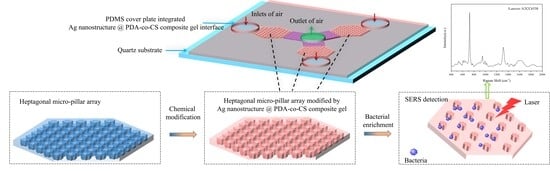
Microfluidic Biochip Integrated with Composite Gel Composed of Silver Nanostructure @ Polydopamine–co–Chitosan for Rapid Detection of Airborne Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
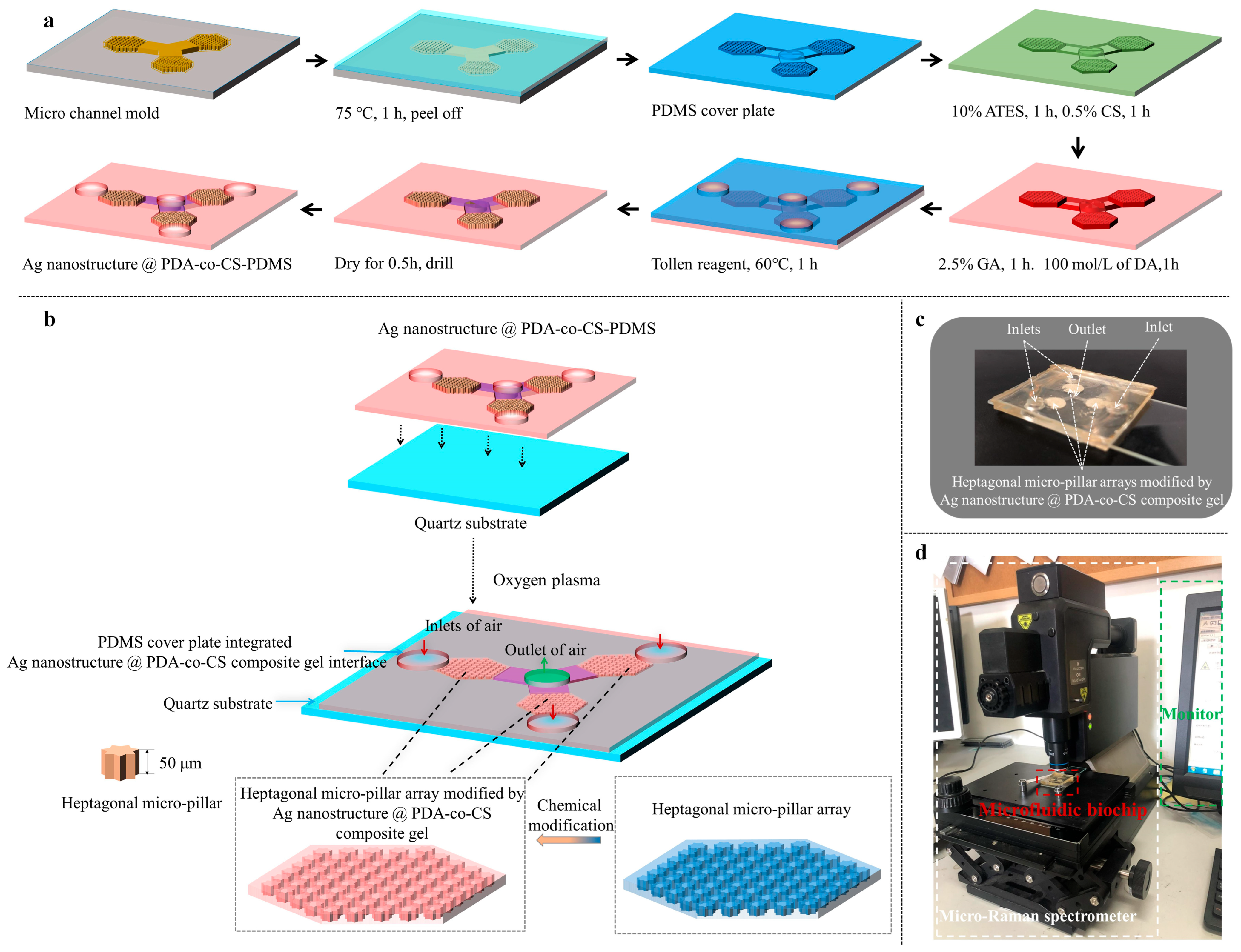
2.2. Preparation of the Microfluidic Biochip
2.3. Experimental Setup for Bacterial Enrichment
2.4. Capture and Enrichment of S. aureus in Synthetic Aerosol by the Microfluidic Biochip
2.5. In Situ SERS Detection of S. aureus, E. coli, and Bacillus cereus in Synthetic Aerosols
2.6. Data Processing
3. Results
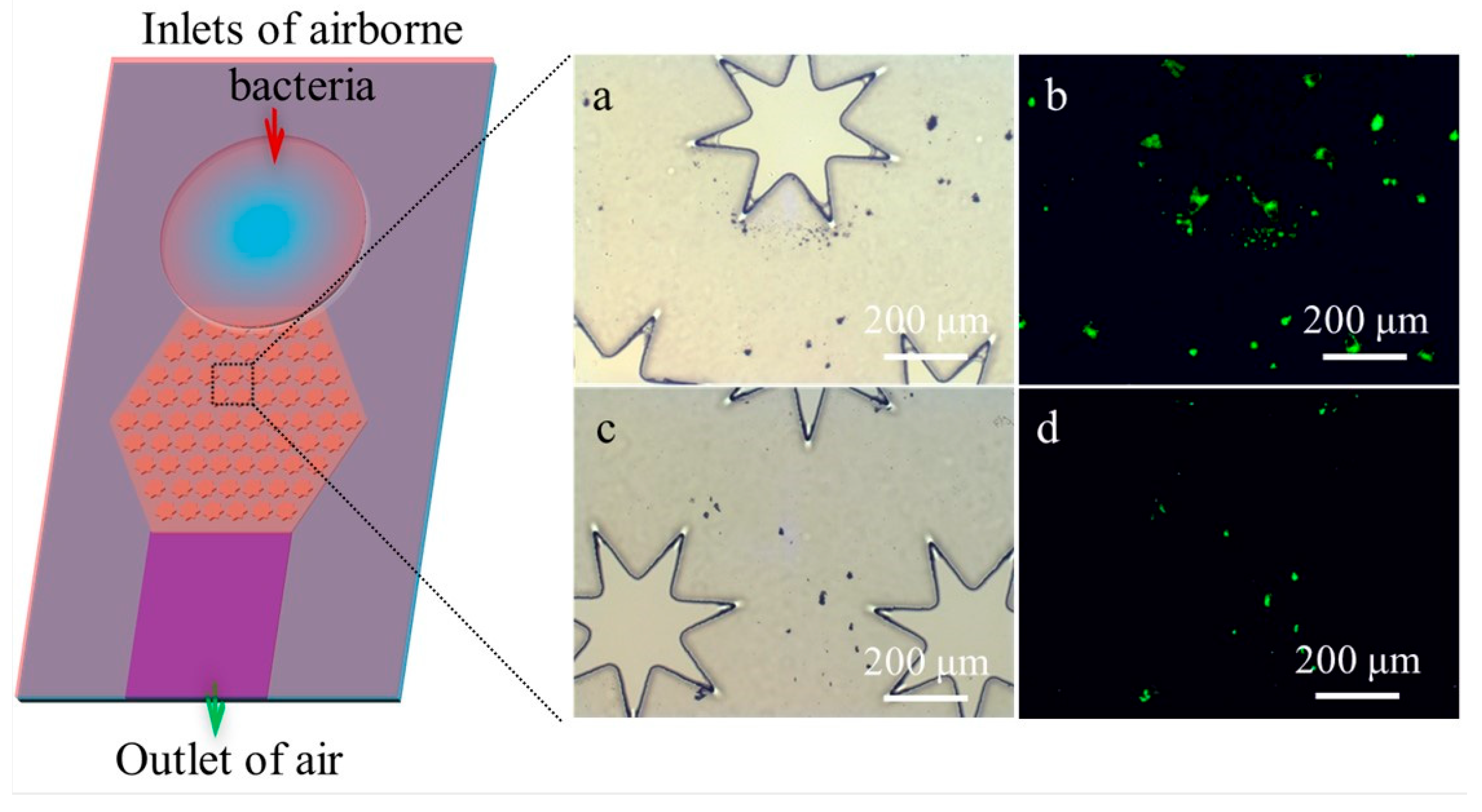
3.1. Design of the Microfluidic Biochip
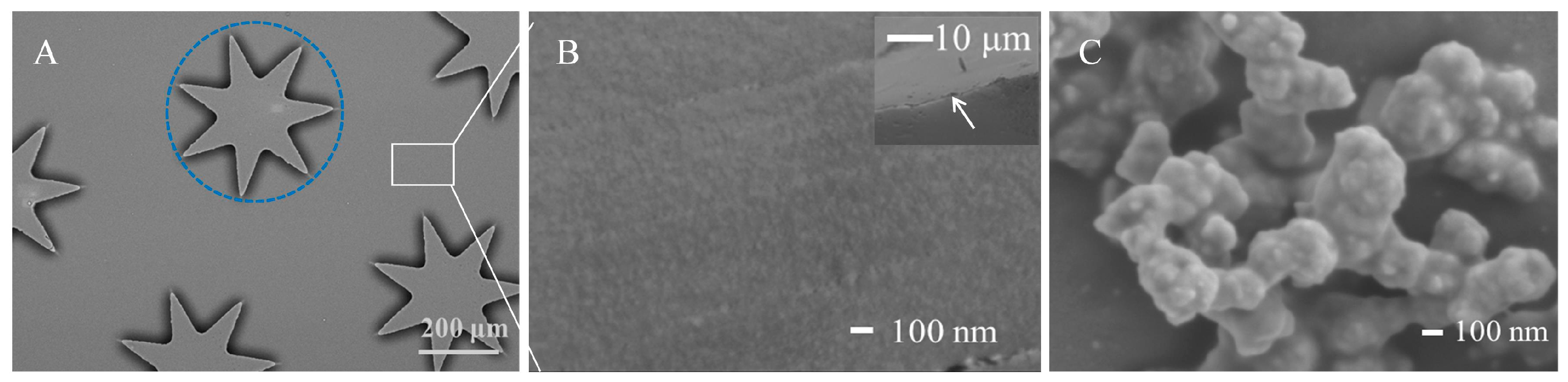
3.2. Characterization of the Ag Nanostructure @ PDA-co-CS Composite Gel Interface
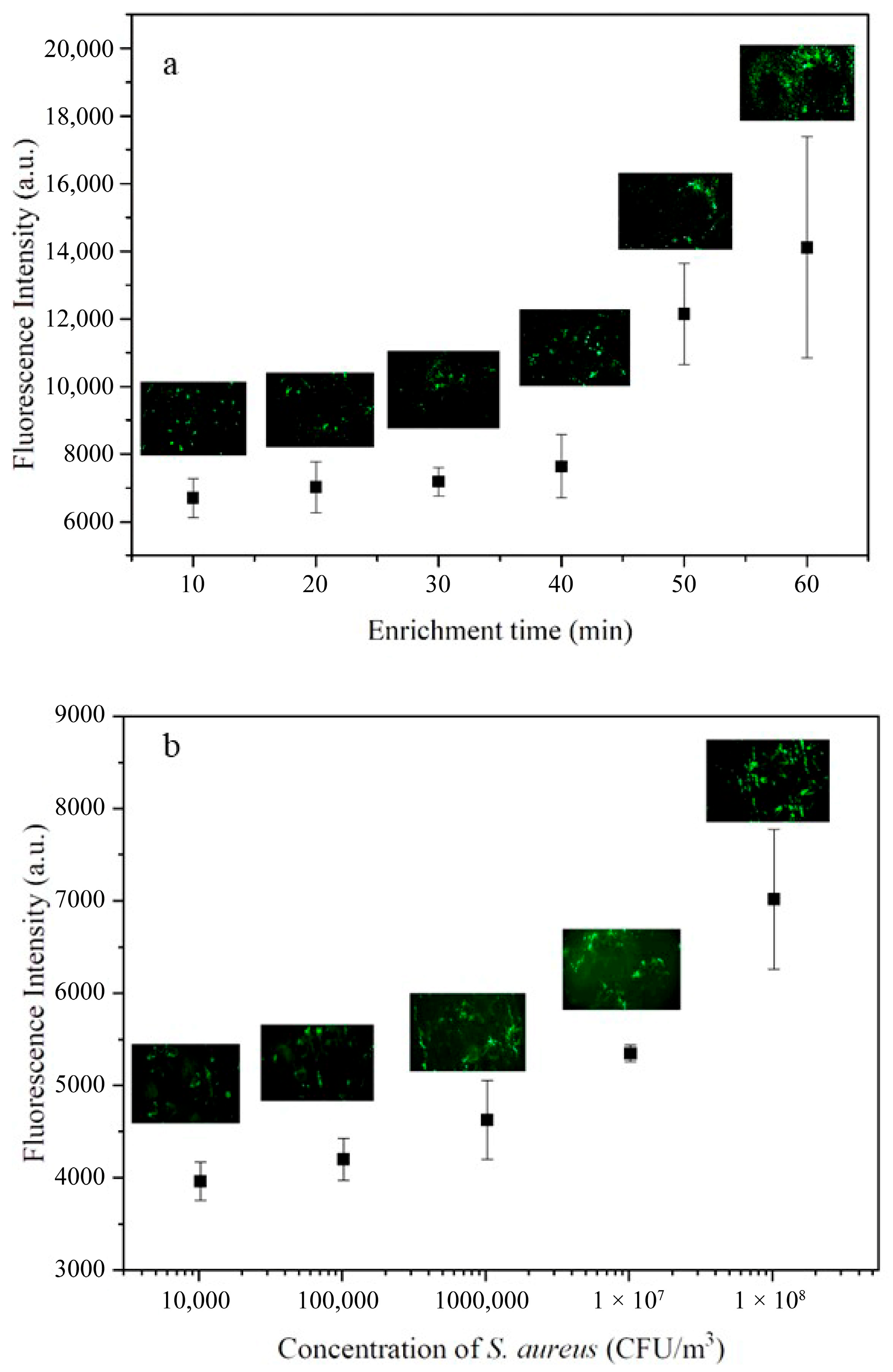
3.3. Capture Efficiency of S. aureus from Synthetic Aerosols Using the Microfluidic Biochip
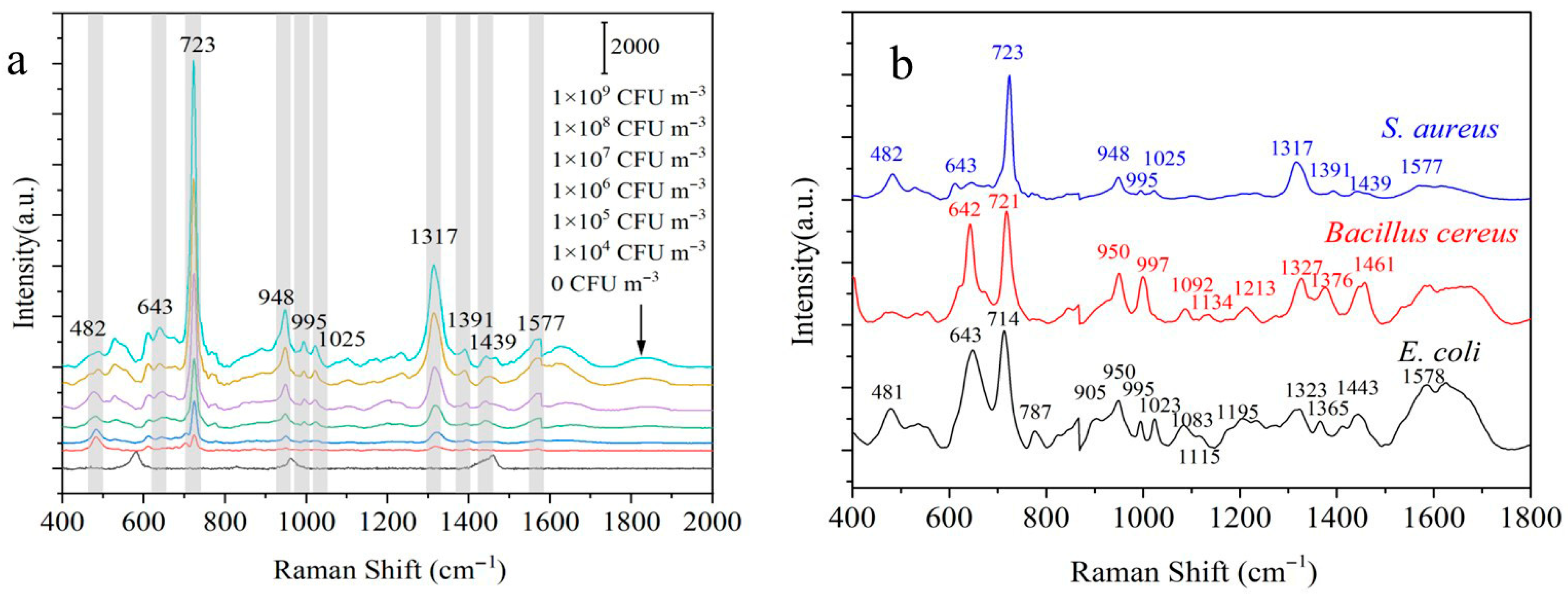
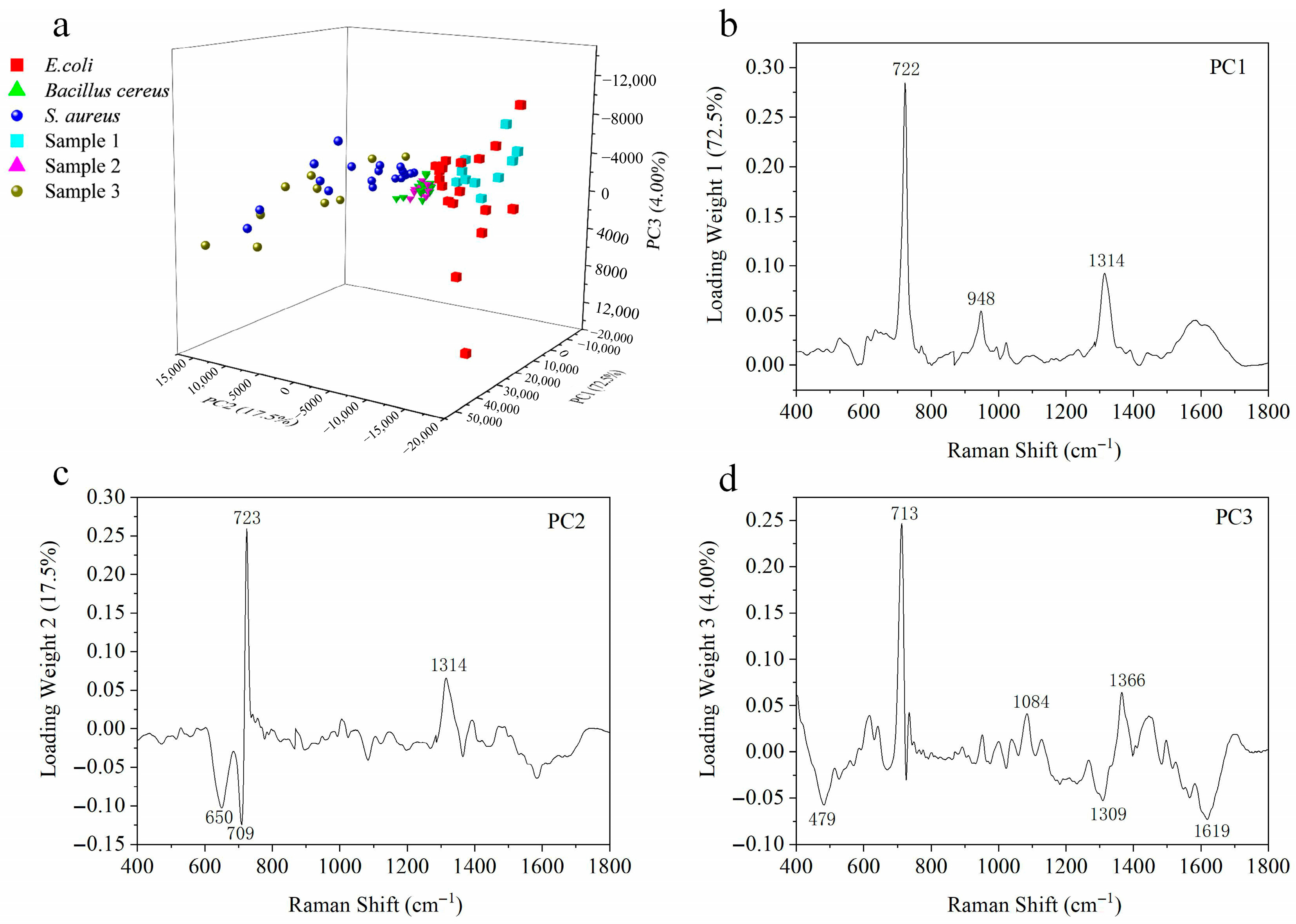
3.4. In Situ SERS Detection of S. aureus, E. coli, and Bacillus cereus in Synthetic Aerosols
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, K.; Lee, T.; Choi, E.; Yang, J.; Shukla, S.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, X.; Wang, T.; Wang, B.; Li, C.; Zeng, G. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. [Google Scholar] [CrossRef]
- Fronczek, C.; Yoon, J. Biosensors for Monitoring Airborne Pathogens. J. Lab. Autom. 2015, 20, 390–410. [Google Scholar] [CrossRef]
- Yan, S.; Qing, L.; Bing, L.; Miao, Z.; Wujun, C.; Daijun, Z.; Chao, W.; Xing, D. Airborne microbes: Sampling, detection, and inactivation. Crit. Rev. Biotechnol. 2025, 45, 556–590. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, S.; Zhang, M.; Wang, C.; Xing, D. Air sampling and ATP bioluminescence for quantitative detection of airborne microbes. Talanta 2024, 274, 126025. