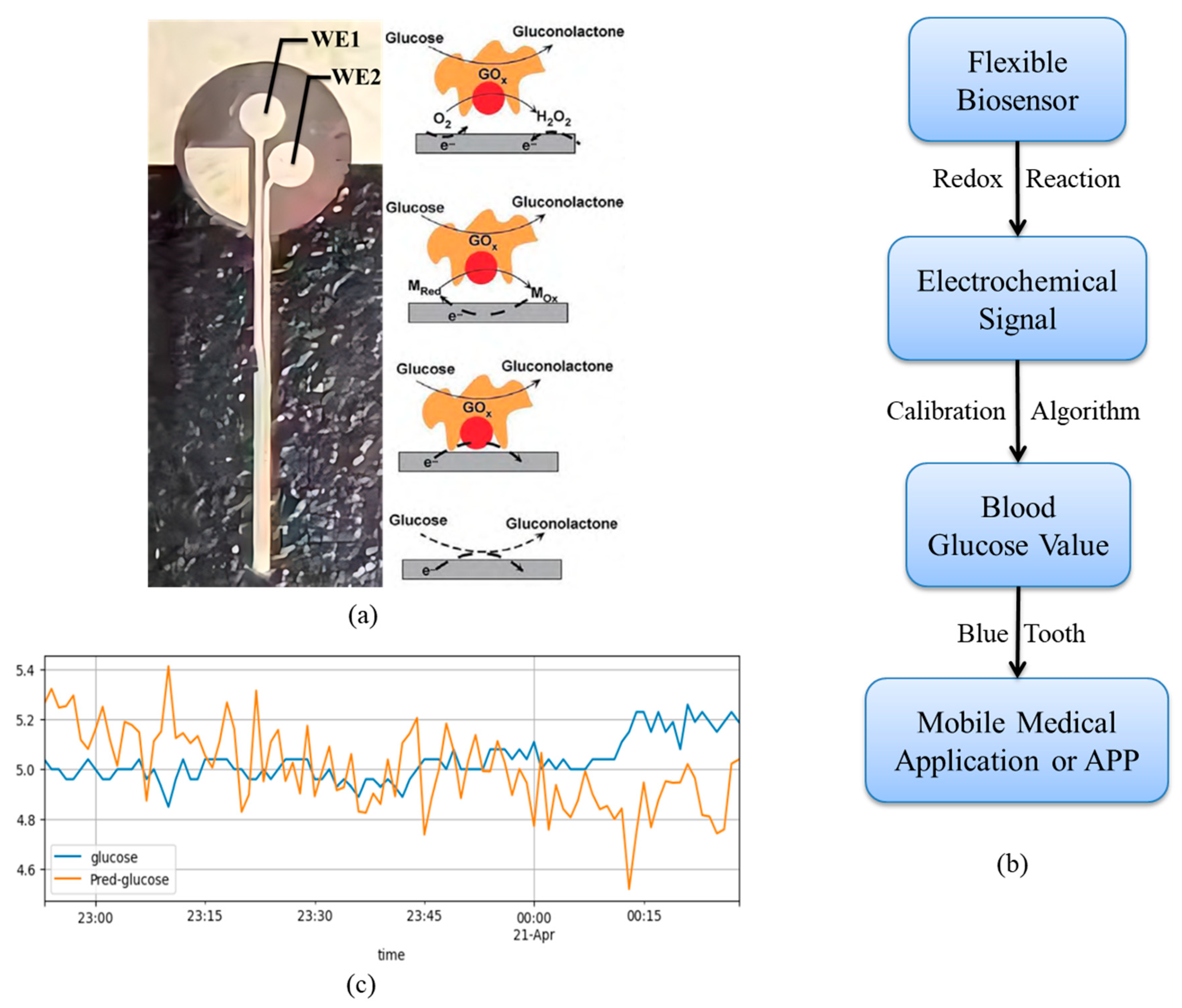
1. Introduction
Diabetes mellitus is a metabolic disorder characterized by persistent hyperglycemia [
1], with its incidence continuing to rise globally. According to a 2021 report by the International Diabetes Federation (IDF) [
2], the number of adults with diabetes worldwide has reached 537 million. In 2021 alone, over 6.7 million people died from diabetes and its complications, accounting for 12.2% of all global deaths.
Blood glucose monitoring is critical in the comprehensive management of diabetes. By implementing glycemic control strategies to manage hyperglycemia, the incidence of diabetes complications can be effectively reduced [
3,
4]. Traditional fingerstick blood glucose monitoring (BGM) is convenient to use but only provides data at a single time point, which may lead to inappropriate treatment decisions [
5]. Continuous glucose monitoring (CGM), however, offers comprehensive, real-time blood glucose data, enabling patients to achieve precise glycemic control [
6]. The American Diabetes Association (ADA) first included CGM in its diagnostic and treatment guidelines in 2023 [
7]. Numerous studies have demonstrated that CGM significantly improves blood glucose control and reduces the risk of complications [
8,
9,
10,
11].
With the widespread adoption of calibration-free technology, there is an increasing demand for stability and accuracy in blood glucose prediction, necessitating algorithm optimization to enable real-time prediction and early warning. Blood glucose prediction relies on CGMS data (interstitial fluid glucose concentration or enzyme electrode current) combined with glucose-influencing factors (diet, insulin, physical activity). However, current algorithms face challenges: heterogeneity in performance across glucose ranges (MARD 8% for hyperglycemia > 180 mg/dL vs. 10% for hypoglycemia < 70 mg/dL) and inter-individual variability due to physiological differences (e.g., metabolic rate affecting glucose transmembrane equilibrium).
Blood glucose prediction can be viewed as a time-series analysis problem. Time-series methods fall into descriptive (data visualization) and statistical paradigms, with the latter including frequency domain analysis [
12] and time domain analysis [
13]. Frequency domain methods (e.g., Fourier analysis [
14], maximum entropy spectral estimation [
15]) have high computational complexity and poor interpretability, while time domain analysis (e.g., AR [
16], ARMA [
17], ARIMA [
18]) has become mainstream due to its intuitive statistical framework.
Although traditional statistical methods have achieved certain results in blood glucose prediction, their linear predictive mechanisms make it difficult to effectively capture nonlinear characteristics during dynamic blood glucose changes. The emergence of machine learning and deep learning technologies has effectively addressed this issue, and these techniques are now extensively applied in medical research [
19,
20,
21,
22,
23].
