FGAN@PB NP Nanozyme-Based Colorimetric–Photothermal Dual-Mode Immunosensor for Malachite Green Detection
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Instruments
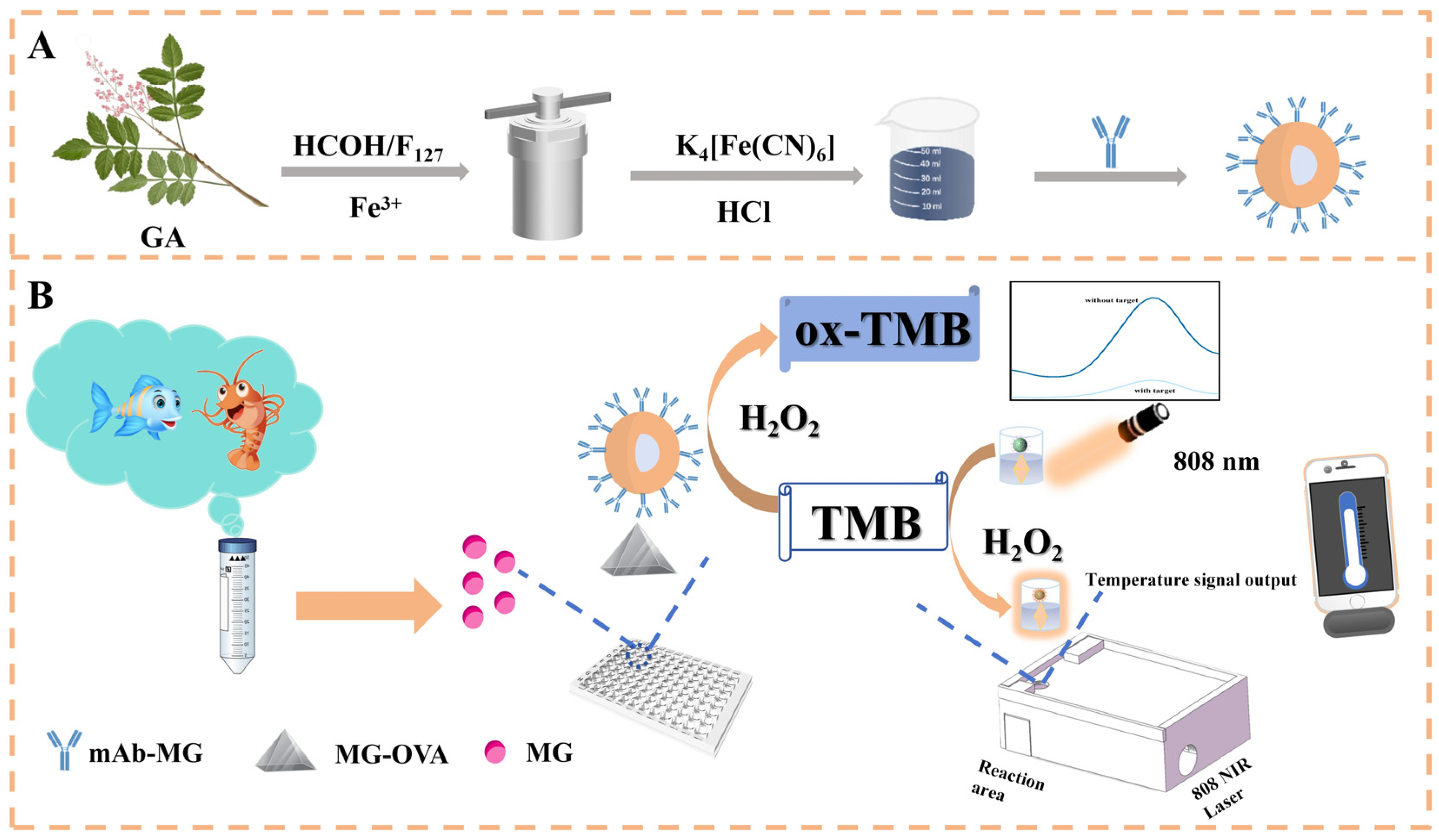
2.2. Preparation and Characterization of FGAN@PB NP Nanozyme
2.3. Catalytic Activity and Steady-State Kinetic Testing of FGAN@PB NP Nanozyme
2.4. Photothermal Properties of FGAN@PB NP Nanozyme
2.5. Preparation of FGAN@PB@Ab1 Probe
2.6. Procedure of the Colorimetric–Photothermal Immunosensor for Malachite Green Detection
2.7. Detection of Actual Samples
2.8. Validation by LC-MS/MS
3. Results and Analysis
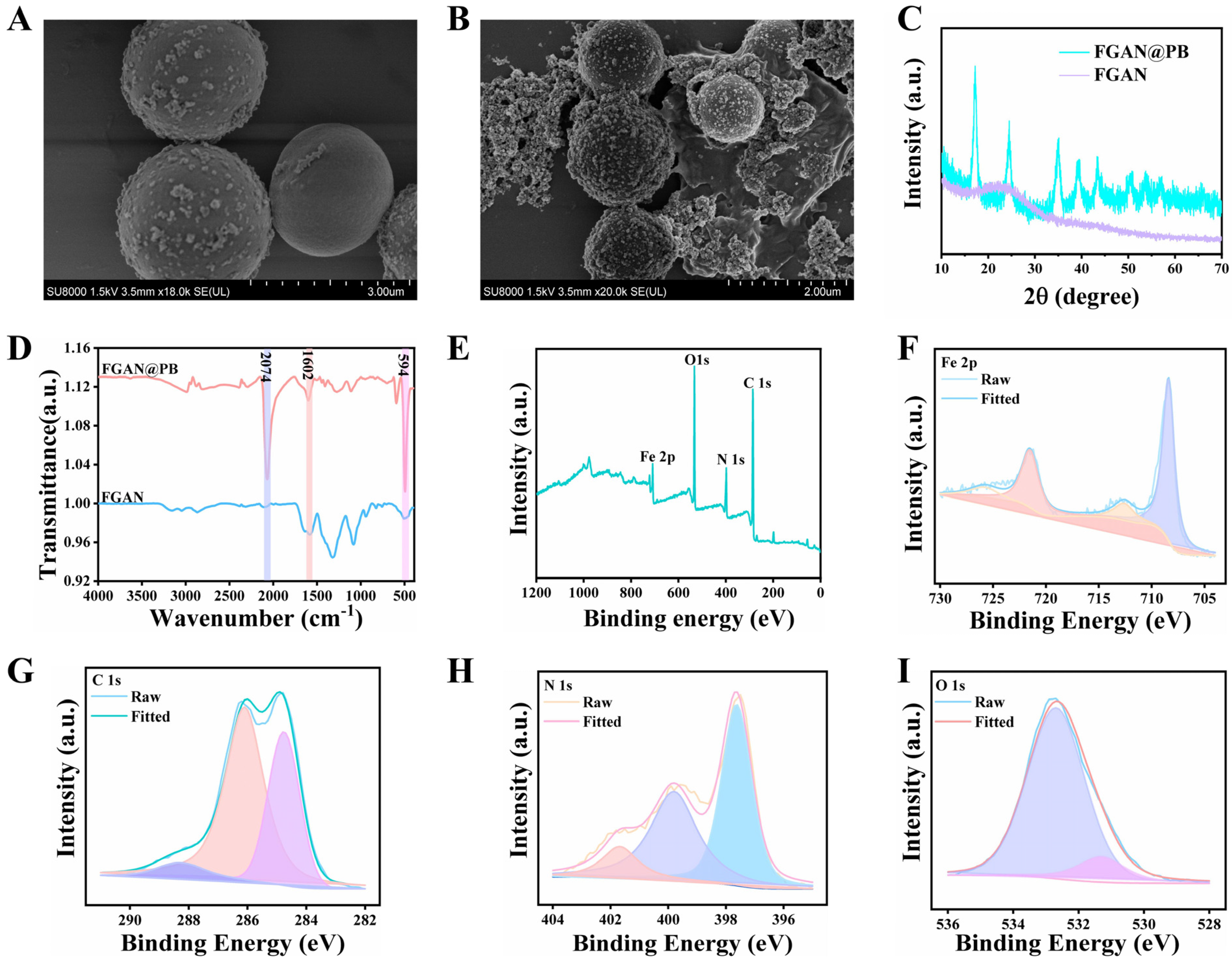
3.1. Synthesis and Characterization of FGAN@PB NPs
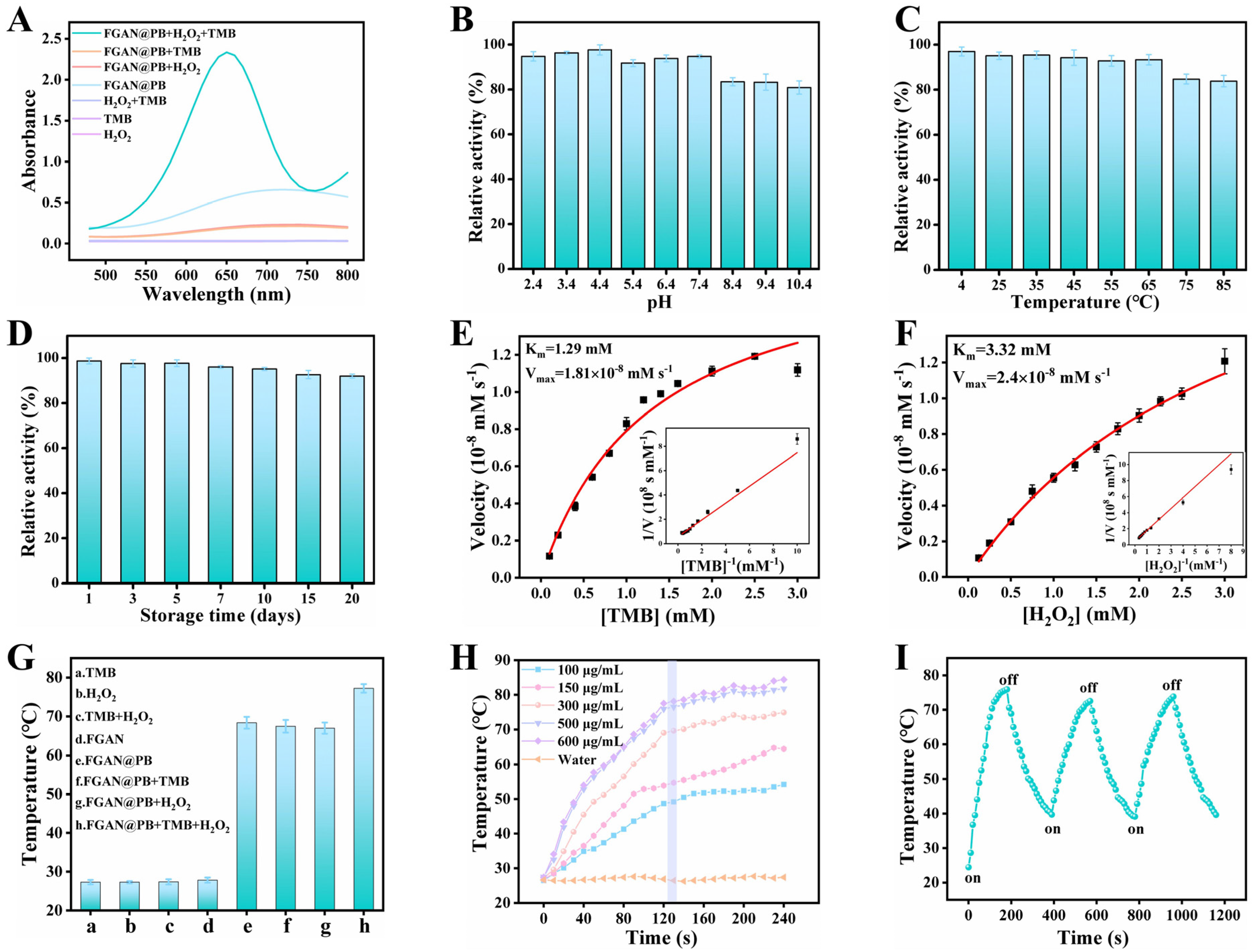
3.2. Enzyme-Mimicking Catalytic Activity and Steady-State Kinetic Analysis of FGAN@PB NPs
3.3. Investigation of the Photothermal Properties of FGAN@PB NPs
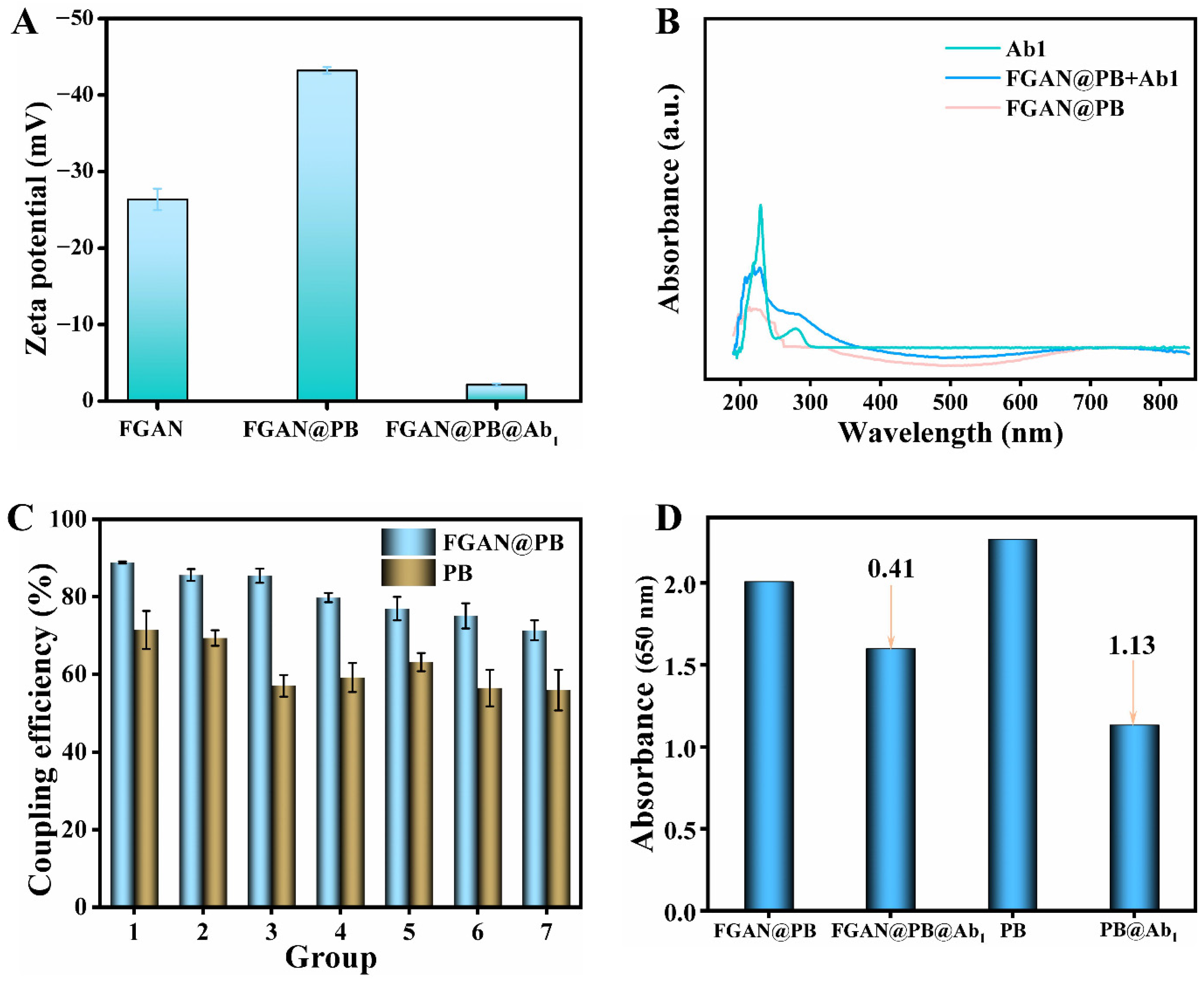
3.4. Evaluation of the Conjugation of FGAN@PB NPs with Antibodies
3.5. Optimization of Key Parameters for the Immunosensor
3.6. Performance of the Dual-Mode Immunosensor
3.7. Detection of MG in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abed Almonem, K.I.; El-Ashgar, N.M.; Gahal, A.A. Applications of potentiometric sensors for the determination of malachite green dye in real samples. Sens. Int. 2022, 3, 100186. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Chen, X.; Long, H.; Zhang, M.; Tang, Z.; He, Y.; Zhang, L.; Le, T. Enhanced Sensitivity and Accuracy of Tb3+-Functionalized Zirconium-Based Bimetallic MOF for Visual Detection of Malachite Green in Fish. Foods 2024, 13, 2855. [Google Scholar] [CrossRef]
- Wu, M.F.; Xu, N.; Li, S.; Huang, Y.L.; Wu, M.H.; Li, J.D.; Chen, R.S.; Xiong, W.M.; Li, Y.J.; Lei, H.T.; et al. A hapten design strategy to enhance the selectivity of monoclonal antibodies against malachite green. Front. Sustain. Food Syst. 2024, 8, 1490750. [Google Scholar] [CrossRef]
- Parekh, J.; Munjapara, A.; Pandya, D.; Bhimani, A.; Bishoyi, A.; Patra, S.; Jebaliya, H. Developing new benzilic acid-based hydrophobic deep eutectic solvents: Study towards dye extraction and antimicrobial efficiency. Int. J. Environ. Anal. Chem. 2025, 1–17. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Zhao, F.; Lin, Y.; Zheng, S.; Han, S. Selection and Application of ssDNA Aptamers for Fluorescence Biosensing Detection of Malachite Green. Foods 2022, 11, 801. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, D.; Wen, Y.; Wang, M.; Huang, J.; Lian, Z.; Li, J. Molecularly Imprinted SERS Plasmonic Sensor for the Detection of Malachite Green. Biosensors 2025, 15, 329. [Google Scholar] [CrossRef]