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Q.; Liu, Z.; Liu, R.; Xing, K.; Zhang, M.; Zhang, X.; Xu, J.; Jia, Q.; Gao, W.; et al. Gel-confined fabrication of fully bio-based filtration membrane for green capture and rapid detection of airborne microbes. J. Colloid Interface Sci. 2024, 670, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Whitby, C.; Colbeck, I.; Dumbrell, A.J.; Ferguson, R.M.W. Rapid In-Field Detection of Airborne Pathogens Using Loop-Mediated Isothermal Amplification (LAMP). Microorganisms 2024, 12, 2578. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Heo, K.J.; Kim, I.H.; Shin, J.H.; Jung, J.H. From airborne bacteria to imaging chip: A fully integrated system for on-site bioaerosol monitoring. Sens. Actuators B Chem. 2025, 426, 137084. [Google Scholar] [CrossRef]
- Rastmanesh, A.; Boruah, J.S.; Lee, M.S.; Park, S. On-Site Bioaerosol Sampling and Airborne Microorganism Detection Technologies. Biosensors 2024, 14, 122. [Google Scholar] [CrossRef]
- Kant, K.; Shahbazi, M.; Dave, V.; Ngo, T.; Chidambara, V.; Than, L.; Bang, D.; Wolff, A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef]
- Wang, L.; Qi, W.; Liu, Y.; Essien, D.; Zhang, Q.; Lin, J. Recent Advances on Bioaerosol Collection and Detection in Microfluidic Chips. Anal. Chem. 2021, 93, 9013–9022. [Google Scholar] [CrossRef]
- Feng, X.; Hu, P.; Jin, T.; Fang, J.; Tang, F.; Jiang, H.; Lu, C. On-site monitoring of airborne pathogens: Recent advances in bioaerosol collection and rapid detection. Aerobiologia 2024, 40, 303–341. [Google Scholar] [CrossRef]
- Yoo, S.J.; Oh, J.; Hong, S.J.; Kim, M.; Hwang, J.; Kim, Y.J. Microfluidics-based condensation bioaerosol sampler for multipoint airborne virus monitoring. Biosens. Bioelectron. 2024, 264, 116658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, L.; Wang, H.; Wang, L. Application of Microfluidic Chips in the Detection of Airborne Microorganisms. Micromachines 2022, 13, 1576. [Google Scholar] [CrossRef] [PubMed]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2019, 144, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Salafi, T.; Zeming, K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef]