In the field of blood glucose prediction, traditional machine learning methods such as Support Vector Regression, Random Forests, and Decision Trees [
24] have been widely adopted with significant success. Reference [
25] integrated the XGBoost-enhanced random forest regression model with feature engineering methods to construct a multi-parameter blood glucose prediction system. This model demonstrated outstanding performance on the Ohio T1DM dataset within a 30 min prediction time window, achieving a root mean square error (RMSE) accuracy of 20.38 mg/dL. Reference [
26] developed a prediction framework based on support vector regression. By systematically evaluating the impact of different input combinations on predictive performance, it demonstrated the importance of multi-parameter integration for enhancing prediction accuracy. The results indicated that incorporating all available physiological parameters not only significantly improved short-term prediction accuracy but also markedly enhanced the reliability and safety of most long-term predictions. This finding provides crucial theoretical support for optimizing clinical blood glucose management systems. Furthermore, leveraging the superior performance of deep learning in time-series prediction, Ref. [
27] constructed a deep CNN framework. This demonstrated that deep network architectures possess stronger feature representation capabilities than shallow networks, enabling more precise fitting of complex nonlinear distribution patterns in blood glucose data. Reference [
28] achieved root mean square errors of 20.7 ± 3.2 mg/dL (30 min prediction) and 33.6 ± 3.2 mg/dL (60 min prediction) using a recurrent neural network model on the Ohio T1DM dataset. Reference [
29] achieved superior predictive performance on the same dataset using an artificial neural network architecture, with RMSEs of 19.33 mg/dL and 31.72 mg/dL for 30 min and 60 min predictions, respectively. Comparative analysis indicates that deep learning-based prediction models significantly outperform traditional machine learning methods in predictive accuracy. This performance improvement can be attributed to the enhanced feature extraction capabilities of deep learning models and their effective modeling of temporal dependencies.
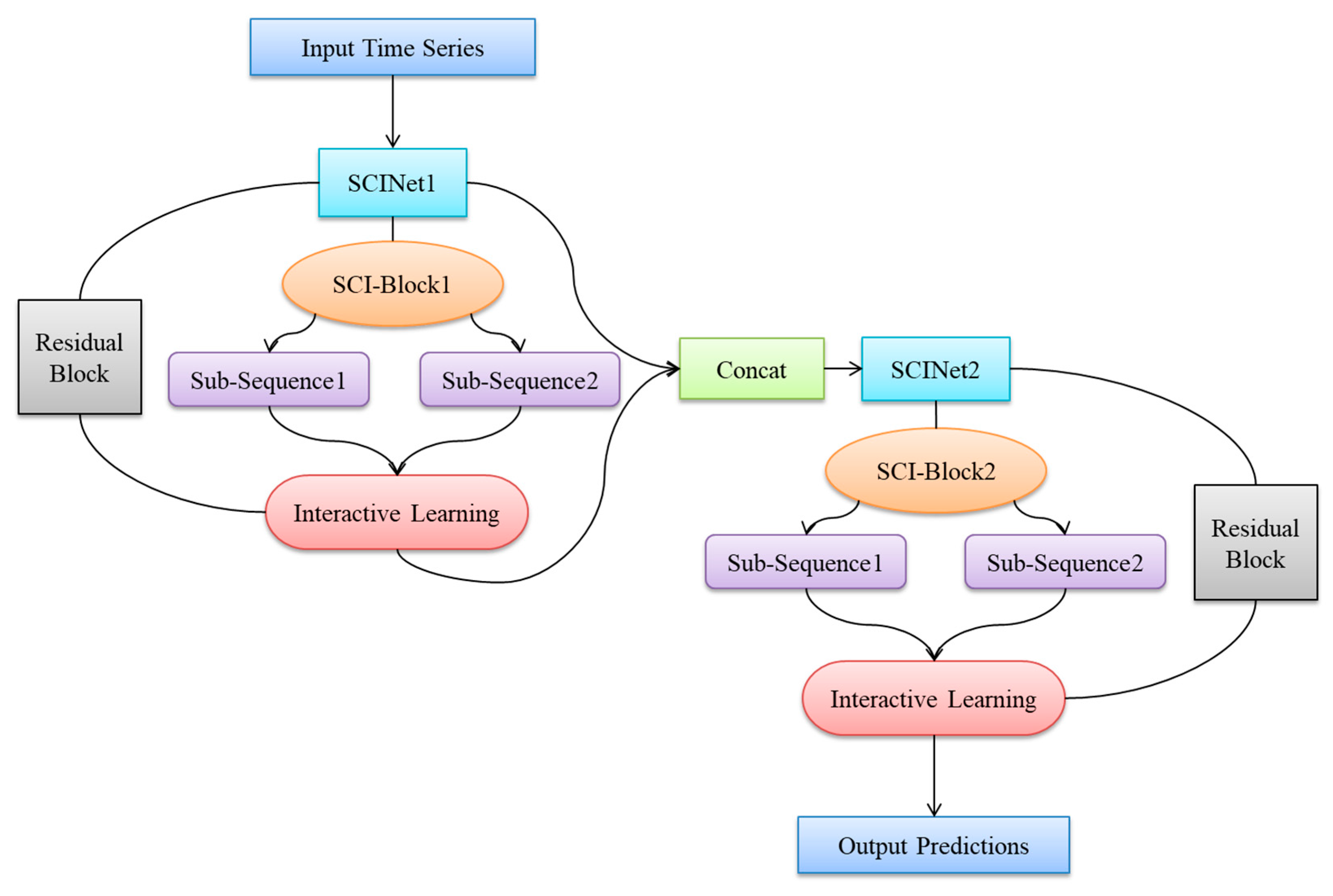
In this paper, addressing the issues of low prediction accuracy, complex input features, and poor generalization performance in existing blood glucose prediction methods, we propose a blood glucose prediction model based on a stacked two-layer Simplified Complex Interval Neural Network (SCINet). First, we theoretically demonstrate its advantages in time-series data prediction. Subsequently, we elaborate on the architectural design of the blood glucose prediction model based on the stacked two-layer SCINet. Subsequently, experiments are conducted using continuous glucose monitoring data from real patients at Yixing People’s Hospital. Before constructing training and testing datasets, the model’s raw input features are optimized through variable selection and data preprocessing. The model is then trained using multiple continuous glucose datasets as the training dataset, and through multiple experiments, it is adjusted to achieve optimal hyperparameter configurations. Finally, the model’s predictive performance was validated on the test dataset and compared against multiple time-series forecasting algorithms. This demonstrated the superiority of the proposed dual-layer SCINet-stacked blood glucose prediction model in terms of prediction accuracy and model complexity. The innovation of this paper is primarily reflected in the following three aspects:
- (1)
First application of a double-layer SCINet stacking architecture for predicting blood glucose levels in hospitalized patients, with optimized residual module and feature interaction strategies;
- (2)
Experiments conducted using real-world hospitalized patient datasets incorporating sensor parameters, addressing the limitations of single-scenario public datasets;
- (3)
The proposed model demonstrates superior predictive performance compared to models based on other time-series forecasting algorithms across various prediction horizons.
