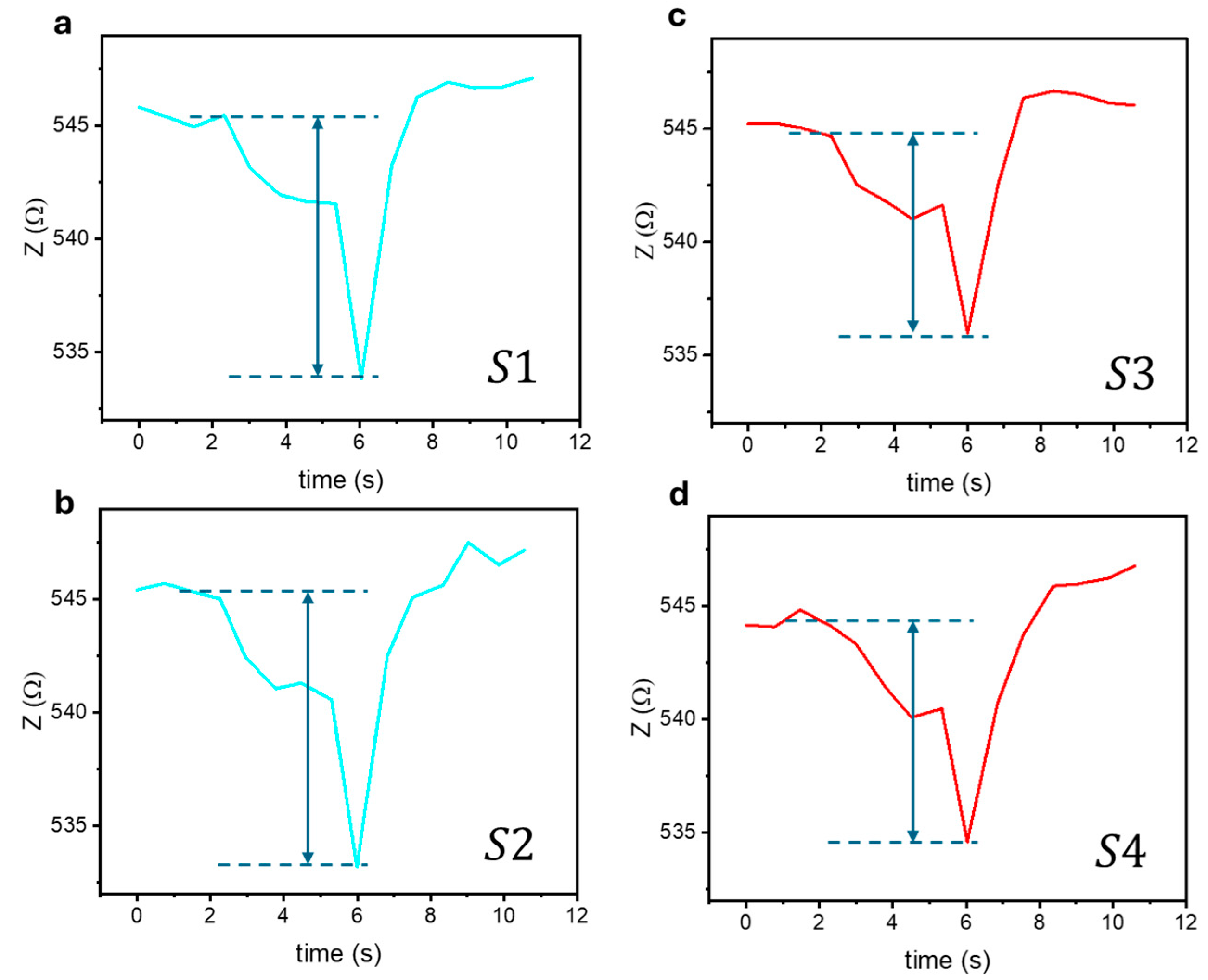
4.2. Biosensing Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef]
- Ge, X.; Mohapatra, J.; Silva, E.; He, G.; Gong, L.; Lyu, T.; Madhogaria, R.P.; Zhao, X.; Cheng, Y.; Al-Enizi, A.M.; et al. Metal–Organic Framework as a New Type of Magnetothermally-Triggered On-Demand Release Carrier. Small 2024, 20, 2306940. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Jalal, N.; Mehrbod, P.; Shojaei, S.; Labouta, H.I.; Mokarram, P.; Afkhami, A.; Madrakian, T.; Los, M.J.; Schaafsma, D.; Giersig, M.; et al. Magnetic nanomaterials in microfluidic sensors for virus detection: A review. ACS Appl. Nano Mater. 2021, 4, 4307–4328. [Google Scholar] [CrossRef]
- Huang, H.T.; Garu, P.; Li, C.H.; Chang, W.C.; Chen, B.W.; Sung, S.Y.; Lee, C.M.; Chen, J.Y.; Hsieh, T.F.; Sheu, W.J.; et al. Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. Sensors 2019, 19, 400022. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Magnetic Nanoparticle Biosensors. WIREs Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, G. Advances in Giant Magnetoresistance Biosensors with Magnetic Nanoparticle Tags: Review and Outlook. IEEE Trans. Magn. 2008, 44, 1687–1702. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Im, J.; Kim, M.; Kim, T.; Wang, S.X.; Kim, D.; Lee, J.-R. Magnetic Supercluster Particles for Highly Sensitive Magnetic Biosensing of Proteins. Microchim. Acta 2022, 189, 256. [Google Scholar] [CrossRef]
- Jimenez, V.O.; Hwang, K.Y.; Nguyen, D.; Rahman, Y.; Albrecht, C.; Senator, B.; Thiabgoh, O.; Devkota, J.; Bui, V.D.A.; Lam, D.S.; et al. Magnetoimpedance Biosensors and Real-Time Healthcare Monitors: Progress, Opportunities, and Challenges. Biosensors 2022, 12, 517. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef] [PubMed]
- Devkota, J.; Mai, T.T.T.; Stojak, K.; Ha, P.T.; Pham, H.N.; Nguyen, X.P.; Mukherjee, P.; Srikanth, H.; Phan, M.H. Synthesis, Inductive Heating, and Magnetoimpedance-Based Detection of Multifunctional Fe3O4 Nanoconjugates. Sens. Actuators B Chem. 2014, 190, 715–722. [Google Scholar] [CrossRef]
- Melnikov, G.Y.; Lepalovskij, V.N.; Svalov, A.V.; Safronov, A.P.; Kurlyandskaya, G.V. Magnetoimpedance thin film sensor for detecting of stray fields of magnetic particles in blood vessel. Sensors 2021, 21, 3621. [Google Scholar] [CrossRef]