- Lee, I.; Jeon, E.; Lee, J. On-site bioaerosol sampling and detection in microfluidic platforms. TrAC Trends Anal. Chem. 2023, 158, 116880. [Google Scholar] [CrossRef]
- Yang, N.; Song, W.; Xiao, Y.; Xia, M.; Xiao, L.; Li, T.; Zhang, Z.; Yu, N.; Zhang, X. Minimum Minutes Machine-Learning Microfluidic Microbe Monitoring Method (M7). ACS Nano 2024, 18, 4862–4870. [Google Scholar] [CrossRef]
- Jing, W.; Zhao, W.; Liu, S.; Li, L.; Tsai, C.; Fan, X.; Wu, W.; Li, J.; Yang, X.; Sui, G. Microfluidic Device for Efficient Airborne Bacteria Capture and Enrichment. Anal. Chem. 2013, 85, 5255–5262. [Google Scholar] [CrossRef]
- Cheng, J.; Nie, M.; An, Y.; Chen, Z.; Tong, Y. Microfluidic Chip-based Enrichment and Nucleic Acid Extraction for Quantitative Detection of Mycobacterium Smegmatis in Aerosols. Curr. Microbiol. 2024, 82, 42. [Google Scholar] [CrossRef]
- Bian, X.; Lan, Y.; Wang, B.; Zhang, Y.; Liu, B.; Yang, P.; Zhang, W.; Qiao, L. Microfluidic Air Sampler for Highly Efficient Bacterial Aerosol Collection and Identification. Anal. Chem. 2016, 88, 11504–11512. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, I.; Song, M.; Charmchi, M.; Sun, H. A microfluidics-based on-chip impinger for airborne particle collection. Lab Chip 2016, 16, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.; Yao, Y.; Jing, W.; Liu, S.; Sui, G. A novel microfluidic module for rapid detection of airborne and waterborne pathogens. Sens. Actuators B Chem. 2018, 258, 1138–1145. [Google Scholar] [CrossRef]
- Sui, G.; Wang, J.; Lee, C.; Lu, W.; Lee, S.; Leyton, J.; Wu, A.; Tseng, H. Solution-Phase Surface Modification in Intact Poly(dimethylsiloxane) Microfluidic Channels. Anal. Chem. 2006, 78, 5543–5551. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Liu, Y.; Feng, X.; Dong, Q.; Li, J.; Wang, J.; Zhao, C. A concise detection strategy of Staphylococcus aureus using N-Succinyl-Chitosan-dopped bacteria-imprinted composite film and AIE fluorescence sensor. J. Hazard. Mater. 2022, 423, 126934. [Google Scholar] [CrossRef]
- Samyn, P. Polydopamine and Cellulose: Two Biomaterials with Excellent Compatibility and Applicability. Polym. Rev. 2021, 61, 814–865. [Google Scholar] [CrossRef]