3. Experiment
3.1. Dataset
The Ohio T1DM dataset contains only three types of features: blood glucose values, insulin doses, and carbohydrate intake. It does not include performance parameters of the CGM sensors themselves, making it impossible to model the impact of sensor status on blood glucose measurement accuracy. Additionally, the dataset lacks frequent medical interventions during monitoring (e.g., intravenous insulin administration, emergency blood glucose management), and blood glucose fluctuations are relatively mild. This prevents simulation of blood glucose changes under complex interventions experienced by hospitalized patients (e.g., stress-induced hyperglycemia in postoperative patients, hypoglycemia rescue in critically ill patients). The experimental data in this paper were derived from clinical data of actual hospitalized patients at Yixing People’s Hospital (all subjects provided informed consent to address ethical concerns). Data were collected using the Snuo i3 CGM sensor (approved for market release by China’s National Medical Products Administration in March 2023, registration certificate number: Guoxie Zhuanzhun 20233070435). All patients underwent continuous monitoring during hospitalization (April–May 2025):—Daily sampling: 1 reading per minute from 8:00 to 20:00 and every 10 min from 20:00 to 8:00 the following day. Each monitoring session lasted 7–14 days (matching the sensor’s maximum wear duration). During monitoring, medical interventions such as dietary intake (carbohydrate consumption per meal) and insulin injection doses (rapid-acting/long-acting) were recorded, reflecting more complex blood glucose fluctuations in patients. A single fingerstick blood glucose measurement (gold standard) was collected before breakfast daily for CGM data calibration. Post-calibration data achieved MARD < 10%, meeting clinical requirements. Through variable screening, this study incorporated sensor_k, WE1, and Battery Voltage as input features. The sensor performance dimensions listed in
Table 1—sensor_r (electrode linearity coefficient), sensor_k (electrode reaction rate), WE1 (working current), WE2 (contrast current), and Battery Voltage—are not present in the public dataset.
Table 2 presents the statistical characteristics of the subjects.
3.2. Variable Selection
When training the blood glucose prediction model proposed in this paper, blood glucose levels are undoubtedly selected as the sole prediction target. However, we know that human blood glucose levels are influenced by multiple factors, and the interrelationships among these factors are highly complex. If only blood glucose values are used as input features for model training, the model will fail to learn the characteristics of other variables affecting blood glucose levels. Even if the predicted results appear satisfactory, the model will lack interpretability. Furthermore, selecting only one variable other than blood glucose as an input feature would prevent the model from learning the intricate relationships among all variables affecting blood glucose fluctuations. This would not only diminish the model’s predictive accuracy but also limit its applicability to predicting blood glucose levels for a specific subset of individuals. Therefore, to enhance both interpretability and generalization, it is necessary to screen all features listed in
Table 1 and select multiple covariates as input features for the model.
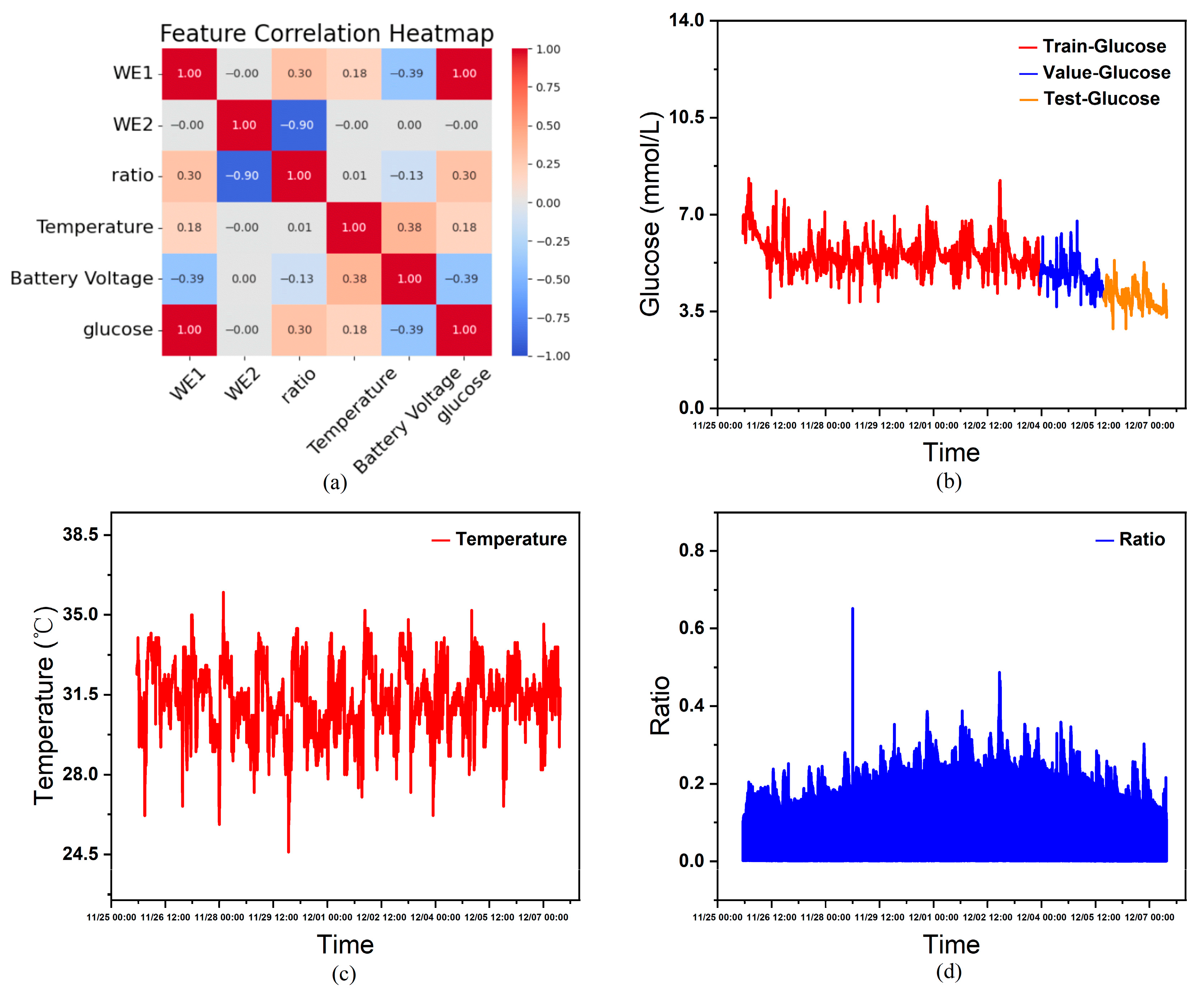
Performing correlation heatmap analysis using the features in
Table 1 as variables, this study excluded constant variables from the analysis since they exert minimal influence on other variables within the single-subject dataset. As shown in
Figure 4a, the correlation heatmap derived from statistical analysis indicates that, except for WE2, all non-constant variables exhibit a certain degree of correlation with blood glucose levels. Furthermore, considering that features such as age, gender, and diabetes type significantly influence human blood glucose fluctuations, although these constant variables cannot aid model training in an individual’s continuous glucose data, when combining continuous glucose data from multiple individuals as the training set, the actual circumstances and CGM devices worn by each person vary. Inputting these characteristics helps the model learn the complex relationships between them and blood glucose fluctuations, thereby influencing the model’s prediction outcomes. Therefore, this paper ultimately selects age, sex (0 = male, 1 = female), type (0 = healthy, 1 = Type 1 diabetes, 2 = Type 2 diabetes), sensor_k, WE1 (nA), Temperature (°C), and Battery Voltage (V) as covariate input features, glucose (mmol/L) as the prediction target, and time as the temporal index.