- Giouroudi, I.; Kokkinis, G. Recent Advances in Magnetic Microfluidic Biosensors. Nanomaterials 2017, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Chen, Y.-T.; Chinwangso, P.; Nekrashevich, I.; Dannangoda, G.C.; Singh, A.; Jamison, A.C.; Zenasni, O.; Rusakova, I.A.; Martirosyan, K.S.; et al. Magnetic Sensing Potential of Fe3O4 Nanocubes Exceeds That of Fe3O4 Nanospheres. ACS Omega 2017, 2, 8010–8019. [Google Scholar] [CrossRef] [PubMed]
- Devkota, J.; Kokkinis, G.; Berris, T.; Jamalieh, M.; Cardoso, S.; Cardoso, F.; Srikanth, H.; Phan, M.H.; Giouroudi, I. A Novel Approach for Detection and Quantification of Magnetic Nanomarkers Using a Spin Valve GMR-Integrated Microfluidic Sensor. Sensors 2015, 15, 32027–32042. [Google Scholar] [CrossRef]
- Giouroudi, I.; Keplinger, F. Microfluidic Biosensing Systems Using Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 18535–18556. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Üzek, R.; Sari, E.; Merkoçi, A. Optical-Based (Bio) Sensing Systems Using Magnetic Nanoparticles. Magnetochemistry 2019, 5, 59. [Google Scholar] [CrossRef]
- Cleuziou, J.-P.; Wernsdorfer, W.; Ondarçuhu, T.; Monthioux, M. Electrical Detection of Individual Magnetic Nanoparticles Encapsulated in Carbon Nanotubes. Nanomaterials 2006, 6, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, N.; Duschka, R.L.; Ahlborg, M.; Bringout, G.; Debbeler, C.; Graeser, M.; Kaethner, C.; Lüdtke-Buzug, K.; Medimagh, H.; Stelzner, J.; et al. Magnetic Particle Imaging: Current Developments and Future Directions. Int. J. Nanomed. 2015, 10, 3097–3114. [Google Scholar] [CrossRef] [PubMed]
- Moritake, S.; Taira, S.; Sugiura, Y.; Setou, M.; Ichiyanagi, Y. Magnetic Nanoparticle-Based Mass Spectrometry for the Detection of Biomolecules in Cultured Cells. J. Nanosci. Nanotechnol. 2009, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lovchinsky, I.; Sushkov, A.O.; Urbach, E.; de Leon, N.P.; Choi, S.; De Greve, K.; Evans, R.; Gertner, R.; Bersin, E.; Müller, C.; et al. Nuclear Magnetic Resonance Detection and Spectroscopy of Single Proteins Using Quantum Logic. Science 2016, 351, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, T.; Zhong, Y.; Li, R.; Deng, W.; Xiao, X.; Xu, Y.; Zhang, J.; Hu, X.; Wang, Y. A Review of Nanomaterials for Biosensing Applications. J. Mater. Chem. B 2024, 12, 1168–1193. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sun, J.; Li, B.D.; Przybysz, A.; Kosel, J. Magnetic sensors-A review and recent technologies. Eng. Res. Express 2021, 3, 022005. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.; Pu, H. Magnetic Impedance Biosensor: A Review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef]
- Devkota, J.; Wang, C.; Ruiz, A.; Mohapat, S.; Mukherjee, P.; Srikanth, H.; Phan, M.-H. Detection of Low-Concentration Superparamagnetic Nanoparticles Using an Integrated RF Magnetic Biosensor. J. Appl. Phys. 2013, 113, 104701. [Google Scholar] [CrossRef]
- Barrera, G.; Celegato, F.; Vassallo, M.; Martella, D.; Coïsson, M.; Olivetti, E.S.; Martino, L.; Sözeri, H.; Manzin, A.; Tiberto, P. Microfluidic Detection of SPIONs and Co-Ferrite Ferrofluid Using Amorphous Wire Magneto-Impedance Sensor. Sensors 2024, 24, 4902. [Google Scholar] [CrossRef]
- Sayad, A.; Skafidas, E.; Kwan, P. Magneto-Impedance Biosensor Sensitivity: Effect and Enhancement. Sensors 2020, 20, 5213. [Google Scholar] [CrossRef] [PubMed Central]
- Thiabgoh, O.; Eggers, T.; Phan, M.-H. A New Contactless Magneto-LC Resonance Technology for Real-Time Respiratory Motion Monitoring. Sens. Actuators A Phys. 2017, 265, 120–126. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Jimenez, V.O.; Muchharla, B.; Eggers, T.; Le, A.-T.; Lam, V.D.; Phan, M.-H. A Novel Magnetic Respiratory Sensor for Human Healthcare. Appl. Sci. 2021, 11, 3585. [Google Scholar] [CrossRef]
- Nguyen, D.; Huynh, P.K.; Bui, V.D.A.; Hwang, K.Y.; Jain, N.; Nguyen, C.; Minh, L.H.N.; Truong, L.V.; Nguyen, X.T.; Nguyen, D.H.; et al. Real-Time Magnetic Tracking and Diagnosis of COVID-19 via Machine Learning. arXiv 2023, arXiv:2311.00737. [Google Scholar]
- Landau, L.D.; Lifshits, E.M. Electrodynamics of Continuous Media, 2nd ed.; Pergamon: Oxford, MS, USA, 1984. [Google Scholar]
- Zangwill, A. Modern Electrodynamics; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Johnson, R.C. (Ed.) Antenna Engineering Handbook, 3rd ed.; McGraw-Hill, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Nagaoka, H. The Inductance Coefficients of Solenoids. J. Coll. Sci. Imp. Univ. Tokyo 1909, 27, 18. [Google Scholar]