- Ai, Y.; Wang, C.; Pan, Y.; Videen, G. Optical trapping-surface enhanced Raman spectroscopy (OT-SERS) for sensing single bioaerosol particles in air. Aerosol Sci. Technol. 2025, 59, 635–648. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, L.; Wang, Y.; Shao, J.; Wang, L.; Wang, H.; Qian, H.; Yao, W. Label-free detection of the foodborne pathogens of Enterobacteriaceae by surface-enhanced Raman spectroscopy. Anal. Methods 2013, 5, 946–952. [Google Scholar] [CrossRef]
- Chawla, K.; Bankapur, A.; Acharya, M.; D’Souza, J.S.; Chidangil, S. A micro-Raman and chemometric study of urinary tract infection-causing bacterial pathogens in mixed cultures. Anal. Bioanal. Chem. 2019, 411, 3165–3177. [Google Scholar] [CrossRef]
- Zhang, W.; Man, P.; Wang, M.; Shi, Y.; Xu, Y.; Li, Z.; Yang, C.; Man, B. Roles of graphene nanogap for the AgNFs electrodeposition on the woven Cu net as flexible substrate and its application in SERS. Carbon 2018, 133, 300–305. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Sun, Y.; Wu, H.; Luo, L.; Song, Y. Recent Advances in Surface-Enhanced Raman Scattering for Pathogenic Bacteria Detection: A Review. Sensors 2025, 25, 1370. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Zhang, X.; Cheng, H.; Xu, D.; Feng, Y.; Sui, G.; Fu, H.; Valev, V.; Zhang, L.; Chen, J. Klarite as a label-free SERS-based assay: A promising approach for atmospheric bioaerosol detection. Analyst 2020, 145, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Sun, J.; Liang, R.; Guo, H.; Wang, L.; Sun, X. Application progress of microfluidics-integrated biosensing platforms in the detection of foodborne pathogens. Trends Food Sci. Technol. 2021, 116, 115–129. [Google Scholar] [CrossRef]
- Pu, H.; Xiao, W.; Sun, D. SERS-microfluidic systems: A potential platform for rapid analysis of food contaminants. Trends Food Sci. Technol. 2017, 70, 114–126. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Dong, X.; Huang, Q. A review: Research progress of SERS-based sensors for agricultural applications. Trends Food Sci. Technol. 2022, 128, 90–101. [Google Scholar] [CrossRef]