- Yang, L.; Tang, J.; Guo, S.; Park, E.; Chen, L.; Jung, Y.M. Highly efficient surface-enhanced Raman scatting performance of the Na2Ti3O7-Au chip for facile thiram or malachite green sensing. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137051. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Pan, Z.; Li, D. Review of Methods for the Detection and Determination of Malachite Green and Leuco-Malachite Green in Aquaculture. Crit. Rev. Anal. Chem. 2019, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, N.; Varenina, I.; Kolanović, B.S.; Oraić, D.; Zrnčić, S. Malachite green residues in farmed fish in Croatia. Food Control 2012, 26, 393–396. [Google Scholar] [CrossRef]
- Shen, Y.D.; Deng, X.F.; Xu, Z.L.; Wang, Y.; Lei, H.T.; Wang, H.; Yang, J.Y.; Xiao, Z.L.; Sun, Y.M. Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme-linked immunosorbent assay. Anal. Chim. Acta 2011, 707, 148–154. [Google Scholar] [CrossRef]
- Pan, Y.C.; Chen, Y.T.; Pang, H.H.; Prayadrat, C.; Huang, S.C.; Yang, H.W. RNA aptamer-packaged virus-like particles for label-free, rapid, and on-site fluorescence detection of malachite green in aquatic products. Biosens. Bioelectron. 2025, 288, 117796. [Google Scholar] [CrossRef]
- Jiao, Y.; Miao, X.W.; Liu, X.N.; Gao, Y.F.; Guo, J.M.; Liu, C.; Wang, L.Z.; Qian, T.W.; Wang, X.; Hong, S.S. Innovative engineering of red-emissive carbon dots for profoundly sensitive and recyclable determination of malachite green in fish tissue. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2026, 344, 126631. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Tang, Y.; Li, X.; Zhang, X.; Li, C.; Xu, S. Magnetic solid-phase extraction based on Fe3O4/graphene oxide nanoparticles for the determination of malachite green and crystal violet in environmental water samples by HPLC. Int. J. Environ. Anal. Chem. 2018, 98, 215–228. [Google Scholar] [CrossRef]
- Li, L.; Lin, Z.-Z.; Chen, X.-M.; Zhang, H.-Y.; Lin, Y.-D.; Lai, Z.-Z.; Huang, Z.-Y. Molecularly imprinted polymers for extraction of malachite green from fish samples prior to its determination by HPLC. Microchim. Acta 2015, 182, 1791–1796. [Google Scholar] [CrossRef]
- Nebot, C.; Iglesias, A.; Barreiro, R.; Miranda, J.M.; Vázquez, B.; Franco, C.M.; Cepeda, A. A simple and rapid method for the identification and quantification of malachite green and its metabolite in hake by HPLC–MS/MS. Food Control 2013, 31, 102–107. [Google Scholar] [CrossRef]
- Hussain Hakami, A.A.; Ahmed, M.A.; Khan, M.A.; AlOthman, Z.A.; Rafatullah, M.; Islam, M.A.; Siddiqui, M.R. Quantitative Analysis of Malachite Green in Environmental Samples Using Liquid Chromatography-Mass Spectrometry. Water 2021, 13, 2864. [Google Scholar] [CrossRef]