- Coillot, C.; Leroy, P. Induction Magnetometers Principle, Modeling, and Ways of Improvement, Magnetic Sensors—Principles and Applications. In Magnetic Sensors—Principles and Applications; Kuang, K., Ed.; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Phan, M.-H.; Peng, H.-X. Giant Magnetoimpedance Materials: Fundamentals and Applications. Prog. Mater. Sci. 2008, 53, 323–420. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Deng, L.; Lee, J.M.; Resendez, K.M.; Fuller, M.; Hoijang, S.; Robles-Hernandez, F.; Chu, C.-W.; Litvinov, D.; Hadjiev, V.G.; et al. Magnetic Tunability via Control of Crystallinity and Size in Polycrystalline Iron Oxide Nanoparticles. Small 2024, 20, 2402940. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Select 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.T.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.L.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef] [PubMed]
- Hsing, I.-M.; Xu, Y.; Zhao, W. Micro- and Nano- Magnetic Particles for Applications in Biosensing. Electroanalysis 2007, 19, 755–768. [Google Scholar] [CrossRef]
- Miyagawa, A.; Okada, T. Biosensing Strategies Based on Particle Behavior. Chemosensors 2023, 11, 172. [Google Scholar] [CrossRef]
- Koh, I.; Josephson, L. Magnetic Nanoparticle Sensors. Sensors 2009, 9, 8130–8145. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Cham-Fai Leung, K. Tuning the Grain Size and Particle Size of Superparamagnetic Fe3O4 Microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Jamshaid, T.; Neto, E.T.T.; Eissa, M.M.; Zine, N.; Kunita, M.H.; El-Salhi, A.E.; Elaissari, A. Magnetic Particles: From Preparation to Lab-on-a-Chip, Biosensors, Microsystems and Microfluidics Applications. TrAC Trends Anal. Chem. 2016, 79, 344–362. [Google Scholar] [CrossRef]
- Gijs, M.A.M.; Lacharme, F.; Lehmann, U. Microfluidic Applications of Magnetic Particles for Biological Analysis and Catalysis. Chem. Rev. 2010, 110, 1518–1563. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, L.; Lei, C.; Zhang, J.; Li, D.; Zhou, Z.M.; Bao, C.C.; Hu, H.Y.; Chen, X.; Cui, F.; et al. Giant Magnetoimpedance-Based Microchannel System for Quick and Parallel Genotyping of Human Papilloma Virus Type 16/18. Appl. Phys. Lett. 2010, 97, 043702. [Google Scholar] [CrossRef]
