Nucleic Acid Nanomaterial-Mediated Single-Cell Encapsulation and Its Application
Abstract
1. Introduction
2. Development of Cell Encapsulation Materials
3. Design and Construction of DNA-Mediated Cell Encapsulation
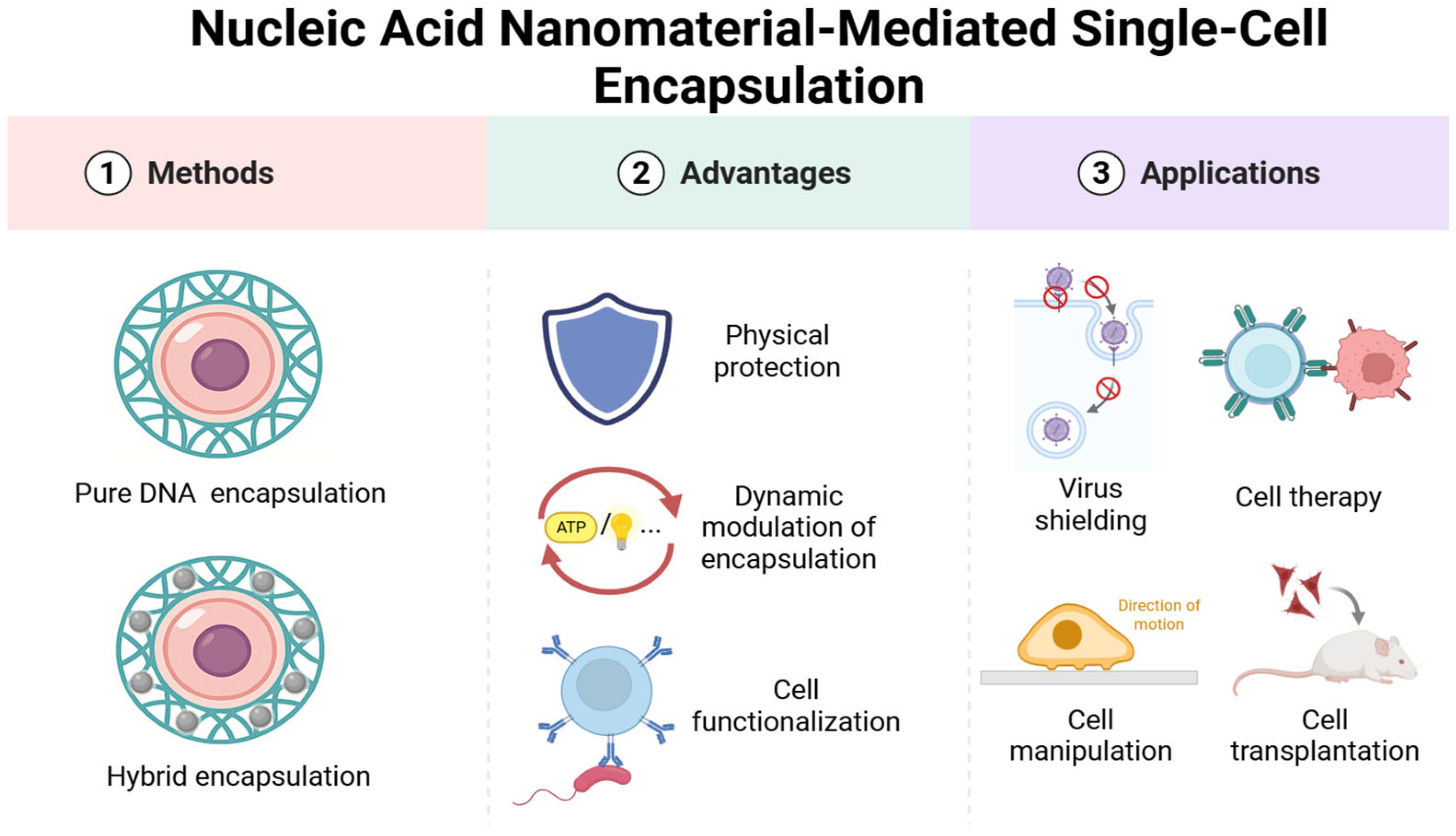
3.1. Nucleic Acid-Mediated Single-Cell Encapsulation Classification
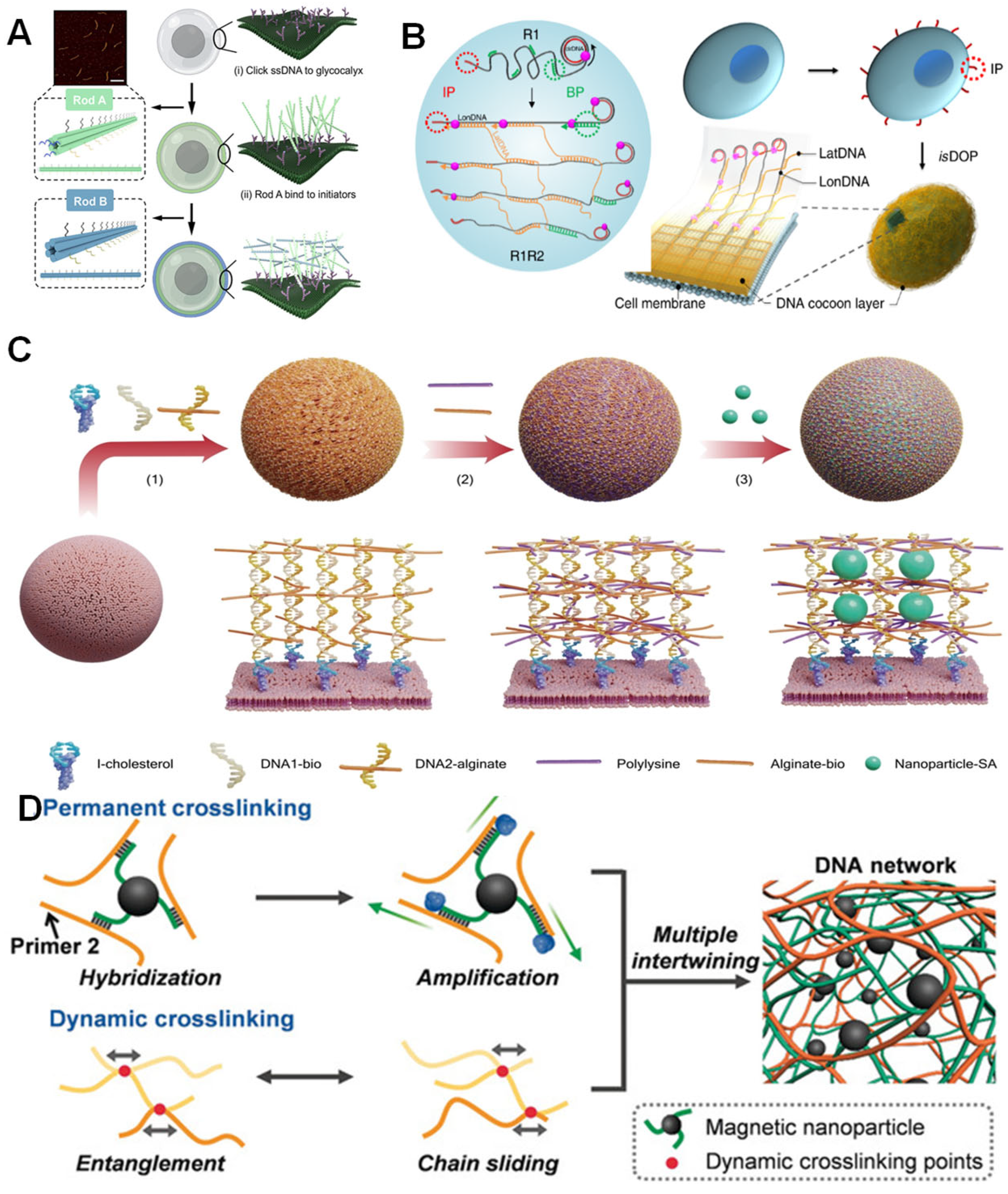
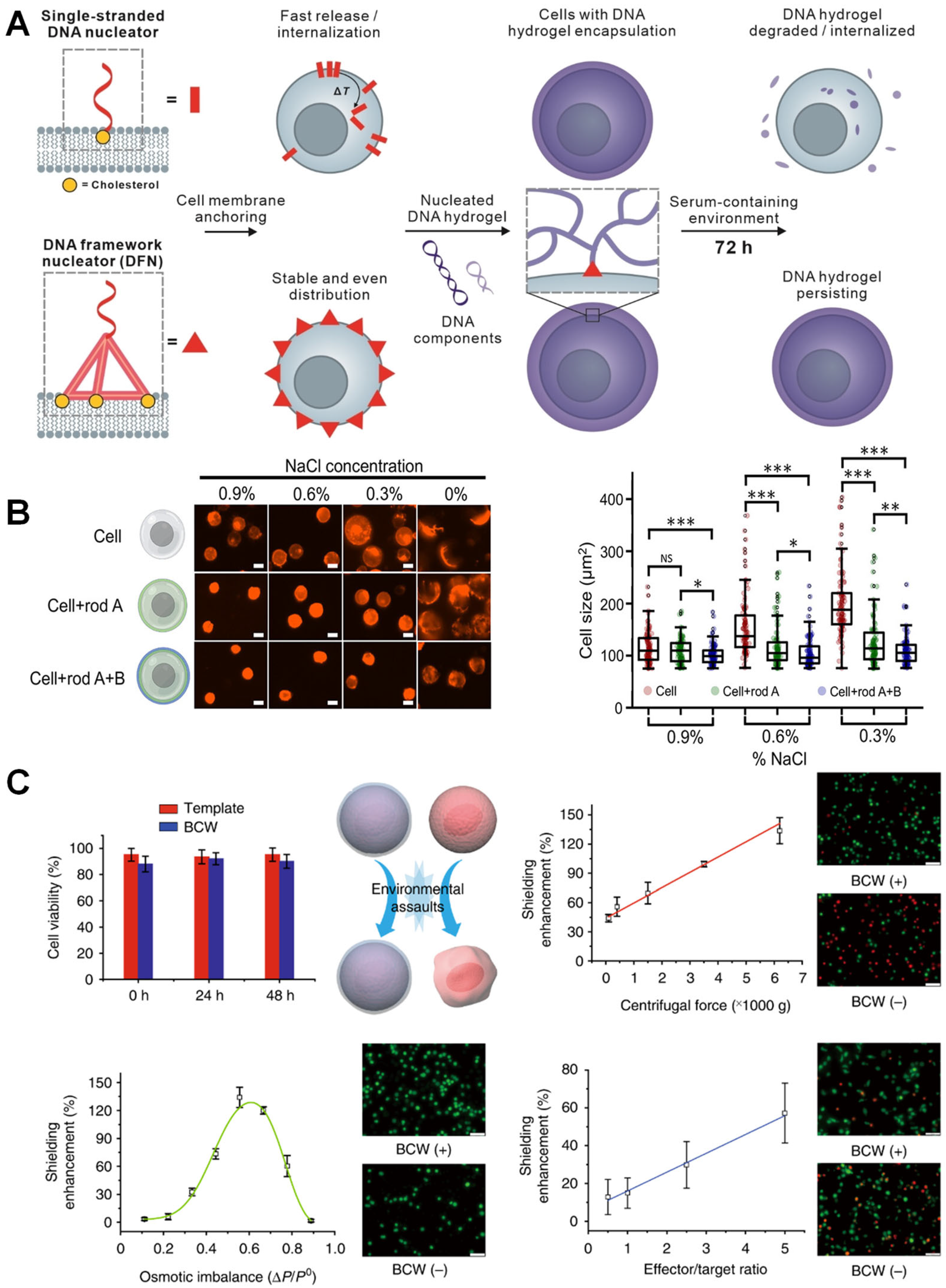
3.2. Pure DNA Material-Mediated Encapsulation Strategy
3.3. DNA-Based Composite Co-Encapsulation System with Other Substances
4. Advantages of DNA-Mediated Cell Encapsulation
4.1. Basic Physical Protection
4.2. Dynamic Response of Single-Cell Encapsulation
4.3. Functionalization of Single-Cell Encapsulation
5. Biomedical Applications of DNA-Mediated Single-Cell Encapsulation
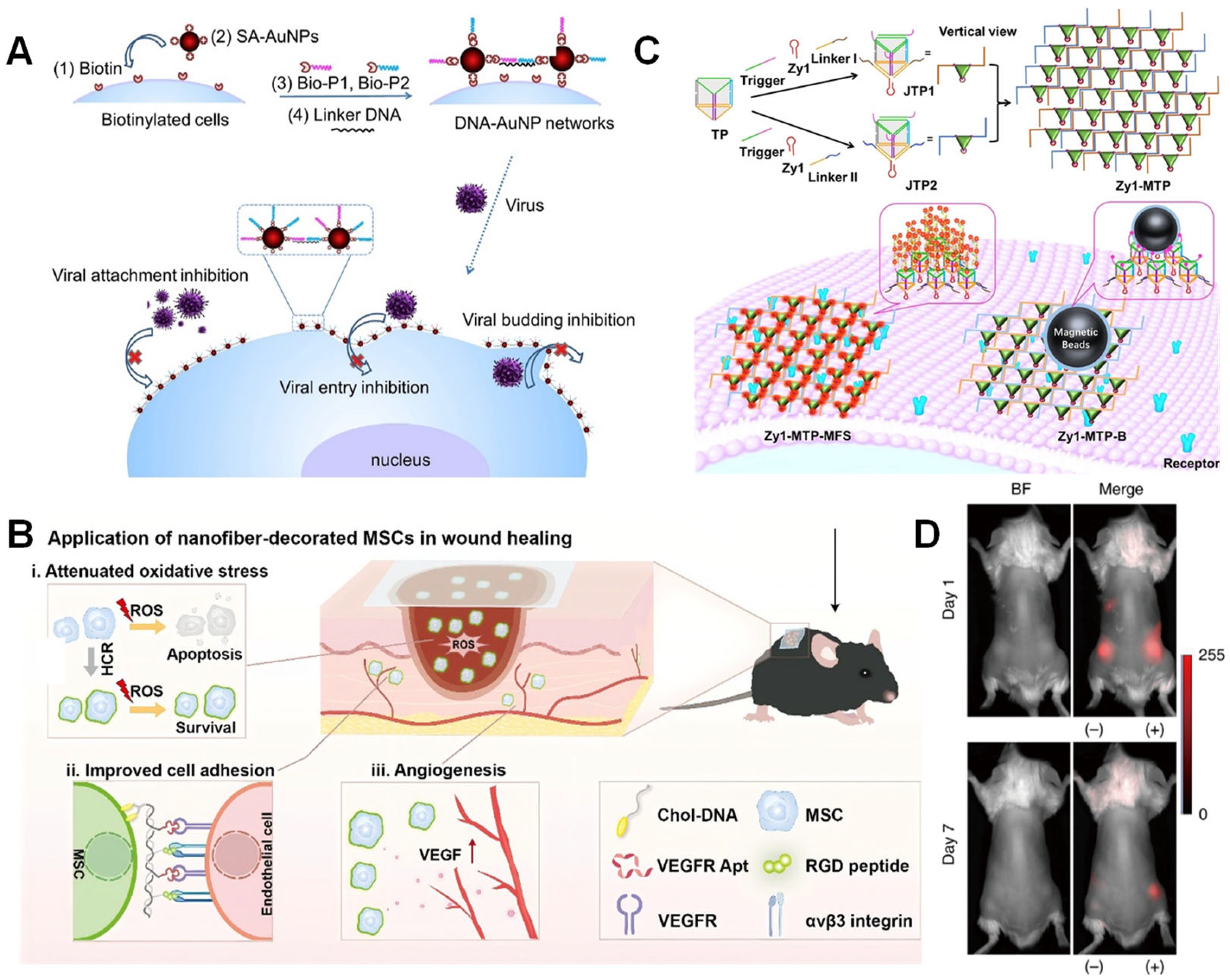
5.1. Antiviral Physical Barrier Technology
5.2. Cell Therapy
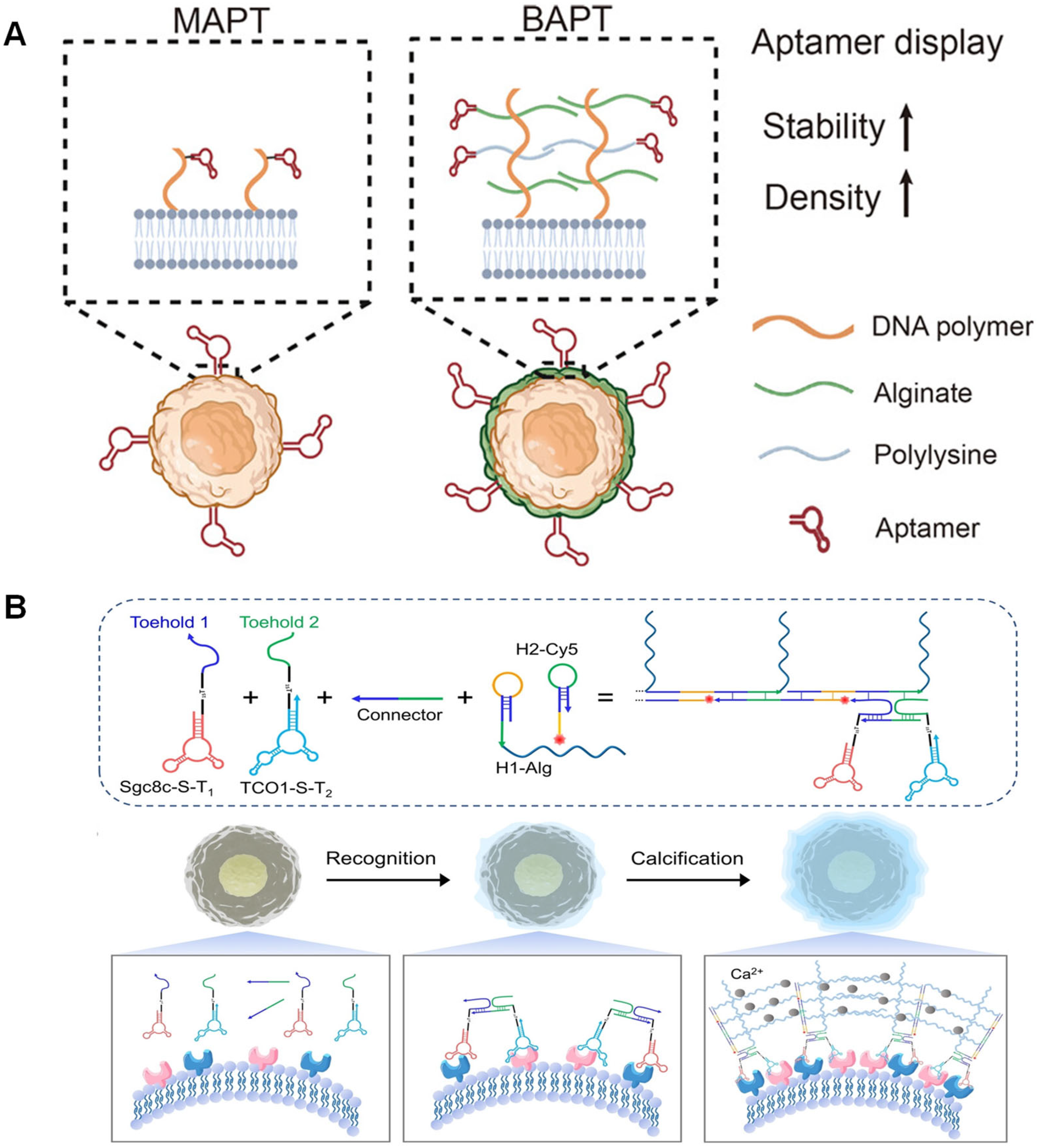
5.3. Precise Encapsulation and Capture of Tumor Cells
5.4. Cell Transplantation
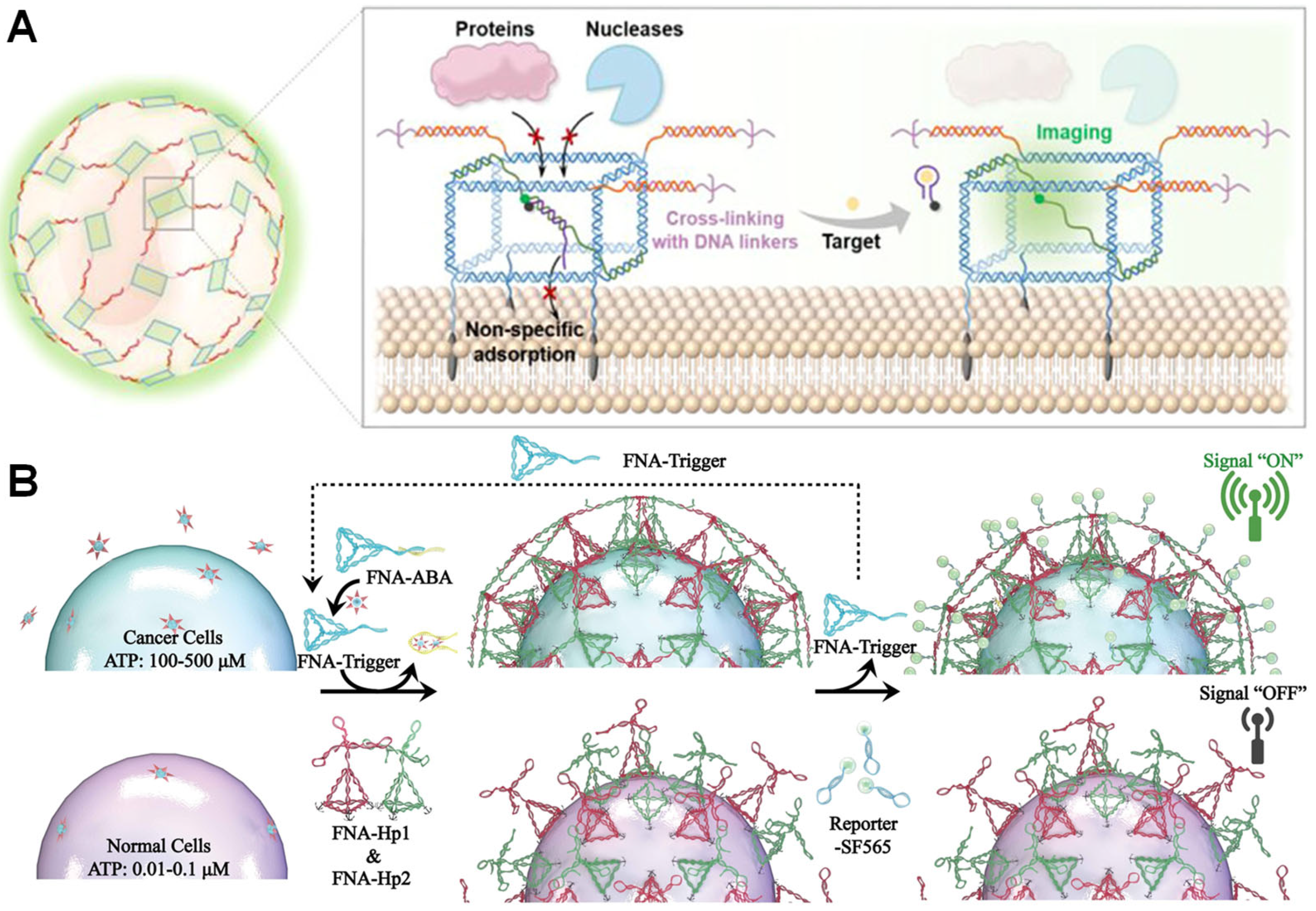
5.5. Applications of Biosensing
5.5.1. Stable Membrane Interface Encapsulation for In Situ Monitoring
5.5.2. Signal-Triggered Smart Encapsulation and Sensing Regulation
6. Challenges and Future
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIN6 | Mouse insulinoma cell lines 6 |
| PLL | Poly-L-lysine |
| TNFα | Tumor necrosis factor α |
| MSCs | Mesenchymal stem cells |
| ZIF-8 | Zeolite imidazolate framework-8 |
| HCR | Hybridization chain reaction |
| BCW | Biocompatible wrapping |
| IP | Initial primer |
| DFN | DNA framework nucleators |
| TP | DNA trihedra |
| BP | Branched polymers |
| NPs | Nanoparticles |
| MNPs | Magnetic nanoparticles |
| ssDNA | Single-stranded DNA |
| CTCs | Circulating tumor cells |
| NK | Natural killer |
| RFP | Red fluorescent protein |
| H | Hairpin structures |
| LonDNA | Longitudinal DNA |
| LatDNA | Latitude DNA |
| LbL | Layer-by-layer |
| ROS | Reactive oxygen species |
| RGD | Arg-Gly-Asp |
| CN2 | Carry nanoparticles |
| ES | Recognition sequences |
References
- Camp, J.G.; Wollny, D.; Treutlein, B. Single-cell genomics to guide human stem cell and tissue engineering. Nat. Methods 2018, 15, 661–667. [Google Scholar] [CrossRef]
- Lindström, S.; Andersson-Svahn, H. Overview of single-cell analyses: Microdevices and applications. Lab Chip 2010, 10, 3363–3372. [Google Scholar] [CrossRef]
- Stuart, T.; Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 2019, 20, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Tanay, A.; Regev, A. Scaling single-cell genomics from phenomenology to mechanism. Nature 2017, 541, 331–338. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.-H.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Wen, L.; Li, G.; Huang, T.; Geng, W.; Pei, H.; Yang, J.; Zhu, M.; Zhang, P.; Hou, R.; Tian, G.; et al. Single-cell technologies: From research to application. Innovation 2022, 3, 100342. [Google Scholar] [CrossRef]
- Hasturk, O.; Kaplan, D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2019, 95, 3–31. [Google Scholar] [CrossRef]