- Xie, F.; Liu, W.; Yan, W.; Han, Z.; Yan, D.; Lin, X. Rapid detection of malachite green and leucomalachite green in aquatic products based on nanopore electrodes. Microchem. J. 2025, 216, 114707. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Z.; Du, X.; Xie, J.; Ding, Z. Novel aptamer fluorescence assays for malachite green and leucomalachite green detection. Microchem. J. 2024, 205, 111391. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Shen, Y.-D.; Li, Y.-J.; Dong, J.-X.; Xu, Z.-L.; Yang, J.-Y.; Sun, Y.-M.; Xiao, Z.-L. Bispecific monoclonal antibody-based multianalyte ELISA for furaltadone metabolite, malachite green, and leucomalachite green in aquatic products. J. Agric. Food Chem. 2016, 64, 8054–8061. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.X.; Zhang, J.R.; Gu, L.; Tang, Y.Y.; Zhang, X.; Huang, X.Y.; Shen, X.S.; Zhai, W.L.; Fodjo, E.K.; Kong, C. Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish. Biosensors 2023, 13, 38. [Google Scholar] [CrossRef]
- Ziyang, H.; Shaoen, Z.; Qingzhou, C.; Hong, L.; Jianxin, S.; Kaiqiang, W.; Huiying, W.; Limin, C. Novel solid-phase extraction promotes simultaneous colloidal gold immunochromatographic assay of malachite green, leuco-malachite green, chloramphenicol, and semi-carbazone metabolites in aquatic products. Food Chem. 2025, 465, 142118. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0308814624037683?via%3Dihub (accessed on 12 October 2025). [CrossRef] [PubMed]