- García-Arribas, A.; Martínez, F.; Fernández, E.; Ozaeta, I.; Kurlyandskaya, G.V.; Svalov, A.V.; Berganzo, J.; Barandiaran, J.M. GMI Detection of Magnetic-Particle Concentration in Continuous Flow. Sens. Actuators A Phys. 2011, 172, 103–108. [Google Scholar] [CrossRef]
- Fodil, K.; Denoual, M.; Dolabdjian, C.; Treizebre, A.; Senez, V. In-Flow Detection of Ultra-Small Magnetic Particles by an Integrated Giant Magnetic Impedance Sensor. Appl. Phys. Lett. 2016, 108, 173701. [Google Scholar] [CrossRef]
- Melnikov, G.Y.; Lepalovskij, V.N.; Svalov, A.V.; Safronov, A.P.; Kurlyandskaya, G.V. Magnetoimpedance Thin Film Sensor for Detecting of Stray Fields of Magnetic Particles in Blood Vessel. Sensors 2021, 21, 3621. [Google Scholar] [CrossRef] [PubMed]


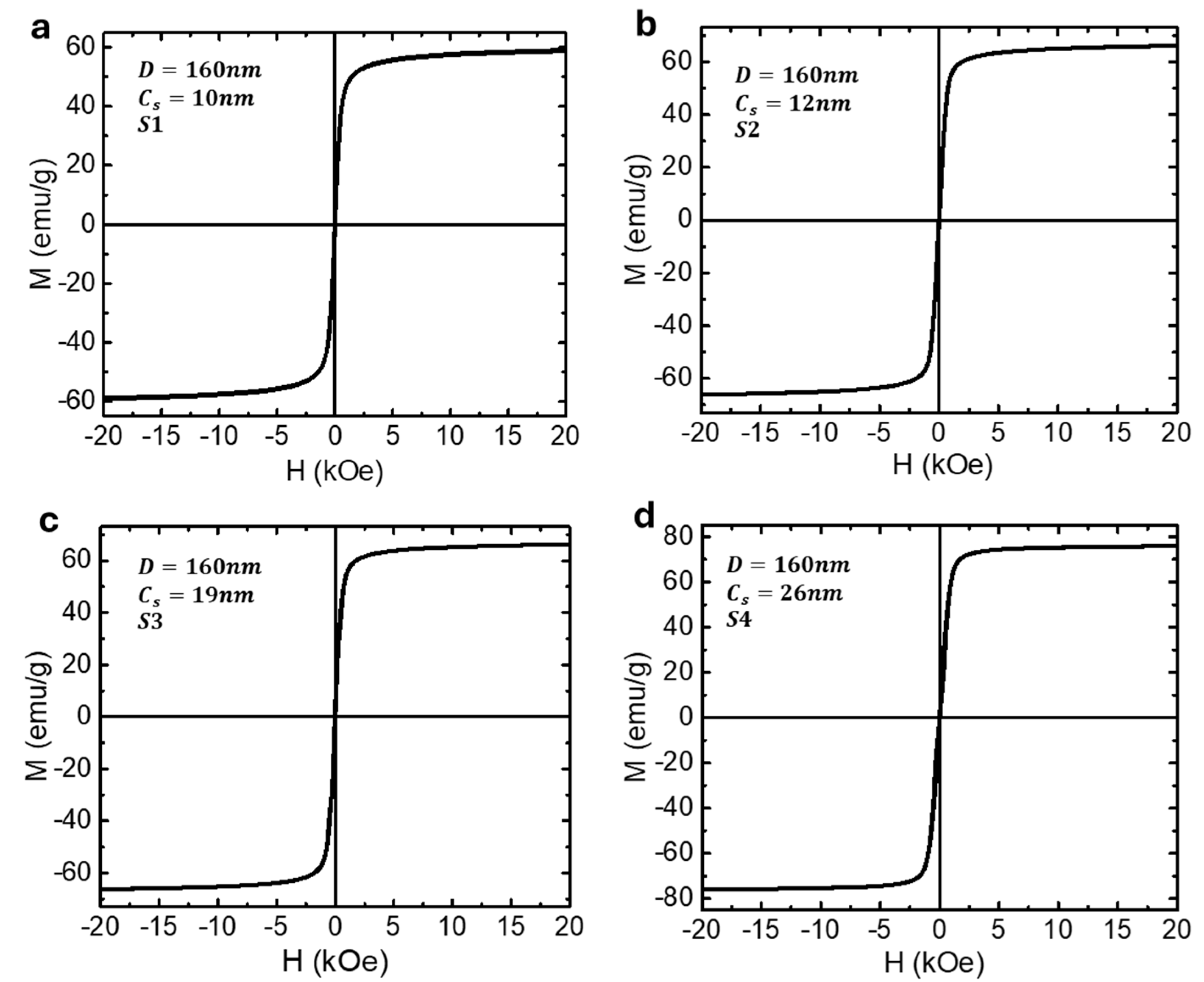


| Sample | |||||
|---|---|---|---|---|---|
| S1 (SPM) | 160 | 10 | 58.9 | 0 | 14,298 |
| S2 (SPM) | 160 | 12 | 66.1 | 0 | 14,669 |
| S3 (FM) | 160 | 19 | 66.3 | ~5 | 8685 |
| S4 (FM) | 160 | 26 | 75.6 | ~10 | 11,294 |
| Sample | |||||
|---|---|---|---|---|---|
| S1 (SPM) | 160 | 10 | 58.9 | 0 | 14,298 |
| S5 (SPM) | 271 | 10 | 62.1 | 0 | 14,673 |
| S6 (SPM) | 375 | 10 | 60.7 | 0 | 13,707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, K.Y.; Brown, D.; Attanayake, S.B.; Luu, D.; Nguyen, M.D.; Lee, T.R.; Phan, M.-H. Signal Differentiation of Moving Magnetic Nanoparticles for Enhanced Biodetection and Diagnostics. Biosensors 2025, 15, 116. https://doi.org/10.3390/bios15020116
Hwang KY, Brown D, Attanayake SB, Luu D, Nguyen MD, Lee TR, Phan M-H. Signal Differentiation of Moving Magnetic Nanoparticles for Enhanced Biodetection and Diagnostics. Biosensors. 2025; 15(2):116. https://doi.org/10.3390/bios15020116
Chicago/Turabian StyleHwang, Kee Young, Dakota Brown, Supun B. Attanayake, Dan Luu, Minh Dang Nguyen, T. Randall Lee, and Manh-Huong Phan. 2025. "Signal Differentiation of Moving Magnetic Nanoparticles for Enhanced Biodetection and Diagnostics" Biosensors 15, no. 2: 116. https://doi.org/10.3390/bios15020116
APA StyleHwang, K. Y., Brown, D., Attanayake, S. B., Luu, D., Nguyen, M. D., Lee, T. R., & Phan, M.-H. (2025). Signal Differentiation of Moving Magnetic Nanoparticles for Enhanced Biodetection and Diagnostics. Biosensors, 15(2), 116. https://doi.org/10.3390/bios15020116