- Lian, S.; Li, X.; Lv, X. Recent Developments in SERS Microfluidic Chips: From Fundamentals to Biosensing Applications. ACS Appl. Mater. Interfaces 2025, 17, 10193–10230. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Jung, J. Fully integrated optofluidic SERS platform for real-time and continuous characterization of airborne microorganisms. Biosens. Bioelectron. 2020, 169, 112611. [Google Scholar] [CrossRef]
- Su, X.; Ren, R.; Wu, Y.; Li, S.; Ge, C.; Liu, L.; Xu, Y. Study of biochip integrated with microelectrodes modified by poly-dopamine-co-chitosan composite gel for separation, enrichment and detection of microbes in the aerosol. Biosens. Bioelectron. 2021, 176, 112931. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.; Li, W.; Zhu, H.; Yeap, S.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Ge, X.; Fang, L.; Wang, K.; Ren, F.; Lu, X. Mussel-Inspired Contact-Active Antibacterial Hydrogel with High Cell Affinity, Toughness, and Recoverability. Adv. Funct. Mater. 2019, 29, 1805964. [Google Scholar] [CrossRef]
- Usman, M.; Tang, J.; Li, F.; Lai, J.; Liu, Q.; Liu, W.; Wang, L. Recent advances in surface enhanced Raman spectroscopy for bacterial pathogen identifications. J. Adv. Res. 2022, 51, 31–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Wu, S.; Liu, N.; Peng, C.; Chan, T.; Hsu, C.; Wang, J.; Wang, Y. Highly Raman-Enhancing Substrates Based on Silver Nanoparticle Arrays with Tunable Sub-10 nm Gaps. Adv. Mater. 2006, 18, 491–495. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.; Feng, H.; Dong, J.; Wang, C.; Liu, Q. Bionic SERS chip with super-hydrophobic and plasmonic micro/nano dual structure. Photon. Res. 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wang, R.; Wang, C.; Zhao, H.; Zheng, X.; Liao, X.; Cheng, L. A microfluidic chip based on an ITO support modified with Ag-Au nanocomposites for SERS based determination of melamine. Microchim. Acta 2017, 184, 279–287. [Google Scholar] [CrossRef]