- Li, N.; Guo, Y.; Wu, M.; Wang, A.; Guo, Y.; Li, Y. High-performance electrochemical immunosensor for ultrasensitive detection of malachite green in food matrices using MOF-derived nanocomposites. Microchim. Acta 2025, 192, 310. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-X.; Jia, B.-Z.; Wang, H.; Sun, Y.-M.; Xu, X.-Y.; Wei, X.-Q.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L.; Luo, L. Prussian blue nanoparticles-enabled sensitive and accurate ratiometric fluorescence immunoassay for histamine. Food Chem. 2022, 376, 131907. [Google Scholar] [CrossRef]
- Tong, L.; Wu, L.; Zai, Y.; Zhang, Y.; Su, E.; Gu, N. based colorimetric glucose sensor using Prussian blue nanoparticles as mimic peroxidase. Biosens. Bioelectron. 2023, 219, 114787. [Google Scholar] [CrossRef]
- Arshad, F.; Arrigan, D.W.M.; Ahmed, M.U. Recent Developments in Nanozyme Based Sensors for Detection of Clinical Biomarkers—A Review. IEEE Sens. J. 2022, 22, 15622–15634. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Yin, X.; Wang, S.; Tian, Y.; Shu, R.; Jia, C.; Chen, Y.; Sun, J.; Zhang, D.; et al. Nature-inspired nanozymes as signal markers for in-situ signal amplification strategy: A portable dual-colorimetric immunochromatographic analysis based on smartphone. Biosens. Bioelectron. 2022, 210, 114289. [Google Scholar] [CrossRef]
- GB/T 19857-2005; Determination of Malachite Green and Crystal Violet Residues in Aquatic Product. China Standard Press: Beijing, China, 2005.