- Lu, Z.-C.; Zhang, R.; Liu, H.-Z.; Zhou, J.-X.; Su, H.-F. Nanoarmor: Cytoprotection for single living cells. Trends Biotechnol. 2024, 42, 91–103. [Google Scholar] [CrossRef]
- Freimark, D.; Pino-Grace, P.; Pohl, S.; Weber, C.; Wallrapp, C.; Geigle, P.; Portner, R.; Czermak, P. Use of Encapsulated Stem Cells to Overcome the Bottleneck of Cell Availability for Cell Therapy Approaches. Transfus. Med. Hemother. 2010, 37, 66–73. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Krishnan, R.; Alexander, M.; Robles, L.; Foster, C.E., 3rd; Lakey, J.R. Islet and stem cell encapsulation for clinical transplantation. Rev. Diabet. Stud. 2014, 11, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, e2201247. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 8, e1800939. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Swaminathan, V.; Malkovskiy, A.; Santhanam, S.; McConnell, K.; George, P.M. Single-Cell Encapsulation via Click-Chemistry Alters Production of Paracrine Factors from Neural Progenitor Cells. Adv. Sci. 2020, 7, 1902573. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Hatami, J.; Mano, J.F. Coating Strategies Using Layer-by-layer Deposition for Cell Encapsulation. Chem. Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Tilokee, E.L.; Latham, N.; McNeill, B.; Lam, B.-K.; Ruel, M.; Suuronen, E.J.; Courtman, D.W.; Stewart, D.J.; Davis, D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 2014, 35, 133–142. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Son, Y.M.; Lee, N.E. Hydrogel Encapsulation of Cells in Core–Shell Microcapsules for Cell Delivery. Adv. Healthc. Mater. 2015, 4, 1537–1544. [Google Scholar] [CrossRef]
- Rogan, H.; Ilagan, F.; Yang, F. Comparing Single Cell Versus Pellet Encapsulation of Mesenchymal Stem Cells in Three-Dimensional Hydrogels for Cartilage Regeneration. Tissue Eng. Part A 2019, 25, 1404–1412. [Google Scholar] [CrossRef]
- Si, H.; Chen, Y.; Jiang, K.; Ma, K.; Ramsey, E.; Oakey, J.; Sun, M.; Jiang, Z. Deterministic Single-Cell Encapsulation in PEG Norbornene Microgels for Promoting Anti-Inflammatory Response and Therapeutic Delivery of Mesenchymal Stromal Cells. Adv. Healthc. Mater. 2024, 13, e2304386. [Google Scholar] [CrossRef]
- Yoshino, T.; Tanaka, T.; Nakamura, S.; Negishi, R.; Hosokawa, M.; Matsunaga, T. Manipulation of a Single Circulating Tumor Cell Using Visualization of Hydrogel Encapsulation toward Single-Cell Whole-Genome Amplification. Anal. Chem. 2016, 88, 7230–7237. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Lv, K.; Xin, J.; Wang, Y.; Zhao, J.; Hu, W.; Xiao, C.; Zhu, K.; Zhu, L.; et al. Surface-Anchored Nanogel Coating Endows Stem Cells with Stress Resistance and Reparative Potency via Turning Down the Cytokine-Receptor Binding Pathways. Adv. Sci. 2021, 8, 2003348. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, N.; Rheem, H.B.; Kim, B.J.; Park, J.H.; Choi, I.S. A Decade of Advances in Single-Cell Nanocoating for Mammalian Cells. Adv. Healthc. Mater. 2021, 10, 2100347. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, K.; Luo, S.; Li, F.; Zuo, X.; Fan, C.; Li, Q. Programmable DNA Hydrogels as Artificial Extracellular Matrix. Small 2022, 18, 2107640. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, K.; Liao, R.; Suja, V.C.; Kapate, N.; Lu, A.; Gao, Y.; Mitragotri, S. Materials for Cell Surface Engineering. Adv. Mater. 2023, 36, 2210059. [Google Scholar] [CrossRef]