- He, X.; Ge, C.; Zheng, X.; Tang, B.; Chen, L.; Li, S.; Wang, L.; Zhang, L.; Xu, Y. Rapid identification of alpha-fetoprotein in serum by a microfluidic SERS chip integrated with Ag/Au Nanocomposites. Sens. Actuators B Chem. 2020, 317, 128196. [Google Scholar] [CrossRef]
- Liu, J.; Si, T.; Zhang, Z. Mussel-inspired immobilization of silver nanoparticles toward sponge for rapid swabbing extraction and SERS detection of trace inorganic explosives. Talanta 2019, 204, 189–197. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; He, Y.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of Ag@TiO2 electrospinning nanofibrous felts as SERS substrate for direct and sensitive bacterial detection. Sens. Actuators B Chem. 2018, 273, 600–609. [Google Scholar] [CrossRef]
- Liu, T.; Ho, J.; Wei, J.; Cheng, W.; Chen, I.; Shiue, J.; Wang, H.; Wang, J.; Wang, Y.; Lin, J. Label-free and culture-free microbe detection by three dimensional hot-junctions of flexible Raman-enhancing nanohybrid platelets. J. Mater. Chem. B 2014, 2, 1136–1143. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Zhao, H.; Li, S.; Chen, L. Design and preparation of centrifugal microfluidic chip integrated with SERS detection for rapid diagnostics. Talanta 2019, 194, 903–909. [Google Scholar] [CrossRef]
- Su, L.; Zhang, P.; Zheng, D.; Wang, Y.; Zhong, R. Rapid detection of Escherichia coli and Salmonella typhimurium by surface-enhanced Raman scattering. Optoelectron. Lett. 2015, 11, 157–160. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Shi, L.; Shao, L.; Fu, P.; Wang, K.; Xiao, R.; Wang, S.; Gu, B. Rapid identification and antibiotic susceptibility test of pathogens in blood based on magnetic separation and surface-enhanced Raman scattering. Microchim. Acta 2019, 186, 475. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, G.; Ma, Q.; Wang, H.; Du, M.; Wang, C.; Xie, X.; Li, T.; Chen, S. Rapid detection of airborne protein from Mycobacterium tuberculosis using a biosensor detection system. Analyst 2022, 147, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qian, S.; Guo, Y.; Geng, Y.; Guo, B.; Chen, Z.; Yang, R.; Chen, X.; Guo, Z.; Liu, S. Integrated