Figure 4c and
Figure 4d show the curves of temperature and current ratio over time, respectively.
3.3. Data Preprocessing
The collected data, being real clinical data, are susceptible to various objective and subjective factors, such as issues in data recording and transmission, calibration algorithm errors, or human instability. Therefore, the presence of missing values and outliers in the data sample is unavoidable. Regarding missing values, since the model performs time-series predictions, omitting missing values also results in temporal gaps. This omission adversely affects model training. Considering that human blood glucose levels do not undergo drastic changes within short timeframes, this paper employs forward imputation to fill missing values. Considering that overly smoothed blood glucose time-series data may overlook hypoglycemic and hyperglycemic events, while directly learning from completely unfiltered data may lead to inaccurate model predictions, handling outliers requires a balance between these two extremes. Therefore, this paper adopts a filtering approach to process the raw data for outliers, employing a low-pass filter to eliminate outliers from the original data.
Specifically, among 25 patients, 3 cases (1 with type 1 diabetes and 2 with type 2 diabetes) had missing data, resulting in a missing rate of 5.2–7.8% (remaining patients had a missing rate < 2%); Missing values were imputed using the previous value method. After applying a 5 Hz low-pass filter, data completeness reached 98.3%. Outliers (exceeding the 2.8–22.2 mmol/L range) constituted < 0.5% and were excluded. Both processing methods preserved the characteristics of the original blood glucose data while enhancing its smoothness and accuracy to some extent. Inputting this processed data into the model improved the performance of the blood glucose prediction model.
The raw data for each sample is collected with millisecond precision. However, since hospitals primarily receive data via Bluetooth and local area networks, transmission errors inevitably occur during this process, resulting in variations in the time intervals between samples. Given the stringent requirement for temporal continuity in time-series analysis, coupled with significant differences in the data provided by different users, and the fact that each sensor is worn for no longer than 14 days, this study adopts a sampling frequency of one minute. This decision is grounded in practical considerations: human blood glucose levels do not undergo substantial fluctuations within second-long intervals.
Due to variations in patient age, diabetes type, and differences in CGM device sensitivity (sensor_k) and working current (WE1), this study integrates multi-patient data to train a global model. This approach enables the model to learn associations between individual differences and glucose fluctuations, thereby enhancing its generalization capability. For the preprocessed data, the first 70% is used as the training set for the model. Half of the remaining 30% is allocated as the validation set for model training, while the other half serves as the test set after training completion.
Figure 4b illustrates the partitioned continuous glucose data.
3.4. Model Training
Reference [
31] only performs supervised training on the final output layer without constraining intermediate layer features, which may cause the temporal characteristics learned in intermediate layers to deviate from physiological glucose patterns (e.g., predicting blood glucose values beyond clinically reasonable ranges). Therefore, an “intermediate supervision loss” (MAE loss, weighted at 0.3) is added after the first layer output in the dual-layer SCINet, with the final output layer loss weighted at 0.7. This ensures that the short-term features extracted by the first layer align with physiological blood glucose fluctuations (e.g., postprandial blood glucose increase within 15 min not exceeding 5 mmol/L). The total loss function is as follows:
During training, MAE demonstrates greater robustness to outliers in blood glucose data (such as sudden hyperglycemia), preventing extreme values from unduly disrupting model training. It optimizes model parameters by minimizing the loss function. Concurrently, MARD—a commonly used clinical metric mentioned in the introduction—serves as one of the evaluation indicators. Together with MAE, R2, and accuracy, it is employed in the results analysis:
Accuracy rate: Percentage of samples with a difference between predicted and actual blood glucose values < 0.5 mmol/L.
In this case, is the number of samples, is the actual value, and is the predicted value.