- He, H.; Yuan, Y.; Wu, Y.; Lu, J.; Yang, X.; Lu, K.; Liu, A.; Cao, Z.; Sun, M.; Yu, M.; et al. Exoskeleton Partial-Coated Stem Cells for Infarcted Myocardium Restoring. Adv. Mater. 2023, 35, e2307169. [Google Scholar] [CrossRef]
- He, X.; Gong, G.; Chen, M.; Zhang, H.; Zhang, Y.; Richardson, J.J.; Chan, W.Y.; He, Y.; Guo, J. Metal-Phenolic Nanocloaks on Cancer Cells Potentiate STING Pathway Activation for Synergistic Cancer Immunotherapy. Angew. Chem. Int. Ed. 2024, 63, e202314501. [Google Scholar] [CrossRef]
- Youn, W.; Kim, J.Y.; Park, J.; Kim, N.; Choi, H.; Cho, H.; Choi, I.S. Single-Cell Nanoencapsulation: From Passive to Active Shells. Adv. Mater. 2020, 32, 1907001. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, B.S.N.; Cheng, Y.; Zhao, Y. Artificial Polymerizations in Living Organisms for Biomedical Applications. Angew. Chem. Int. Ed. 2024, 63, e202410579. [Google Scholar]
- Hui Chong, L.S.; Zhang, J.; Bhat, K.S.; Yong, D.; Song, J. Bioinspired cell-in-shell systems in biomedical engineering and beyond: Comparative overview and prospects. Biomaterials 2021, 266, 120473. [Google Scholar] [CrossRef]
- Jia, S.; Lv, H.; Li, Q.; Fan, C.; Wang, F. DNA-based biocomputing circuits and their biomedical applications. Nat. Rev. Bioeng. 2025, 3, 535–548. [Google Scholar] [CrossRef]
- Madsen, M.; Gothelf, K.V. Chemistries for DNA Nanotechnology. Chem. Rev. 2019, 119, 6384–6458. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. DNA in a material world. Nature 2003, 421, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Neild, A.; deMello, A.; Liu, A.Q.; Ai, Y. The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef]
- Jiang, H.; Pan, V.; Vivek, S.; Weeks, E.R.; Ke, Y. Programmable DNA Hydrogels Assembled from Multidomain DNA Strands. Chembiochem 2016, 17, 1156–1162. [Google Scholar] [CrossRef]
- Qi, H.; Ghodousi, M.; Du, Y.; Grun, C.; Bae, H.; Yin, P.; Khademhosseini, A. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 2013, 4, 2275. [Google Scholar] [CrossRef]
- Spinks, G.M.; Martino, N.D.; Naficy, S.; Shepherd, D.J.; Foroughi, J. Dual high-stroke and high-work capacity artificial muscles inspired by DNA supercoiling. Sci. Robot. 2021, 6, eabf4788. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, C.; Lv, C.; Sun, Y.; Zhang, M.; Zhao, Y.; Yang, L.; Han, D.; Tan, W. Construction of a Multiple-Aptamer-Based DNA Logic Device on Live Cell Membranes via Associative Toehold Activation for Accurate Cancer Cell Identification. J. Am. Chem. Soc. 2019, 141, 12738–12743. [Google Scholar] [CrossRef]