- Cao, L.Y.; Liu, Y.L.; Zhang, B.H.; Lu, L.H. In situ Controllable Growth of Prussian Blue Nanocubes on Reduced Graphene Oxide: Facile Synthesis and Their Application as Enhanced Nanoelectrocatalyst for H2O2 Reduction. ACS Appl. Mater. Interfaces 2010, 2, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Yang, M.T.; Zhang, Z.Y.; Wi-Afedzi, T.; Lin, Y.F. Prussian Blue analogue supported on sulfur-doped carbon nitride as an enhanced heterogeneous catalyst for activating peroxymonosulfate. J. Colloid Interface Sci. 2018, 529, 161–170. [Google Scholar] [CrossRef]
- Daud, H.; Ghani, A.; Iqbal, D.N.; Ahmad, N.; Nazir, S.; Muhammad, M.J.; Hussain, E.A.; Nazir, A.; Iqbal, M. Preparation and characterization of guar gum based biopolymeric hydrogels for controlled release of antihypertensive drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, H.; Liu, X.; Duan, S.; Shen, Y.; Zhang, Q.; Shu, Z.; Xiao, A.; Wang, J. An electrochemical immunosensor based on prussian blue@ zeolitic imidazolate framework-8 nanocomposites probe for the detection of deoxynivalenol in grain products. Food Chem. 2023, 405, 134842. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z. A carbon dots–based nanoprobe for intracellular Fe3+ detection. Mater. Today Chem. 2019, 13, 121–127. [Google Scholar] [CrossRef]
- Chen, T.; Yao, T.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhu, S.; Wang, Z. An injectable hydrogel for simultaneous photothermal therapy and photodynamic therapy with ultrahigh efficiency based on carbon dots and modified cellulose nanocrystals. Adv. Funct. Mater. 2021, 31, 2106079. [Google Scholar] [CrossRef]
- Liang, H.; Liu, Y.; Qileng, A.; Shen, H.; Liu, W.; Xu, Z.; Liu, Y. PEI-coated Prussian blue nanocubes as pH-Switchable nanozyme: Broad-pH-responsive immunoassay for illegal additive. Biosens. Bioelectron. 2023, 219, 114797. [Google Scholar] [CrossRef]
- Ding, L.; Shao, X.; Wang, M.; Zhang, H.; Lu, L. Dual-mode immunoassay for diethylstilbestrol based on peroxidase activity and photothermal effect of black phosphorus-gold nanoparticle nanohybrids. Anal. Chim. Acta 2021, 1187, 339171. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Liu, C.; Du, D.; Lin, Y.; Zhu, C. Fe–N–C Single-Atom Nanozymes for the Intracellular Hydrogen Peroxide Detection. Anal. Chem. 2019, 91, 11994–11999. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Liu, M.; Liu, F.; Bian, S.; Du, S.; Zhu, S.; Wang, H. Biominerized gold-Hemin@MOF composites with peroxidase-like and gold catalysis activities: A high-throughput colorimetric immunoassay for alpha-fetoprotein in blood by ELISA and gold-catalytic silver staining. Sens. Actuators B Chem. 2018, 266, 543–552. [Google Scholar] [CrossRef]