The model makes predictions on the validation set and, through multiple iterations, progressively reduces the error between predicted and actual values to bring the predictions closer to the true blood glucose concentrations. The Adam optimizer is employed to update model parameters, enhancing training efficiency and model performance. An early stopping strategy is implemented during training: training ceases when the loss function fails to decrease for 100 consecutive epochs on the validation set, thereby preventing model overfitting. Finally, MARD, R2, accuracy, and MAE are collectively employed for result analysis.
4. Results and Discussion
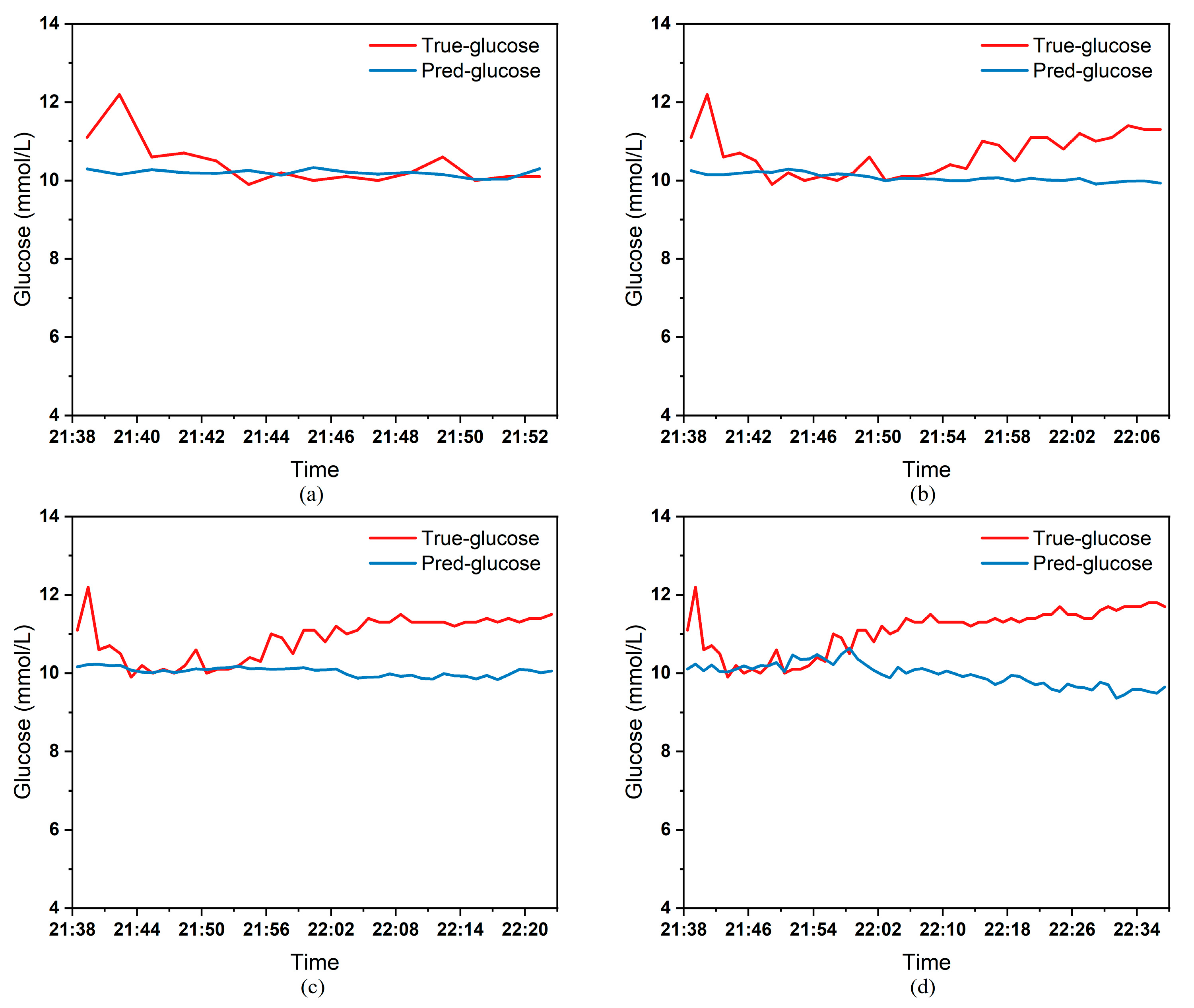
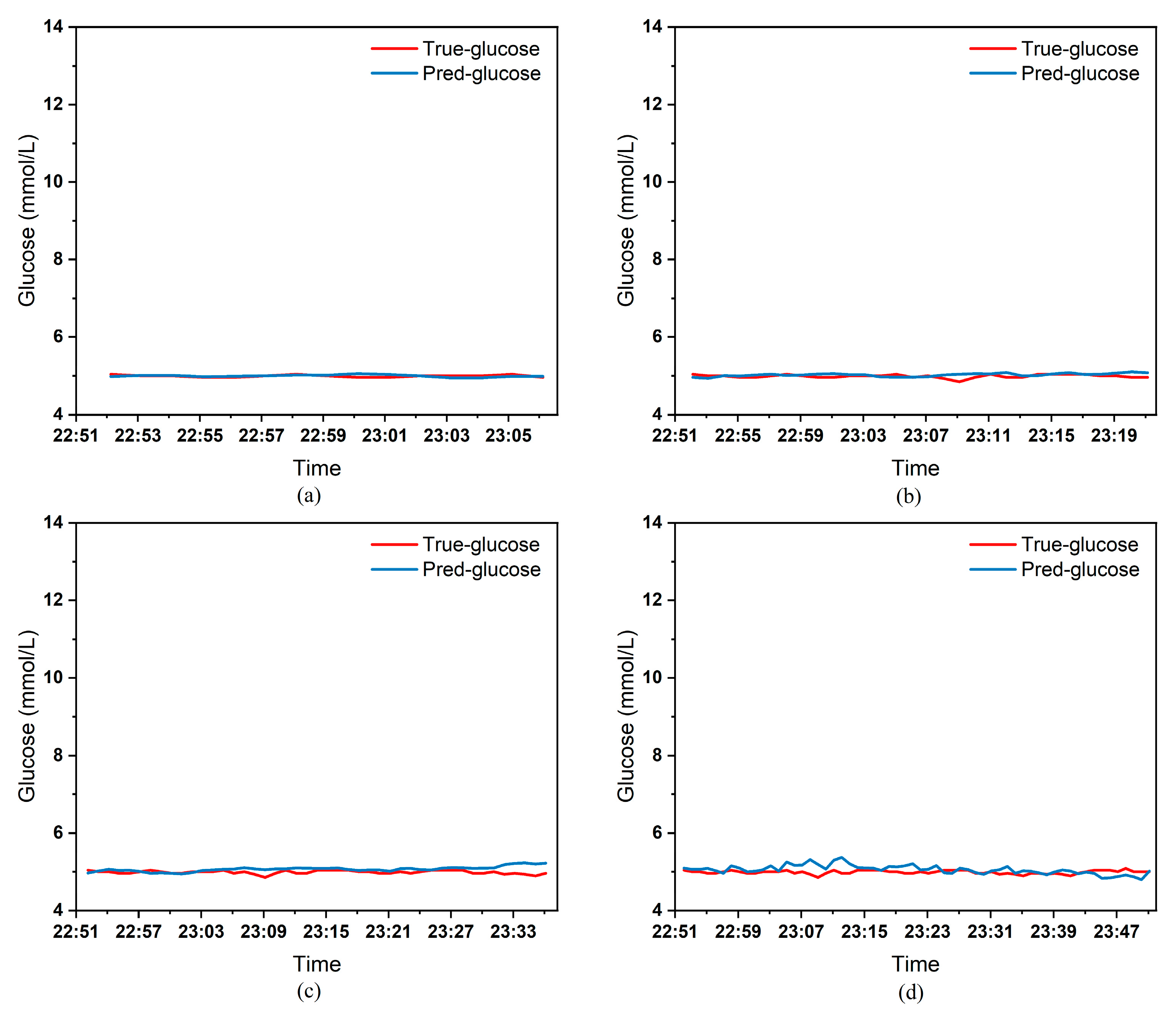
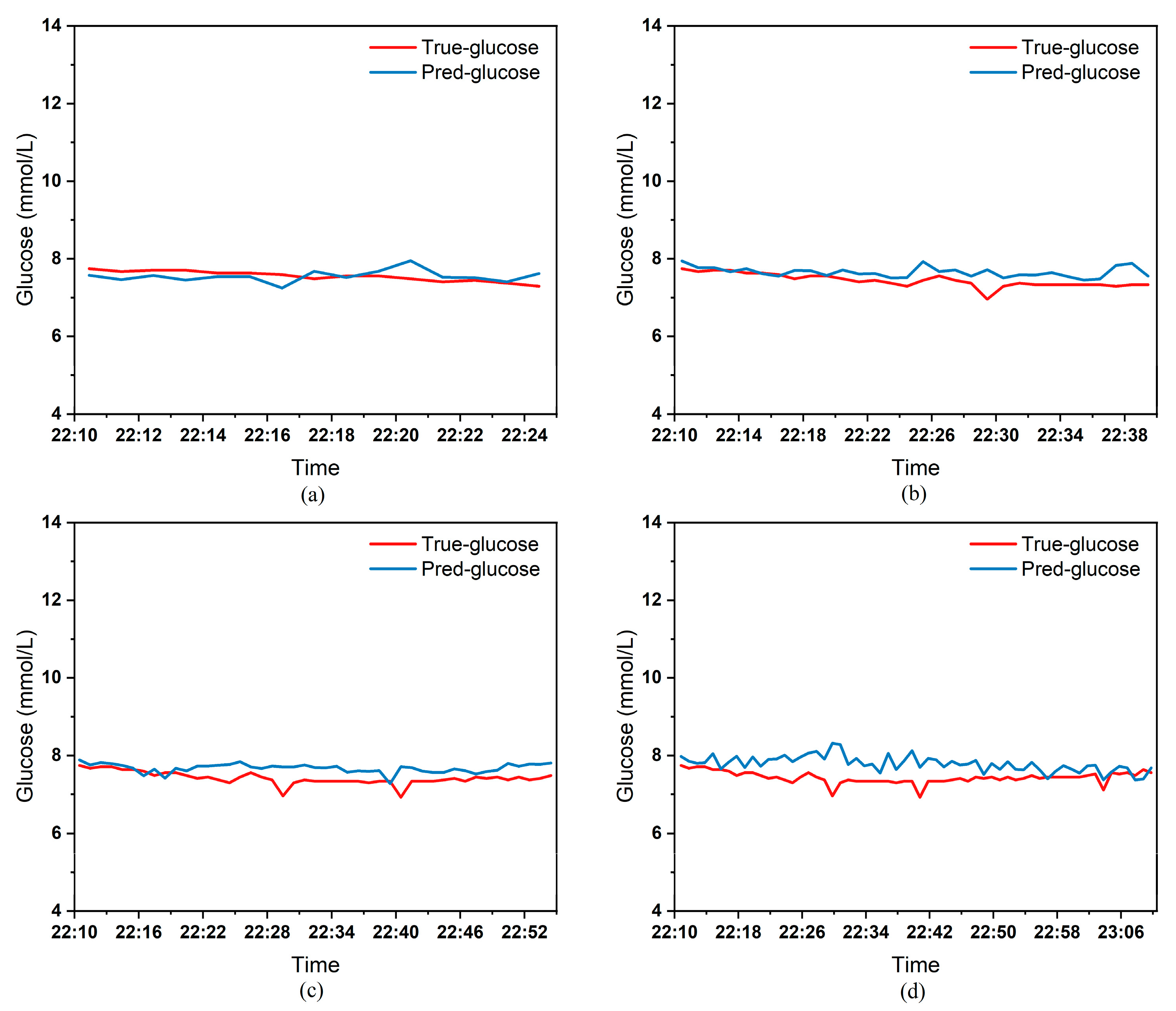
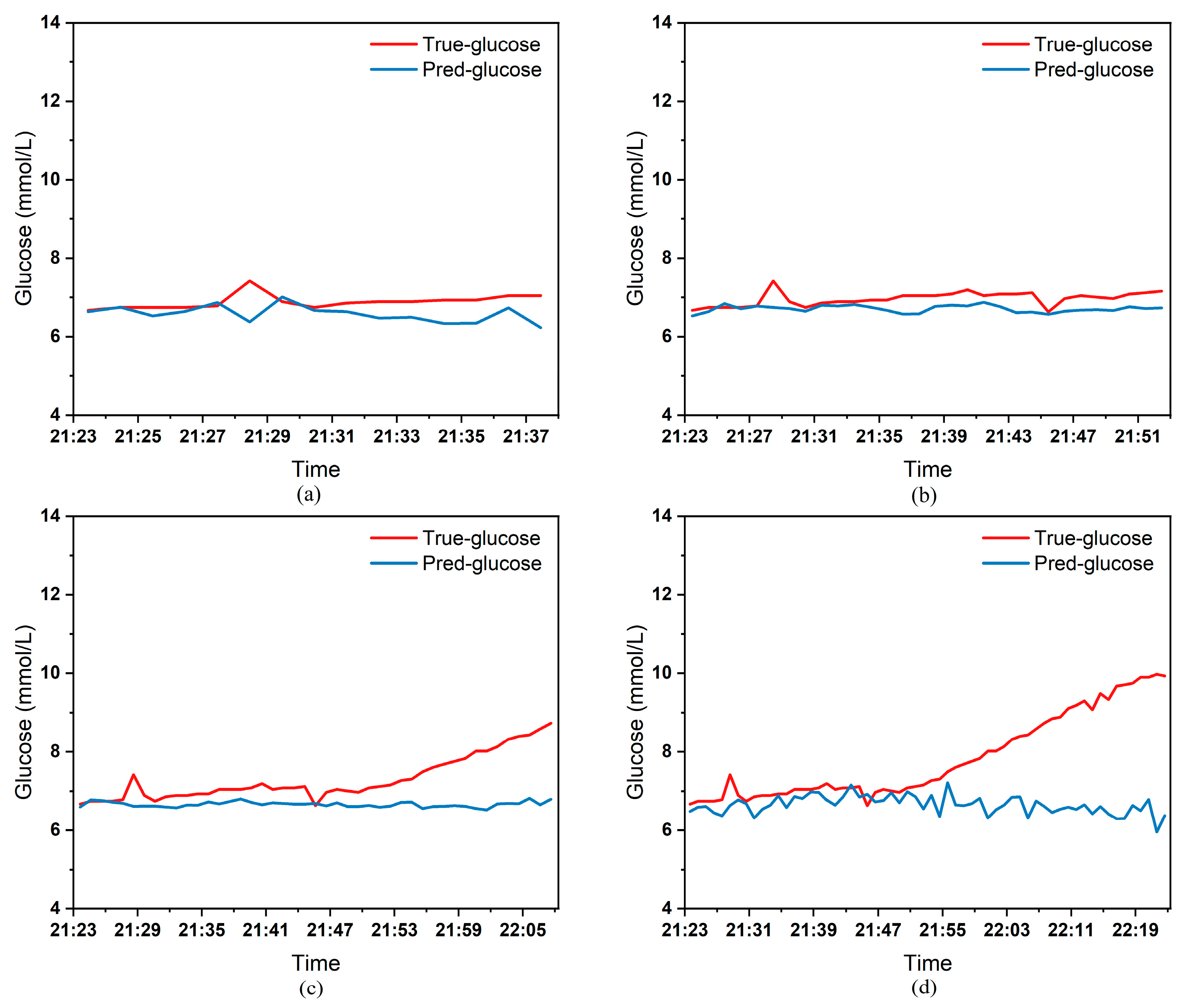
To clearly demonstrate the performance of the proposed model in blood glucose prediction tasks, this paper selected five diabetic patients and plotted comparison graphs of predicted versus actual blood glucose values across different prediction time horizons.
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9, respectively, show the comparison between the predicted glucose curves and the actual glucose curves for patients 16, 18, 20, 22, and 24 over the next 15 min, 30 min, 45 min, and 60 min. The horizontal axis represents time, and the vertical axis represents glucose levels (mmol/L). The figures reveal that within the 15 min prediction window, the model’s predicted curve most closely aligns with