- Ting, L.R.L.; García-Muelas, R.; Martín, A.J.; Veenstra, F.L.P.; Chen, S.T.J.; Peng, Y.; Per, E.Y.X.; Pablo-García, S.; López, N.; Pérez-Ramírez, J.; et al. Electrochemical Reduction of Carbon Dioxide to 1-Butanol on Oxide-Derived Copper. Angew. Chem. Int. Ed. 2020, 59, 21072–21079. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; Zhao, Z.; Zhang, X.; Tan, W. Engineering a Second-Order DNA Logic-Gated Nanorobot to Sense and Release on Live Cell Membranes for Multiplexed Diagnosis and Synergistic Therapy. Angew. Chem. Int. Ed. 2021, 60, 15816–15820. [Google Scholar]
- Yin, Y.; Xie, W.; Xiong, M.; Gao, Y.; Liu, Q.; Han, D.; Ke, G.; Zhang, X.B. FINDER: A Fluidly Confined CRISPR-Based DNA Reporter on Living Cell Membranes for Rapid and Sensitive Cancer Cell Identification. Angew. Chem. Int. Ed. 2023, 62, e202309837. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Balaguer, P.; Nicolas, J.-C.; Lopez, F.; Esteve, J.-P.; Sukhorukov, G.B.; Winterhalter, M.; Richard-Foy, H.; Fournier, D. Protection of mammalian cell used in biosensors by coating with a polyelectrolyte shell. Biosens. Bioelectron. 2006, 21, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, G.; Guan, T.; Zhang, X.; Khosrozadeh, A.; Xing, M.; Kong, J. Manipulable Permeability of Nanogel Encapsulation on Cells Exerts Protective Effect against TNF-α-Induced Apoptosis. ACS Biomater. Sci. Eng. 2018, 4, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, Y.; Wang, W.; Hao, H.; Tang, M.; Zhang, Z.; Yang, J.; Zheng, Y.; Shi, X. Transglutaminase-Catalyzed Encapsulation of Individual Mammalian Cells with Biocompatible and Cytoprotective Gelatin Nanoshells. ACS Biomater. Sci. Eng. 2020, 6, 2336–2345. [Google Scholar] [CrossRef]
- Pires-Santos, M.; Carreira, M.; Morais, B.P.; Perfeito, F.G.; Oliveira, M.B.; Monteiro, C.F.; Nadine, S.; Mano, J.F. Single-Cell Liquid-Core Microcapsules for Biomedical Applications. Adv. Healthc. Mater. 2025, 24, e2403808. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Goli, P.L.; Stewart-Clark, S.S.; Lvov, Y.M.; Mills, D.K. Nanoencapsulation of Stem Cells within Polyelectrolyte Multilayer Shells. Macromol. Biosci. 2007, 7, 877–882. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.-S.; Kim, J.-W.; Lee, E.-Y.; Rhee, M.; You, Y.-H.; Khang, G.; Park, C.-G.; Yoon, K.-H. Suppression of Fibrotic Reactions of Chitosan-Alginate Microcapsules Containing Porcine Islets by Dexamethasone Surface Coating. Endocrinol. Metab. 2021, 36, 146–156. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Swelling and smoothing of polyelectrolyte multilayers by salt. Langmuir 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Kamperman, T.; Karperien, M.; Le Gac, S.; Leijten, J. Single-Cell Microgels: Technology, Challenges, and Applications. Trends Biotechnol. 2018, 36, 850–865. [Google Scholar] [CrossRef]
- Guo, W.Y.; Wang, W.H.; Xu, P.Y.; Kankala, R.K.; Chen, A.Z. Decellularised extracellular matrix-based injectable hydrogels for tissue engineering applications. Biomater. Transl. 2024, 5, 114–128. [Google Scholar] [PubMed]