Fiber-Optic SPR Sensor with Cyclone Sampling for Rapid On-Site Bioaerosol Pathogen Detection. ACS Sens. 2025, 10, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, P.; Chang, Y.; Liu, M. Total Bioaerosol Detection by Split Aptamer-Based Electrochemical Nanosensor Chips. Anal. Chem. 2022, 94, 16752–16758. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Q.; Li, T.; Mao, H.; Wang, Y.; Jiang, F.; Wang, S.; Yu, N.; Yang, N. A multi-scale microbial aerosol detection method based on the microfluidic AC impedance sensor. Microchem. J. 2025, 215, 114240. [Google Scholar] [CrossRef]
- Yang, N.; Li, T.; Dong, S.; Zhang, S.; Jia, Y.; Mao, H.; Zhang, Z.; Zhang, F.; Pan, X.; Zhang, X.; et al. Detection of airborne pathogens with single photon counting and a real-time spectrometer on microfluidics. Lab Chip 2022, 22, 4995–5007. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Jing, W.; Liu, S.; Zhang, D.; Sui, G. First airborne pathogen direct analysis system. Analyst 2016, 141, 1637–1640. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, Q.; Zhao, W.; Liu, S.; Sui, G. Microfluidic System for Rapid Detection of Airborne Pathogenic Fungal Spores. ACS Sens. 2018, 3, 2095–2103. [Google Scholar] [CrossRef]
| Biochip Structures | Amount of S. aureus in Tail Gas Absorber (CFU) | Capture Efficiency (%) |
|---|---|---|
| Without biochip (blank experiment) | 7900 ± 361 | - |
| Without micro–nano structures | 7000 ± 629 | 11.4 ± 4.4 |
| Heptagonal micropillar array | 1080 ± 76 | 86.3 ± 1.4 |
| Heptagonal micropillar array modified with PDA-co-CS composite gel | 176 ± 34 | 97.8 ± 0.5 |
| Heptagonal micropillar array modified with Ag nanostructure @ PDA-co-CS composite gel | 0 ± 0 | >99.9 ± 0.0 |
| Function of the Chip | Capture Principles | Capture Efficiency | Flow Rate (L·min−1) | Detection Method | Detection Limit | Time (min) | Ref |
|---|---|---|---|---|---|---|---|
| Enrichment | Horizontal inertial centrifugal force and vertical turbulence | >99.9% | 0.004 | - | - | 20 | [21] |