| Sample | Colorimetric Mode | Photothermal Mode | LC-MS/MS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spiked Level (ng/mL or g) | Measured (ng/mL or g) (X ±SD) a | Recovery (%) | CVs (%) | Measured (ng/mL or g) (X ± SD) a | Recovery (%) | CVs(%) | Measured (ng/mL or g) (X ± SD) a | Recovery (%) | |
| aquaculture water | 5 | 5.1 ± 0.3 | 102.1 | 4.9 | 4.7 ± 0.3 | 94.6 | 5.0 | 5.3 ± 0.1 | 105.7 |
| 10 | 9.4 ± 0.4 | 94.2 | 3.8 | 9.5 ± 0.7 | 95.1 | 7.5 | 10.4 ± 0.2 | 103.5 | |
| 15 | 14.1 ± 0.2 | 94.1 | 1.2 | 14.4 ± 1.0 | 95.8 | 6.7 | 16.0 ± 0.3 | 106.6 | |
| mandarin fish | 5 | 4.7 ± 0.2 | 93.2 | 3.3 | 4.7 ± 0.2 | 93.0 | 5.0 | 4.9 ± 0.2 | 98.4 |
| 10 | 10.3 ± 0.6 | 102.6 | 5.7 | 10.0 ± 0.8 | 99.5 | 7.6 | 9.5 ± 0.2 | 94.7 | |
| 15 | 14.7 ± 0.7 | 97.9 | 4.8 | 14.8 ± 0.7 | 98.4 | 4.5 | 14.7 ± 0.3 | 98.2 | |
| bass | 5 | 5.0 ± 0.5 | 100.6 | 10.4 | 4.5 ± 0.6 | 89.9 | 12.6 | 4.9 ± 0.2 | 98.0 |
| 10 | 9.1 ± 0.4 | 91.4 | 3.7 | 10.0 ± 0.6 | 99.9 | 5.9 | 9.5 ± 0.2 | 95.2 | |
| 15 | 14.7 ± 0.6 | 97.7 | 4.16 | 12.5 ± 0.6 | 83.3 | 4.5 | 15.1 ± 0.7 | 100.5 | |
| grass carp | 5 | 4.3 ± 0.2 | 86.4 | 5.1 | 4.1 ± 0.4 | 82.9 | 8.7 | 4.8 ± 0.1 | 95.5 |
| 10 | 9.3 ± 0.5 | 93.1 | 5.3 | 8.7 ± 0.9 | 87.1 | 10.4 | 9.5 ± 0.6 | 94.9 | |
| 15 | 14.0 ± 0.8 | 93.1 | 5.7 | 14.6 ± 1.0 | 97.4 | 6.7 | 13.7 ± 0.3 | 91.6 | |
| Penaeus vannamei | 5 | 4.3 ± 0.5 | 86.5 | 10.4 | 4.2 ± 0.5 | 84.5 | 12.5 | 4.7 ± 0.1 | 94.2 |
| 10 | 9.9 ± 1.2 | 98.6 | 12.5 | 9.8 ± 0.7 | 97.8 | 7.6 | 10.2 ± 0.2 | 101.8 | |
| 15 | 13.3 ± 0.9 | 88.3 | 7.1 | 14.2 ± 0.6 | 94.7 | 4.2 | 14.9 ± 0.3 | 99.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-F.; Li, J.-M.; Li, S.; Wu, M.-H.; Chen, R.-S.; Liu, Y.-C.; Liu, J.-N.; Xu, Z.-L.; Yang, Y.-C.; Li, J.-D.; et al. FGAN@PB NP Nanozyme-Based Colorimetric–Photothermal Dual-Mode Immunosensor for Malachite Green Detection. Biosensors 2025, 15, 719. https://doi.org/10.3390/bios15110719
Wu M-F, Li J-M, Li S, Wu M-H, Chen R-S, Liu Y-C, Liu J-N, Xu Z-L, Yang Y-C, Li J-D, et al. FGAN@PB NP Nanozyme-Based Colorimetric–Photothermal Dual-Mode Immunosensor for Malachite Green Detection. Biosensors. 2025; 15(11):719. https://doi.org/10.3390/bios15110719
Chicago/Turabian StyleWu, Min-Fu, Jing-Min Li, Sha Li, Min-Hua Wu, Ri-Sheng Chen, Yan-Can Liu, Jian-Nan Liu, Zhen-Lin Xu, Yi-Chao Yang, Jia-Dong Li, and et al. 2025. "FGAN@PB NP Nanozyme-Based Colorimetric–Photothermal Dual-Mode Immunosensor for Malachite Green Detection" Biosensors 15, no. 11: 719. https://doi.org/10.3390/bios15110719
APA StyleWu, M.-F., Li, J.-M., Li, S., Wu, M.-H., Chen, R.-S., Liu, Y.-C., Liu, J.-N., Xu, Z.-L., Yang, Y.-C., Li, J.-D., Lei, Q.-Y., Zhan, S.-M., & Luo, L. (2025). FGAN@PB NP Nanozyme-Based Colorimetric–Photothermal Dual-Mode Immunosensor for Malachite Green Detection. Biosensors, 15(11), 719. https://doi.org/10.3390/bios15110719