- Xue, C.; Chen, L.; Wang, N.; Chen, H.; Xu, W.; Xi, Z.; Sun, Q.; Kang, R.; Xie, L.; Liu, X. Stimuli-responsive hydrogels for bone tissue engineering. Biomater. Transl. 2024, 5, 257–273. [Google Scholar] [PubMed]
- Yang, H.; Zheng, M.; Zhang, Y.; Li, C.; Lai, J.H.C.; Zhang, Q.; Chan, K.W.; Wang, H.; Zhao, X.; Yang, Z.; et al. Enhanced angiogenesis in porous poly(epsilon-caprolactone) scaffolds fortified with methacrylated hyaluronic acid hydrogel after subcutaneous transplantation. Biomater. Transl. 2024, 5, 59–68. [Google Scholar] [PubMed]
- Mao, A.S.; Shin, J.-W.; Utech, S.; Wang, H.; Uzun, O.; Li, W.; Cooper, M.; Hu, Y.; Zhang, L.; Weitz, D.A.; et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat. Mater. 2016, 16, 236–243. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, J.; Amini, S.; Ju, Y.; Agola, J.O.; Zimpel, A.; Shang, J.; Noureddine, A.; Caruso, F.; Wuttke, S.; et al. SupraCells: Living Mammalian Cells Protected within Functional Modular Nanoparticle-Based Exoskeletons. Adv. Mater. 2019, 31, e1900545. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Kong, F.; Cao, J.; Wang, L.; Zhu, W.; Brinker, C.J. Bioinspired Cell Silicification: From Extracellular to Intracellular. J. Am. Chem. Soc. 2021, 143, 6305–6322. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, D.; Lee, J.; Choi, I.S. Cell-in-Shell Hybrids: Chemical Nanoencapsulation of Individual Cells. Acc. Chem. Res. 2016, 49, 792–800. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yang, X.-Y.; Zhang, B.-B.; Ninane, N.; Busscher, H.J.; Hu, Z.-Y.; Delneuville, C.; Jiang, N.; Xie, H.; et al. Single-cell yolk-shell nanoencapsulation for long-term viability with size-dependent permeability and molecular recognition. Natl. Sci. Rev. 2021, 8, nwaa097. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, R.; Wang, J.; Yao, C.; Yang, D. Construction of Polymeric DNA Network and Application for Cell Manipulation. Chin. J. Chem. 2023, 41, 1875–1887. [Google Scholar] [CrossRef]
- Li, L.; Yin, J.; Ma, W.; Tang, L.; Zou, J.; Yang, L.; Du, T.; Zhao, Y.; Wang, L.; Yang, Z.; et al. A DNA origami device spatially controls CD95 signalling to induce immune tolerance in rheumatoid arthritis. Nat. Mater. 2024, 23, 993–1001. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Y.; Xie, X.; Li, Q.; Liu, X.; Wang, L.; Li, J.; Wu, J.; Fan, C. DNA-Programmed Stem Cell Niches via Orthogonal Extracellular Vesicle-Cell Communications. Adv. Mater. 2023, 35, e2302323. [Google Scholar] [CrossRef]
- Wu, S.; Shang, Y.; Yan, Y.; Zhou, A.; Bing, T.; Zhao, Z.; Tan, W. Aptamer-Based Enforced Phosphatase-Recruiting Chimeras Inhibit Receptor Tyrosine Kinase Signal Transduction. J. Am. Chem. Soc. 2024, 146, 22445–22454. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Shen, X.; Tang, W.; Yang, D. Emerging Trends in DNA Nanotechnology-Enabled Cell Surface Engineering. JACS Au 2025, 5, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Wickham, S.F.; Shih, W.M.; Perrault, S.D. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano 2014, 8, 8765–8775. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hayes, P.R.; Ren, X.; Taylor, R.E. Synthetic Cell Armor Made of DNA Origami. Nano Lett. 2023, 23, 7076–7085. [Google Scholar] [CrossRef]