the patient’s actual glucose curve. As the prediction time extends, a time-lag error begins to emerge between predicted and actual values, with prediction accuracy gradually declining. By the 60 min prediction window, the model’s predicted curve exhibits the poorest fit with the patient’s actual glucose curve.
The model also exhibits varying performance across different patient datasets. As shown in
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9, the fitting accuracy between the predicted glucose curves and actual glucose curves differs significantly for these five patients under identical prediction durations. For instance, under the same prediction period, patients 18 (
Figure 6) and 24 (
Figure 9) demonstrate substantial lag errors between predicted and actual values, indicating poor alignment between the predicted and actual glucose curves. In contrast, Patient 20 (
Figure 7) exhibits consistently good alignment between the predicted and actual glucose curves across all prediction durations. Macro-level analysis reveals that the overall trends of glucose prediction curves and actual glucose curves are largely consistent across patients. Although micro-level deviations exist, the absolute error between the model’s predicted glucose values and actual values at each time point is not substantial.
Table 3 presents the Mean Absolute Error (MAE) between the model’s predicted blood glucose values and actual values for multiple patients across different prediction durations. In fact, as the prediction duration increases, factors influencing blood glucose fluctuations become more complex and difficult to predict. The model struggles to accurately capture long-term fluctuation trends, leading to a gradual increase in the error between predicted and actual values.
Table 3 clearly demonstrates that within a 15 min prediction horizon, the mean MAE between predicted and actual blood glucose values across multiple patients was only 0.2365 mmol/L—the smallest error among the four prediction horizons. In contrast, at the 60 min prediction horizon, the mean MAE reached 0.9376 mmol/L. Compared to the 15 min prediction results, this indicates that the model achieves superior prediction accuracy within shorter forecasting periods.
Additionally, variations in prediction accuracy across different patients may stem from multiple factors. On one hand, each patient exhibits distinct patterns of blood glucose fluctuations, with some experiencing significantly greater amplitude and frequency of fluctuations than others, which undoubtedly increases predictive complexity. On the other hand, constrained by device limitations and human operational factors, the quality of raw data collected from different patients in this study also varies considerably. A small number of patients exhibited relatively high rates of missing glucose data, which adversely affected the time-series prediction task. These variations in data quality caused the same model to demonstrate differing performance across different patients’ datasets.