| Detection | - | - | - | A silicon nanowire field-effect-transistor biosensor | 4 × 104 particles mL−1 | >10 | [52] |
| Detection | - | 97.9% (cyclone air sampler) | - | SPR/LSPR 1 | 297.3 TCID50/mLbuffer | ~30 | [53] |
| Detection | - | - | - | Electrochemical method | <600 CFU m−3 | ~70 | [54] |
| Separation, detection | Inertial separation | - | 0.014 | AC impedance sensor | 1 × 103 CFU mL−1 | <20 | [55] |
| Focus, separation, detection | Dual-sheath flow-focusing | - | 0.012 | Dynamic transmission spectroscopy analysis, single-photon measurement | - | - | [56] |
| Enrichment, detection | Filtration | >99% | 0.071 | LAMP 2 | 109 CFU m−3 | >60 | [23] |
| Enrichment, detection | Chaotic vortex flow | - | 0.004 | LAMP | - | 70 | [57] |
| Enrichment, detection | Chaotic vortex flow | - | - | LAMP | ~300 CFU m−3 | 180 | [58] |
| Enrichment, in-situ detection | Chaotic vortex flow and adsorption of Ag nanostructure @ PDA-co-CS composite gel | >99.9% | 1.8 | SERS 3 | 105 CFU m−3 | ≤25 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; He, X.; Ge, C.; Wang, Y.; Xu, Y. Microfluidic Biochip Integrated with Composite Gel Composed of Silver Nanostructure @ Polydopamine–co–Chitosan for Rapid Detection of Airborne Bacteria. Biosensors 2025, 15, 720. https://doi.org/10.3390/bios15110720
Su X, He X, Ge C, Wang Y, Xu Y. Microfluidic Biochip Integrated with Composite Gel Composed of Silver Nanostructure @ Polydopamine–co–Chitosan for Rapid Detection of Airborne Bacteria. Biosensors. 2025; 15(11):720. https://doi.org/10.3390/bios15110720
Chicago/Turabian StyleSu, Xi, Xinyu He, Chuang Ge, Yipei Wang, and Yi Xu. 2025. "Microfluidic Biochip Integrated with Composite Gel Composed of Silver Nanostructure @ Polydopamine–co–Chitosan for Rapid Detection of Airborne Bacteria" Biosensors 15, no. 11: 720. https://doi.org/10.3390/bios15110720
APA StyleSu, X., He, X., Ge, C., Wang, Y., & Xu, Y. (2025). Microfluidic Biochip Integrated with Composite Gel Composed of Silver Nanostructure @ Polydopamine–co–Chitosan for Rapid Detection of Airborne Bacteria. Biosensors, 15(11), 720. https://doi.org/10.3390/bios15110720