- Gao, T.; Chen, T.; Feng, C.; He, X.; Mu, C.; Anzai, J.-i.; Li, G. Design and fabrication of flexible DNA polymer cocoons to encapsulate live cells. Nat. Commun. 2019, 10, 2946. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, Y.; Wang, K.; Wei, Y.; Li, Q.; Zuo, X.; Li, B.; Li, J.; Wang, L.; Fan, C.; et al. Directing the Encapsulation of Single Cells with DNA Framework Nucleator-Based Hydrogel Growth. Angew. Chem. Int. Ed. 2024, 63, e202319907. [Google Scholar]
- Guo, Z.; Zhang, L.; Yang, Q.; Peng, R.; Yuan, X.; Xu, L.; Wang, Z.; Chen, F.; Huang, H.; Liu, Q.; et al. Manipulation of Multiple Cell–Cell Interactions by Tunable DNA Scaffold Networks. Angew. Chem. Int. Ed. 2021, 61, e202111151. [Google Scholar]
- Lee, K.; Davis, B.; Wang, X.; Mirg, S.; Wen, C.; Abune, L.; Peterson, B.E.; Han, L.; Chen, H.; Wang, H.; et al. Nanoparticle-Decorated Biomimetic Extracellular Matrix for Cell Nanoencapsulation and Regulation. Angew. Chem. Int. Ed. 2023, 62, e202306583. [Google Scholar] [CrossRef]
- Tang, J.; Yao, C.; Gu, Z.; Jung, S.; Luo, D.; Yang, D. Super-Soft and Super-Elastic DNA Robot with Magnetically Driven Navigational Locomotion for Cell Delivery in Confined Space. Angew. Chem. Int. Ed. 2020, 59, 2490–2495. [Google Scholar]
- Shi, P.; Zhao, N.; Coyne, J.; Wang, Y. DNA-templated synthesis of biomimetic cell wall for nanoencapsulation and protection of mammalian cells. Nat. Commun. 2019, 10, 2223. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Nam, K.; Kim, Y.M.; Yang, K.; Roh, Y.H. DNA-Assisted Smart Nanocarriers: Progress, Challenges, and Opportunities. ACS Nano 2021, 15, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ye, D.; Zuo, X.; Li, J.; Wang, J.; Liu, H.; Hwang, M.T.; Chao, J.; Su, S.; Wang, L.; et al. DNA Hydrogel with Aptamer-Toehold-Based Recognition, Cloaking, and Decloaking of Circulating Tumor Cells for Live Cell Analysis. Nano Lett. 2017, 17, 5193–5198. [Google Scholar] [CrossRef]
- You, M.; Peng, L.; Shao, N.; Zhang, L.; Qiu, L.; Cui, C.; Tan, W. DNA "nano-claw": Logic-based autonomous cancer targeting and therapy. J. Am. Chem. Soc. 2014, 136, 1256–1259. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Wu, Q.; Yan, Q.; Sun, J.; Liang, F.; Liu, Y.; Wang, H. Organization of Protein Tyrosine Kinase-7 on Cell Membranes Characterized by Aptamer Probe-Based STORM Imaging. Anal. Chem. 2021, 93, 936–945. [Google Scholar] [CrossRef]
- He, L.; Chen, F.; Zhang, D.; Xie, S.; Xu, S.; Wang, Z.; Zhang, L.; Cui, C.; Liu, Y.; Tan, W. Transducing Complex Biomolecular Interactions by Temperature-Output Artificial DNA Signaling Networks. J. Am. Chem. Soc. 2020, 142, 14234–14239. [Google Scholar] [CrossRef]
- Sun, S.; Yang, S.; Hu, X.; Zheng, C.; Song, H.; Wang, L.; Shen, Z.; Wu, Z.S. Combination of Immunomagnetic Separation with Aptamer-Mediated Double Rolling Circle Amplification for Highly Sensitive Circulating Tumor Cell Detection. ACS Sens. 2020, 5, 3870–3878. [Google Scholar] [CrossRef]