Table 3 reveals that across the four prediction time horizons, Patient 19 exhibited the worst model performance among all patients, with a mean MAE of 2.1826 mmol/L between predicted and actual blood glucose values. Conversely, Patient 20 demonstrated excellent model performance, achieving a mean MAE of only 0.0729 mmol/L. Overall, however, the MAE between predicted and actual blood glucose values for each patient across different prediction time horizons was generally maintained below 1.0 mmol/L, sufficiently demonstrating the model’s reliability for blood glucose prediction tasks. (Annotation: True value refers to CGM-calibrated blood glucose, validated by daily fingertip blood tests MARD < 10%.)
Table 4 presents the mean values of the model evaluation metrics mentioned in
Section 3.4, further demonstrating the superiority of the model designed in this paper. To avoid patient identity disclosure, the personalized model was tested on 5 anonymized patients (1 with Type 1 diabetes, 4 with Type 2 diabetes) whose demographic characteristics are consistent with the overall cohort (
Table 2). Their age, BMI, and monitoring duration fall within the 25th–75th percentiles of the total subjects, ensuring representativeness (Their anonymized demographic data are as follows: age range 42–68 years (mean 55.2 ± 9.8), BMI range 23.1–27.8 kg/m
2 (mean 25.3 ± 1.6), average monitoring duration 9.5 ± 1.8 days. These patients were chosen to cover diverse glucose fluctuation patterns (mild/moderate/severe) to verify generalizability.
Table 5 shows the results of the personalized model comparison experiments. The personalized model exhibits superior performance across all prediction durations but requires separate training for each patient. In clinical applications, a trade-off must be made between “prediction accuracy” and “training efficiency.”
The performance improvement of the personalized model is not an isolated case. For the remaining 5 diabetic patients not explicitly shown in
Table 5, the personalized model also reduced the MAE by 15.2–17.5% (15 min: 0.201–0.228 mmol/L → 0.168–0.191 mmol/L; 60 min: 0.892–1.056 mmol/L → 0.735–0.884 mmol/L) and MARD by 0.8–1.6% compared to the global model. This consistency confirms that the personalized model’s advantage in capturing patient-specific glucose patterns is generalizable across diverse patients, rather than limited to individual cases.
This paper also conducted comparative experiments on multiple time-series prediction models, including both AI and non-AI models. The experiments compared the blood glucose prediction results of these models across different time horizons.
Table 6 presents the mean absolute error (MAE) between the predicted and actual blood glucose levels for multiple patients over the next 15, 30, 45, and 60 min. Based on the experimental results presented in
Table 6, the MAE of the model designed in this paper (DS-SCINet) is significantly lower than that of non-AI models, validating the advantages of AI models in blood glucose prediction. Additionally, it can be observed that within the 15 min prediction window, the performance of the blood glucose prediction models based on CNNLSTM and TCN is comparable to that of the model designed in this paper. However, as the prediction time increases, the model designed in this paper continues to maintain a smaller prediction error. This indicates that the model can still fully capture the complex relationship between blood glucose fluctuations and various factors affecting blood glucose changes over longer prediction periods, demonstrating the superiority of the proposed model in terms of prediction accuracy.
The 0.4 mmol/L reduction in MAE (60 min prediction: Kalman filter 1.3254 mmol/L vs. DS-SCINet 0.9376 mmol/L,
Table 6) has tangible clinical significance, particularly in scenarios where precise glucose prediction guides intervention decisions.
First, in hypoglycemia warning (a critical clinical priority), suppose the hospital starts intervention when the patient’s blood glucose is < 3.9 mmol/L. If the actual glucose is 3.5 mmol/L (hypoglycemic), the Kalman filter may predict 3.9 mmol/L (error + 0.4 mmol/L)—failing to trigger timely intervention—while DS-SCINet predicts 3.5 mmol/L (accurate), enabling early action (e.g., consuming 15 g carbohydrates) to prevent severe hypoglycemia (≤3.0 mmol/L), which is associated with seizures or cognitive impairment.
Second, in target glucose control for vulnerable populations (e.g., critically ill patients, pregnant women with gestational diabetes). A 0.4 mmol/L error could misclassify glucose status: if actual glucose is 7.4 mmol/L (mild hyperglycemia), the Kalman filter may predict 7.0 mmol/L (within target, no intervention), while DS-SCINet predicts 7.4 mmol/L (triggering insulin dose adjustment). This avoids delayed correction of hyperglycemia, which is linked to increased infection risk and longer hospital stays. For patients with stable glucose (e.g., 5.0 mmol/L), the 0.4 mmol/L error (5.0 vs. 5.4 mmol/L) may not require immediate intervention, but consistent small errors can accumulate over 6–12 h, leading to suboptimal long-term glycemic control (e.g., HbA1c elevation).
Limitations should be noted: this study’s clinical impact is inferred from error reduction and existing guidelines, not prospective clinical trials. Future work will validate DS-SCINet in a randomized controlled trial (RCT) to measure changes in intervention frequency, hypoglycemia/hyperglycemia incidence, and patient-reported outcomes (e.g., quality of life).
5. Conclusions
This paper introduces a blood glucose prediction model constructed using SCINet. While detailing the model design process, theoretical analysis clarifies SCINet’s significant advantages in handling time-series data. Subsequently, the SCINet architecture undergoes further refinement, leading to the design of a blood glucose prediction model based on a stacked dual-layer SCINet. This enhancement significantly boosts the model’s capability to learn complex nonlinear features in blood glucose data. To validate the proposed model’s performance, continuous glucose monitoring data from real patients at Yixing People’s Hospital were used as the experimental dataset. The dataset was divided into training, validation, and test sets. The model was trained using the training set, with optimal hyperparameters selected through iterative experiments on the validation set. Model evaluation was conducted on the test set. Specifically, correlation experiments were conducted to screen human characteristics captured by CGM sensors, identifying key features influencing blood glucose fluctuations. The selected input features for the model include age, gender, diabetes type, sensor sensitivity, sensor operating current, temperature, battery voltage, and calibrated blood glucose value. Subsequently, various anomalies present in the raw data were appropriately addressed. Next, the model training strategy was briefly outlined. Finally, model performance was evaluated on the test dataset. The experimental results demonstrate that the proposed blood glucose prediction model maintains low prediction errors when forecasting both time-varying glucose fluctuations and glucose changes across different patients. Furthermore, comparative experiments with existing time-series prediction models on the same dataset conclusively show that the proposed model outperforms existing models in both prediction accuracy and stability.