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional Aptamer-Embedded Nanomaterials for Diagnostics and Therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560. [Google Scholar] [CrossRef]
- Wang, X.; Jia, B.; Lee, K.; Davis, B.; Wen, C.; Wang, Y.; Zheng, H.; Wang, Y. Biomimetic Bacterial Capsule for Enhanced Aptamer Display and Cell Recognition. J. Am. Chem. Soc. 2023, 146, 868–877. [Google Scholar] [CrossRef]
- Yu, X.; Lin, W.; Lai, Q.; Song, J.; Liu, Y.; Su, R.; Niu, Q.; Yang, L.; Yang, C.; Zhang, H.; et al. ARMOR: Auto-Assembled Resilient Biomimetic Calcified Ornaments for Selective Cell Protection by Dual-Aptamer-Driven Hybridization Chain Reaction. Angew. Chem. Int. Ed. 2023, 62, e202301083. [Google Scholar] [CrossRef]
- Yang, S.; Youn, W.; Rheem, H.B.; Han, S.Y.; Kim, N.; Han, S.; Schattling, P.; Städler, B.; Choi, I.S. Construction of Liposome-Based Extracellular Artificial Organelles on Individual Living Cells. Angew. Chem. Int. Ed. 2024, 64, e202415823. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Yang, X.; Wan, X.; Wu, W.; Zhen, S.; Li, Y.; Luo, L.; Huang, C. Characteristics of DNA-AuNP networks on cell membranes and real-time movies for viral infection. Data Brief 2016, 6, 652–660. [Google Scholar] [CrossRef]
- Li, C.M.; Zheng, L.L.; Yang, X.X.; Wan, X.Y.; Wu, W.B.; Zhen, S.J.; Li, Y.F.; Luo, L.F.; Huang, C.Z. DNA-AuNP networks on cell membranes as a protective barrier to inhibit viral attachment, entry and budding. Biomaterials 2016, 77, 216–226. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, R.; Li, Z.; Ma, S.; Wu, Y.; Liu, F.; Han, Q.; Li, J.; Zhao, R.C.; Jiang, Q.; et al. Mesenchymal Stem Cells Engineered by Multicomponent Coassembled DNA Nanofibers for Enhanced Wound Healing. Nano Lett. 2024, 24, 13955–13964. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, J.; Zhou, Y.; Xiang, D.; Luan, Y.; Wang, Q.; Huang, J.; Liu, J.; Yang, X.; Wang, K. Spatially Controlled DNA Frameworks for Sensitive Detection and Specific Isolation of Tumor Cells. Angew. Chem. Int. Ed. 2024, 63, e202411382. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Zeng, L.; Yin, W.; Xu, Y.; Chen, J.; Liu, S.Y.; Zou, X.; He, Z.; Dai, Z. Cell-Selective Encapsulation within Metal–Organic Framework Shells via Precursor-Functionalized Aptamer Identification for Whole-Cell Cancer Vaccine. Small Methods 2022, 6, e2101391. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, S.; Li, Q.; Guo, J.; Li, J.; Qu, F. Probe-Encapsulated and Cell-Membrane-Anchored DNA Nanotoolboxes Enable In Situ Imaging of Extracellular Biochemical Components in Complex Biological Media. Anal. Chem. 2025, 97, 19265–19274. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, Y.; Xue, Y.; Fang, B.; Li, Y.; Zhu, X.; Du, Y.; Peng, P. NETosis-Inspired Cell Surface-Constrained Framework Nucleic Acids Traps (FNATs) for Cascaded Extracellular Recognition and Cellular Behavior Modulation. Angew. Chem. Int. Ed. 2024, 63, e202319908. [Google Scholar] [CrossRef]
- Xie, X.; Ma, W.; Zhan, Y.; Zhang, Q.; Wang, C.; Zhu, H. Methods to Improve the Stability of Nucleic Acid-Based Nanomaterials. Curr. Drug Metab. 2023, 24, 315–326. [Google Scholar] [CrossRef]
- Tu, T.; Huan, S.; Ke, G.; Zhang, X. Functional Xeno Nucleic Acids for Biomedical Application. Chem. Res. Chin. Univ. 2022, 38, 912–918. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, R.; Wen, T.; Qin, Y.; Wang, Y.; Luo, X.; Liu, J.-W. Recent advances in functional nucleic acid decorated nanomaterials for cancer imaging and therapy. Biomed. Pharmacother. 2024, 174, 116546. [Google Scholar] [CrossRef]
- Paludan, S.R. Activation and regulation of DNA-driven immune responses. Microbiol. Mol. Biol. Rev. 2015, 79, 225–241. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Y. Synthetic DNA for Cell-Surface Engineering. Angew. Chem. Int. Ed. 2021, 60, 11580–11591. [Google Scholar]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev Biol. 2020, 36, 191–218. [Google Scholar]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef]
- Marras, S.A.; Gold, B.; Kramer, F.R.; Smith, I.; Tyagi, S. Real-time measurement of in vitro transcription. Nucleic Acids Res. 2004, 32, e72. [Google Scholar] [CrossRef] [PubMed]
- Sago, C.D.; Kalathoor, S.; Fitzgerald, J.P.; Lando, G.N.; Djeddar, N.; Bryksin, A.V.; Dahlman, J.E. Barcoding chemical modifications into nucleic acids improves drug stability in vivo. J. Mater. Chem. B 2018, 6, 7197–7203. [Google Scholar] [CrossRef] [PubMed]
- Urata, H.; Ogura, E.; Shinohara, K.; Ueda, Y.; Akagi, M. Synthesis and properties of mirror-image DNA. Nucleic Acids Res. 1992, 20, 3325–3332. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Ma, W.; Zhan, Y.; Mao, C.; Shao, X.; Lin, Y. Multi-targeted Antisense Oligonucleotide Delivery by a Framework Nucleic Acid for Inhibiting Biofilm Formation and Virulence. Nano-Micro Lett. 2020, 12, 74. [Google Scholar] [CrossRef]
- Kitada, T.; DiAndreth, B.; Teague, B.; Weiss, R. Programming gene and engineered-cell therapies with synthetic biology. Science 2018, 359, eaad1067. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Huang, M.; Jiang, X.; Chen, P.; Guo, Z.; Zhang, K. Nucleic Acid Nanomaterial-Mediated Single-Cell Encapsulation and Its Application. Biosensors 2025, 15, 712. https://doi.org/10.3390/bios15110712
Qiu Y, Huang M, Jiang X, Chen P, Guo Z, Zhang K. Nucleic Acid Nanomaterial-Mediated Single-Cell Encapsulation and Its Application. Biosensors. 2025; 15(11):712. https://doi.org/10.3390/bios15110712
Chicago/Turabian StyleQiu, Yue, Mengyu Huang, Xiaotong Jiang, Peiru Chen, Zhenzhen Guo, and Kaixiang Zhang. 2025. "Nucleic Acid Nanomaterial-Mediated Single-Cell Encapsulation and Its Application" Biosensors 15, no. 11: 712. https://doi.org/10.3390/bios15110712
APA StyleQiu, Y., Huang, M., Jiang, X., Chen, P., Guo, Z., & Zhang, K. (2025). Nucleic Acid Nanomaterial-Mediated Single-Cell Encapsulation and Its Application. Biosensors, 15(11), 712. https://doi.org/10.3390/bios15110712




